Abstract
Tumour growth promotes the expansion of CD4+ CD25+ FoxP3+ regulatory T cells (Tregs) which suppress various arms of immune responses and might therefore contribute to tumour immunosurveillance. In this study, we found an inverse correlation between circulating Treg frequencies and phosphoantigen-induced γδ T-cell proliferation in cancer patients, which prompted us to address the role of Tregs in controlling the γδ T-cell arm of innate immune responses. In vitro, human Treg–peripheral blood mononuclear cell (PBMC) co-cultures strongly inhibited phosphoantigen-induced proliferation of γδ T cells and depletion of Tregs restored the impaired phosphoantigen-induced γδ T-cell proliferation of cancer patients. Tregs did not suppress other effector functions of γδ T cells such as cytokine production or cytotoxicity. Our experiments indicate that Tregs do not mediate their suppressive activity via a cell–cell contact-dependent mechanism, but rather secrete a soluble non-proteinaceous factor, which is independent of known soluble factors interacting with amino acid depletion (e.g. arginase-diminished arginine and indolamine 2,3-dioxygenase-diminished tryptophan) or nitric oxide (NO) production. However, the proliferative activity of αβ T cells was not affected by this cell–cell contact-independent suppressive activity induced by Tregs. In conclusion, these findings indicate a potential new mechanism by which Tregs can specifically suppress γδ T cells and highlight the strategy of combining Treg inhibition with subsequent γδ T-cell activation to enhance γδ T cell-mediated immunotherapy.
Keywords: cancer, γδ T cells, innate immunity, regulatory T cells (Tregs)
Introduction
Regulatory T cells (Tregs) play an important role in the maintenance of immune tolerance and have been shown to suppress immune responses to infectious agents, transplants and tumours in both the priming and the effector phases.1–3 The ability of Tregs to regulate immunity has prompted attempts to stimulate Tregs to treat autoimmunity and induce transplant tolerance on the one hand or to dampen Tregs to increase immunity to pathogens and tumours on the other hand. Current evidence suggests the existence of several phenotypically distinct Treg populations [naturally occurring CD4+ CD25+ FoxP3+ Tregs, induced Tregs such as type 1 T regulatory (Tr1) cells and T helper type 3 (Th3) cells, CD4− CD8− DN αβ T cells, CD8+ CD28− Tregs, mesenchymal stem cell-induced Tregs and others].4,5 Among the different types of Treg, the classic Tregs are thymus-derived CD4+ CD25+ FoxP3+ T lymphocytes (termed Tregs throughout this study), representing 5–10% of total peripheral blood CD4+ T cells in humans, and are usually phenotypically characterized by the constitutive expression of CD25, transcription factor FoxP3, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and glucocorticoid-induced tumour necrosis factor receptor (TNFR)-related protein (GITR) and, as recently revealed, by low expression of CD127 [interleukin-7 receptor α (IL-7Rα)].6 There is now considerable evidence that FoxP3 is a key control molecule for Treg development and function, and is an excellent marker for the study of Tregs.7 Tregs inhibit proliferation and other effector functions of various immune cells of both the adaptive (CD4+ and CD8+αβ T cells and B cells) and the innate [natural killer (NK) cells, NKT cells, monocytes/macrophages and dendritic cells (DCs)] immune system.8–14 Multiple suppressive mechanisms of Tregs, including direct (cell–cell contact dependent) and indirect (soluble factors) mechanisms, have been proposed; the relative importance of these mechanisms may depend on the experimental in vitro or in vivo model. Possible inhibitory effects of Tregs include (1) cell–cell contact-dependent effects produced by membrane-bound transforming growth factor (TGF)-β or intracellular transport of cAMP via gap junctions, (2) secretion or induction of immunosuppressive cytokines such as TGF-β and IL-10, (3) competitive consumption of IL-2, (4) down-regulation of immunostimulatory B7 family members (CD80 and CD86) or induction of immunosuppressive B7 family members (e.g. B7-H4) on antigen-presenting cells (APCs), (5) CTLA-4- or prostaglandin E2 (PGE2)-mediated induction of indoleamine 2,3-dioxygenase (IDO)-expressing APCs, (6) depletion of arginine by induction of arginase in myeloid cells, and (7) induction of nitric oxide (NO) by activation of inducible NO synthase (iNOS).15–19 However, none of the proposed mechanisms can explain all aspects of Treg-mediated suppression and it is possible that various combinations of several mechanisms or undiscovered modes of action are operating.
Among innate effector lymphocytes, γδ T cells represent a unique subset of unconventional lymphocytes, because they display features of both conventional αβ T cells and NK cells.20,21 With increasing knowledge regarding their antigen specificity and function, human γδ T cells have attracted more and more interest for clinical immunotherapy approaches. Vγ9Vδ2 T cells, which represent the vast majority of human circulating γδ T cells (50–70% of γδ T cells and 0·5–7% of CD3+ T cells, respectively), recognize phosphorylated, non-peptide compounds (so-called phosphoantigens), which naturally occur in the microbial non-mevalonate pathway of isoprenoid biosynthesis (the Rohmer pathway).22,23 The capacity of Vγ9Vδ2 T cells to be activated by these naturally occurring phosphoantigens facilitates a specific immune response to numerous microbial pathogens through targeting of a distinctive and essential metabolic route in these organisms. In addition to these natural phosphoantigens, several synthetic compounds have been identified which selectively stimulate Vγ9Vδ2 T cells either by mimicking the highly potent natural phosphoantigens [e.g. bromohydrinpyrophosphate (BrHPP)] or by inducing accumulation of cross-reactive endogenous mevalonate metabolites [e.g. isopentenylpyrophosphate (IPP)] via inhibition of the mevalonate pathway (aminobisphosphonates).24–26γδ T cells not only recognize microbial antigens, but are also capable of exerting significant major histocompatibility complex (MHC)-unrestricted anti-tumour activity against a broad spectrum of tumour cells in vitro.21,27 Previous studies by our group and recent data from Dieli et al. have provided additional evidence that selective stimulation of γδ T cells by phosphoantigens can induce anti-tumour activity in vivo.28,29 However, γδ T cell-mediated immunotherapy is limited by the fact that phosphoantigen-induced proliferation of γδ T cells has been shown to be frequently impaired in patients with haematological malignancies.28 This kind of γδ T-cell anergy has also been described in certain chronic infectious diseases such as HIV-infection or tuberculosis, although the cause of this γδ T-cell anergy has not yet been discovered.30,31
To date, the role of Tregs in hampering γδ T-cell function has not been investigated. In this study we provide the first direct evidence that Tregs potently suppress phosphoantigen-induced γδ T-cell proliferation via a novel cell–cell contact-independent mechanism. In the light of this finding, we speculate that the increased Treg:γδ T-cell ratio in cancer patients may contribute to the state of apparent immunological unresponsiveness to phosphoantigens found in many types of cancer. Thus, our data are compatible with the hypothesis that eliminating Tregs in cancer patients might constitute a rational approach to stimulating not only the adaptive immune response to tumour-associated antigens, but also the γδ T-cell arm of the innate immune system.
