Abstract
There is a clear link between obesity and metabolic disorders; however, little is known about the effect of obesity on immune function, particularly during an infection. We have previously reported that diet-induced obese mice are more susceptible to morbidity and mortality during influenza infection than lean mice. Obese mice displayed aberrant innate immune responses characterized by minimal induction of interferon (IFN)-α/β, delayed expression of pro-inflammatory cytokines and chemokines, and impaired natural killer cell cytotoxicity. To further examine the abnormal immune response of diet-induced obese mice, we analysed the cellularity of their lungs during influenza virus infection. We found delayed mononuclear cell entry with a marked decrease in dendritic cells (DCs) throughout the infection. Given the critical role of the DC in activating the cell-mediated immune response, we also analysed the functional capacity of DCs from obese mice. We found that, while obesity did not interfere with antigen uptake and migration, it did impair DC antigen presentation. This was probably attributable to an altered cytokine milieu, as interleukin (IL)-2, IL-12, and IL-6 were differentially regulated in the obese mice. Overall, this did not impact the total number of virus-specific CD8+ T cells that were elicited, but did affect the number and frequency of CD3+ and CD8+ T cells in the lung. Thus, obesity interferes with cellular responses during influenza infection, leading to alterations in the T-cell population that ultimately may be detrimental to the host.
Keywords: dendritic cells, influenza, obesity
Introduction
Obesity is clearly associated with metabolic disturbances, such as insulin resistance and non-alcoholic fatty liver disease.1,2 What is less well studied is the impact of obesity on immune function during infection. In humans, obesity has been shown to be a risk factor for infections, poor wound healing,3,4 and decreased response to vaccination.5,6 Additionally, obese individuals have changes in the circulating T-cell population, displaying an increased frequency of CD4+, but a reduced frequency of CD8+ T cells.7 Studies in genetically obese mice demonstrate decreased resistance to infections8,9 as well as reduced cell-mediated cytotoxicity and impaired dendritic cell (DC) function10–12 Diet-induced obese (DIO) mice have impaired antigen presentation,13 as well as altered mitogen-stimulated proliferation of splenocytes,14 and diminished natural killer (NK) cell function.15
During infection with influenza virus, DCs and macrophages are the initiators and modulators of the immune response.16 DCs, in particular, are efficient at endocytosing viral antigen and processing it into peptide fragments which are then presented on major histocompatibility complex (MHC) molecules. Presentation of antigen by the DCs to T cells occurs following migration of the DCs to the draining lymph node (LN) through which T cells are circulating.16 This function is performed primarily by conventional DCs (cDCs), whereas plasmacytoid DCs (pDCs) have limited antigen-presenting capacity, but secrete high levels of interferon (IFN)-α in the inflamed tissue.17,18 Both DC subtypes reside in peripheral tissues in an immature state and mature after exposure to inflammatory stimuli, but high expression of B220 on pDCs allows them to be differentiated from cDCs.
In our previous study19 we demonstrated that DIO mice infected with influenza virus had increased morbidity and mortality. The immune response in the obese mice was characterized by minimal expression of type I IFN and delayed expression of pro-inflammatory cytokines and chemokines. These results suggested that obese mice have improper recruitment of mononuclear cells to the influenza-infected lungs and therefore activation of ensuing responses would be impaired.
In this paper, we demonstrate that recruitment of mononuclear cells to the lung during influenza infection is delayed in DIO mice, and the number of DCs remains significantly lower throughout infection. Despite this, DCs are capable of taking up antigen and migrating to the LN, but their ability to present antigen to T cells is impaired. As a result, the total number of CD8+ T cells is reduced in obese mice, but they are capable of eliciting a quantitatively normal virus-specific CD8+ T-cell response.
Materials and methods
Animals
Weanling C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). All mice were housed in the University of North Carolina Animal Facility, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. Animals were maintained under protocols approved by the Institutional Animal Use and Care Committee. Mice were randomized to receive either a low-fat/no-sucrose diet or a high-fat/high-sucrose diet for 22 weeks. Mice were housed four per cage with free access to food and water.
Diets
The diets, which have been previously described,20 were obtained from Research Diets, Inc., (New Brunswick, NJ).
Virus and infection
The mouse-adapted strain of influenza A/Puerto Rico/8/34 (American Type Culture Collection) was propagated in the allantoic fluid of fertilized chicken eggs and the viral titre was determined by standard plaque assay on Madin Darby canine kidney (MDCK) cells. After 22 weeks on the diets, mice were anaesthetized with an intramuscular injection of a ketamine (0·6 mg/kg)/xylazine (0·35 mg/kg) solution and infected intranasally with 0·05 ml of 50 plaque-forming unit (PFU) influenza A/PR8 virus diluted in phosphate-buffered saline (PBS).
Lung cell isolation and staining for flow cytometry
Lungs were removed, incubated in a collagenase solution, and then processed into single-cell suspensions by mechanical agitation of a Stomacher (Seward, West Sussex, UK) and strained through a 40-μm nylon filter. Cells were subjected to red blood cell (RBC) lysis using ACK lysis buffer for 5 min at room temperature, washed, and counted. At least 5 × 105 cells were stained with fluorescein isothiocyanate (FITC)-anti-CD11b, allophycocyanin (APC)-anti-CD11c, phycoerythrin (PE) Cy7-anti-B220 (eBioscience, San Diego, CA) and peridinin-chlorophyll-protein complex (PerCP)-anti-CD8 and analysed by FACSCaliber (BD Biosciences, San Jose, CA). Intracellular staining was performed on total lung cells from influenza-infected mice that were incubated for 4–6 hr with Brefeldin A (BD Biosciences). Cells were then Fc-blocked with anti-FcγII/III, surface stained, and permeabilized for IL-6 staining. An irrelevant PE-immunoglobulin G (IgG) (Sigma, St Louis, MO) was used as a staining control, and non-specific staining was subtracted from the values presented. Antibodies were obtained from BD Biosciences unless otherwise noted. Gates were set for alveolar macrophages (AMs), DCs, and monocytes based on previous reports.21–23 Briefly, gates were set based on CD11b and CD11c expression. AMs were identified by CD11chigh/CD11blow expression, DCs by intermediate levels of CD11b and CD11c, and monocytes by expression of CD11b but no CD11c. cDCs and pDCs were identified from the gated DC population as CD11c+ B220− and CD11c+ B220+, respectively.
