Abstract
An efficient immune response against tumours depends on a well-orchestrated function of integrated components, but is finally exerted by effector tumour-infiltrating lymphocytes (TIL). We have previously reported that homophilic CEACAM1 interactions inhibit the specific killing and interferon-γ (IFN-γ) release activities of natural killer cells and TIL. In this study a model for the investigation of melanoma cells surviving long coincubation with antigen-specific TIL is reported. It is demonstrated that the surviving melanoma cells increase their surface CEACAM1 expression, which in turn confers enhanced resistance against fresh TIL. Furthermore, it is shown that this is an active process, driven by specific immune recognition and is at least partially mediated by lymphocyte-derived IFN-γ. Similar results were observed with antigen-restricted TIL, either autologous or allogeneic, as well as with natural killer cells. The enhanced CEACAM1 expression depends, however, on the presence of IFN-γ and sharply drops within 48 hr. This may be a mechanism that allows tumour cells to transiently develop a more resistant phenotype upon recognition by specific lymphocytes. Therefore, this work substantiates the melanoma-promoting role of CEACAM1 and marks it as an attractive target for novel immunotherapeutic interventions.
Keywords: cell surface molecules, T cells, tolerance/suppression/anergy, tumour immunity
Introduction
The interaction between the immune system and tumours is complex, especially because it may be persistent. This can lead to superimposed effects of selection, cell alterations and acquisition of new attributes that affect the total balance. Delineation of the subsequent effects of persistent tumour–host interactions that favour tumour growth is crucial for the development of novel anti-cancer regimens.
CEACAM1 is a transmembrane glycoprotein that belongs to the carcinoembryonic antigen family (part of the immunoglobulin superfamily), which encompasses several forms of proteins with different biochemical properties, all encoded on chromosome 19.1 The CEACAM proteins share a common basic structure of sequentially ordered, different immunoglobulin-like domain(s). They may appear in two forms of cytosolic tail, a long form containing immunodominant tyrosine-based inhibitory motif (ITIM) and a short form devoid of ITIM.2 These proteins are able to interact with each other.2 For example, CEACAM1 interacts homophilically with CEACAM13,4 and heterophilically with CEACAM5 but not with CEACAM6.5 CEACAM1 is broadly distributed on a variety of cells, e.g. epithelial cells, melanoma and immune cells.1 Many different functions have been attributed to the CEACAM1 protein. The CEACAM1 protein exhibits antiproliferative properties in carcinomas of the colon6 and prostate.7 Additional data support the central involvement of CEACAM1 in angiogenesis,8 metastasis9 and insulin clearance.10 CEACAM1 also has a role in the modulation of innate and adaptive immune responses because it is expressed on some lymphocyte subpopulations, such as CD16– natural killer (NK)11,12 and activated T cells.12 T-cell inhibition through engagement of CEACAM1 has been demonstrated by direct T-cell receptor (TCR) cross-linking13 and via binding of Neisseria opacity associated (opa) proteins.14 The inhibitory effect of CEACAM1 is exerted by the recruitment of Src homology domain 2-containing tyrosine phosphatase-1 (SHP-1) to the cytosolic ITIM sequences.15 Lymphocytes express only the CEACAM1 isoform that bears a long cytosolic tail.11 We have previously shown that the CEACAM1 homophilic interactions16–18 and the CEACAM1 heterophilic interactions with CEACAM519 inhibit NK-mediated killing independently of major histocompatibility complex (MHC) class I recognition. CEACAM1-mediated inhibition of NK cell function was dependent on the cytosolic tail.16 CEACAM1 homophilic interactions seem important in some cases of metastatic melanoma because increased CEACAM1 expression on NK cells derived from patients has been observed compared with cells from healthy donors.16 Indeed, a strong association has been reported between CEACAM1 expression on primary cutaneous melanoma lesions9 or on primary non-small cell lung cancer20,21 and the development of metastatic disease and poor survival.
Attention to treatments based on adoptive cell transfer (ACT) of tumour-infiltrating lymphocytes (TIL) has increased, especially in view of a recent clinical trial of TIL ACT treatment in 35 patients with refractory metastatic melanoma. In this specific study, up to 50% of the patients exhibited a significant clinical response, with some of the patients remarkably achieving a complete response.22 However, although these results are encouraging, TIL-based treatment is still far from its full potential. We have recently demonstrated that CEACAM1 expression on TIL increases following clinical ACT expansion protocols for refractory melanoma patients.23 The combination of CEACAM1 inhibitory effect on TIL,23 increased CEACAM1 expression during expansion protocols23 and common CEACAM1 expression on melanoma16,23 suggests that CEACAM1 homophilic interactions may hinder TIL function in vivo and hence diminish the efficacy of TIL ACT treatments. We have previously reported a preliminary observation that melanoma cells surviving long coculture in vitro with specific TIL express higher levels of CEACAM1, while no similar change could be observed on the surface of the attacking TIL.23
The objective of this study is to characterize the dynamic expression of CEACAM1 by melanoma cells during an ongoing attack by tumour-reactive lymphocytes in vitro and to investigate the underlying mechanisms. We shall demonstrate that CEACAM1 up-regulation by surviving melanoma cells is an active process, driven by specific immune recognition and dependent on lymphocyte-derived interferon-γ (IFN-γ). Enhanced CEACAM1 expression protects the surviving melanoma cells against subsequent attack by fresh lymphocytes. However, it depends on the continuous presence of IFN-γ, because it drops sharply to basal levels following elimination of IFN-γ. Our laboratory findings therefore suggest that melanoma cells can actively develop a transient phenotype of enhanced resistance during lymphocyte-mediated attack by coupling CEACAM1 expression with responsiveness to IFN-γ.
Materials and methods
Cells and media
The TIL clones used in this study were: L2D8 [directed against the gp100-derived peptide 209–217 in complex with human leucocyte antigen (HLA) -A*02] and JKF6 (directed against the MART1-derived peptide 27–35 in complex with HLA-A*02). Human melanoma cell lines were the HLA-A2-positive 526mel (HLA-A*02, -A*03, -B*15, -Cw*03) and 624mel (HLA-A*02, -A*03), the HLA-A2-negative 938mel (HLA-A*0101, -A*2402, -B*0702, -B*0801, -Cw*0701, -Cw*0702) and LB33B1. In addition, the NK92 cell line [American Type Culture Collection (ATCC), Manassas, VA] and 721.221 Epstein–Barr virus-transformed B-cell lymphoma (ATCC) were used. Melanoma cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Minneapolis, MN) supplemented with: 10% heat inactivated fetal calf serum; 1 mm penicillin–streptomycin; 1 mm non-essential amino acids; 1 mm l-glutamine and 1 mm sodium pyruvate (all from Gibco). The NK92 cell line was grown as suggested by the ATCC. Complete RPMI-1640 (Gibco) medium included 10% heat inactivated fetal calf serum, 1 mm penicillin–streptomycin, 1 mm non-essential amino acids, 1 mm l-glutamine and 1 mm sodium pyruvate.
Primary melanoma and TIL bulk cultures
Primary melanoma cultures were developed from specimens as previously described,24 and included the MEL05 cells (HLA-A*29, -B*1402, -B*3502, -Cw*04, -Cw*0802) and MEL09 cells (HLA-A*02, -A*26, -B*51, -B*38, -Cw*14, -Cw*12). Primary TIL bulk cultures were obtained from surgically excised melanoma specimens and included: TIL001, TIL01, TIL02, TIL05F1, TIL05F10 and TIL09. The TIL bulk cultures were grown as formerly reported.24 TIL05F1 and TIL05F10 served as reactive TIL in assays against autologous MEL05 melanoma cells and TIL09 against autologous MEL09 cells. TIL001, TIL01 and TIL02 do not recognize any of the melanoma cells used in this study and have therefore been used as inert TIL controls for specificity. These TIL, however, can be stimulated with non-specific TCR stimulation (data not shown).
