Abstract
Single-stranded versus multimeric phosphorothioate-modified CpG oligodeoxynucleotides (ODNs) undergo differential endosomal trafficking upon uptake into plasmacytoid dendritic cells (pDCs), correlating with Toll-like receptor-9-dependent pDC maturation/activation (single-stranded B-type CpG ODN) or interferon-α (IFN-α) induction (multimeric A-type CpG ODN), respectively. As was recently shown, IFN-α production, other than by CpG ODNs, can also be induced in a sequence-independent manner by phosphodiester (PD) ODNs multimerized by 3′ poly-guanosine (poly-G) tails. We investigate here the type of endosomal trafficking responsible for IFN-α induction by natural PD ODN ligands. We show that 3′ extension with poly-G tails leads to multimerization of single-stranded PD ODNs and to enhanced cellular uptake into pDCs. While monomeric PD ODNs, which induce CpG-dependent Toll-like receptor-9 stimulation and pDC maturation/activation, localized to late endosomal/lysosomal compartments, the poly-G multimerized PD ODNs, which induce CpG-independent IFN-α production, located to vesicles with a distinct, ‘early’ endosomal phenotype. We conclude that poly-G-mediated multimerization of natural PD ODNs allows for sequence-independent, Toll-like receptor-9-dependent IFN-α induction in pDCs by combining three distinct effects: relative protection of sensitive PD ODNs from extracellular and intracellular DNase degradation, enhanced cellular uptake and preferential early endosomal compartmentation.
Keywords: interferon-α production, plasmacytoid dendritic cells, phosphodiester DNA, sequence independence, Toll-like receptor-9
Introduction
In the course of infection or incomplete clearance of cell damage, immune cells sense nucleic acids either via cytosolic RNA and DNA receptors1–3 or via endosomally expressed Toll-like receptors (TLRs).4 Endosomal TLRs comprise TLR-9 which recognizes single-stranded (ss) phosphorothioate-modified (PS) CpG DNA5,6 or natural, random sequence phosphodiester (PD) DNA,7 TLR-7/8 which recognizes ss RNA8,9 and TLR-3 which recognizes double-stranded (ds) RNA.10 Both TLR-7 and TLR-9 are highly expressed in plasmacytoid DCs (pDCs), and stimulation of pDCs but not of myeloid DCs (mDCs) with appropriate TLR-7 and TLR-9 ligands leads to induction of large amounts of interferon-α (IFN-α) in a myeloid differentiation primary response protein 88 (MyD88) and IFN regulatory factor (IRF-7)-dependent manner.11 The TLR-induced IFN-α production from pDCs, also termed natural IFN-producing cells,12 plays a critical role in immune responses against a variety of pathogens and is also implicated in the pathogenesis of various types of autoimmune diseases.13 Elucidating the molecular mechanism of TLR-9-dependent IFN-α induction by DNA ligands in pDCs is therefore crucial for the understanding and treatment of such diseases.
The stimulatory effect of bacterial or viral genomic DNA on TLR-9, usually attributed to unmethylated CpG motifs, which are underrepresented and selectively methylated in mammalian genomes,14 is thought to be mimicked by short synthetic oligodeoxynucleotides (ODNs) carrying CpG-motifs (CpG ODNs).15 In contrast to natural PD ODNs, the PS-modified ODNs not only are relatively DNase resistant but also display efficient cellular uptake and endosomal translocation.16,17 Therefore, PS-modified ODNs have commonly been used to elucidate sequence requirements for the activation of TLR-9. These studies led to the definition of canonical CpG sequence motifs and to the description of different classes of TLR-9-activating CpG ODNs, each triggering distinct biological responses.15 The B-type CpG ODN 1668 (CpG-B ODN), for example, leads to robust proinflammatory cytokine production and DC maturation/activation in mixed DC cultures, but causes only marginal induction of IFN-α in pDCs.15 The A-type CpG ODN (CpG-A ODN), in contrast, is a potent inducer of IFN-α but is less efficient at proinflammatory cytokine production or DC maturation.