FITC uptake and DC migration to lymph nodes
Anaesthetized mice were given 250 μg of FITC-ovalbumin (OVA) (Molecular Probes, Carlsbad, CA) intranasally in 50 μl of PBS. Control mice were given unconjugated OVA which served as a gating control for FITC-OVA+ cells. Mediastinal LNs were harvested 24 hr later and processed into single-cell suspensions as described above. Prior to filtering and RBC lysis, cells were re-suspended in calcium- and magnesium-free Hanks’ balanced salt solution (HBSS) containing 10 mm ethylenediaminetetraacetic acid (EDTA) and incubated at room temperature with agitation for 5 min. Cells (1 × 105) were stained in fluorescence-activated cell sorter (FACS)-EDTA buffer24 using the antibodies already described, and analysed by FACSCalibur. DCs were identified by CD11c expression.
Quantification of LN mRNA cytokine levels
Total RNA was isolated from the LN at various days post-infection (p.i.) using the TRIzol method (Invitrogen, Carlsbad, CA). Reverse transcription was carried out using the Superscript II First Strand Synthesis kit (Invitrogen) using oligo (dT) primers. mRNA levels for murine IL-2, IL-12 and GAPDH were determined using quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR). Fluorescent reporters were detected using the Bio-Rad (Hercules, CA) iCycler PCR machine and primers and probes were designed using primer express 1.5 (Applied Biosystems, Foster City, CA). The levels of mRNA for GAPDH were determined for all samples and were used to normalize gene expression. All data are expressed as fold induction over lean controls at day 3 p.i.
Antigen presentation assay
Spleens from uninfected mice were removed and processed into single-cell suspensions by mechanical agitation as described above and pooled (n = 4–5 mice/group). To isolate as many DCs as possible, cell suspensions were treated with EDTA in a similar manner to that described for the FITC-OVA experiment. Cells were then counted and 1 × 108 cells were used to isolate DCs using the DC enrichment kit from Dynal Biotech (Carlsbad, CA). The purity of the CD11c+ population was assessed by flow cytometry and found to be 76%. About 20% of the population was composed of CD11c− B220+ cells, probably B cells,25 which have been shown to be poor stimulators of T cells when antigen is not continuously present in the stimulation culture.26
T cells were isolated from spleens of lean mice that had been infected with influenza 7 days previously. Single-cell suspensions were incubated on plastic for 1 hr and non-adherent cells were collected. This population was shown to be greater than 92% pure by FACS analysis of CD3+ cells.
DCs were incubated at a multiplicity of infection (MOI) of 2 with 56° heat-inactivated influenza A/PR8 for 2 hr followed by extensive washing to remove any excess virus. Serial dilutions of 0·1 ml of DCs, starting at 1 × 106 cells/ml, were plated with 1 × 105 T cells in a 96-well round-bottom microplate. The resulting DC to T-cell ratios were 1 : 1, 1 : 2 and 1 : 4. All samples were prepared in triplicate and incubated for 2 hr at 37°, followed by the addition of Golgi Plug (BD Biosciences) and incubation for an additional 4 hr. Prior to surface staining, Fc receptors were blocked with anti-FcγII/III. Cells were then stained with FITC-anti-CD3, APC-anti-CD5 and PerCp-anti-CD8 (BD Biosciences), followed by fixation and permeabilization for subsequent intracellular staining with PE-anti-IFN-γ. An irrelevant PE-IgG (Sigma) was used as a staining control.
Tetramer staining for antigen-specific T-cell analysis
The tetramer probes were conjugated by the NIH Tetramer Facility at Emory University (Atlanta, GA). The NP366-74 (influenza) peptide was synthesized by the OSU Peptide Synthesis facility (Columbus, OH). The NP366-74 probe consists of four peptides (ASNENMETN) conjugated to a single H-2Db molecule and labelled with commercially manufactured streptavidin-PE.
Lungs were removed and processed into single-cell suspensions as described above. For staining, 1 × 106 cells were used. Cells were Fc-blocked, and then stained with the DbNP366-PE (ASNENMETM) tetramer, FITC-anti-CD3, APC-anti-CD4 and PerCp-anti-CD8 for 45 min at 4° and analysed by flow cytometry. Uninfected mice were used as a staining control and the percentage of non-specific binding was subtracted from values obtained from infected mice.
Enzyme-linked immunosorbent spot-forming cell assay (ELISPOT)
Lungs were lavaged three times with PBS 7 days after infection to obtain bronchoaveolar lavage fluid (BALF). RBCs were lysed, cells were counted, and Lyt-2+ CD8+ T cells were isolated by positive selection using magnetic beads (Dynal Biotech), according to the manufacturer’s directions. Ninety-six-well filtration plates were coated with capture anti-mouse IFN-γ monoclonal antibody (mAb), washed, and blocked according to the manufacturer’s protocol (BD Biosciences). Mitomycin C-treated splenocytes, infected with influenza A/PR8, were used as antigen-presenting cells and 105 cells were added to wells in duplicate. Isolated lung CD8+ cells were added to each well at a concentration of 104 cells/well. Plates were incubated for 12 hr at 37° in 5% CO2. Splenocytes and T cells alone served as negative controls. After washing with PBS/0·1% Tween-20, a biotinylated IFN-γ mAb was added, followed by streptavidin-alkaline phosphatase. The developed spots were then counted manually.
Statistics
Statistical analyses were performed using jmp Statistical Software (SAS Institute, Cary, NC). All results are expressed as mean ± standard error of the mean (SEM). Statistical significance was calculated using Tukey–Kramer honestly significant differences (HSD) or Wilcoxon signed ranks test, with α = 0·05 and P < 0·05, respectively.