Patients, materials and methods
Patients
Blood samples were drawn from 42 healthy donors and 336 patients with different types of cancer [multiple myeloma (MM), n= 198; B-cell non-Hodgkin lymphoma (B-NHL), n= 65; metastatic renal cell cancer (mRCC), n= 22; metastatic malignant melanoma (mMEL), n= 40; metastatic colorectal cancer (mCRC), n= 7; metastatic breast cancer (mBC), n= 4] and 19 patients with monoclonal gammopathy of unknown significance (MGUS) having obtained their written informed consent. Ethical approval for this study was obtained from the Ethics Committee of the University of Würzburg. All patients included in this study had not received any cancer-specific or immunosuppressive (i.e. steroid) treatment for at least 3 months and had a performance status Eastern Cooperative Oncology Group (ECOG) 0-1. Peripheral blood mononuclear cells (PBMC) were analysed by flow cytometry and the number of Vδ2+ T cells and Tregs (CD4+ CD25+ FoxP3+ Tregs) was assessed as described below.
Reagents
The following compounds, monoclonal antibodies (mAbs), assays and kits were used in this study: BrHPP (kindly provided by Innate Pharma, Marseilles, France), recombinant human IL-2 (Proleukin; Novartis, Basel, Switzerland), the anti-human FoxP3 Staining Kit (eBiosciences, San Diego, CA), interferon (IFN)-γ secretion assay, CD4+ CD25+ Treg isolation kit and anti-PE microbeads (all from Miltenyi Biotec, Bergisch Gladbach, Germany), recombinant human (rh) cytomegalovirus (CMV) pp65 protein (Miltenyi Biotec) 1-methyl-l-tryptophan, phytohaemagglutinin (PHA), the PKH26 red fluorescent cell linker mini kit (Sigma-Aldrich, Taufkirchen, Germany), anti-TGF-β mAb, recombinant human TGF-β soluble receptor II (sRII), anti-IL-10 mAb, human latency-associated petide (LAP; R&D Systems, Wiesbaden, Germany), proteinase K (Merck, Darmstadt, Germany), ToPro-3 iodide, the Vybrant®CFDA SE cell tracer kit (Molecular Probes, Eugene, OR) granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-4, IL-15, IL-1β, IL-6, TNF-α (Tebu-Bio, Offenbach, Germany), prostaglandin E2 (Biomol, Hamburg, Germany), iTAg Tetramer/PE-HLA-A*0201 CMV pp65 (NLVPMVATV; Beckmann Coulter Immunomics Operations, Villepinte Roissy, France), NG-monomethyl-l-arginine (l-NMMA) and Nω-hydroxy-nor-l-arginine (nor-NOHA; Axxora, Grünberg, Germany).
Flow cytometric analysis
Cells were harvested after the indicated* culture periods and analysed by two- or three-colour flow cytometry (FACScan flow cytometer; Becton Dickinson, Heidelberg, Germany) using the cellquest program (Becton Dickinson). Cells were stained with the appropriate concentrations of following mABs: fluorescein isothiocyanate (FITC)-conjugated anti-pan γδ T-cell receptor (TCR), anti-Vδ2 TCR, anti-Vγ9 TCR, anti-CD8, anti-CD4, phycoerythrin (PE)-conjugated anti-CD14, anti-CD25, anti-CD3, anti-pan γδ TCR, anti-CD69 (Beckman Coulter, Krefeld, Germany), anti-CD25, anti-αβ TCR (Miltenyi Biotec), anti-FoxP3 (eBiosciences) and peridinin chlorophyll protein (PerCP)-conjugated anti-CD3 (Becton Dickinson). Lymphocytes were gated using forward/sideward scatter analysis.
Isolation of CD4+ CD25+ FoxP3+ Tregs
CD4+ CD25+ Tregs were purified from fresh PBMC either by magnetic cell separation (Miltenyi Biotec) according to the manufacturer’s instructions or by using a cell sorter cytometer (FACSVantage; Becton Dickinson) and cultivated in RPMI-1640 medium supplemented with 10% pooled human AB serum (PAN Biotech, Aidenbach, Germany), 2 mmol/l l-glutamine and 100 U/ml IL-2 before further use. The purity of Tregs was assessed by flow cytometry using mAb against CD4 and CD25 (Becton Dickinson). In addition, FoxP3 expression in isolated CD4+ CD25+ cells was determined using the anti-human FoxP3 Staining Kit (eBiosciences).
γδ T-cell proliferation assay
The γδ T-cell proliferation assay was described previously.25 Briefly, 1 × 105 PBMC from healthy donors were cultivated in triplicate in 100 μl of RPMI-1640 medium per well (Biochrom, Berlin, Germany), 10% pooled human AB serum, and 100 U/ml IL-2 in 96-well round-bottom microtitre plates (Nunc, Wiesbaden, Germany). For determination of phosphoantigen-induced γδ T-cell proliferation, BrHPP (Innate Pharma) was added in escalating concentrations on day 0. Cells were harvested on day 7 and double or triple stained with FITC, PE or PerCP Cy5·5-conjugated monoclonal CD3, TCR pan γδ, TCR Vγ9 or TCR Vδ2 (Beckman Coulter) antibodies. Using a FACScan supported with cellquest acquisition and data analysis software (Becton Dickinson), 1 × 104 cells from each sample were analysed. The lymphocytes were gated using forward/sideward scatter analysis. The amplification rate (A) was calculated as A = (%γδ day 7 × total cell number day 7)/(%γδ day 0 × total cell number day 0). A more than 50-fold amplification rate was considered as proliferation positive. To deplete CD25+ cells, PBMC were labelled with anti-CD25 PE mAb (Beckman Coulter) and further incubated with anti-PE microbeads (Miltenyi Biotec). The increase in γδ T cell numbers was calculated by analysing the relative and absolute numbers of viable cells per well by cytofluorometric identification of γδ T cells using fluorescence-activated cell sorter (FACS) analysis on day 7 of culture. In some experiments, γδ T cell-enriched fractions of PBMC were used after depletion of αβ T cells, B cells, NK cells or monocytes using PE-conjugated anti-αβ TCR, anti-CD19, anti-CD56 or anti-CD14 mAb and anti-PE microbeads (Miltenyi Biotec).
To determine the suppressive effects of Tregs on phosphoantigen-induced γδ T-cell proliferation, 2 × 104 PBMC treated with a single dose of BrHPP (1 μm) were co-cultured with purified CD4+ CD25+ FoxP3+ Tregs or supernatants from overnight IL-2 activated (100 U/ml) Tregs at indicated ratios. Where indicated, Tregs were additionally activated with PHA (2·5 μg/ml). To further investigate the suppressive mechanisms, Tregs or supernatants from Tregs were treated with anti-IL-10 (25 ng/ml), TGF-β sRII (150 ng/ml), LAP (800 ng/ml), l-NMMA (0·5 mm, 2 mm), nor-NOHA (0·1 mm, 0·5 mm) or 1-methyl-l-tryptophan (1 mm, 2 mm, 5 mm) or proteins in the supernatants were inactivated by heat (15 min at 56° or 99°) or by proteinase K digestion (2 hr at 37°; inactivation of enzyme for 15 min at 99°). Transwell experiments were performed in Nunc Tissue Culture Inserts (0·2 μm pore size; Nunc). PBMC (1 × 105) were cultured in the upper chamber, and purified CD4+ CD25+ Tregs at indicated ratios were placed in the bottom chamber. Each γδ T-cell proliferation assay was carried out in RPMI-1640 medium + 10% AB serum + IL-2 (100 U/ml).