Antibodies
The following purified monoclonal antibodies (mAbs) were used: Kat4c mAb directed against human CEACAM1, -3, -5, -6, -8 (Dako, Glostrup, Denmark), NC8 mAb directed against human CEACAM1 [kind gift of Dr Miguel Lopez-Botet, Universitad Pompeu Fabra (DCEXS), Barcelona, Spain], rabbit anti-human polyclonal anti-CEACAM (Dako), control immunoglobulin G (IgG) (Serotec, Oxford, UK), pan anti-human MHC class I mAb W6/32, anti-human CD99 mAb 12E7 and mAb anti-human IFN-γ (BD Pharmingen, San Diego, CA). The conjugated mAbs used in this work were: fluorescein isothiocyanate (FITC)-conjugated Kat4c (Dako), phycoerythrin (PE)-conjugated anti-MCSP (Miltenyi Biotec, Bergisch-Gladbach, Germany), allophycocyanin-conjugated anti-CD56 (R&D Systems, Minneapolis, MN) and biotin-conjugated anti human IFN-γ (Pharmingen). Secondary antibodies included FITC-conjugated goat anti-mouse IgG F(ab′)2 (ICN, Irvine, CA) and PE-conjugated goat anti human IgG (Jackson Immunoresearch, West Grove, PA).
Immunoglobulin fusion proteins
The CEACAM1–immunoglobulin fusion proteins (kind gift of Dr Ofer Mandelboim, Lautenberg Centre for General and Tumour Immunology, Hadassah Medical School, Jerusalem, Israel) were produced and utilized according to a previous report.17 Briefly, the complementary DNA of the extracellular portion of each receptor was amplified by polymerase chain reaction and cloned in frame into a vector containing the Fc portion of human immunoglobulin. Constructs were then transfected into COS-7 cells. The immunoglobulin fusion proteins were purified on a protein G column, divided into aliquots, stored at −20° and routinely analysed for degradation by sodium dodecyl sulphate–polyacrylamide gel electrophoresis.
Long coculture assays and treatment with recombinant IFN-γ
Long coculture assays were performed in 48-well plates. Twenty thousand melanoma cells were allowed to adhere firmly to the well for 4 hr in complete RPMI-1640. Media were removed and 50 000 TIL, clones or bulk cultures, were added in 600 μl complete medium per well. Plates were cultured in a humidified 5% CO2 incubator for 4 days. Monitoring of cells and supernatants was conducted daily; therefore, multiple well replicates for each time-point were prepared in advance at day 0. Once a day, culture medium containing both supernatants and floating cells was collected from all designated well replicates and centrifuged. The IFN-γ was quantified in the supernatants and cell phenotype was determined by fluorescence-acitvated cell sorting (FACS). The remaining adherent cells were gently washed with phosphate-buffered saline, trypsinized and analysed by FACS. Adherent cells were comprised chiefly of viable melanoma cells, with less than 10% non-viable cells, and were therefore considered as ‘surviving cells’. Floating cells, on the other hand, were comprised chiefly of viable TIL and some melanoma cells that were mostly propidium iodide (PI)-positive. Surviving melanoma cells could be easily differentiated from residual TIL in flow cytometry based on forward scatter (FSC) over side scatter (SSC) parameters, as verified by staining for the melanoma-specific antigen MCSP and CD3.23 The presence of various markers on surviving cells was expressed as the ‘Ratio’ of staining median fluorescence intensity (MFI) on surviving cells divided by the MFI of the same staining on the same cells cultured in the absence of TIL. In conditioned medium experiments, the expression ratio of various molecules was calculated by dividing the MFI of cells incubated in conditioned medium or control conditioned medium by cells incubated in regular culture medium. Neutralizing anti-IFN-γ mAb was used in a final concentration of 10μg/ml. The concentration of recombinant IFN-γ (Chemicon International, Temecula, CA) included in the culture did not exceed 50 ng/ml.
Cytotoxicity assays and quantification of IFN-γ secretion
Cytotoxicity assays were performed by PI costaining of carboxy-fluorescein diacetate succinimidyl ester (CFSE)-labelled target cells; 5 × 106 target cells were labelled with 2·5 μm CFSE, which strongly labels cells and allows differentiation between target and effector cells. Ten thousand labelled target cells were coincubated in 96 U-shaped microplates with given amounts of effector cells for 5 hr in a humidified 5% CO2 incubator. Killing rate was assessed by the percentage of PI costaining cells out of gated CFSE-labelled cells. In all experiments the percentage of PI-positive CFSE-labelled cells in wells cultured without effectors did not exceed 15%. The amount of IFN-γ in supernatants was evaluated by standardized enzyme-linked immunosorbent assay (R&D Systems).
Generation of CEACAM1-silenced melanoma cells
Generation of CEACAM1-silenced melanoma cells was carried out using commercially available small interfering RNA (siRNA) target sequences cloned in the MISSIOM™ short hairpin RNA system (lentiviral plasmids pLKO.1-puro) (Sigma-Aldrich, Rehovot, Israel) and lentiviral expression system. Viral particles were produced by transient liposome-mediated cotransfection (FuGENE6, Roche, Indianapolis, IN) of 293T cells with the vectors pCMVΔ8.9, pVSVG and each vector containing the designated siRNA sequence (Sigma-Aldrich). Culture medium was replaced after 24 hr with fresh complete RPMI-1640 medium, and incubated for an additional 24 hr. The medium containing viral particles was collected and filtered through a 0·45-μm syringe filter. Infection was carried out using the filtered medium along with 1 μg/ml polybren on melanoma cells. Virus-containing medium was removed after 6 hr and replaced with fresh medium. Double infections were performed by generation of single-clone viral particles in a concentrated volume, and introduced together onto the target cells. This procedure was performed twice, on sequential days. Selection medium of 0·5 μg/ml puromycin, in complete RPMI-1640, was introduced 3 days after infection. Cells were grown in selection medium for at least 2 weeks, before being used for experiments. The sequence of the scrambled control siRNA was not specified by the manufacturer. The following are the names of the clones and their respective accession numbers as specified by the manufacturer (Sigma-Aldrich): Clone ID: NM_001712.2-881s1c1 Accession Number(s): NM_001024912.1, NM_001712.3; Clone ID: NM_001712.2-757s1c1 Accession Number(s): NM_001024912.1, NM_001712.3; and Clone ID: NM_001712.2-1248s1c1 Accession Number(s): NM_001024912.1, NM_001712.3. The DI #1 cells were generated by transfection with the sequences 881 and 757. The DI #2 cells were generated by transfection with the sequences 881 and 1248. The control RNA cells were generated by transfecting twice with the scrambled sequence.
Results
Melanoma cells surviving TIL-mediated attack increase the expression of CEACAM1 and MHC class I but not CD99
We have previously reported that CEACAM1 expression is up-regulated on 526mel melanoma cells surviving attack by a MART1-specific TIL clone (JKF6), while it remains unaltered on the JKF6 cells.23 To further investigate this phenomenon we expanded the experimental setup to include melanoma cell lines (HLA-A2-positive 526mel cells and HLA-A2-negative 938mel cells) and TIL clones (HLA-A2-restricted L2D8 or JKF6 clones and HLA-mismatched TIL001, all from allogeneic sources), as well as primary melanoma (MEL05) and TIL cognate cultures (TIL05F1 or TIL05F10). Analysis included MHC class I and CD99 in addition to CEACAM1. Supernatants, floating cells and adherent cells were collected daily from designated wells for analysis. The experimental methodology is detailed in the Materials and methods section.