Results
Reduced cellularity in influenza-infected lungs of obese mice
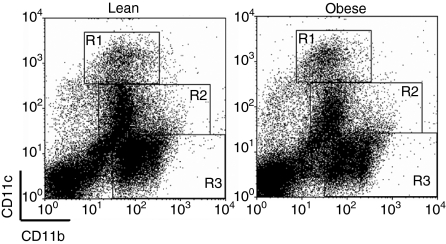
Previous work from our laboratory indicated that innate immune responses were dysregulated during influenza infection in diet-induced obese mice. While viral titres were similar between the groups during infection, we observed minimal type I IFN expression as well as a delay in IL-6 production in obese mice. To determine the reason for this impairment, we infected lean and obese mice and analysed the cellularity of the lung at days 0, 3 and 6 p.i. Lung populations were identified by their CD11b and CD11c expression, as previously described (Fig. 1).21,23 As expected, the total number of cells in the lungs of lean mice increased during infection as a result of mononuclear cell infiltrate (Table 1). However, the progressive infiltration of mononuclear cells was not observed in obese mice until day 6 p.i. While the number of monocytes and DCs had doubled by day 3 p.i. and continued to increase until day 6 in lean mice, there was little change in these cell populations in obese mice until day 6 p.i., yet the number of DCs remained significantly lower. Additionally, the population of cells not expressing CD11c or CD11b, which probably includes naïve T cells27 and epithelial cells,28 was similar between the groups at days 0 and 3, but was significantly lower in obese mice at day 6 p.i. These data indicate that the temporal infiltration of monocytes and lymphocytes during influenza infection is affected by obesity, and therefore may result in impairment of subsequent responses.
Figure 1.
Gating strategy to identify cell populations in lungs. Single-cell suspensions were isolated from whole lung at day 3 post-infection (p.i.) and levels of CD11b and CD11c expression were used to identify alveolar macrophages (R1), dendritic cells (R2) and monocytes/interstitial macrophages (R3).
Table 1.
Cell population in the lung during infection
| Cell type | Phenotype | Mean number of cells × 105 (SEM × 105) | ||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | ||
| Total cells | Lean | 105 (12·6) | 118 (11·9) | 294 (35·0) |
| Obese | 91·5 (15·4) | *67·2 (8·6) | 188 (32·8) | |
| Alveolar macrophages | Lean | 6·4 (0·69) | 5·9 (0·49) | 13·2 (2·3) |
| Obese | *3·1 (0·96) | *4·4 (0·31) | 7·9 (1·6) | |
| Monocytes/interstitialmacrophages | Lean | 18·8 (3·1) | 44·7 (5·2) | 80·8 (14·9) |
| Obese | 17·8 (2·4) | *18·9 (3·0) | 59·4 (14·1) | |
| Dendritic cells | Lean | 15·6 (2·2) | 25·8 (1·7) | 90·0 (12·3) |
| Obese | 16·0 (2·4) | *11·6 (1·2) | *55·6 (9·3) | |
| CD11b– CD11c– | Lean | 62·0 (7·2) | 41·0 (4·0) | 101 (13·6) |
| Obese | 54·1 (9·7 | 31·4 (3·6) | *59·1 (10·2) | |
Cell populations from total lung were identified by CD11b and CD11c expression (Fig. 1) at various time-points post-infection (p.i.). Data are expressed as mean (standard error of mean (SEM)) of three to six animals per group per time-point.
Significantly different from lean mice; P ≤ 0·05.
Enhanced IL-6 production by mononuclear cells of obese mice
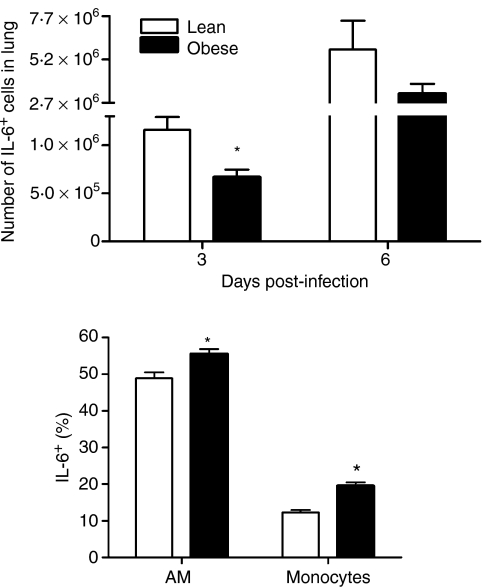
Given the difference in lung cellularity, we determined if the delay in IL-6 production previously observed was attributable to the reduction in cell number or to an inability of the mononuclear cells to produce IL-6. As shown in Fig. 2(a), the total number of IL-6+ cells was significantly reduced at day 3 p.i. in obese mice. Interestingly, this reduction appeared to be caused solely by the decrease in cell number. When the leucocyte populations were analysed for IL-6 production, AMs and monocytes from lungs of obese mice were found to have a higher proportion of IL-6+ cells (Fig. 2b). Furthermore, the amount IL-6 produced by the AMs and monocytes from obese mice was also greater [AMs: lean mean fluorescence intensity (MFI) = 50·9 ± 1·7 versus obese MFI = 58·9 ± 1·2; monocytes: lean MFI = 40 ± 0·9 versus obese MFI = 44·2 ± 0·8; P < 0·05]. By day 6, cells from both lean and obese mice produced high levels of IL-6 (AMs: lean MFI = 106 ± 24·8 versus obese MFI = 68 ± 6·7; monocytes: lean MFI = 196 ± 45 versus obese MFI = 182 ± 45) and the total number of IL-6+ cells was equivalent (Fig. 2a). These data indicate that, while mononuclear cells are slower to enter the lungs of obese mice following infection, resident cells may be hyper-activated early after infection in obese mice.
Figure 2.
Interleukin (IL)-6+ cells in the lung during infection. Single-cell suspensions from influenza-infected lungs were surface-stained with anti-CD11b and anti-CD11c following a 4–6-hr incubation with Brefeldin A. (a). Total number of IL-6+ cells in the lung. (b) The per cent IL-6 production at day 3 p.i. was determined for alveolar macrophage (AM) and monocyte populations using the gating strategy described earlier (see Fig. 1). Data are expressed as the mean ± standard error of the mean for five to six animals per group per time-point. *Significantly different from lean mice; P ≤ 0·05.