We have recently shown that the CpG-motif dependency of TLR-9 activation is a feature that characterizes PS-modified ODNs.7 It is based on the exclusive ability of CpG motifs to overcome the TLR-9 inhibitory quality of PS-modified 2′ deoxyribose sugar backbones. In contrast, PD ODNs, whose base-free PD 2′ deoxyribose sugar backbones possess basal TLR-9 stimulatory potential, activate TLR-9 sequence independently upon enhanced translocation into endosomes; this is achieved by multimerization either via 3′ poly-G extension or the cationic lipid N-[1-(2,3-dioleoyloxy)]-N,N,N-trimethylammonium propan methylsulphate (DOTAP). Importantly, TLR-9 activation by 3′ poly-G-multimerized or DOTAP-complexed PD ODNs not only induces proinflammatory cytokine production and pDC maturation/activation in mixed DC cultures (Flt3 ligand-DCs) but also IFN-α production in pDCs.7
The TLR-9-dependent IFN-α induction by PS-modified CpG ODNs has been demonstrated to rely on the spatiotemporal regulation of ligand interaction with TLR-9 in pDCs. Single-stranded PS-modified CpG-B ODNs, which contain a canonical CpG motif (GTCGTT), translocate within minutes to late endosomal/lysosomal compartments of pDCs,18,19 where they interact with the TLR-9/MyD88/tumour-necrosis factor receptor-associated factor 6 (TRAF6)/IL-1 receptor-associated kinase 1/4 (IRAK1/4) complex for nuclear factor-κB activation. In contrast, CpG-A ODNs have a palindromic PD core sequence with two CpG motifs flanked by PS-modified 5′ and 3′ poly-G tails, which favours multimerization via Hoogsteen base-pairing and G-tetrad formation. They preferentially locate to early endosomal compartments, even hours after uptake into pDCs,18,19 presumably because of their multimeric structure which contains both globular and linear components.20 In early endosomes, multimeric CpG-A ODNs interact with TLR-9 which, in contrast to late endosomal TLR-9, specifically associates with the MyD88/IRF-7 signalling complex to induce production of IFN-α.18 Therefore, subtle endosomal routing of CpG ODNs appears to determine IFN-α production in pDCs. Accordingly, complex formation between ss CpG-B ODNs and DOTAP or polymyxin B (PMXB), which produces higher-order structures, not only leads to retention in early endosomal compartments but also to potent IFN-α induction.18,19,21 The tertiary structure of CpG ODN ligands (monomeric versus multimeric) seems to determine the type of endosomal routing and, therefore, the character of the pDC response.
Here we explored whether the functional tie, which links the multimeric tertiary structure of PS CpG ODNs with early endosomal routing and IFN-α production, also applied for IFN-inducing natural PD ODN ligands. We report that 3′ poly-G extension led to multimerization of ss PD ODNs, which efficiently induced selective early endosomal compartmentation and so drove TLR-9-dependent but sequence-independent IFN-α induction in pDCs.
Materials and methods
Oligodeoxynucleotides
CpG-A ODN 2216 (GsGsGGGACGATCGTCGsGsGsGsGsG) was obtained from Coley Pharmaceuticals (Wellesley, MA); where s represents a phosporothioate linkage. All other ODNs (CpG-B ODN 1668: TCCATGACGTTCCTGATGCT; non-CpG ODN AP-1: GCTTGATGACTCAGCCG GAA; non-CpG ODN pTC: TCTCTCTCTCTCTCTCTCTCTCTCT) were commercially synthesized by TIB Molbiol (Berlin, Germany).
Mice
Male wild-type C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) and male TLR9−/− mice (provided by S. Akira) were bred at our specific pathogen-free animal facility according to German federal regulations and institutional guidelines.
Polyacrylamide gel electrophoresis
For electrophoresis, 1·5 nmol ODNs, suspended in loading buffer [1 × Tris-borate-EDTA (TBE), 50% formamide], were run on a denaturing polyacrylamide gel (15% polyacrylamide, 8 m urea, 1 × TBE) using a constant electrical field of 40 V/cm. For visualization of DNA, the gel was fixed for 30 min in 25% methanol/10% acetic acid, incubated overnight in Stains-All solution [0·5‰ Stains All (Sigma-Aldrich, Schnelldorf, Germany), 50% formamide/H2O] and then washed in 50% formamide/H2O until the background staining faded.