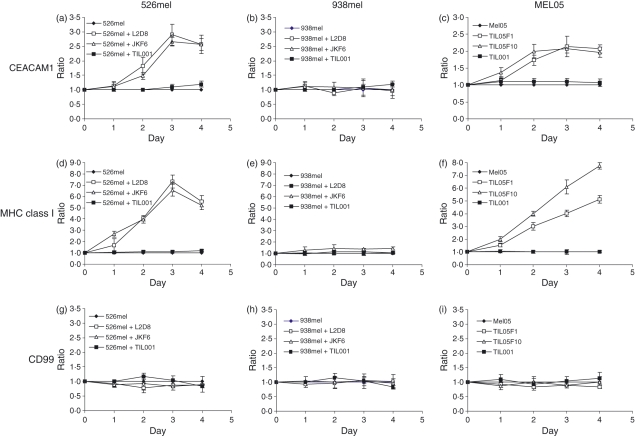
A three-fold increase in the CEACAM1 expression ratio (from baseline MFI of 100 to around 300) was observed on the surface of 526mel cells surviving specific attack by reactive L2D8 or JKF6 TIL clones on day 3 (Fig. 1a). In another study that used renal cell carcinoma with allogeneic CD8 cells a similar observation was reported.25 No similar phenomenon could be observed when the 526mel cells were coincubated with the inert TIL001 cells (Fig. 1a). Therefore, specific recognition is probably required, and the possibilities of metabolic stress as a result of the mere presence of TIL or of independent non-TCR-mediated interactions are not favoured as alternative explanations. Supporting this suggestion, when the HLA-A2-negative 938mel cells (MFI of CEACAM1 expression baseline = 35), which are not recognized by the L2D8, JKF6 or TIL001 cells, were used, the CEACAM1 expression ratio did not change (Fig. 1b). Importantly, a two-fold enhancement in the CEACAM1 expression ratio (from a baseline MFI of 45 to around 90) was also observed in primary MEL05 cells surviving specific attack by the cognate reactive TIL05F1 or TIL05F10 cells, but not with the inert TIL001 (Fig. 1c). It is still impossible to conclude at this stage whether this occurrence represents the selection of melanoma cells with stronger CEACAM1 expression or whether the expression pattern of CEACAM1 is actively altered by the surviving cells.
Figure 1.
CEACAM1 expression is increased on melanoma cells surviving specific tumour-infiltrating lymphocyte (TIL)-mediated attack. Melanoma cells lines 526mel and 938mel were cocultured for 4 days either with reactive JKF6 or L2D8 TIL clones or with inert TIL001 cells. Primary MEL05 cells were cocultured for 4 days either with reactive TIL05F1 or TIL05F10 or with inert TIL001 cells. Every day, the surviving melanoma cells were isolated and analysed by flow cytometry for expression CEACAM1 (a–c), major histocompatibility complex (MHC) class I (d–f) or CD99 (g–i). Discrimination of melanoma cells from remnants of TIL was verified by costaining for MCSP (MCSP-positive cells). The y-axis represents the ratio of expression, which was calculated daily as [mean fluorescence intensity (MFI) of a given staining on surviving melanoma cells] / (MFI with the same monoclonal antibody on melanoma cells cultured without TIL). Background was a ratio of 1. The figure shows the mean results of five independent experiments.
A similar pattern of increased expression ratio on melanoma cells surviving specific TIL-mediated attack was observed with MHC class I. The ratio of MHC class I expression was increased by up to seven-fold with similar kinetics over time on the surface 526mel cells incubated with reactive L2D8 or JKF6 cells but not with inert TIL001 cells (Fig. 1d). No similar phenomenon could be documented when the HLA-A2-negative 938mel cells were used (Fig. 1e). MHC class I up-regulation was observed with primary MEL05 cells surviving specific attack by the autologous TIL05F1 or TIL05F10 cells (Fig. 1f). The increased expression ratios of CEACAM1 and MHC class I on surviving melanoma cells did not seem to be incidental because no significant change in the expression ratio of CD99 could be seen in any of the settings (Figs 1g–i). These observations suggest that this process is driven by the specific recognition of melanoma cells by TIL. Similar results were observed with similar kinetics with another melanoma cell line and reactive or inert TIL (data not shown).
There is no correlation between TIL killing activity and CEACAM1 enhancement by surviving melanoma cells
Two main explanations may account for this immune recognition-dependent phenomenon: (1) selection of specific melanoma subpopulations that express higher levels of CEACAM1, (2) active up-regulation. During the long coincubation setup described above, the killing and IFN-γ secretion activities of both L2D8 and JKF6 were tested daily. Both TIL clones exhibited specific secretion of IFN-γ in the presence of 526mel but not 938mel (Fig. 2a,c). Surprisingly, however, a considerable difference in the killing activity was observed, as L2D8 displayed only moderate cytotoxic activity around 10%, while JKF6 demonstrated strong cytotoxic activity of more than 30% (Fig. 2b and d). Importantly, there were no significant differences in the enhancement patterns of CEACAM1 or MHC class I on 526mel cells surviving either one of the TIL clones (Fig. 1a,d). The specificity of L2D8 and JKF6 was verified because minor or no killing or secretion of IFN-γ was observed in the presence of 938mel cells (Fig. 2a–d). This could imply that killing activity may have less significance in causing up-regulation of CEACAM1 and MHC class I on surviving 526mel cells.
Figure 2.
Enhanced CEACAM1 expression correlates with cytokine secretion but not with killing activity. Various tumour-infiltrating lymphocytes (TIL; indicated in the left) were cocultured either with cognate melanoma cells or with irrelevant melanoma cells for 4 days. The amount of interferon-γ (IFN-γ) secreted into the medium (a,c,e) and TIL killing activity (b,d,f) were monitored daily, as well as the expression ratios of CEACAM1, major histocompatibility complex (MHC) class I and CD99 on surviving MEL09 melanoma cells (g). The expression profile on surviving 526mel cells is described in Fig. 1(a,d,g). Background is a ratio of 1. The figure shows one representative experiment out of five performed.
Therefore similar experiments were conducted with another primary TIL bulk culture (TIL09) against autologous MEL09 cells. In this cognate pair, a prominent killing activity could be seen (Fig. 2f), but no IFN-γ could be detected (Fig. 2e). Specificity of TIL09 was verified against HLA-mismatched 526mel cells because no effector functions were identified (Fig. 2e,f). In this case, however, despite the efficient recognition and killing activity exhibited by TIL09, no change either in the pattern of CEACAM1 or MHC class I could be revealed among the surviving MEL09 cells (Fig. 2g). Similar results were obtained with additional bulk cultures (data not shown). These results show that immune recognition of the melanoma cells was mandatory for enhancement of CEACAM1 and MHC class I on surviving cells (Figs 1 and 2). However, there was no correlation between killing activity and this occurrence (Fig. 2b and d; Fig. 1a and d; Fig. 2e–g). It seems that under these conditions a simple selection process of melanoma subpopulations with higher CEACAM1 expression by killing does not play a significant role. Nevertheless, it implies that effector functions other than cytotoxicity may play a role in enhancing CEACAM1 and MHC class I on surviving melanoma cells. Indeed, this was observed only when IFN-γ was secreted, as shown by L2D8 (Fig. 2a) and JKF6 (Fig. 2c), but not by TIL09 (Fig. 2e).