Obese mice have fewer lung-migrating DCs during infection
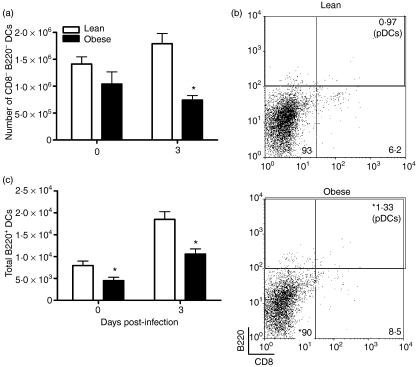
DCs are a critical link between the innate and cell-mediated immune responses. There are several distinct subsets of DC, including cDCs and pDCs. Belz et al. have demonstrated there are other major subsets within the cDC population which include the CD8− B220− CD11c+ cells (DN). These cells appear to play a major role in antigen uptake in the lungs and subsequent migration to the LN. Within the LN, migrating DN cells can present antigen to T cells, as well as transfer antigen to LN-resident DCs (CD8+ B220− CD11c+) for additional presentation.18 As the total number of DCs remained significantly lower in obese mice throughout infection, we hypothesized that the number of DN DCs would also be lower, thus indicating that obese mice may have reduced antigen trafficking to the LN. Therefore, we determined the number of DN cells in the lungs of lean and obese mice prior to and during infection. Prior to infection we found no difference in the number of resident DN DCs between lean and obese mice (Fig. 3a). By day 3 p.i., however, the total number of DN DCs was lower in obese mice and this was not simply a result of a reduction in total DC cell numbers as the percentage of DN cells in the DC population was also significantly lower in obese mice at day 3 p.i. (Fig. 3b). This suggests that infiltration of new DN DCs does not occur in obese mice early during infection, which could lead to reduced antigen accumulation in the LN. In contrast to the DN DC population, the percentage of pDCs (CD11c+ B220+) during infection was greater in obese mice compared with lean mice (Fig. 3b). Despite the amplified increase in the percentage of pDCs, obese mice had significantly lower total numbers of pDCs in their lungs at day 3 p.i. than lean mice, although the total number remained low in both groups (Fig. 3c).
Figure 3.
Population of double-negative (DN) dendritic cells (DCs) and plasmacytoid dendritic cells (pDCs) in the total DC population during influenza virus infection. In the lung, flow cytometry was used to identify the DC subsets within the DC population. (a) Total number of DN DCs in the lung. DN cells were identified as CD8− B220− at days 0 and 3 post-infection (p.i.). Data are expressed as the mean ± standard error of the mean (SEM), with n = 3–6 animals per group. *Significantly different from lean mice; P < 0·05. (b) Gating strategy to identify the proportion of DN DCs and pDCs. Values listed represent the mean percentage in the DC population. The per cent pDCs includes all B220+ cells in the DC gate. *Significantly different from lean mice; P < 0·05 (c) Based on the percentage of pDCs found in (b), the total number of pDCs in the lung was calculated. Data are expressed as the mean ± SEM, with n = 3–6 animals per group. *Significantly different from lean mice; P < 0·05.
DCs accumulate in the LN of obese mice following OVA instillation
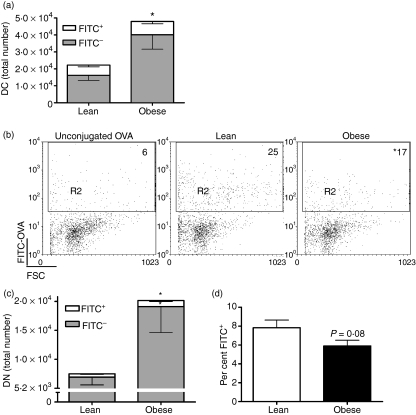
To determine whether DCs transport antigen normally in obese mice, we instilled FITC-OVA intranasally and measured accumulation of FITC+ in CD11c+ cells in LNs after 24 hr. Surprisingly, we found that the total number of CD11c+ cells was higher in LNs from obese mice (Fig. 4a). With the increase in the total number of DCs we expected to see more antigen in the LNs of obese mice. However, we found that obese mice had an equivalent number of FITC+ DCs to lean mice (Fig. 4a). Accordingly, the percentage of the DC population that contained FITC+ was lower in obese mice (Fig. 4b).
Figure 4.
Migration of lung dendritic cells (DCs) to the lymph nodes (LNs). Mice were intranasally instilled with fluorescein isothiocyanate (FITC)-ovalbumin (OVA) or unconjugated OVA (unstained control) and 24 hr later mesenteric lymph nodes (MLNs) were harvested. Flow cytometry was used to analyse the cell populations in the LNs. DCs in the LNs were identified as CD11c+. (a) Total number of FITC+ DCs in the LNs. Data are expressed as the mean ± standard error of the mean (SEM) of three experiments with three to five animals per group per experiment. (b) Percentage of FITC+ cells in the LN DC population. The values presented account for the per cent non-specific staining shown in the first panel and are the mean of three experiments with three to five animals per group per experiment. *P ≤ 0·05. (c) Total number of FITC+ DN DCs. Data are expressed as the mean ± SEM of three experiments with three to five animals per group per experiment. *Significantly different from lean mice; P ≤ 0·05. (d) Percentage of FITC+ DN DCs within the DN DC population. Data are expressed as the mean ± SEM of three experiments with three to five animals per group per experiment. FSC, forward scatter.
As the proportion of lung DN DCs decreased following infection in obese mice (Fig. 2b), it was possible that more DN cells had phagocytosed antigen and migrated to the LNs soon after infection. We determined whether this was the case after FITC-OVA instillation. When we analysed the population of DCs in the LNs we found more DN DCs in obese mice (Fig. 4c), although the proportion within the DC population was similar between the groups (data not shown). Additionally, the number of DN DCs that contained FITC was greater in obese mice (Fig. 4c). In both groups a significant proportion of the DN DCs were FITC− (Fig. 4d). These data indicate that DCs in obese mice are fully capable of migrating to the LNs following antigen uptake.