Preparation of DCs
Bone marrow cells were harvested from mouse femurs and tibias and cultured for 8 days in complete RPMI [RPMI-1640 with l-glutamine, heat-inactivated 10% fetal calf serum (FCS), 100 μg/ml streptomycin and 50 μm 2′ mercaptoethanol; all from PAA Laboratories (Cölbe, Germany)] conditioned with recombinant murine Flt3-ligand (Flt3L; WEHI, Melbourne, Australia).
Cell sorting
For confocal microscopy, pDCs were enriched from Flt3L-cultured bone marrow-derived DCs using magnetic-activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) as described previously.18 In short, collected cells were incubated with pDC-specific rat monoclonal α-120G8-Biotin antibody22 and α-biotin microbeads (Miltenyi Biotec) and separated into pDCs (positively selected cells) and mDCs (flow-through cells) using a MACS column (Miltenyi Biotec). For cell stimulation, pDCs were highly enriched by fluorescence-acitvated cell sorting (FACS; using a FACS Aria; BD Biosciences, Heidelberg, Germany) after staining with α-120G8-fluorescein isothiocyanate and α-B220-phycoerythrin (BD Biosciences) antibodies. Live/dead discrimination was performed with propidium iodide (Invitrogen, Karlsruhe, Germany). The purity of the FACS-sorted cells was controlled on a CyAn ADP Lx (Dako, Glostrup, Denmark) and found to be > 99%.
Cell stimulation
The Flt3L-DCs were suspended in 500 μl RPMI-1640 with 10% FCS, 50 μm 2′ mercaptoethanol on 24-well plates and incubated for the indicated times and indicated concentrations of ODNs. For measurement of cytokine induction, culture supernatants were collected for analysis using an enzyme-linked immunosorbent assay specific for mouse IFN-α (compiled from rat anti-mouse IFN-α antibody), rabbit anti-mouse IFN-α antibody (both Tebu-Bio, Offenbach, Germany), POX-donkey anti-rabbit immunoglobulin G antibody (Jackson Laboratories). For complex formation with DOTAP (Roche, Penzberg, Germany), ODNs were suspended in 50 μl Opti-Mem (Invitrogen), combined with 50 μl DOTAP solution (10 μg in Opti-Mem), incubated for 15 min at room temperature and added to cells. For complex formation with PMXB (Sigma-Aldrich), ODNs were suspended in 50 μl tissue culture medium, combined with 50 μl PMXB solution (0·5 mg in tissue culture medium), incubated for 30 min at room temperature and added to cells (essentially as described in ref. 19).
ODN uptake
Uptake of ODN was measured as described previously.23 In brief, 0·5 × 106 Flt3L-DCs were incubated with Cy5-labelled ODNs, DOTAP complexes of ODNs or PMXB complexes of ODNs in 500 μl complete RPMI for 30 or 90 min. Cells were harvested, washed with ice-cold phosphate-buffered saline (PBS), incubated with 12·5 mg/ml dextran sulphate (Sigma- Aldrich) for 10 min on ice (to remove ODNs bound to the cell surface), washed in PBS, fixed with 2% paraformaldehyde and analysed by FACS.
Confocal imaging
For analysis of live cells, 0·4 × 106/ml pDC were incubated with 2 μm fluorescent ODNs (labelled with Cy3 or Cy5) in 250 μl RPMI + 10% FCS on eight-well ibiTreat μ-slides (Ibidi, Munich, Germany) for 45 min at 37°, then washed once with PBS and used for microscopy in a chamber heated to 37°. For analysis of antibody-stained cells, 0·4 × 106/ml pDC were incubated with 2 μm fluorescent ODNs (Cy5) in 250 μl RPMI + 10% FCS on 96-well plates for 45 min, washed three times with ice-cold PBS, fixed in PBS containing 1% paraformaldehyde at room temperature for 15 min and permeabilized/blocked with 0·25% saponin, 1% bovine serum albumin in PBS for 30 min. Labelling was performed with rat anti-LAMP-1 antibody (BD Biosciences) at 1 μg/ml (1 hr) and goat anti-rat immunoglobulin G Alexa546 antibody (Invitrogen) at 10 μg/ml (30 min) in 0·25% saponin, 1% bovine serum albumin/PBS. Fixed cells were washed once with PBS, resuspended in 250 μl PBS on eight-well ibiTreat μ-slides and used for microscopy. Confocal images were acquired using a Leica SP5 confocal microscope (Leica microsystems GmbH, Wetzlar, Germany), 63 ×/1·4 NA objective, with the pinhole set to 1 airy unit in each channel. Dual colour images were acquired using a sequential acquisition mode to avoid cross-excitation.