Conditioned medium causes up-regulation of CEACAM1 on melanoma cells
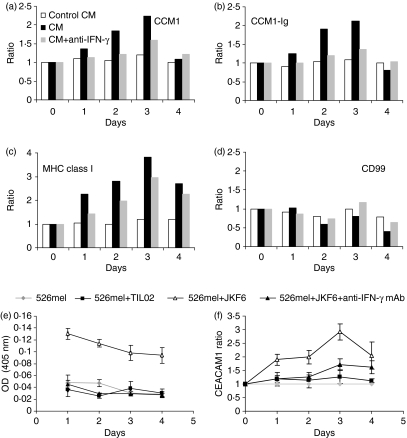
Since enhancement of CEACAM1 depends on immune recognition (Fig. 1) and is probably independent of killing activity (Fig. 2), we hypothesized that secreted proteins might induce CEACAM1 expression. Therefore, conditioned media were collected on the first or second days of long coculture experiments of either reactive JKF6 cells (CM) or inert TIL001 cells (Control CM) with 526mel cells. Fresh 526mel cells were cultured for 4 days either with fresh medium, CM or Control CM. Melanoma cells were stained daily for the expression of CEACAM1, MHC class I and CD99, as well as for CEACAM1-binding capacity with the CEACAM1–immunoglobulin fusion protein. Strikingly, the staining ratios of CEACAM1, CEACAM1–immunoglobulin and of MHC class I on 526mel cells cultured in CM increased over time, reaching a maximum on day 3 (Fig. 3a–c). The Control CM had little or no effect (Fig. 3a–c). Importantly, the CD99 ratio did not change significantly, attesting to the specificity of CEACAM1 and MHC class I up-regulation (Fig. 3d). Similar results were gathered with CM generated via the coincubation of other reactive TIL, such as TIL05F1 or TIL05F10 with the cognate MEL05 cells, but not of TIL09 with MEL09 (data not shown). This demonstrates that a soluble factor derived from lymphocytes following specific target recognition induces CEACAM1 and MHC class I expression, but not CD99. Indeed, dilution of CM with Control CM abolished the induction of CEACAM1 and MHC class I expression. The induction of CEACAM1 was completely eliminated when CM was diluted to 20% whereas some increased expression ratio of MHC class I was still evident even when CM was diluted to 3% (data not shown). Incubation of TIL or clones in the presence of CM did not change CEACAM1 expression (data not shown) nor did it alter TIL cytotoxic or IFN-γ secretion activities (data not shown).
Figure 3.
Interferon-γ (IFN-γ) is responsible for the up-regulation of CEACAM1 on melanoma cells. 526mel cells were incubated only with conditioned medium of 526mel incubated with inert TIL001 cells (Control CM – white bars), or of 526mel incubated with JKF6 cells with or without neutralizing anti-IFN-γ monoclonal antibody (CM alone – black bars or CM plus anti-IFN-γ– grey bars). The expression of various molecules was tested, as indicated in each graph (a–c). (d) The CEACAM1 binding capacity to CEACAM1-Ig. The y-axis shows the expression ratio as detailed in the Materials and methods section. The figure shows one representative experiment out of four performed. (e–f) A prolonged coincubation of 526mel cells either alone or with inert TIL02 cells, or with reactive JKF6 cells in the presence or absence of neutralizing anti-IFN-γ monoclonal antibody, was performed. (e) The neutralization of IFN-γ in the culture medium as measured by enzyme-linked immunosorbent assay and (f) shows the abrogation of enhanced CEACAM1 expression ratio on surviving cells. Error bars represent deviation within the experiment. The figure shows one representative experiment out of three performed.
IFN-γ is responsible for up-regulation of CEACAM1 on surviving melanoma cells
The findings described above, including the coupling of CEACAM1 induction with MHC class I (Fig. 1), which is up-regulated by IFN-γ,26 suggest that this process is triggered by IFN-γ. Indeed, when a neutralizing anti-IFN-γ mAb was added to the CM, both induction of CEACAM1 expression and binding capacity were considerably impaired (Fig. 3a,c– grey bars). Induction of MHC class I was impaired as well, but to a lesser extent (Fig. 3b). The expression of CD99 did not change in the presence of CM with or without anti-IFN-γ (Fig. 3d). These results show that IFN-γ is responsible for up-regulation of CEACAM1 and concur with previous reports regarding the regulation of CEACAM1 expression by IFN-γ.25,27
Next, we neutralized IFN-γ during the long coculture assays. 526mel cells were incubated either alone, with inert TIL02 cells or with reactive JKF6 cells in the presence or absence of anti-IFN-γ mAb. Daily analysis of supernatants revealed the specific secretion of IFN-γ from reactive JKF6 cells, which could not be detected when the inert TIL02 cells were used (Fig. 3e). Notably, no IFN-γ could be detected in the supernatants of the 526mel cells cocultured with JKF6 cells in the presence of anti-IFN-γ mAb (Fig. 3e). A striking eradication of the up-regulation of CEACAM1 on 526mel cells surviving JKF6 was observed when anti-IFN-γ mAb was included in the assay (Fig. 3f). These results provide evidence that the specific secretion of IFN-γ from TIL is responsible for this phenomenon.
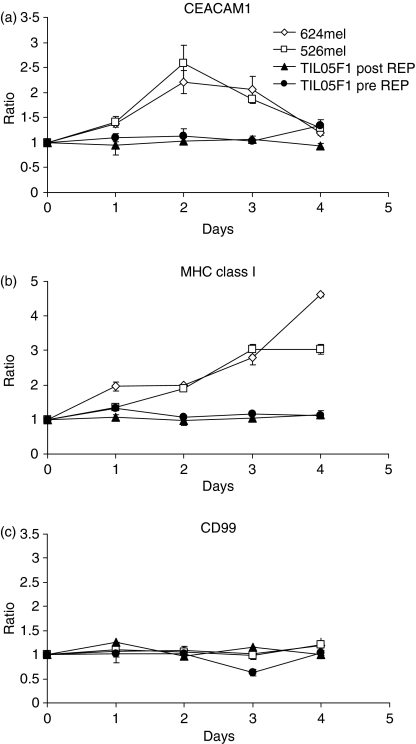
Further, addition of purified recombinant human IFN-γ to various melanoma cell lines, such as 526mel and 624mel, resulted in increased CEACAM1, but not CD99, expression even in the absence of TIL (Fig. 4a,c). The MHC class I up-regulation was observed as expected (Fig. 4b). Purified recombinant human IFN-γ was also added to isolated primary TIL cultures before rapid expansion (TIL05F1 pre-REP) or after rapid expansion (TIL05F1 post-REP). Surprisingly, however, neither CEACAM1 nor MHC class I was induced even in the presence of high IFN-γ concentrations of 50 ng/ml (Fig. 4a–c).
Figure 4.
Recombinant interferon-γ (IFN-γ) induces functional CEACAM1 up-regulation on melanoma cells but not on tumour-infiltrating lymphocytes (TIL). Various melanoma cell lines (526mel, 624mel) or TIL, pre- or post-rapid expansion protocol, were incubated for 4 days in the presence of 50 ng/ml recombinant IFN-γ. The expression analysis of various molecules was tested over time, as indicated above each graph. The y-axis shows the expression ratio as detailed in the Materials and methods section. The figure shows one representative experiment out of four performed.
CEACAM1 is up-regulated on melanoma cells following natural killer-mediated attack
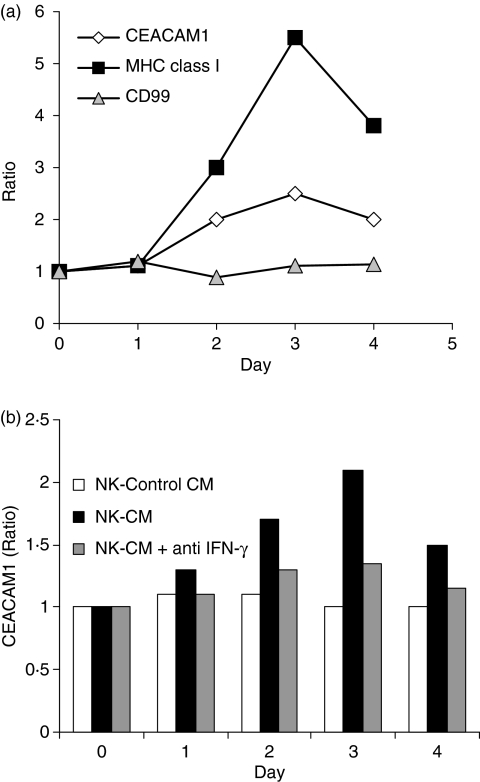
Natural killer (NK) cells are a major source of IFN-γ, which is secreted upon interaction with tumours and virus-infected cells.28 We utilized the NK92 cell line, which has intact cytotoxic and IFN-γ-release activities, in long coculture assays. LB33melB1 cells, which display some degree of susceptibility to NK cells,29 were used as target cells. Long coculture assays were performed as described above. The surviving adherent melanoma cells were stained daily either for CEACAM1, MHC class I or CD99 with concomitant costaining for CD56 to allow differentiation of residual NK92 cells (CD56-positive) from the surviving melanoma cells (CD56-negative, data not shown). The ratio of expression was calculated as described above. Remarkably, both expression ratios of CEACAM1 and MHC class I were increased on the surface of the LB33melB1 (Fig. 5a). There was no significant change in the ratio of CD99 (Fig. 5a). Furthermore, conditioned medium from NK cells coincubated with non-melanoma cells such as 721.221 B-lymphoma cells induced similar CEACAM1 expression on melanoma cell lines such as 526mel (Fig. 5b), 938mel and MEL05 (data not shown). Experiments with conditioned media and neutralizing anti-IFN-γ mAb, similar to those described above, have yielded similar results, demonstrating that this occurrence is also attributed to NK-derived IFN-γ (Fig. 5b). Specific recognition of tumour cells by any effector lymphocyte with the capability to secret IFN-γ may therefore result in up-regulation of CEACAM1 on the surviving cells.