Impaired antigen presentation by DCs from obese mice
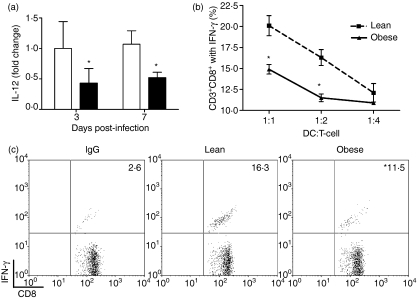
The capacity of the DC to stimulate the appropriate antigen-specific T-cell responses relies on its ability not only to migrate to the LN following antigen uptake but also to present antigen in conjunction with the production of polarizing cytokines such as IL-12.29 We observed decreased IL-12 expression in LNs from obese mice during influenza infection (Fig. 5a). The initial onset of T-cell proliferation during influenza A/PR8 infection began at around day 3 p.i.30 and thus the reduction in IL-12 expression at day 3 p.i. in the LNs suggested that DC stimulation of CD8+ T cells in obese mice may be impaired, despite efficient migration to the LN.
Figure 5.
Dendritic cells (DCs) from obese mice have impaired stimulatory function. (a) Lymph node (LN) interleukin (IL)-12 mRNA expression was quantified by quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) using total RNA. Values are normalized to GAPDH and are expressed as the mean fold increase ± standard error of the mean (SEM) over lean controls at day 3 post-infection (p.i.) (n = 6–8 mice per group). *Significantly different from lean mice; P ≤ 0·05. (b) An antigen presentation assay was performed using T cells isolated from spleens of influenza-infected lean mice at day 7 p.i. These cells were incubated with varying numbers of influenza-loaded DCs isolated from spleens of uninfected lean and obese mice for 6 hr. The percentage of interferon (IFN)-γ+ CD8+ T cells in the CD3+ lymphocyte population was determined by intracellular cytokine staining and flow cytometry. Data are expressed as the mean ± SEM and are representative of two separate experiments (n = 4–5 pooled mice per group per experiment). (c) The flow cytometry gating strategy used to determine the percentage of splenic IFN-γ+ CD8+ T cells within the CD8+ T-cell population. The 1 : 2 DC:T-cell ratio is shown. Values presented have been corrected for the per cent non-specific immunoglobulin G (IgG) staining and are representative of two separate experiments (n = 4–5 pooled mice per group per experiment). *P ≤ 0·05.
To address whether DCs from obese mice were able to stimulate T cells, we negatively selected CD11c+ cells from spleens of uninfected lean and obese mice and stimulated T cells derived from spleens of influenza-primed lean mice. Infection of DCs with heat-inactivated influenza has been shown to effectively induce DC maturation and subsequent stimulation of T-cell proliferation, as the virus retains fusogenic capacity but is non-replicative.31 IFN-γ production by CD8+ T cells was used to determine the effectiveness of the DCs to present antigen. As shown in Fig. 5(b), influenza-pulsed DCs from lean mice effectively presented antigen to CD8+ T cells and elicited an IFN-γ response. However, there was a 30% reduction in the proportion of CD8+ T cells that produced IFN-γ from obese mice when DCs were present at a 1 : 1 or 1 : 2 ratio relative to T cells. Representative flow cytometry gates for the 1 : 2 DC:T-cell ratio are shown in Fig. 5(c). While we cannot rule out an inability of the DCs to phagocytose the virus as a reason for this difference, the FITC-OVA data suggest that antigen uptake by DCs occurs normally and therefore is probably not a factor in the reduced ability of the DCs to stimulate CD8+ T-cell IFN-γ production.
Obese mice have altered antigen-specific CD8 T-cell responses during infection
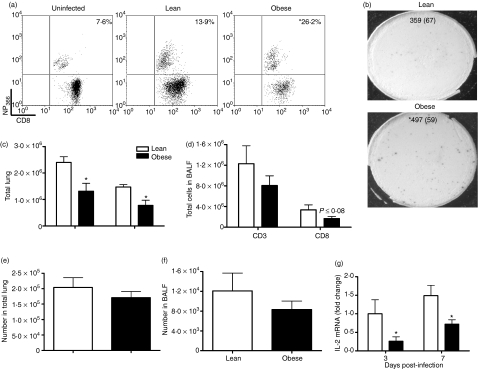
The reduced effectiveness of DCs from obese mice to stimulate CD8 T cells suggested that DC MHC class I presentation was impaired and also suggested that obese mice may have altered T-cell responses. To address this issue, we analysed T cells in the lung at day 7 p.i. to determine the magnitude of the antigen-specific response in influenza-infected obese mice. Additionally we evaluated MHC class I expression on the DCs during infection. Virus-specific CD8+ T cells were identified by binding of the DbNP366-PE tetramer, a peptide derived from the viral nucleoprotein which has been shown to be an epitope recognized by a high percentage of CD8+ T cells.32 Although we found no difference in DC MHC class I expression between lean and obese mice (data not shown), we did find differences in their antigen-specific CD8+ T-cell response. Surprisingly, we found that the frequency of NP366-specific CD8+ T cells was significantly higher in obese compared with lean mice (Fig. 6a). In agreement with previous studies,33 about 14% of CD8 T cells from lean mice were NP366-specific, whereas in obese mice, 26% of CD8+ were influenza-specific. This increase was also seen when the proportion of antigen-specific CD8 T cells from BALF was determined by IFN-γ ELISPOT (Fig. 6b). In this assay, splenocytes were pulsed with influenza virus and therefore presented a variety of viral peptides. We found a 40% increase in the frequency of influenza-specific CD8+ T cells in obese mice. The similarity of the response between the two assays indicates that obese mice have a general increase in the percentage of antigen-specific CD8+ T cells. Additionally, the ELISPOT indicated that antiviral responses were functional in the effector CD8+ T cells.
Figure 6.