CMV pp65-specific αβ T-cell proliferation assay
Immature dendritic cells (iDCs) were derived with GM-CSF (800 U/ml) and rhIL-4 (800 U/ml) after CD14-positive magnetic antibody cell sorting (MACS; purity > 98%) from fresh PBMC using anti-CD14 PE (Beckmann Coulter) and anti-PE microbeads (Miltenyi Biotec) according to the manufacturer’s instructions. Further iDCs were loaded with CMV pp65 recombinant protein (20 μl per 1 × 107 cells) (Miltenyi Biotec) and incubated in RPMI-1640 medium + 10% AB serum and fresh cytokines (800 U/ml IL-4 and 800 U/ml GM-CSF) overnight, and then a maturing cocktail was added to the DCs (2 ng/ml IL-1β, 1 μg/ml PE2, 10 ng/ml TNF-α and 5 ng/ml IL-6). On the next day, mature DCs were washed twice with 1 × Hanks’ balanced salt solution (HBSS), re-suspended in RPMI-1640 medium supplemented with 10% AB serum and 100 U/ml IL-2, and co-cultivated either with autologous PBMC (ratio mDCs:PBMC = 1 : 10) alone or together with autologous purified Tregs or supernatants from overnight-cultivated Tregs at indicated ratios. Proliferation of CMV pp65-specific CD8+αβ T cells was determined on day 7 by flow cytometry using FITC-conjugated anti-CD8 (Beckman Coulter) and iTAg Tetramer/PE-HLA-A*0201 CMV pp65 (NLVPMVATV; Beckmann Coulter Immunomics Operations).
Cytokine and cytotoxicity assay
Freshly purified Tregs were activated for 16 hr with 100 U/ml IL-2. Pre-activated Tregs or supernatant from pre-activated Tregs were co-cultured with 2 × 104 autologous PBMC at indicated ratios for 24 hr and stimulated with BrHPP (1 μm) or medium alone as control for a further 16 hr. They were then analysed either for expression of activation marker CD69 by flow cytometry (FACScan) or for IFN-γ production using the IFN-γ Secretion Assay Detection Kit (Miltenyi Biotec) according to the manufacturer’s instructions.
A standard cytotoxicity assay was performed.32 In brief, the target cell line (Daudi) was labelled with 2 μm PKH26 according to the manufacturer’s manual (Sigma, St Louis, MO) and 5 × 103 cells/well were incubated in 96-well V-shaped microtitre plates in triplicates with a phosphoantigen-reactive Vγ9Vδ2 T-cell line at the indicated effector:target (E:T) ratios. After 4 hr, cells were harvested, brought up to 400 μl with 1 × phosphate-buffered saline (PBS), stained with the nucleic acid-specific ToPro-3 iodide stain (0·5 μm; 15 min on ice) and further analysed by flow cytometry using FL-2 and FL-4, respectively (FACS Calibur; Becton Dickinson). Percentages of cells killed by cytotoxic activity were calculated as (upper right)/(upper and lower right) × 100 followed by subtraction of the percentage of spontaneous death in the target cells-only control tube. In some experiments, purified CD4+ CD25+ Tregs or the CD4+ CD25− fraction of PBMC was added at the indicated ratios.
Cell lines
γδ T-cell lines were established by culturing 1 × 105 freshly isolated PBMC per well in 96-well microtitre plates in standard medium (RPMI-1640 medium supplemented with 10% pooled AB human serum, 2 mmol/l l-glutamine, and 100 U/ml rhIL2) with a single dose of BrHPP (1 μm). Cells were periodically re-stimulated with IL-2 (100 U/ml) every 3–4 days and after 2 weeks more than 90% of the cells expressed the Vγ9Vδ2 TCR, as determined by flow cytometry. For proliferation assays, γδ T-cell lines were αβ T cells depleted using anti-αβ TCR PE and anti-PE microbeads (Miltenyi Biotec) on days 7–8 and kept without IL-2 overnight. Further γδ T cells were labelled with 1 μm carboxyfluorescein succinimidyl ester (CFSE; 1 × 106 cells/ml pre-warmed PBS; 15 min at 37°), washed once with PBS and incubated with medium alone or BrHPP and co-cultured with autologous purified Tregs. On day 6, proliferation was assessed by three-colour staining additionally using anti-pan γδ TCR-PE and anti-CD3-PerCP.
The Daudi cell line was obtained from DSMZ (Braunschweig, Germany) and was grown in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) and 2 mmol/l l-glutamine.
Mixed lymphocyte reaction (MLR)
To determine the suppressive activity of Tregs on αβ T cells, a mixed lymphocyte reaction (MLR) was performed. PBMC (1 × 105 cells/well) from healthy donors were labelled for 15 min with 1 μm CFSE as described above. Further PBMC were co-cultured with allogenic iDCs (1 × 104 cells/well) alone or together with supernatant from overnight-cultivated autologous Tregs (ratio PBMC:Tregs = 1 : 1). Proliferation of αβ T cells was determined by assessing CFSE dilution on day 6 by three-colour staining additionally using anti-αβ TCR-PE and anti-CD3-PerCP.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). The two-sided Student t-test for independent samples was used to determine statistical significance of detected differences. P<0·05 was considered significant.
Results
Impaired phosphoantigen-induced γδ T-cell proliferation in cancer patients
We have recently shown that phosphoantigen-induced proliferation of γδ T cells in vitro is frequently impaired in patients with lymphoid malignancies.28 To confirm and extend this initial observation, we investigated the proliferative response of peripheral blood γδ T cells to phosphoantigen (BrHPP) and IL-2 (100 U/ml) from 336 consecutive patients with different types of cancer (MM, n= 198; B-NHL, n= 65; mRCC, n= 22; mMel, n= 40; mBC, n= 4; mCRC, n= 7). In an age-matched group of healthy donors (n= 42), 95% showed significant in vitro proliferation of γδ T cells in response to phosphoantigen/IL-2, but only 42% of cancer patients were responsive. Figure 1 shows representative cancer patients with maintained and impaired phosphoantigen-induced γδ T-cell proliferation, respectively (49% versus 1·1%γδ T cells after 1 week of culture). Cancer-associated impaired proliferative function of γδ T cells was observed in lymphoid malignancies (71% of MM and 42% of B-NHL) as well as in various advanced solid cancers (55% of mRCC, 27% of mMel, 50% of mBC and 29% of mCRC) (Table 1). Interestingly, individuals with MGUS (n= 19), a premalignant precursor of MM, also showed an increased frequency of impaired phosphoantigen-induced γδ T-cell proliferation compared with healthy individuals (47% versus 5%; data not shown). Impaired proliferative function of γδ T cells might therefore be a general phenomenon in patients with cancer and certain premalignant conditions.
Figure 1.
Proliferative response of γδ T cells to phosphoantigen/interleukin (IL)-2. Peripheral blood mononuclear cells (PBMC) from cancer patients were incubated with phosphoantigen (1 μm bromohydrinpyrophosphate) and IL-2 (100 U/ml), harvested on day 7, stained with anti-pan γδ T-cell receptor (TCR) fluorescein isothiocyanate (FITC) and anti-CD3 phycoerythrin (PE) and analysed by flow cytometry. Fluorescence-activated cell sorter (FACS) analysis of γδ T cells from one representative cancer patient (no. 236) with maintained and one representative cancer patient (no. 004) with impaired phosphoantigen-induced proliferation showed 49% versus 1·1%γδ T cells in CD3+ T cells, respectively.