Figure 5.
CEACAM1 expression is increased on melanoma cells that survive natural killer (NK)-mediated attack. (a) A prolonged coincubation of LB33mel.B1 with the NK92 cell line was performed. Discrimination of melanoma cells from remnants of NK92 cells was verified by costaining for CD56 (CD56-negative cells). The expression analysis of various molecules was tested over time, as indicated above each graph. The y-axis shows the expression ratio as detailed in the Materials and methods section. The figure shows one representative experiment out of three performed. (b) 526mel cells were incubated in the conditioned medium of NK92 and NK-sensitive 721.221 cells (NK-CM) with or without blocking anti-interferon-γ (IFN-γ) monoclonal antibody. The conditioned medium of NK92 and NK-resistant 938mel (NK-Control CM) was used as control. The y-axis shows the expression ration of CEACAM1 and the figure shows one representative experiment out of three performed.
Enhanced CEACAM1 expression on surviving melanoma cells depends on the continuous presence of IFN-γ
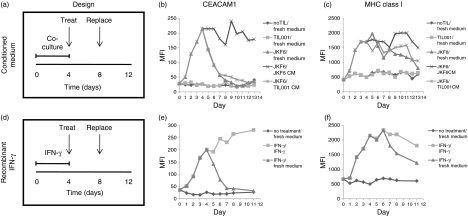
To test the persistence of enhanced CEACAM1 expression, 526mel melanoma cells were cocultured for 4 days either with JKF6, inert TIL001 or without TIL. The surviving melanoma cells were isolated after the fourth day, washed thoroughly and re-seeded in new culture plates either in fresh medium, conditioned medium of JKF6 and 526mel (CM) or conditioned medium of TIL001 and 526mel (Control CM). After an additional 4 days, half of the medium was carefully replaced with fresh medium of the same type (Fig. 6a, experimental design). Cells were analysed daily for expression of CEACAM1 and MHC class I during the first 4 days of coculture as well as during the additional 9 days after re-seeding (Fig. 6b,c). The basal expression level of CEACAM1 and MHC class I was determined before the coculture. As expected, a significant up-regulation in the expression of both CEACAM1 and MHC class I was observed during coculture only with JKF6 (Fig. 6b,c). The enhanced CEACAM1 expression documented on melanoma cells that survived coculture with JKF6 declined by 70% within 2 days after re-dispersion and incubation in fresh medium or Control CM, and returned to baseline over an additional 2 days (Fig. 6b). Enhanced CEACAM1 expression persisted only in CM (Fig. 6b). In contrast, high levels of MHC class I were maintained for 9 days in fresh medium or Control CM, although a gradual decrease could be observed starting from day 8 (Fig. 6c). Enhanced MHC class I expression continued in the presence of CM and remained high after day 8 as well (Fig. 6c). No significant changes could be observed in CEACAM1 or MHC class I expression levels in control cells over time (Fig. 6b,c). CM induced expression of CEACAM1 and MHC class I on melanoma cells that were not pre-exposed to JKF6 (data not shown). Monitoring of cell density revealed that all cells exposed to either JKF6 cells or to CM were growth inhibited but viable (data not shown). This concurs with the already described attributes of IFN-γ on cell cycle arrest.30,31
Figure 6.
Enhanced CEACAM1 expression on surviving cells is dependent on the continuous presence of interferon-γ (IFN-γ). (a) Experimental design of kinetics after coculture includes: Time (in days); ‘Treat’ refers to isolation, washing and re-seeding of surviving cells in designated media; ‘Replace’ refers to replacement of half of the medium with fresh medium of the same kind. (b,c) Expression analyses of CEACAM1 (b) and major histocompatibility coplex (MHC) class I (c) in surviving cells isolated at each day. Each type of treatment includes the conditions of coculture followed by the kind of medium in which surviving cells were re-seeded, as indicated in the legend, and separated by a slanted line. (d) Experimental design of kinetics after incubation with recombinant IFN-γ includes similar components as (a). (e,f) Expression analysis of CEACAM1 (e) and MHC class I (f) in cells isolated at each day. Composition of each treatment is similar to that in (b,c). The figure shows one representative experiment out of three performed.
A similar experimental design was employed with recombinant IFN-γ. Melanoma cells were incubated in culture medium with or without recombinant IFN-γ for 4 days. Afterwards, cells were washed thoroughly and re-seeded in new culture plates in the presence or absence of IFN-γ for a further 7 days. Half of the medium was replaced with fresh appropriate medium 4 days after re-seeding (Fig. 6d). An expected up-regulation in both CEACAM1 and MHC class I expression levels was documented (Fig. 6e,f). However, CEACAM1 expression rapidly declined after IFN-γ was washed, at a rate of a 70% decrease during the first 2 days and a return to basal levels after two more days (Fig. 6e). The declining rate of CEACAM1 was similar to the rate observed following washout of the CM (compare with Fig. 6b). In the continuing presence of IFN-γ, CEACAM1 expression was even further increased (Fig. 6e). In contrast, enhanced MHC class I expression was persistent for the first 2 days after removal of IFN-γ (Fig. 6f). Then, a slow decrease over the next 5 days was measured, but the expression level was still clearly above basal MHC class I levels (Fig. 6f). Concentration of IFN-γ in the medium did not exhibit a significant decrease over time (data not shown). Notably, a slight decrease in CEACAM1 and MHC class I expression in the presence of CM was observed on day 8, and was followed by an increase at day 9 (Fig. 6b,c). However, half of the medium was replaced at day 8 so this observation is probably related to the general culture conditions.
These results show that enhanced CEACAM1 expression depends on the continuous presence of IFN-γ, as opposed to a more stable enhancement of MHC class I.
Increased CEACAM1 expression on surviving melanoma cells contributes to enhanced resistance to specific TIL-mediated killing
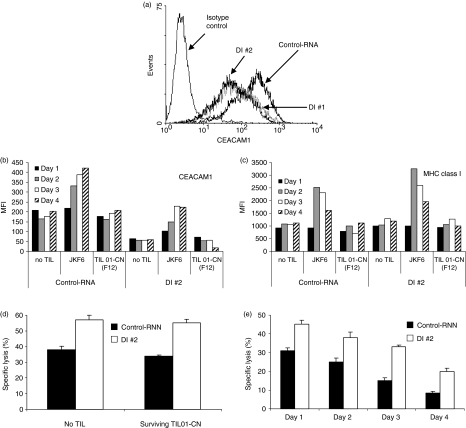
To assess the protective contribution of CEACAM1 to surviving melanoma cells, we generated 526mel cells that stably express siRNA targeted at CEACAM1, as described in the Materials and methods section. Several different target sequences have been selected for silencing of CEACAM1. Figure 7(a) shows CEACAM1 expression in two different representative clones (DI #1 and DI #2), each transfected with different target sequences, as compared to 526mel cells transfected with scrambled control sequence (Control-RNA).