Increased frequency of antigen-specific CD8+ T cells in lungs of obese mice. (a) Percentage of NP366+ cells in the lung CD8+ T-cell population at day 7 post-infection (p.i.). Lungs were processed into single-cell suspensions, incubated with the DbNP366-PE (ASNENMETM) tetramer, and analysed by flow cytometry. Values presented are means (n = 6–8 per group) that have been corrected for the per cent non-specific NP366 staining shown in the first panel. *P ≤ 0·05. (b) Percentage of antigen-specific CD8+ T cells from bronchoalveolar lavage fluid (BALF) at day 7 p.i. An interferon (IFN)-γ enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) was performed with 104 CD8+ T cells obtained from BALF by negative selection and incubated with 105 influenza-pulsed splenocytes from lean, uninfected mice. Wells containing only splenocytes or T cells were used as negative controls. Values are presented as the mean (standard error of the mean (SEM)). *P ≤ 0·05. (c, d) Flow cytometry was used to enumerate the total numbers of CD3+ and CD8+ cells in (c) the lung and (d) BALF at day 7 p.i. Data are expressed as mean ± SEM (n = 6–8 per group). *Significantly different from lean mice; P ≤ 0·05. (e, f) The total number of antigen-specific CD8+ T cells in (e) the lung and (f) BALF at day 7 p.i. was calculated by multiplying the percentage of antigen-specific CD8+ T cells determined by flow cytometry (a) and ELISPOT (b) by the total number of CD8+ T cells in the CD3+ population (c and d). Data are expressed as mean ± SEM (n = 6–8 per group). *Significantly different from lean mice; P ≤ 0·05. (g) IL-2 mRNA expression was quantified by quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) using total RNA extracted from lymph nodes (LNs) at days 3 and 7 p.i. Values are normalized to GAPDH and are expressed as fold change from lean mice at day 3 p.i. Values are mean ± SEM; n = 6–8. *Significantly different from lean mice; P ≤ 0·05.
Despite a higher proportion of antigen-specific CD8+ T cells, obese mice did not have more of these cells in their lungs. When the cell populations in the lung were enumerated at day 7 p.i., we found that obese mice had significantly fewer CD8+ T cells than lean mice and the total T-cell population was also reduced in this group (Fig. 6c). A similar trend was seen in the BALF, although the differences were not significant (Fig. 6d). Given the lower number of CD8+ T cells in obese mice, the total number of CD8+ T cells specific for antigen is comparable between the groups (Fig. 6e,f).
The decrease in the number of CD8+ T cells may be related to a decrease in IL-2 production as IL-2 supports CD8+ T-cell proliferation.34 Indeed, we found that, at day 3 and 7 p.i., expression of IL-2 in the LNs of obese mice was significantly diminished (Fig. 6g).
Discussion
Here, we demonstrate that obesity results in impairment in DC recruitment and function during influenza infection. Our previous studies showed that influenza infection in obese mice resulted in delayed pro-inflammatory responses and inhibited type I IFN and chemokine expression.19 This suggested that infiltration of cells into the lungs during infection may be altered in obese mice. Indeed, we saw a delay in the recruitment of monocytes and DCs in the lungs of obese mice, with a sustained decrease in DC numbers throughout infection.
Two major groups of DC exist, pDCs and cDCs, and these can be further subdivided based on the expression of cell surface makers.35 pDCs express B220 and are major producers of IFN-α/β.36 We found that the percentage of pDCs in the DC population of obese mice increased during infection and made up a significantly larger percentage of the population than in lean mice, but there was still a lower total number of pDCs in the lungs of obese mice. Therefore, a reduction in the number of pDCs during infection may explain the diminished expression of IFN-α we previously observed in influenza-infected obese mice.19 Given the low number of pDCs, however, it is also likely that the low level of IFN-α/β expression during influenza infection in obese mice is a result of impairment in production by respiratory epithelial cells.37
In contrast, the proportion of the cDC DN subset decreased during infection in obese mice. cDCs are very efficient at stimulating T-cell responses following migration to the LNs. Studies by Carbone et al. have demonstrated that cDCs with the DN phenotype bring antigen from the lungs to the LNs, where it can be transferred to LN-resident DCs or be presented by the DN DCs themselves.18,38 The decrease in the number of DN cells in the lungs of obese mice could have been a result of these cells already migrating out to the LNs with no new DCs coming in, whereas lean mice maintain a homeostatic level in the lung.
Indeed, we did observe a lack of recruitment of cDCs into the lungs of obese mice. This may be attributable to the reduction in CCL2 [monocyte chemotactic protein (MCP-1)] and CCL5 (RANTES) expression we have previously reported at day 3 p.i. in obese mice.19 Immature DCs respond to these ligands through interactions with receptors on the cell surface which promotes chemotaxis of the immature cell to the inflamed tissue.39 The lack of chemokine induction may also explain the reduced recruitment of monocytes/macrophages to the lung. Additionally, given that obese mice have lower tumour necrosis factor (TNF)-α expression at day 3 p.i.,19 differentiation of DCs from monocyte precursors could also be impaired.40
It is unclear what effect the lack of increase in lung DCs by day 3 p.i. has on the trafficking of antigen to the LNs. Legge et al. showed that accelerated migration to the LNs during influenza infection only occurs within the first 24–48 hr p.i.30,41 However, new DCs continue to enter the LNs, but whether these cells are lung derived and play a role in T-cell responses is unknown.41,42 When we assessed the migratory function of lung-derived DCs, we found that obesity resulted in comparable antigen trafficking to the LNs 24 hr after intranasal FITC-OVA instillation. Therefore, accelerated migration following initial antigenic insult is intact in obese mice. These results are similar to those of another study that used ob/ob mice and demonstrated normal migratory capacity of Langherans cells out of the epidermis in response to TNF-α-induced migration.12 Although migration of DCs from the lung was similar in lean and obese mice, obese mice had an augmented number of FITC− DCs in their lymph nodes. This suggests that obese mice may have an increased number of DCs in their LNs at baseline. Indeed, studies by Verwaerde et al.13 and Macia et al.12 indicate that this increase is a result of greater baseline cellularity. Verwaerde et al. demonstrated that the number of splenic DCs was greater in diet-induced obese mice prior to OVA immunization; however, this difference was abolished 5 days following antigenic stimulation as both groups had increased the number of DCs to a comparable level. Similarly, Macia et al. found higher numbers of Langerhans cells in the epidermis of ob/ob mice compared with lean mice.12 Whether these resident DCs affect the type of response that ensues remains to be determined.