Table 1.
In vitro proliferation of γδ T cells in response to phosphoantigen/interleukin (IL)-2
| + | − | |
|---|---|---|
| Healthy donors | 40/42 (95%) | 2/42 (5%) |
| MM | 57/198 (29%) | 141/198 (71%) |
| B-NHL | 38/65 (58%) | 27/65 (42%) |
| mRCC | 10/22 (45%) | 12/22 (55%) |
| mMel | 29/40 (73%) | 11/40 (27%) |
| mBC | 2/4 (50%) | 2/4 (50%) |
| mCRC | 5/7 (71%) | 2/7 (29%) |
| All patients | 141/336 (42%) | 195/336 (58%) |
MM, multiple myeloma; B-NHL, B-cell non-Hodgkin lymphoma; mRCC, metastatic renal cell cancer; mMel, metastatic malignant melanoma; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer. The amplification rate (A) was calculated as A = (%γδ day 7 × total cell number day 7)/(%γδ day 0 × total cell number day 0). A > 50-fold amplification rate was considered as positive (+) for phosphoantigen-induced γδ T-cell proliferation.
Phosphoantigen-induced γδ T-cell proliferation inversely correlates with Treg frequencies in cancer patients
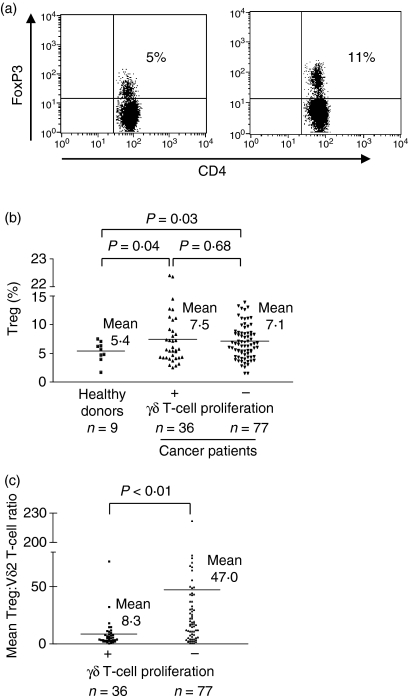
To investigate the potential role of Tregs in impaired γδ T-cell function in cancer patients, we first determined the frequencies of circulating CD4+ CD25+ FoxP3+ Tregs by flow cytometry in cancer patients (n= 113) and healthy controls (n= 9). Consistent with a series of recent reports, cancer patients showed significantly increased frequencies of Tregs compared with healthy controls (Fig. 2a,b). However, there was no statistically significant difference in Treg frequencies between cancer patients with impaired and maintained phosphoantigen-induced γδ T-cell proliferation (Fig. 2b). As using the ratio of Tregs to a given effector lymphocyte subset is a more comprehensive approach to determining the functional significance of Treg-mediated suppression, we compared the ratio of circulating Tregs to the phosphoantigen-sensitive Vδ2+ subset of γδ T cells in the peripheral blood of both cohorts of cancer patients (impaired versus preserved phosphoantigen-induced γδ T-cell proliferation). In this analysis, the Treg:Vδ2+ ratio was significantly higher in cancer patients with impaired phosphoantigen-induced proliferative response (mean Treg:Vδ2+ ratio in cancer patients without γδ T-cell proliferation: 47·0; mean Treg:Vδ2+ ratio in cancer patients with preserved γδ T-cell proliferation: 8·3; P=0·000246), suggesting that an inverse correlation exists between the Treg:Vδ2+ ratio and phosphoantigen-induced γδ T-cell proliferation in cancer patients (Fig. 2c). Importantly, there was no statistically significant difference in relative or absolute numbers of Vδ2+ T cells between cohorts, indicating that the impaired γδ T-cell proliferation in cancer patients is not attributable to a reduced frequency of this T-cell subset (data not shown). Hence, CD4+ CD25+ FoxP3+ Tregs might contribute to the impaired Vγ9Vδ2 T-cell proliferation in cancer patients and the Treg:Vδ2+ ratio from peripheral blood can predict the capacity of γδ T cells to proliferate in response to phosphoantigens.
Figure 2.
Comparison of circulating CD4+ FoxP3+ regulatory T cell (Treg) frequencies in healthy donors and cancer patients. Peripheral blood mononuclear cells (PBMC) from healthy donors and cancer patients were stained with anti-CD4 fluorescein isothiocyanate (FITC), anti-CD3 peridinin chlorophyll protein (PerCP) and anti-FoxP3 phycoerythrin (PE) and analysed by flow cytometry. To determine the frequencies of Tregs, CD4/CD3 double-positive cells were gated and further analysed for FoxP3 expression. (a) Representative fluorescence-activated cell sorter (FACS) analysis of Tregs from one healthy donor and one cancer patient, showing 5% versus 11% Tregs in PBMC, respectively. (b) Percentages of CD4+ CD25+ FoxP3+ Tregs in CD4+ T cells from nine healthy donors (mean ratio: 5·4) and 113 cancer patients, with maintained (n= 36; mean ratio: 7·5) and impaired (n= 77; mean ratio: 7·1) phosphoantigen-induced proliferation of γδ T cells. (c) Ratio of Tregs to the phosphoantigen-sensitive Vδ2+ subset of γδ T cells in the peripheral blood of cancer patients with preserved (mean: 47·0) and impaired (mean: 8·3) phosphoantigen-induced γδ T-cell proliferation. Student’s t-test was used to determine statistical significance of detected differences. P<0·05 was considered significant.
Tregs inhibit phosphoantigen-induced γδ T-cell proliferation in vitro
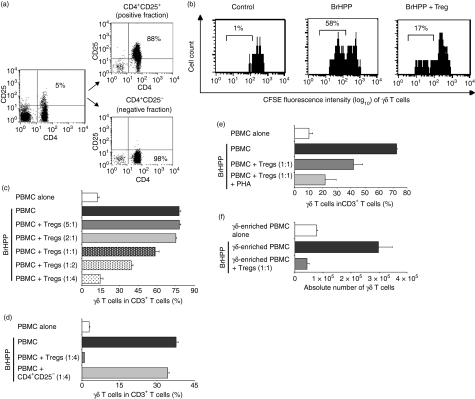
To directly assess the capacity of Tregs to interfere with γδ T-cell proliferation, CD4+ CD25+ Tregs were purified by cell sorting or magnetic cell separation from healthy donors (purity ∼90%) and co-cultured at different ratios with either freshly isolated PBMC or purified γδ T-cell lines from the same donor (Fig. 3a). In contrast to CD4+ CD25− T cells, purified CD4+ CD25+ Tregs exhibited a cell dose-dependent inhibition of phosphoantigen-induced γδ T-cell proliferation, as shown by CFSE assay and by assessment of γδ T-cell proliferation after 1 week of culture (Fig. 3b–d). Consistent with the findings in cancer patients, at lower Treg:PBMC cell ratios than 1 : 1 the suppressive effect of Tregs began to diminish. The inhibitory effect of Tregs on γδ T-cell proliferation could not be overcome by higher doses of cytokines important for γδ T-cell proliferation, such IL-2 (up to 1000 U/ml) or IL-15, indicating that competitive consumption of cytokines is not responsible for Treg-mediated inhibition of γδ T-cell proliferation (data not shown). Titration experiments demonstrated that the suppressive effect of Tregs on γδ T-cell proliferation could be enhanced by using more stimulated Tregs (Tregs stimulated with PHA + IL-2 instead of IL-2 alone) (Fig. 3e). To exclude indirect effects of Tregs on γδ T-cell proliferation via a third cell population within PBMC, γδ T cell-enriched PBMC after depletion of αβ T cells, B cells, NK cells or monocytes were co-cultured with Tregs. The results of these experiments demonstrated that inhibition of phosphoantigen-induced proliferation of γδ T cells by Tregs is maintained after depletion of these PBMC subpopulations and suppression occurs at real γδ T-cell:Treg ratios of 1 : 1 to 1 : 5 (Fig. 3f). Similar ratios have been reported in the literature for Treg-mediated inhibition of other effector lymphocyte subsets.10–12
Figure 3.