Figure 7.
Increased CEACAM1 expression contributes to enhanced resistance. Stable transfection of 526mel cells with small interfering RNA for CEACAM1 (DI #1 and DI #2) or scrambled (Control-RNA) were generated as described in the Materials and methods section. (a) Relative CEACAM1 expression on Control-RNA cells, DI #1 and DI #2 cells as compared to isotype control staining; (b,c) prolonged coincubation of melanoma cell lines Control-RNA or DI #2 was performed either alone or with reactive JKF6 or inert TIL01 cells. Surviving cells were analysed for the expression of CEACAM1 (b) or major histocompatibility complex (MHC) class I (c) over time. The y-axis shows the mean fluorescence intensity (MFI) of each staining; (d–e) Control-RNA and DI #2 melanoma cell lines surviving either TIL01-CN or no tumour-infiltrating lymphocytes (TIL) (d) or JKF6 (e) were isolated and tested in cytotoxicity assays against fresh JKF6 cells; (d) shows the results only for day 1. Error bars represent deviation within the experiment. Differences were statistically significant in all tests with P < 0·01 between the killing of Control-RNA and DI #2. The figure shows one representative experiment out of five performed.
Various 526mel transfectants were divided into two groups: (1) prelabelled with CFSE or (2) no pre-label. Each group was concomitantly coincubated with reactive JKF6 cells, inert TIL01-CN cells or in the absence of any TIL for 4 days. Reactivity of TIL was monitored by the detection of IFN-γ in the culture supernatant (data not shown). Surviving cells were obtained daily from all experimental setups. CEACAM1 and MHC class I expression were monitored as described above using the unlabelled cells. As observed in other experiments, both CEACAM1 and MHC class I expression increased only in the presence of reactive JKF6 cells (Fig. 7b,c). Importantly, although initial CEACAM1 expression in silenced 526mel cells was lower, the CEACAM1 up-regulation rate on surviving cells was similar to the rate observed with Control-RNA cells (Fig. 7b). Maximal CEACAM1 intensity on surviving DI #1 and DI #2 cells was around 50% of the Control-RNA cells (Fig. 7b). CEACAM1 silencing did not affect MHC class I expression or up-regulation on surviving cells, as compared to the Control-RNA cells (Fig. 7c). The presence of the inert TIL01-CN cells had little or no effect (Fig. 7b,c). There were no changes in the expression of CD99 (data not shown).
The surviving CFSE-labelled 526mel cells were isolated and tested for susceptibility to fresh JKF6 in killing assays. The protective effect of CEACAM1 was evident by the enhanced susceptibility of DI #2 cells, as compared to the Control-RNA cells, to elimination by JKF6 (Fig. 7d). There was no significant difference between melanoma cells that were incubated alone and melanoma cells that survived incubation with inert TIL01-CN cells (Fig. 7d). Similar results were gathered daily throughout the long coincubation experiment (data not shown). Remarkably, Control-RNA cells that survived JKF6 gradually became more resistant to killing by fresh JKF6 cells (Fig. 7e). Therefore, the contribution of CEACAM1 to resistance could not be calculated by simply comparing the susceptibility to fresh TIL of melanoma cells that survive JKF6 to melanoma cells that survive inert TIL01-CN or no TIL. The surviving DI #2 cells displayed similar behaviour, as they also gradually became more resistant (Fig. 7e). Importantly, in all cases the DI #2 cells were more efficiently killed than Control-RNA cells (Fig. 7e, P < 0·01). This shows that the intensity of CEACAM1 expression affects tumour protection. The resistance to killing by fresh JKF6 cells increased over time, as indicated by the Relative Resistance Index (RRI; Table 1). Interestingly, there was no significant difference between Control-RNA and DI #2 cells in the rate at which the RRI increased (Table 1). It seems that in this specific model the feature of tumour cells becoming more resistant during attack occurs at a certain rate. The rate of this process is independent of CEACAM1 initial expression level (Fig. 7a,b, Table 1). Yet, the rate at which CEACAM1 is increased on Control-RNA or DI #2 surviving cells is similar (Fig. 7b), and correlates with the rate of RRI increment. The increasing resistance of surviving cells to fresh TIL may be attributed to several mechanisms on top of increased CEACAM1 expression. Quantification of secreted IFN-γ concentrations was performed concomitantly with the cytotoxicity assays and demonstrated similar results (data not shown). Comparable results were also obtained with DI #1 cells, which diminished the possibility of a non-specific epiphenomenon (data not shown). These results clearly indicate that tumour cells actively become more resistant during specific attack by reactive lymphocytes. This may be attributed to a combination of putative mechanisms, including CEACAM1 expression level, that directly affect resistance.
Table 1.
Increasing resistance of surviving cells to attack by fresh JKF6 cells
| Day | 526mel/Control-RNA | 526mel/DI #2 | ||
|---|---|---|---|---|
| RRI JKF6 | RRI TIL01 | RRI JKF6 | RRI TIL01 | |
| 1 | 0·24 | 0·00 | 0·24 | 0·02 |
| 2 | 0·32 | 0·0 | 0·31 | 0·04 |
| 3 | 0·53 | 0·06 | 0·45 | 0·00 |
| 4 | 0·78 | 0·11 | 0·65 | 0·04 |
Table presents the Relative Resistance Index (RRI) of 526mel cells stably transfected either with scrambled sequence (Control-RNA) or with small interfering RNA to CEACAM1 (DI #2). Melanoma cells that survived prolonged incubation either with TIL01 or with JKF6 were further tested at each time-point in a cytotoxicity assay against fresh JKF6 cells. RRI represents the relative resistance to killing of melanoma cells surviving tumour-infiltrating lymphocytes (TIL) compared to normal melanoma cells and was calculated as follows: 1 – [(fresh JKF6 killing rate of melanoma cells precultured with JKF6 or with TIL01)/(fresh JKF6 killing rate of melanoma cells cultured without any TIL)]. The increase in RRI of melanoma cells in the presence of JKF6 was statistically significant every day as compared to the day before (P < 0·05). There was no statistically significant difference in parallel RRI between 526mel/Control-RNA and 526mel/DI #2 cells (P = 0·17). The table shows one representative experiment out of three performed.
Discussion
Tumour transformation occurs continuously in vivo so hosts are equipped with various monitoring mechanisms, including the immune system. Normally, immunosurveillance rapidly leads to elimination of the transformed cells, but once tumour masses form, the interrelationship with the immune system changes substantially. The interactions become prolonged and tumours can impose local immunosuppressive conditions,32,33 effectively evade immunosurveillance34–38 and even launch counterattacks on tumour-infiltrating lymphocytes.32,33,39,40 Yamshchikov et al.41 demonstrated ‘immune editing’ by the tumour and the consequent adaptation of the dominant immune response in vivo in a long-term survivor of melanoma. Nevertheless, immunotherapy still holds great promise for melanoma therapy. It seems that a new generation of cell-based therapies may encompass the cutting edge of future immunotherapy. However, relapse is not uncommon even following impressive shrinkage in tumour load after TIL treatment. Indeed, one of our patients (05-KG) (whose TIL and cognate melanoma cells were used in this work), exhibited just such a dramatic course, as massive lysis of all evident tumour (lung, mediastinal lymph nodes, liver and spleen) was observed following treatment with TIL, to remarkably achieve complete response. Unfortunately, however, 2 months afterwards the disease recurred with an aggressive variant and the patient died despite maximal treatment within an additional month. Many reasons may account for this event, such as immune selection or tumour cell adaptation.