The inability of DCs from obese mice to properly stimulate T cells may be a result of altered cytokine production. This was shown in ob/ob mice, where the altered cytokine milieu resulted in less potent stimulation of allogeneic T cells by bone marrow-derived DCs, despite similar expression of costimulatory molecules.12 In our study, the dampened expression of IL-12 in the LNs of obese mice suggests that DCs from these mice have limited IL-12 production. This may contribute to the reduction in T-cell responses, given that IL-12 acts directly on CD8+ T cells to augment cytolytic activity and IFN-γ production.16,43 Similarly, the overproduction of IL-6 in the AM and monocyte population of obese mice could impair DC presentation. Exaggerated IL-6 production can inhibit monocyte differentiation into DCs and prevent their maturation.44,45 Previous reports indicate that IL-6 can switch DC progenitor commitment away from a DC towards a phenotype that can phagocytose but cannot present antigen.45 Additionally, IL-6 blocks MHC class II, CD80 and CD86 expression on DCs, but does not affect MHC class I.44 Therefore, the impairment in antigen presentation, without a reduction in MHC class I expression, we find in DCs from obese mice could be attributable to the fact that their macrophages are producing more IL-6 than those of lean mice.
In spite of a reduced ability of the DCs from obese mice to stimulate CD8+ T cells from lean mice, influenza-specific CD8+ T-cell responses in vivo remained relatively intact in obese mice. Because of the essentiality of an effective immune response, the immune system has evolved to dynamically eradicate infection when components of the response are deficient.33,46 In this study we found that, while influenza-infected obese mice had fewer CD8+ T cells infiltrating the lung, the frequency of cells that were virus-specific was higher, which resulted in a total number of antigen-specific cells that was comparable to that of lean mice. The CD8+ T-cell response elicited by obese mice suggests that more of the CD8+ T-cell precursor pool is being utilized to clear virus.47 Although this is effective for clearing a primary influenza infection, it may result in a compromised memory response.33,47,48
The decrease in total CD8+ T cells in obese mice could be a result of reduced trafficking to the lungs or reduced proliferation. IL-2 from CD4+ T cells plays a significant role in CD8+ T-cell proliferation34 and its expression is inversely correlated with suppressor of cytokine signalling protein 3 (SOCS3) expression.49 SOCS proteins are a family of cytokine-inducible negative-feedback inhibitors that target cytokine receptors and cytoplasmic signalling adaptor molecules. In diet-induced obesity, SOCS3 up-regulation has been suggested as a mediator of the impaired signal transducer and activator of transcription 3 (STAT3) DNA binding that occurs in T cells from these mice.50 Therefore, it is possible that the reduction in CD8+ T-cell number we observed in obese mice was attributable to SOCS3-inhibited proliferation via prevention of IL-2 expression. Another possibility is that failure to present antigen efficiently resulted in lower T-cell activation and proliferation.30
In conclusion, we demonstrated that diet-induced obesity results in selective impairment of DC functions. Our data indicate that, during influenza infection, obesity leads to delayed recruitment of mononuclear cells to the infected lung. The number of DCs, in particular, is noticeably low throughout infection. While migration of antigen-loaded DCs to the LNs is normal in obese mice, the ability of the DCs to present antigen to CD8+ T cells is impaired. This may be attributable to lack of costimulation by the DCs. The outcome of these alterations was an elevated frequency of virus-specific CD8+ T-cell response in obese mice. Further studies are needed to elucidate the exact mechanism behind these changes in obese mice and to determine whether these alterations result in defective memory responses.
Acknowledgments
The authors thank Erik Karlsson and Jacki Mays for their excellent technical assistance. This work was supported, in part, by grants from the NIH to the Clinical Nutrition Research Unit (DK56350).
Abbreviations
- AM
alveolar macrophage
- BALF
bronchoalveolar lavage fluid
- cDC
conventional dendritic cell
- DC
dendritic cell
- DIO
diet-induced obesity
- DN
double negative
- LN
lymph node
- p.i.
post-infection
- pDC
plasmacytoid dendritic cell
- PFU
plaque-forming unit
Conflict of interest
There are no conflicts of interest.
References
- 1.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Perlemuter GBA, Cassard-Doulcier AM, Naveau S. Nonalcoholic fatty liver disease: from pathogenesis to patient care. Nat Clin Pract Endocrinol Metab. 2007;3:458–69. doi: 10.1038/ncpendmet0505. [DOI] [PubMed] [Google Scholar]
- 3.Norris S, Provo B, Stotts N. Physiology of wound healing and risk factors that impede the healing process. AACN Clin Issues Crit Care Nurs. 1990;1:545–52. doi: 10.4037/15597768-1990-3010. [DOI] [PubMed] [Google Scholar]
- 4.Massie J, Heller J, Abitbol J, McPherson D, Garfin S. Postoperative posterior spinal wound infections. Clin Orthop Relat Res. 1992;284:99–108. [PubMed] [Google Scholar]
- 5.Eliakim A, Swindt C, Zaldivar F, Casali P, Cooper DM. Reduced tetanus antibody titers in overweight children. Autoimmunity. 2006;39:137–41. doi: 10.1080/08916930600597326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber DJ RW, Samsa GP, Santimaw JE, Lemon SM. Obesity as a predictor of poor antibody response to hepatitis B plasma vaccine. JAMA. 1985;254:3187–9. [PubMed] [Google Scholar]
- 7.O’Rourke RW, Kay T, Scholz MH, Diggs B, Jobe BA, Lewinsohn DM, Bakke AC. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg. 2005;15:1463–8. doi: 10.1381/096089205774859308. [DOI] [PubMed] [Google Scholar]
- 8.Ikejima S, Sasaki S, Sashinami H, et al. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005;54:182–9. doi: 10.2337/diabetes.54.1.182. [DOI] [PubMed] [Google Scholar]
- 9.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J Immunol. 2002;168:4018–24. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 10.Cousin B, André M, Casteilla L, Pénicaud L. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. J Cell Phys. 2001;186:380–6. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1038>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Meade C, Sheena J, Mertin J. Immunological changes associated with the obob (obese) genotype. Proc Nutr Soc. 1978;37:38A. [PubMed] [Google Scholar]