Inhibitory effects of purified regulatory T cells (Tregs) on phosphoantigen-induced γδ T-cell proliferation. (a) CD4+ CD25+ Tregs were isolated from peripheral blood mononuclear cells (PBMC) by using a FACSVantage cell sorter cytometer. After sorting, the CD4+ CD25+ fraction and the CD4+ CD25− fraction were reanalysed to confirm enrichment and purity (88% versus 98%, respectively) as shown for one representative donor. (b) After depletion of αβ T cells a carboxyfluorescein succinimidyl ester (CFSE)-labelled γδ T-cell line was incubated with medium alone or bromohydrinpyrophosphate (BrHPP, 1 μm) and co-cultured with autologous purified Tregs at a ratio of 1 : 2·5. On day 6, cells were analysed by flow cytometry. γδ T-cell receptor (TCR)/CD3 double-positive T cells were gated, and loss of CFSE represented proliferation of gated cells. The percentage of proliferating cells is indicated for one representative donor. (c, d) PBMC were incubated with medium alone or BrHPP (1 μm) and co-cultured (c) with autologous purified Tregs at the indicated ratios or (d) with the CD4+ CD25− T-cell fraction. On day 7, proliferation of γδ T cells was analysed by flow cytometry. (e) Stimulation of Tregs with phytohaemagglutinin (PHA; 2·5 μg/ml). (f) Preserved inhibition of phosphoantigen-induced γδ T-cell proliferation by Tregs after depletion of other PBMC populations [αβ T cells, B cells, natural killer (NK) cells and monocytes]. Representative results are shown after depletion of αβ T cells within PBMC. Similar results were obtained after depletion of B cells, NK cells or monocytes. After depletion of αβ T cells, 2 × 104γδ T cell-enriched PBMC (25%γδ T cells in PBMC) were incubated with medium alone or BrHPP (1 μm) and co-cultured with autologous Tregs at a ratio of 1 : 1, which corresponds to a γδ T-cell:Treg ratio of 1 : 4. On day 7, proliferation of γδ T cells was analysed by flow cytometry, and absolute γδ T-cell numbers are shown. Each bar represents mean values ± standard deviation of triplicate cultures from one representative of at least three independent healthy donors.
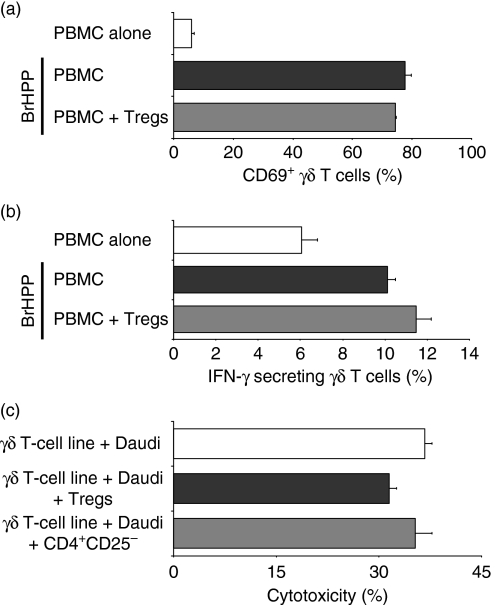
In contrast with the broad inhibitory effects of Tregs described for other lymphocyte subsets, the suppressive effect of Tregs on γδ T cells was restricted to proliferation. As shown in Fig. 4, neither pre-activated Tregs themselves nor supernatant from pre-activated Tregs (data not shown) significantly affected phosphoantigen-induced up-regulation of an activation marker (CD69) (Fig. 4a) or cytokine production (IFN-γ) (Fig. 4b) of naïve γδ T cells. The functional capacity of non-naïve γδ T cells (BrHPP-induced γδ T-cell lines) to kill the γδ T cell-sensitive tumour cell line Daudi (Fig. 4c) was also not significantly reduced by Tregs.
Figure 4.
Effects of purified regulatory T cells (Tregs) on γδ T-cell function. Fresh peripheral blood mononuclear cells (PBMC) were incubated with bromohydrinpyrophosphate (BrHPP, 1 μm) or medium alone and co-cultured with autologous purified interleukin (IL)-2-activated Tregs at ratio 1 : 1 for 24 hr and further analysed for (a) expression of activation marker CD69 or (b) interferon (IFN)-γ secretion. Each bar represents mean values ± standard deviation (SD) of triplicate cultures from one healthy donor and is representative of two independent experiments. (c) In a standard cytotoxicity assay, a BrHPP-induced γδ effector T-cell line was incubated with the Burkitt lymphoma cell line Daudi alone [effector: target (E:T) ratio = 25 : 1] or together with purified Tregs, or as a control with the CD4+ CD25− fraction of PBMC (Treg or CD4+ CD25− to PBMC ratio = 3 : 1 and E:T ratio = 25 : 1). The percentage cytotoxicity is expressed as percentage specific lysis ± SD of triplicate cultures from one representative donor.
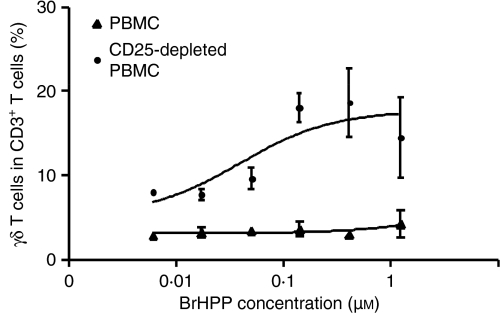
To confirm the inhibitory effects of Tregs on phosphoantigen-induced γδ T-cell proliferation in cancer patients, we depleted Tregs in vitro using anti-CD25 antibodies. As shown for a representative cancer patient who showed impaired γδ T-cell proliferation, depletion of Tregs restored the ability of γδ T cells to proliferate in the presence of phosphoantigen and IL-2 (Fig. 5). In summary, these data strongly support the ability of Tregs to suppress phosphoantigen-induced proliferation of γδ T cells.
Figure 5.