In recent years we have discovered and described an inhibitory mechanism of effector functions of NK cells5,16,17 and TIL23 via CEACAM1. Further, we have shown that clinical preparation protocols of TIL for ACT result in the up-regulation of CEACAM1 on the surface of transferred TIL over time.23 The interactions between TIL and melanoma cells in vivo are prolonged so we have hypothesized that melanoma cells may actively manipulate CEACAM1 expression to evade ongoing TIL-mediated elimination. We developed an in vitro simulation of prolonged interactions by coincubating TIL with melanoma cells for several days and focused on the surviving melanoma cells. Comprehensive experimentation shows that CEACAM1 is gradually up-regulated on melanoma cells that survive in vitro attack by reactive lymphocytes. This effect is directly related to the attack by the TIL, because prolonged coincubation of melanoma cells with inert mismatched TIL failed to induce a similar effect. The effect was observed with established TIL clones or with patient-derived primary bulk TIL cultures, either autologous or allogeneic (partially matched HLA haplotype and antigens), as well as with established melanoma cells lines and primary cultures. Although a selective process cannot be entirely excluded, we have positively identified IFN-γ derived from the attacking immune cells as a causative factor for CEACAM1 up-regulation on surviving melanoma cells. Furthermore, when cytotoxic TIL that lack IFN-γ secretion capability were used, we failed to select for a melanoma subpopulation that expresses higher CEACAM1 levels (Fig. 2).
Interferon-γ is an effector protein of the immune system that is secreted chiefly by NK cells, T helper type 1 cells and cytotoxic T lymphocytes.42 It exhibits pleiotropic effects and alters the expression of multiple gene families.30 Secretion of IFN-γ is believed to create an anti-tumour milieu because it inhibits the growth of many tumour cell types,31 induces proapoptotic death signals,43 hinders angiogenesis44 and polarizes immune response towards a T helper type 1 response.45 In this study we demonstrate that surviving melanoma cells couple the response to IFN-γ, an offensive immune agent, with active up-regulation of a tumour-defensive mechanism to become more resistant to further attack by lymphocytes. These results are in accordance with previous works regarding regulation of CEACAM1 expression by IFN-γ.25,27 Harnessing of host-derived agents by tumours is observed for example in extracellular matrix remodelling and angiogenesis, where the secreted factors are common to both cancer cells and TIL.46 It should be noted that CEACAM1 expression could not be induced on CEACAM1-negative melanoma by IFN-γ (data not shown). The enhanced CEACAM1 levels closely depend on the continuous presence of IFN-γ, as opposed to the more stable enhanced expression of MHC class I, even after IFN-γ has been removed (Fig. 6). Indeed, following elimination of IFN-γ, CEACAM1 expression dramatically falls by approximately 50% approximately every 24 hr. It has been reported by others that IFN-γ regulates CEACAM1 at the transcriptional and posttranscriptional levels.25,27
In conclusion, we demonstrate that melanoma can transiently acquire an enhanced CEACAM1 phenotype, as long as IFN-γ is present. This may allow melanoma cells to ‘sense’ that they are under lymphocyte-mediated attack, and actively respond accordingly. On the other hand, CEACAM1 may potentially transduce signals into the melanoma cells with a yet to be determined cellular effect. Indeed, it has been reported that CEACAM1 may exert some antiproliferative properties in other cell systems.6,7 Therefore, the close dependence on the presence of IFN-γ limits CEACAM1 enhancement to the relevant time of attack only.
The TIL did not up-regulate CEACAM1 when exposed to IFN-γ, either during prolonged coincubation or when subjected to the recombinant protein. Indeed, it has been shown that naïve T cells, which are highly responsive to IFN-γ, down-regulate IFN-γ receptor chain 2 when they become effector cells following in vivo activation and antigen-driven expansion.47 Effector cells are submerged in locally high concentrations of IFN-γ so this may be a mechanism by which immune cells avoid the cell-cycle-arresting properties of IFN-γ. Remarkably, the hyporesponsiveness to IFN-γ concomitantly prevents unnecessary up-regulation of CEACAM1 on effector cells, which would have increased the inhibitory effect.
Interestingly, we have observed that the surviving cells become more resistant despite a more prominent up-regulation in MHC class I expression ratio than in CEACAM1 expression ratio. Several explanations may account for this observation. First, HLA allele expression may be differentially affected by IFN-γ, which may result in a more prominent increase in HLA alleles that do not present immunodominant peptides, such as HLA-C, HLA-E and HLA-G. Alternatively, the MHC class I complex is the target for the TCR, but is concomitantly the target for inhibitory receptors of the killer immunoglobulin-like receptor family (KIRs),48 which are also expressed by some T cells.48 Indeed, we have observed some TIL subpopulations that express LIR1, CD94, KIR2DL1 and KIR2DL2 (unpublished results). This may cause an increased flux of inhibitory signals, especially if HLA expression is differentially regulated by IFN-γ. A third explanation would be a shift from the standard proteasome to immunoproteasome, which may change the pool of peptides presented by MHC class I molecules. The induction of tolerogenic pathways such as indoleamine 2,3-dioxygenase (IDO);49 and additional unidentified mechanisms may also be involved in the emergence of resistant variants, such as down-regulation of certain tumour antigens, adhesion molecules etc. These mechanisms may be either driven by random selection or by specific mechanisms, such as other IFN-γ-sensitive genes. Nevertheless, a protective contribution for enhanced CEACAM1 was still evident, which may exist in parallel with other inhibitory and stimulating alterations caused by IFN-γ (Fig. 7). The added protective effect of enhanced CEACAM1 described here is moderate, probably because the CEACAM1 was only partially silenced (50%, see Fig. 7). The inhibitory signal is thought to be delivered into the attacking lymphocytes following homophilic CEACAM1 interactions through the recruitment of SHP-1 phosphatase to the ITIM sequences15 of the long cytosolic tail.11
In summary, in this work we provide mechanistic evidence that CEACAM1 is actively up-regulated in response to IFN-γ on melanoma cells surviving specific lymphocyte-mediated attack to emerge with transiently enhanced resistance. This dynamic evasion mechanism reveals a potential sophisticated tumour strategy that utilizes an offensive agent as a sensor for the presence of nearby immune attack to actively increase the protection of surviving cells. Recurrence of disease following successful immunotherapeutic treatments, as was observed with patient 05-KG, may be at least partially explained by such a mechanism. The in vitro simulation, however, is only a simplistic approximation of a natural process with substantial complexity. Full delineation of the in vivo relevance of this mechanism to disease recurrence for instance, requires further extensive investigation. Several approaches and immunological tools can be employed, such as the murine experimental B16 melanoma model and xenotransplantation of human CEACAM1-positive melanoma and tumour-reactive lymphocytes in immune-compromised mice. Both of them allow in vivo modelling of tumour–immune cell interactions following establishment of subcutaneous or metastatic melanoma masses and transfer of appropriate lymphocytes. A main advantage for the use of B16 would be the availability of IFN-γ knockout mice that can serve either as hosts or as donors for adoptively transferred lymphocytes. However, it remains to be determined whether B16 expresses CEACAM1 and whether it is similarly regulated by IFN-γ. It has already been demonstrated that CEACAM1 inhibits T-cell function in the mouse.15 Vital in vivo imaging technologies, such as the cooled charged-coupled device (CCCD) camera, may be capable of illustrating the spatiotemporal characteristics of this process. A cellular reporter system that reflects CEACAM1 expression intensity in live cells, such as a fusion construct of CEACAM1 promoter and firefly luciferase can be employed. The contribution of this mechanism to enhanced resistance and recurrence of disease can be directly assessed by elimination of the interferon regulatory factor 1 (IRF1)-responsive element in the CEACAM1 promoter in the transplanted melanoma cells. These cells will express CEACAM1, but will be unable to further modulate CEACAM1 expression. In addition, CEACAM1 knockout mice50 and a conditional CEACAM1 deletion model15 have been lately generated by other groups. Utilization of these powerful tools will considerably advance the mechanistic understanding of CEACAM1 involvement in tumour immunobiology. Finally, these findings point to the important role of CEACAM1 in the development of aggressive melanoma and mark it as an attractive target for novel immunotherapy.