Results
G-tetrad mediated multimerization of natural PD ODNs leads to CpG-motif-independent IFN-α induction
The endosomal compartment which hosts the interaction of TLR-9 with its ligand presumably plays a crucial role for IFN-α induction in pDCs. Multimeric CpG ODNs which activate TLR-9 in early endosomes (e.g. CpG-A ODN) induce high amounts of IFN-α, whereas monomeric CpG ODNs which do so in late endosomes/lysosomes (e.g. CpG-B ODN) preferentially induce pDC maturation/activation but not IFN-α production.18,19
Multimerization of monomeric CpG-B ODNs can be obtained by complex formation with DOTAP18 or PMXB19 and so leads to IFN-α induction, whereas CpG-A ODNs endogenously present as stable multimers because of intermolecular G-tetrad formation, mediated by poly-G nucleotides flanking the 5′ and 3′ ends.20 It has been shown that poly-G-mediated multimerization of DNase-sensitive natural PD ODNs not only conveys relative resistance against DNase-mediated degradation but also improves cellular uptake.7,24,25 Consequently, we first analysed whether 3′ poly-G extension was sufficient for multimer formation and, in combination with enhanced endosomal uptake, would allow for IFN-α induction by natural PD ODNs.
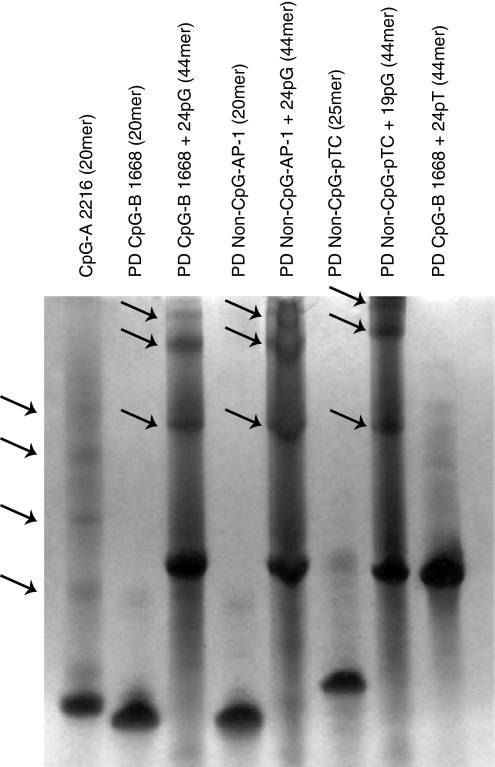
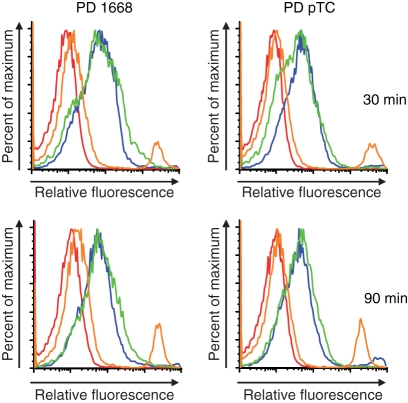
Indeed, polyacrylamide gel electrophoresis revealed that 3′ extension with 24 Gs of both PD CpG-B ODN and PD non-CpG ODNs (PD AP-1 ODN; PD pTC ODN) readily induced formation of stable multimers, comparable to CpG-A ODN multimers, whereas non-extended ODNs as well as ODNs extended with poly-thymidine, poly-adenosine or poly-cytidine tails displayed a monomeric structure (Fig. 1 and data not shown). Multimerization of natural, random sequence PD ODNs can therefore be induced by attaching poly-G tails to the 3′ ends. In analogy to CpG-A ODN,20 these effects are most likely mediated by intermolecular Hoogsteen base pairing between guanosine nucleotides with consequent formation of stable multimers. To confirm the impact of 3′ poly-G extension on PD ODN uptake, we incubated Flt3L-DCs, which contained an average of 20–30% pDCs and 70–80% mDCs (data not shown), with fluorescently labelled PD ODNs. The 3′ poly-G-extended PD ODNs displayed strongly increased uptake by pDCs after 30 and 90 min, similar to DOTAP-complexed PD ODNs. No relevant differences were observed between the uptake characteristics of multimerized PD CpG-B ODNs or PD non-CpG ODNs (see Fig. 5).