Depletion of CD25+ cells restores the capacity of γδ T cells to respond to phosphoantigen. Peripheral blood mononuclear cells (PBMC) from cancer patients were stimulated with bromohydrinpyrophosphate (BrHPP; 0·014; 0·041; 0·123; 0·37 and 1·11 μm) and interleukin (IL)-2 (100 U/ml) before and after in vitro depletion of CD25+ T cells and proliferation of γδ T cells was analysed on day 7 by flow cytometry. Data shown represent mean values ± standard deviation of triplicate cultures from one donor representative of three cancer patients.
Treg suppression of phosphoantigen-induced γδ T-cell proliferation is cell–cell contact independent and mediated by a non-proteinaceous soluble factor
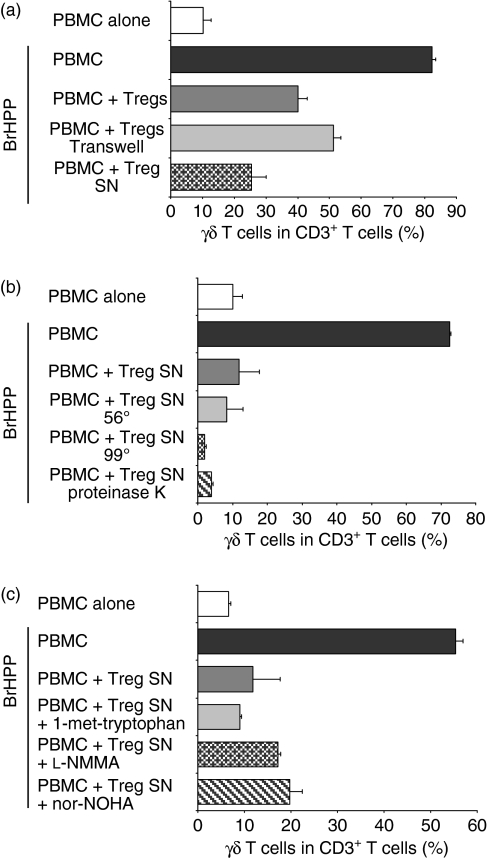
To analyse the mechanisms of Treg-mediated suppression of phosphoantigen-induced γδ T-cell proliferation, we first separated Tregs from γδ T cells by a semi-permeable membrane (Transwell experiments). As shown in Fig. 6(a), the suppressive effect of Tregs on γδ T-cell proliferation by separation of Tregs from γδ T cells was almost completely maintained, suggesting that direct cell-to-cell contacts did not significantly contribute to suppression. Importantly, attempts to transfer suppression of γδ T-cell proliferation using supernatants of purified Tregs were successful, and this suppression could be enhanced by additional stimulation of Tregs using PHA (data not shown), suggesting that a soluble factor secreted by Tregs contributes to suppression (Fig. 6a).
Figure 6.
Mechanisms of regulatory T cell (Treg)-mediated suppression of γδ T-cell proliferation. (a) Peripheral blood mononuclear cells (PBMC) were incubated with bromohydrinpyrophosphate (BrHPP, 1 μm) or medium alone and co-cultured with autologous purified Tregs separated by semi-permeable membranes (Transwells) or with supernatant (SN) from overnight-cultivated and interleukin (IL)-2-stimulated Tregs. (b) Supernatants were heated for 15 min at 56° or 99°, or treated with proteinase K (2 hr at 37°). (c) PBMC incubated with BrHPP were co-cultured with supernatant from overnight-cultivated Tregs alone or treated with 1-methyl-l-tryptophan (1·5 mm), NG-monomethyl-l-arginine (l-NMMA; 2 mm) or Nω-hydroxy-nor-l-arginine (nor-NOHA; 0·5 mm) at ratio 1 : 1 and proliferation of γδ T cells was analysed on day 7 by flow cytometry. Each bar represents mean values ± standard deviation of triplicate cultures from one healthy donor and is representative of four independent experiments.
As the suppressive activity was clearly demonstrable in supernatants of Tregs, we started to characterize the biochemical nature of the soluble factor secreted by Tregs. As shown in Fig. 6(b), the inhibitory effect of the Treg supernatant was maintained even after heat treatment (56° and 99°) and was resistant to protease treatment, suggesting that a non-proteinaceous soluble factor secreted by Tregs is responsible for Treg-mediated suppression of γδ T-cell proliferation. Thus, these data argue against immunosuppressive cytokines such as TGF-β and IL-10 being mediators of the suppressive activity in Treg supernatants. In fact, neutralization of neither TGF-β (by anti-TGF-β mAb, TGF-β soluble receptor II or LAP) nor IL-10 (by anti-IL-10 mAb) restored phosphoantigen-induced γδ T-cell proliferation in the presence of Tregs (data not shown).
Given that non-proteinaceous soluble factors interacting with amino acid metabolism or iNOS activity have been reported to be able to mediate suppressive effects on T-cell proliferation, we next examined whether these factors might be responsible for the cell–cell contact-independent suppression of phosphoantigen-induced γδ T-cell proliferation. However, Tregs maintained their inhibitory effect on γδ T-cell proliferation in the presence of the IDO inhibitor 1-methyl-l-tryptophan, the arginase inhibitor nor-NOHA and the iNOS inhibitor l-NMMA, suggesting that neither depletion of tryptophan nor depletion of arginine nor induction of NO contributed to Treg-mediated suppression of γδ T-cell proliferation (Fig. 6c). Thus, these data argue for a novel cell–cell contact-independent suppressive activity of Tregs on γδ T cells, which is neither dependent on cytokines nor dependent on known non-proteinaceous soluble factors.
Role of Treg-induced soluble factors in inhibition of αβ T-cell proliferation
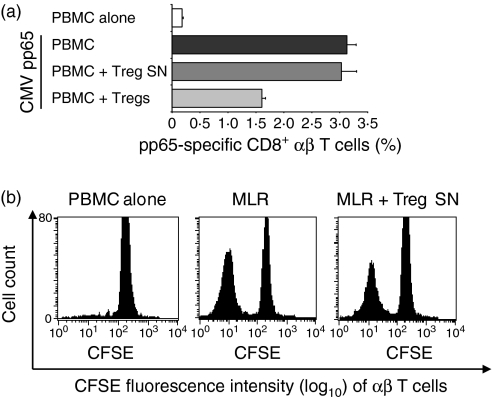
It is widely accepted that Tregs exert their regulatory effect on (CD4+ and CD8+) αβ T cells via cell–cell contact mechanisms. To analyse the effect of Tregs on αβ T cells, polyclonal alloreactive (MLR) and antigen-specific (CMV pp65-specific) proliferation of αβ T cells in the presence of Tregs and Treg supernatant, respectively, was examined. As shown in Fig. 7(a), co-culture of Tregs suppressed the proliferation of CMV pp65-specific CD8+αβ T cells. However, the supernatant of purified Tregs inhibited neither the proliferation of CMV pp65-specific CD8+αβ T cells (Fig. 7a) nor the proliferation of alloreactive αβ T cells (MLR) (Fig. 7b), indicating that the suppressive effects of Tregs on αβ T cells and γδ T cells are mediated by different mechanisms.
Figure 7.