- 12.Macia L, Delacre M, Abboud G, Ouk T-S, Delanoye A, Verwaerde C, Saule P, Wolowczuk I. Impairment of dendritic cell functionality and steady-state number in obese mice. J Immunol. 2006;177:5997–6006. doi: 10.4049/jimmunol.177.9.5997. [DOI] [PubMed] [Google Scholar]
- 13.Verwaerde C, Delanoye A, Macia L, Tailleux A, Wolowczuk I. Influence of high-fat feeding on both naive and antigen-experienced T-cell immune response in DO10.11 mice. Scand J Immunol. 2006;64:457–66. doi: 10.1111/j.1365-3083.2006.01791.x. [DOI] [PubMed] [Google Scholar]
- 14.Mito N, Hosoda T, Kato C, Sato K. Change of cytokine balance in diet-induced obese mice. Metabolism. 2000;49:1295–300. doi: 10.1053/meta.2000.9523. [DOI] [PubMed] [Google Scholar]
- 15.Lamas O, Martinez JA, Marti A. Energy restriction restores the impaired immune response in overweight (cafeteria) rats. J Nutr Biochem. 2004;15:418–25. doi: 10.1016/j.jnutbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y-J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 18.Belz GT SC, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–5. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–43. doi: 10.1093/jn/137.5.1236. [DOI] [PubMed] [Google Scholar]
- 20.Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. Adaptive changes in adipocyte gene expression differ in AKR/J and SWR/J mice during diet-induced obesity. J Nutr. 2002;132:3325–32. doi: 10.1093/jn/132.11.3325. [DOI] [PubMed] [Google Scholar]
- 21.Cleret A, Quesnel-Hellmann A, Mathieu J, Vidal D, Tournier J-N. Resident CD11c + lung cells are impaired by anthrax toxins after spore infection. J Infect Dis. 2006;194:86–94. doi: 10.1086/504686. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Juarrero M, Orme IM. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun. 2001;69:1127–33. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–35. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 24.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2000;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneeberger A, Luhrs P, Kutil R, Steinlein P, Schild H, Schmidt W, Stingl G. Granulocyte-macrophage colony-stimulating factor-based melanoma cell vaccines immunize syngeneic and allogeneic recipients via host dendritic cells. J Immunol. 2003;171:5180–7. doi: 10.4049/jimmunol.171.10.5180. [DOI] [PubMed] [Google Scholar]
- 26.Masten BJ, Lipscomb MF. Comparison of lung dendritic cells and B cells in stimulating naive antigen-specific T cells. J Immunol. 1999;162:1310–7. [PubMed] [Google Scholar]
- 27.Christensen JE, Andreasen SO, Christensen JP, Thomsen AR. CD11b expression as a marker to distinguish between recently activated effector CD8+ T cells and memory cells. Int Immunol. 2001;13:593–600. doi: 10.1093/intimm/13.4.593. [DOI] [PubMed] [Google Scholar]
- 28.Meng G, Wei X, Wu X, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–6. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 29.Gately M, Desai B, Wolitzky A, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–82. [PubMed] [Google Scholar]
- 30.Yoon H, Legge KL, Sung S-sJ, Braciale TJ. Sequential activation of CD8+ T cells in the draining lymph nodes in response to pulmonary virus infection. J Immunol. 2007;179:391–9. doi: 10.4049/jimmunol.179.1.391. [DOI] [PubMed] [Google Scholar]
- 31.Fonteneau J-F, Gilliet M, Larsson M, Dasilva I, Munz C, Liu Y-J, Bhardwaj N. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood. 2003;101:3520–6. doi: 10.1182/blood-2002-10-3063. [DOI] [PubMed] [Google Scholar]
- 32.Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza A virus-specific CD8+ T cell responses. J Immunol. 2001;166:4627–33. doi: 10.4049/jimmunol.166.7.4627. [DOI] [PubMed] [Google Scholar]
- 33.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8+-T-cell memory in CD4+-T-cell-deficient mice. J Virol. 2002;76:12388–93. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cousens L, Orange J, Biron C. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–9. [PubMed] [Google Scholar]
- 35.Shortman K, Liu Y-J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 36.Vremec D, O’Keeffe M, Hochrein H, Fuchsberger M, Caminschi I, Lahoud M, Shortman K. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109:1165–73. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]
- 37.Jewell NA, Vaghefi N, Mertz SE, et al. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J Virol. 2007;81:9790–800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–6. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- 39.Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 2005;16:581–92. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Szabolcs P, Avigan D, Gezelter S, Ciocon D, Moore M, Steinman R, Young J. Dendritic cells and macrophages can mature independently from a human bone marrow-derived, post-colony-forming unit intermediate. Blood. 1996;87:4520–30. [PubMed] [Google Scholar]
- 41.Legge KL, Braciale TJ. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity. 2003;18:265–77. doi: 10.1016/s1074-7613(03)00023-2. [DOI] [PubMed] [Google Scholar]
- 42.Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–48. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- 43.Bhardwaj N, Seder RA, Reddy A, Feldman MV. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J Clin Invest. 1996;98:715–22. doi: 10.1172/JCI118843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park S-J, Nakagawa T, Kitamura H, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–54. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 45.Ratta M, Fagnoni F, Curti A, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–7. doi: 10.1182/blood.v100.1.230. [DOI] [PubMed] [Google Scholar]
- 46.Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty P. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J Exp Med. 1991;174:875–80. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripp R, Sarawar S, Doherty P. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2lAb gene. J Immunol. 1995;155:2955–9. [PubMed] [Google Scholar]
- 48.Tripp R, Hou S, McMickle A, Houston J, Doherty P. Recruitment and proliferation of CD8+ T cells in respiratory virus infections. J Immunol. 1995;154:6013–21. [PubMed] [Google Scholar]
- 49.Yu C-R, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem. 2003;278:29752–9. doi: 10.1074/jbc.M300489200. [DOI] [PubMed] [Google Scholar]
- 50.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–52. doi: 10.4049/jimmunol.176.12.7745. [DOI] [PubMed] [Google Scholar]