Figure 1.
Multimerization of phosphodiester oligodeoxynucleotides (PD ODNs) by 3′ poly-guanosine (G) extension. 1·5 nmol of indicated ODNs were run on a 15% polyacrylamide gel under denaturing conditions. Bands were visualized with STAINS ALL solution. Flashes indicate multimeric forms of CpG-A ODNs or 3′ poly-G-extended PD ODNs.
Figure 5.
PDC uptake of single-stranded (ss) versus multimerized phosphodiester oligodeoxynucleotides (PD ODNs). Wilde-type (WT) Flt3 ligand-DCs (0·5 × 106) were incubated with 2 μm of Cy5-labelled PD ODNs for 30 min or 90 min, harvested and stained with α-120G8-fluorescein iosthiocyanate and α-B220-phycoerythrin antibodies. Double-positive cells (pDCs) were analysed for relative fluorescence intensity, representative of intracellular ODNs, by fluorescence activated cell sorting. Red: PD ODN. Orange: PD ODN + PMXB. Blue: PD ODN + 24pG. Green: PD ODN + DOTAP. Data representative of test independent experiments.
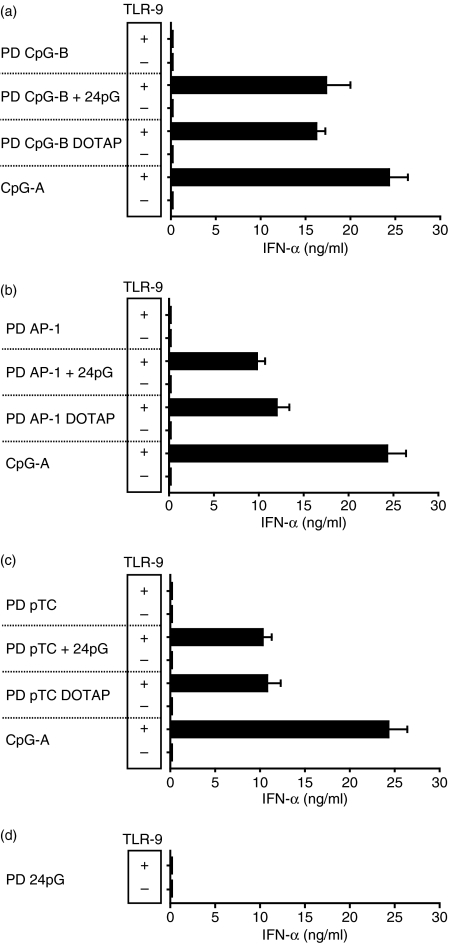
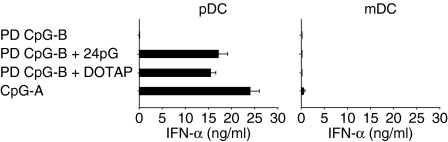
We next explored whether multimerized PD ODNs would, upon uptake into Flt3L-DCs, activate TLR-9 to produce IFN-α. As shown in Fig. 2(a), 3′ poly-G-extended, multimerized PD CpG-B ODNs, in contrast to monomeric PD CpG-B ODNs, induced robust, TLR-9-dependent IFN-α production, similar to prototypic CpG-A ODNs or DOTAP-complexed PD CpG-B ODNs. Interestingly, PD ODNs lacking canonical CpG motifs (PD AP-1 ODN; PD pTC ODN) also displayed TLR-9-dependent IFN-α production upon 3′ poly-G extension (Fig. 2b,c). As shown previously,7 the IFN-α induction capacity of poly-G-extended PD ODNs was, over a wide range of concentrations, comparable to that of PD ODNs complexed to DOTAP. We used PD tails of 24 Gs for these experiments because shorter tails (12 Gs, 4 Gs) were gradually less efficient, whereas longer tails (44 Gs) did not further enhance IFN-α production.7 Importantly, PD poly-G tails alone, with no other sequence attached, did not induce IFN-α production at all (Fig. 2d), providing further evidence that TLR-9-mediated IFN-α induction by PD ODNs is the result of multimerization of the original PD ODN sequence. In general, IFN-α production in Flt3L-DC cultures was restricted to pDCs (Fig. 3). Of note, 3′ poly-G-extended PS-modified CpG-B ODNs or PS non-CpG ODNs failed to activate the IFN-α signalling pathway in Flt3L-DCs (data not shown). Poly-G-mediated multimerization of natural ss PD ODNs therefore allows for TLR-9-dependent IFN-α induction in the absence of canonical CpG motifs. Given that PD ODNs lacking CpG motifs display lower affinity for TLR-9 than PD CpG ODNs,7,26 we propose that enhanced cellular uptake of poly-G-extended PD ODNs provokes increased endosomal ligand concentrations, which, similar to DOTAP-complexed PD ODNs,26 reveals the stimulatory potential of naturally less affine unmodified PD non-CpG ODNs.7
Figure 2.