Effects of purified regulatory T cells (Tregs) on αβ T-cell proliferation. (a) Peripheral blood mononuclear cells (PBMC) from cytomegalovirus (CMV)-seropositive healthy donors were re-stimulated with CMV pp65-loaded mature dendritic cells (DCs) alone or co-cultured with autologous purified Tregs (ratio PBMC:Treg = 1 : 4) or supernatant (SN) from overnight-cultivated Tregs (ratio PBMC:Treg supernatant = 1 : 1). After 1 week, proliferation of CMV pp65-specific CD8+αβ T cells was determined by flow cytometry. Each bar represents mean values ± standard deviation of triplicate cultures from one representative donor. (b) In a mixed lymphocyte reaction (MLR), carboxyfluorescein succinimidyl ester (CFSE)-labelled PBMC were incubated with allogeneic immature dendritic cells (ratio 1 : 10) alone or together with supernatant from overnight-cultivated autologous purified Tregs (ratio PBMC:Treg supernatant = 1 : 1). On day 6, cells were analysed by flow cytometry. αβ T-cell receptor (TCR)/CD3 double-positive T cells were gated, and loss of CFSE represents proliferation of gated cells. One representative donor of three is shown.
Discussion
Tregs have a crucial role in impeding immune surveillance against cancer and hampering the development of effective anti-tumour immunity.3 Increasing evidence supports the existence of increased frequencies of Tregs in both peripheral blood and the tumour microenvironment of solid tumours and haematological malignancies.33 Thus, our results are consistent with a series of recent reports demonstrating increased frequencies of Tregs (characterized as CD4+ CD25+ FoxP3+ T cells) in cancer patients and patients with certain premalignant conditions such as MGUS as compared with healthy individuals.33,34 However, recent data have shown that the ratio of Tregs to effector T cells, rather than the Treg frequency in peripheral blood or tumour specimens, predicts more precisely the inhibitory effects of Tregs on a target cell population (especially for rare effector populations such as γδ T cells) and is a more promising independent prognostic factor for tumour recurrence and survival than the Treg frequency alone.35–37 Our data confirm the predictive value of Treg:effector T-cell ratios, because we found that the Treg:γδ T-cell ratio, unlike the absolute or relative number of circulating Tregs, was negatively correlated with phosphoantigen-induced γδ T-cell proliferation in cancer patients. In addition to this indirect evidence, this study demonstrates for the first time the capacity of Tregs to directly inhibit γδ T-cell proliferation in vitro. Our findings therefore might explain the frequent proliferative anergy of γδ T cells in cancer patients. Although other factors might also play a role in suppression of γδ T-cell function, we have defined Treg-mediated suppression as one major mechanism for impaired γδ T-cell proliferation in cancer patients.38 Therefore, a combination of depletion or attenuation of Tregs and concomitant stimulation of γδ T cells may overcome Treg-mediated suppression and enhance γδ T cell-mediated immunotherapy in cancer patients.
Impaired phosphoantigen-induced proliferation of peripheral γδ T cells has been reported not only in cancer but also in the course of certain chronic infections (HIV, tuberculosis and toxoplasmosis).28,30,39 Several groups demonstrated qualitative and quantitative alterations of the peripheral Vγ9Vδ2 T-cell subset of chronically infected persons including a polyclonal decrease in the absolute number of Vγ9Vδ2 T cells, decreased cytokine (TNF-α and IFN-γ) production and proliferative anergy after stimulation with phosphoantigens in spite of the presence of IL-2.30,40 Although earlier studies suggested a potential role of cytokine dysfunction, increased sensitivity to apoptosis as a consequence of chronic antigenic stimulation, a dysbalance in the expression of killer inhibitory receptors (KIRs) or shedding of NKG2D ligands in down-regulating γδ T-cell effector functions in chronic infection and cancer, our results indicate that Tregs play a major role in the proliferative anergy of Vγ9Vδ2 T cells.38,40–43 In fact, increased frequencies of Tregs were recently observed in patients with chronic active infections, supporting the role of Treg-mediated suppression of Vγ9Vδ2 T cells in chronically infected persons.44,45 Although the role of Tregs in various chronic infections is currently highly controversial for various reasons, a high perforin:Treg ratio in lymphoid tissues of HIV-infected patients has been shown to correlate with non-progressive disease, suggesting that the immune control of virus replication represents a balance between cell-mediated immune response and Treg-mediated counter regulation.46 However, the relevance of Tregs in infection-associated γδ T-cell anergy remains to be established.
Although previous studies have demonstrated a role of Tregs in suppressing other innate lymphocyte subsets such as NK cells and NKT cells in vitro,11,12,47,48 this is the first study to provide mechanistic insights into Treg–γδ T-cell interactions and to formally demonstrate that Tregs display an immunoregulatory effect on human γδ T cells. In contrast to the reported cell–cell contact-dependent suppressive effect of Tregs on αβ T cells, our data show that the inhibitory effect of Tregs on γδ T cells is cell–cell contact independent and restricted to proliferation. Furthermore, Transwell and supernatant experiments suggest that Tregs secrete non-proteinaceous soluble factors which potently inhibit phosphoantigen-induced γδ T-cell proliferation. However, in our experimental system, neither the soluble factors TGF-β, IL-10 and NO, nor IDO- or arginase-dependent immunosuppressive mechanisms appeared to be involved in the Treg-mediated suppressive effect on γδ T-cell proliferation. Further analyses are required to characterize the soluble non-proteinaceous factor secreted by Tregs and experiments to determine the biochemical properties of this factor are currently underway in our laboratory.
There is accumulating evidence that Tregs suppress multiple types of immunocompetent cells and activated Tregs can inhibit a wide range of immune responses through bystander suppression.15 However, our results indicate that Tregs may not only control immune responses via mechanisms targeting various types of immune cells (i.e. cell–cell contact-dependent or TGF-β-mediated mechanisms), but can also mediate suppressive effects through cell type-specific mechanisms such as the inhibition of γδ T-cell proliferation by a soluble non-proteinaceous factor reported here. Thus, our analysis has uncovered a new suppressive function of Tregs that does not affect all lymphocyte subsets and underscores the existence of cell–cell contact-independent and strictly cell–cell contact-dependent suppressive activities of Tregs.
Recent studies have demonstrated that IL-2 signalling is required for thymic development, peripheral expansion, and suppressive activity of Tregs.49 In patients with melanoma and mRCC, the frequency of Tregs was significantly increased after IL-2 treatment, which might explain in part the limited anti-tumour activity of IL-2 administration in cancer patients.50 IL-2 has also been used in human and preclinical non-human primate studies to expand γδ T cells in response to phosphoantigens.28,29,51 However, repetitive applications of phosphoantigen in combination with IL-2 induced less vigorous expansion of γδ T cells in vivo, suggesting a progressive exhaustion of the γδ T-cell proliferative response.28,51 Our findings might provide an explanation for this reduced proliferative response of γδ T cells after successive treatment with phosphoantigen and IL-2, because IL-2-mediated induction of Tregs might counteract the expansion and potential anti-tumour effects of γδ T cells. This emphasizes a major potential disadvantage of IL-2 in clinical γδ T cell-based immunotherapy approaches and therefore has major implications for the design of future clinical trials.
In conclusion, this study demonstrates the capacity of Tregs to inhibit γδ T-cell proliferation in vitro via a cell–cell contact-independent mechanism and thus increased Treg:γδ T-cell ratios may contribute to the frequently impaired γδ T-cell proliferative response in cancer patients. Our results extend recent findings concerning various cellular and molecular events to explain how Tregs suppress immune responses and suggest that immunopharmacological manipulations to deplete Tregs might enhance the effectiveness of γδ T cell-mediated tumour immunotherapy.