Acknowledgments
We are especially grateful to Mr Nechemia and Mrs Chaya Lemelbaum for their massive support, which enabled this research. G.M. is supported by the Talpiot Sheba Researcher-Physician Fund, The Israel Cancer Association through the estate of the late Loewe Minna Margot and the Israel Cancer Research Fund (ICRF). The authors thank Dr Steve Rosenberg for the melanoma cell lines and TIL clones, as well as Dr Tsipi Shoham, Dr Gil Katz and Dr Doron Besser for critically reading the manuscript and for their insightful comments. Also, the authors would like to thank Dr Miguel Lopez-Botet and Dr Ofer Mandelboim for sending valuable reagents.
References
- 1.Beauchemin N, Draber P, Dveksler G, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–9. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 2.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumour marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–34. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 3.Oikawa S, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Homotypic and heterotypic Ca2+-independent cell adhesion activities of biliary glycoprotein, a member of carcinoembryonic antigen family, expressed on CHO cell surface. Biochem Biophys Res Commun. 1992;186:881–7. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- 4.Watt SM, Teixeira AM, Zhou GQ, et al. Homophilic adhesion of human CEACAM1 involves N-terminal domain interactions: structural analysis of the binding site. Blood. 2001;98:1469–79. doi: 10.1182/blood.v98.5.1469. [DOI] [PubMed] [Google Scholar]
- 5.Markel G, Gruda R, Achdout H, et al. The critical role of residues 43R and 44Q of carcinoembryonic antigen cell adhesion molecules-1 in the protection from killing by human NK cells. J Immunol. 2004;173:3732–9. doi: 10.4049/jimmunol.173.6.3732. [DOI] [PubMed] [Google Scholar]
- 6.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–90. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- 7.Busch C, Hanssen TA, Wagener C, OBrink B. Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum Pathol. 2002;33:290–8. doi: 10.1053/hupa.2002.32218. [DOI] [PubMed] [Google Scholar]
- 8.Volpert O, Luo W, Liu TJ, Estrera VT, Logothetis C, Lin SH. Inhibition of prostate tumour angiogenesis by the tumour suppressor CEACAM1. J Biol Chem. 2002;277:35696–702. doi: 10.1074/jbc.M205319200. [DOI] [PubMed] [Google Scholar]
- 9.Thies A, Moll I, Berger J, et al. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol. 2002;20:2530–6. doi: 10.1200/JCO.2002.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Poy MN, Yang Y, Rezaei K, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet. 2002;30:270–6. doi: 10.1038/ng840. [DOI] [PubMed] [Google Scholar]
- 11.Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–74. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Moller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65:740–5. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Morales VM, Christ A, Watt SM, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a) J Immunol. 1999;163:1363–70. [PubMed] [Google Scholar]
- 14.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrest the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–36. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 15.Nagaishi T, Pao L, Lin SH, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–81. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Markel G, Lieberman N, Katz G, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–10. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 17.Markel G, Wolf D, Hanna J, et al. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest. 2002;110:943–53. doi: 10.1172/JCI15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markel G, Mussaffi H, Ling KL, et al. The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood. 2004;103:1770–8. doi: 10.1182/blood-2003-06-2114. [DOI] [PubMed] [Google Scholar]
- 19.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, Mandelboim O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 20.Laack E, Nikbakht H, Peters A, et al. Expression of CEACAM1 in adenocarcinoma of the lung: a factor of independent prognostic significance. J Clin Oncol. 2002;20:4279–84. doi: 10.1200/JCO.2002.08.067. [DOI] [PubMed] [Google Scholar]
- 21.Sienel W, Dango S, Woelfle U, et al. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res. 2003;9:2260–6. [PubMed] [Google Scholar]
- 22.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markel G, Seidman R, Stern N, et al. Inhibition of human tumour infiltrating lymphocytes (TIL) effector functions by the homophilic carcinoembryonic cell adhesion molecule 1 (CEACAM1) interactions. J Immunol. 2006;177:6062–71. doi: 10.4049/jimmunol.177.9.6062. [DOI] [PubMed] [Google Scholar]
- 24.Dudley ME, Wunderlich J, Shelton TE, Even J, Rosenberg SA. Generation of tumour-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kammerer R, Riesenberg R, Weiler C, Lohrmann J, Schleypen J, Zimmermann W. The tumour suppressor gene CEACAM1 is completely but reversibly downregulated in renal cell carcinoma. J Pathol. 2004;204:258–67. doi: 10.1002/path.1657. [DOI] [PubMed] [Google Scholar]
- 26.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369–96. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi H, Okai Y, Paxton RJ, Hefta LJ, Shively JE. Differential regulation of carcinoembryonic antigen and biliary glycoprotein by gamma-interferon. Cancer Res. 1993;53:1612–9. [PubMed] [Google Scholar]
- 28.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–64. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 29.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. Proc Natl Acad Sci U S A. 1997;94:13140–5. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda H, Old LJ, Schreiber RD. The roles of IFNin protection against tumour development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 32.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumour evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Caldwell SA, Greeneltch KM, Yang D, Abrams SI. CTL adoptive immunotherapy concurrently mediates tumour regression and tumour escape. J Immunol. 2006;176:3374–82. doi: 10.4049/jimmunol.176.6.3374. [DOI] [PubMed] [Google Scholar]
- 35.Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. Beta2-microglobulin mutations, HLA class I antigen loss, and tumour progression in melanoma. J Clin Invest. 1998;101:2720–9. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.So T, Takenoyama M, Mizukami M, et al. Haplotype loss of HLA class I antigen as an escape mechanism from immune attack in lung cancer. Cancer Res. 2005;65:5945–52. doi: 10.1158/0008-5472.CAN-04-3787. [DOI] [PubMed] [Google Scholar]
- 37.Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumour evasion of destruction by cytolytic T lymphocytes. J Clin Invest. 2003;111:1487–96. doi: 10.1172/JCI17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowbakht P, Ionescu MC, Rohner A, et al. Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105:3615–22. doi: 10.1182/blood-2004-07-2585. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–82. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber V, Fais S, Iero M, et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 41.Yamshchikov GV, Mullins DW, Chang CC, et al. Sequential immune escape and shifting of T cell responses in a long-term survivor of melanoma. J Immunol. 2005;174:6863–71. doi: 10.4049/jimmunol.174.11.6863. [DOI] [PubMed] [Google Scholar]
- 42.Farrar MA, Schreiber RD. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 43.Egwuagu CE, Li W, Yu CR, Che Mei Lin M, Chan CC, Nakamura T, Chepelinsky AB. Interferon-gamma induces regression of epithelial cell carcinoma: critical roles of IRF-1 and ICSBP transcription factors. Oncogene. 2006;25:3670–9. doi: 10.1038/sj.onc.1209402. [DOI] [PubMed] [Google Scholar]
- 44.Qin Z, Blankenstein T. CD4+ T cell-mediated tumour rejection involves inhibition of angiogenesis that is dependent on IFN-γ receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–86. doi: 10.1016/s1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 45.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 46.Bodey B, Bodey B Jr, Siegel SE, Kaiser HE. Controversies on the prognostic significance of tumour infiltrating leukocytes in solid human tumours. Anticancer Res. 2000;20:1759–68. [PubMed] [Google Scholar]
- 47.Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J Immunol. 2005;174:6791–802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- 48.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol. 2002;20:217–51. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 49.Yasui H, Takai K, Yoshida R, Hayaishi O. Interferon enhances tryptophan metabolism by inducing pulmonary indoleamine 2,3-dioxygenase: its possible occurrence in cancer patients. Proc Natl Acad Sci USA. 1986;83:6622–6. doi: 10.1073/pnas.83.17.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 2006;25:5527–36. doi: 10.1038/sj.onc.1209541. [DOI] [PubMed] [Google Scholar]