Poly-guanosine (G) mediated multimerization of phosphodiester oligodeoxynucleotides (PD ODNs) induces interferon-α (IFN-α) production. Wild-type (WT) or TLR9−/− Flt3 ligand dendritic cells (Flt3L-DCs; 1 × 106/well) were incubated for 18 hr with 3 μm of the indicated ODNs or ODNs + DOTAP (a–d). IFN-α levels were detected in culture supernatants by enzyme-linked immunosorbent assay. Mean values and standard deviations of three independent experiments are shown (Fig. 2a–d).
Figure 3.
Interferon-α (IFN-α) production is restricted to plasmacytoid dendritic cells (pDCs) in mixed Flt3 ligand-cultured bone-marrow-derived dendritic cells. α-120G8/α-B220 double-positive cells (pDCs) were purified from wild-type (WT) Flt3 ligand-cultured bone marrow cells by FACS-ARIA. WT pDCs (0·5 × 106/well) were incubated for 18 hr with indicated ODNs or ODNs + DOTAP. Data are representative of three independent experiments.
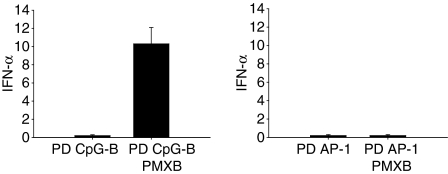
Monomeric PS-modified or PD CpG-B ODNs are poor IFN-α inducers.15 However, when integrated into PMXB complexes to form large microparticles, human pDCs were shown to respond with IFN-α production,27 highlighting the importance of a multimeric TLR-9 ligand structure for IFN-α induction. Although we confirmed these results with murine Flt3L-DCs using PS and PD CpG-B ODNs, we noted that PD non-CpG ODNs in complex with PMXB failed to induce IFN-α production in pDCs (Fig. 4). On the other hand, multimerization via 3′ poly-G extension or complex formation with DOTAP was effective (Fig. 2b,c). The TLR-9 activation potential of a specific ligand obviously depends on both its TLR-9 affinity and its endosomal concentration. Since complex formation with PMXB reportedly does not improve PS ODN uptake,27 we analysed whether the lack of IFN-α induction by less affine PD non-CpG ODNs in complex with PMXB correlated with poor cellular uptake. Indeed, multimerization via poly-G tails or complex formation with DOTAP resulted in strongly enhanced cellular uptake of natural PD ODNs, whereas complex formation with PMXB displayed only marginal effects (Fig. 5). Fluoresence signals obtained for each ODN preparation represented intracellular ODN, because both washing pDCs with dextran sulphate23 and comparison of ODN incubation at 4° (which allows ODN binding to the cell membrane but not endocytosis) and 37° (which allows endocytosis) yielded similar results (data not shown). These data underscore our assumption that one of the determinants that control the ability of PD non-CpG ODNs to induce IFN-α is the efficiency of cellular uptake. As a consequence, increased endosomal concentrations of multimerized PD non-CpG ODN ligands (as obtained by 3′ poly-G extension or DOTAP complex formation but not by PMXB complex formation) allow TLR-9 activation and IFN-α induction despite lower TLR-9 affinity. Monomeric TLR-9 ligands with higher receptor affinity (e.g. PD CpG-B ODN7) therefore acquire the ability to induce IFN-α upon simple multimerization, whereas less affine, monomeric TLR-9 ligands (e.g. PD non-CpG ODN, which display intermediate TLR-9 affinity7) additionally require enhanced endosomal concentrations.