Acknowledgments
We thank Judith Engert for her expert technical assistance and Arne Schäfer for assistance in statistical analysis. This work was supported by Interdisziplinaeres Zentrum für Klinische Forschung Wuerzburg (IZKF, Grant No. 01KS9603) and Deutsche Forschungsgemeinschaft (DFG, Grant No. KFO 124/1-1 TP5).
Footnotes
Wherever indicated is used in the ‘patients, material, and methods’ section, the exact ratios or culture conditions are described in detail in figure legends or results section.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Preserved inhibition of phosphoantigen-induced γδ T cell proliferation by Treg after depletion of different PBMC populations (B cells, NK cells, monocytes). After depletion of different PBMC subsets 2 × 104 γδ T cell enriched PBMC (20–30 % γδ T cells within PBMC) were incubated with medium alone or bromohydrinpyrophosphate (BrHPP 1 μm) and co-cultured with autologous Treg at a ratio of 1 : 1 which corresponds to a γδ T cell/Treg ratios from 1 : 3 to 1 : 5. On day 7 proliferation of γδ T cells were analyzed by flow cytometry and absolute γδ T cell numbers are shown. Each value represents mean values ± SD of triplicate cultures from one representative of at least three independent healthy donors.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Mills KH. Regulatory T cells: friend or foe in immunity to infection. Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 3.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 4.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–7. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 5.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–8. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 6.Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol Immunol. 2006;3:189–95. [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 9.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 10.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+CD25+ regulatory T cells. J Immunol. 2005;175:4180–3. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 11.Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-β-dependent manner. J Exp Med. 2005;202:1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516–20. [PubMed] [Google Scholar]
- 13.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–30. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 15.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Bopp T, Becker C, Klein M, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–10. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–54. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 19.Aiello S, Cassis P, Cassis L, et al. DnIKK2-transfected dendritic cells induce a novel population of inducible nitric oxide synthase-expressing CD4+CD25− cells with tolerogenic properties. Transplantation. 2007;83:474–84. doi: 10.1097/01.tp.0000251808.91901.c3. [DOI] [PubMed] [Google Scholar]
- 20.Hayday AC. [gamma] [delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 21.Kunzmann V, Wilhelm M. Anti-lymphoma effect of γδ T cells. Leuk Lymphoma. 2005;46:671–80. doi: 10.1080/10428190500051893. [DOI] [PubMed] [Google Scholar]
- 22.Jomaa H, Feurle J, Luhs K, Kunzmann V, Tony HP, Herderich M, Wilhelm M. Vγ9/Vδ2 T cell activation induced by bacterial low molecular mass compounds depends on the 1-deoxy-D-xylulose 5-phosphate pathway of isoprenoid biosynthesis. FEMS Immunol Med Microbiol. 1999;25:371–8. doi: 10.1111/j.1574-695X.1999.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 23.Hintz M, Reichenberg A, Altincicek B, et al. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–22. doi: 10.1016/s0014-5793(01)03191-x. [DOI] [PubMed] [Google Scholar]
- 24.Espinosa E, Belmant C, Pont F, et al. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human γδ T cells. J Biol Chem. 2001;276:18337–44. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 25.Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–8. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 26.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 27.Zocchi MR, Poggi A. Role of γδ T lymphocytes in tumor defense. Front Biosci. 2004;9:2588–604. doi: 10.2741/1419. [DOI] [PubMed] [Google Scholar]
- 28.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 29.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poccia F, Boullier S, Lecoeur H, Cochet M, Poquet Y, Colizzi V, Fournie JJ, Gougeon ML. Peripheral V γ 9/V δ 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449–61. [PubMed] [Google Scholar]
- 31.Li B, Rossman MD, Imir T, Oner-Eyuboglu AF, Lee CW, Biancaniello R, Carding SR. Disease-specific changes in γδ T cell repertoire and function in patients with pulmonary tuberculosis. J Immunol. 1996;157:4222–9. [PubMed] [Google Scholar]
- 32.Olin MR, Hwa Choi K, Lee J, Molitor TW. γδ T-lymphocyte cytotoxic activity against Mycobacterium bovis analyzed by flow cytometry. J Immunol Methods. 2005;297:1–11. doi: 10.1016/j.jim.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 34.Beyer M, Kochanek M, Giese T, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107:3940–9. doi: 10.1182/blood-2005-09-3671. [DOI] [PubMed] [Google Scholar]
- 35.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 36.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bui JD, Uppaluri R, Hsieh CS, Schreiber RD. Comparative analysis of regulatory and effector T cells in progressively growing versus rejecting tumors of similar origins. Cancer Res. 2006;66:7301–9. doi: 10.1158/0008-5472.CAN-06-0556. [DOI] [PubMed] [Google Scholar]
- 38.Halary F, Peyrat MA, Champagne E, et al. Control of self-reactive cytotoxic T lymphocytes expressing γδ T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–21. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 39.Rojas RE, Chervenak KA, Thomas J, et al. Vδ2+ γδ T cell function in Mycobacterium tuberculosis- and HIV-1-positive patients in the United States and Uganda: application of a whole-blood assay. J Infect Dis. 2005;192:1806–14. doi: 10.1086/497146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boullier S, Poquet Y, Debord T, Fournie JJ, Gougeon ML. Regulation by cytokines (IL-12, IL-15, IL-4 and IL-10) of the Vγ9Vδ2 T cell response to mycobacterial phosphoantigens in responder and anergic HIV-infected persons. Eur J Immunol. 1999;29:90–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<90::AID-IMMU90>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Ferrarini M, Heltai S, Toninelli E, Sabbadini MG, Pellicciari C, Manfredi AA. Daudi lymphoma killing triggers the programmed death of cytotoxic Vγ9/Vδ2 T lymphocytes. J Immunol. 1995;154:3704–12. [PubMed] [Google Scholar]
- 42.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V γ 9V δ 2 T lymphocytes. J Immunol. 1997;159:6009–17. [PubMed] [Google Scholar]
- 43.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Zhou B, Li M, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol. 2007;123:50–9. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–86. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–17. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trzonkowski P, Szmit E, Mysliwska J, Dobyszuk A, Mysliwski A. CD4+CD25+ T regulatory cells inhibit cytotoxic activity of T CD8+ and NK lymphocytes in the direct cell-to-cell interaction. Clin Immunol. 2004;112:258–67. doi: 10.1016/j.clim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Smyth MJ, Teng MW, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol. 2006;176:1582–7. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 49.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–74. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 50.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–80. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preserved inhibition of phosphoantigen-induced γδ T cell proliferation by Treg after depletion of different PBMC populations (B cells, NK cells, monocytes). After depletion of different PBMC subsets 2 × 104 γδ T cell enriched PBMC (20–30 % γδ T cells within PBMC) were incubated with medium alone or bromohydrinpyrophosphate (BrHPP 1 μm) and co-cultured with autologous Treg at a ratio of 1 : 1 which corresponds to a γδ T cell/Treg ratios from 1 : 3 to 1 : 5. On day 7 proliferation of γδ T cells were analyzed by flow cytometry and absolute γδ T cell numbers are shown. Each value represents mean values ± SD of triplicate cultures from one representative of at least three independent healthy donors.