Figure 4.
Interferon-α (IFN-α) induction by polymyxin B (PMXB) complexes of phosphodiester oligodeoxynucleotides (PD ODNs) is CpG-dependent. Wild-type (WT) Flt3 ligand-dendritic cells (DCs; 1 × 106/well) were incubated for 18 hr with 3 μm of PD ODNs or PD ODNs + polymyxin B (PMXB; 0·5mg). IFN-α levels were detected in culture supernatants by enzyme-linked immunsorbent assay. Mean values and standard deviations of three independent experiments are shown.
Multimerization determines the type of endosomal routing
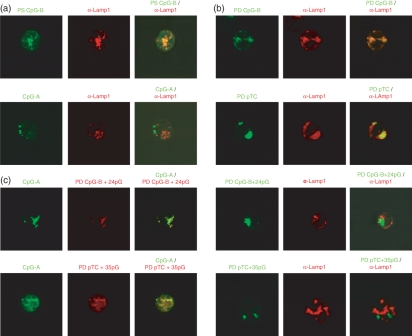
To investigate whether differential endosomal compartmentation of ss PD ODNs versus multimerized PD ODNs accounts for the ability of the latter to induce IFN-α, we analysed the endosomal localization of fluorescently labelled PD ODNs in pDCs enriched from Flt3L-cultured murine bone marrow cells. Using confocal microscopy, we first confirmed that ss PS and PD CpG-B ODN, which induce pDC maturation and activation but not IFN-α production, in murine pDCs primarily localized to LAMP-1-positive, i.e. late endosomal compartments (Fig. 6a,b). In contrast, multimeric CpG-A ODN, a prototypical IFN-α inducer, mainly localized outside these LAMP-1-positive compartments (Fig. 6a). Interestingly, also IFN-α inducing 3′ poly-G extended, thus multimerized CpG-B ODN (PD CpG-B + 24pG ODN) and non-CpG ODN (PD pTC + 35pG ODN) did not localize to LAMP-1-positive compartments (Fig. 6b). Despite rigorous efforts, we failed to confidently identify these as LAMP-1-negative, presumably early endosomal compartments using commercially available early endosomal markers as Dextran or Transferrin (for live cells)17,18 or α-transferrin receptor or α-EEA1 antibodies (for fixed cells16,19,28) so we used colocalization with CpG-A ODN (shown by others to route to early endosomes)18,19 as a surrogate marker for early endosomal localization. Upon 3′ poly-G extension and so multimerization, PD CpG-B ODN (PD CpG-B + 24pG ODN) but not ss PD CpG-B ODN colocalized with IFN-α-inducing CpG-A ODN, as did 3′ poly-G-extended non-CpG ODN (PD pTC + 35pG ODN) (Fig. 6c and data not shown). As expected, all labelled ODNs which colocalized with CpG-A ODN in early endosomes efficiently induced IFN-α, whereas ODNs in LAMP-1-positive compartments did not. It has been previously shown that the ability of poly-G tails to induce G-tetrads is critical for these effects, because disruption of G-tetrad formation and, thus, multimerization in IFN-α inducing ODNs (e.g. CpG-A ODN) by using 7′ deazaguanosine poly-G tails abolishes early endosomal compartmentation and IFN-α production.19,20 Taken together, these data clearly demonstrate that the spatial organization (monomeric versus multimeric) of PD ODN ligands determines the type of intracellular routing and so the potential to induce IFN-α in pDCs. Early endosomal compartmentation of multimeric PD ODN ligands is a critical component of IFN-α induction in pDCs. Overall, we propose that 3′ poly-G extension of PD ODNs leads to IFN-α induction by combining at least three different effects: poly-G-mediated multimer formation conveys relative protection from DNases, which, together with enhanced cellular uptake, leads to increased endosomal ligand concentrations, thus enabling TLR-9 activation even by PD non-CpG ODNs, which display only intermediate TLR-9 affinity.7,21 Finally, multimer formation determines the differential routing of PD ODN ligands to early endosomes, allowing for the activation of the TLR-9/MyD88/IRF-7 signalling pathway of IFN-α induction.
Figure 6.
Poly-guanosine (G)-mediated multimerization of phosphodiester oligodeoxynucleotides (PD ODNs) leads to differential endosomal compartmentation in plasmacytoid dendritic cells (pDCs). The pDCs were enriched from Flt3 ligand cultures using MACS-sorting with α-120G8 antibody. (a) Purified pDCs (0·1 × 106) were first incubated with 2 μm Cy5-labelled PS CpG-B ODN or Cy-5-CpG-A ODN for 45 min and then fixed. Late endosomal/lysosomal compartments were stained with rat anti-LAMP-1/anti-rat immunoglobulin G Alexa 546 antibodies. Fixed cells were analysed by confocal microscopy (left panels: ODN; middle panels: α-LAMP-1; right panels: overlay). (b) Purified pDCs (0·1 × 106) were first incubated with 2 μm Cy5-labelled PD ODN or Cy5-PD ODN + poly-G. Cells were then fixed, stained and analysed as in (a). (Left panels: ODN; middle panels: α-LAMP-1; right panels: overlay). (c) Purified pDCs (0·1 × 106) were coincubated with 2 μm prototypical interferon-α (IFN-α) -inducing CpG-A ODN and either PD CpG-B + 24pG ODN (upper panels) or PD non-CpG pTC + 35pG ODN (lower panels). After 45 min, live cells were washed with phosphate-buffered saline and analysed by confocal microscopy at 37°. (Left panels: CpG-A ODN; middle panels: PD ODN + poly-G; right panels: Overlay). All data are representative of at least three independent experiments.
Discussion
Depending on the mode of TLR-9 activation, pDCs either produce high amounts of IFN-α or mature into fully activated antigen-presenting DCs.15 This functional dichotomy allows pDCs to coordinate aspects of the innate and adaptive immune response to a variety of pathogens.29 It appears that these distinct pDC activation modes are determined by the subcellular compartment which hosts TLR-9 activation.18,19 Upon endocytosis, TLR-9 ligands are transported to mildly acidic early endosomes. These cargo vesicles, migrating towards the cell interior along the microtubular cytoskeleton, then undergo gradual transformation into increasingly acidic late endosomes and eventually fuse with hydrolase-positive, acidic lysosomes, where their content is degraded.17,30 This process seems to be tightly regulated and can take minutes or hours, depending on the physical structure of the DNA cargo.18,19 Since TLR-9 is expressed within endolysosomal compartments, ligand–receptor interaction can potentially occur at any step along the endolysosomal maturation pathway. For example, multimeric CpG-A ODNs, known as prototypical IFN-α inducers, primarily locate to endosomal vesicles expressing an ‘early’ phenotype (as defined by dextran accumulation in murine pDCs18 and by the presence of the transferrin-receptor or of the early endosomal antigen 1 (EEA1) in human pDCs).17,19,28 Activation of TLR-9 within these early endosomal vesicles presumably leads to recruitment of the MyD88/IRF-7 signalling complex and consequently allows induction of high concentrations of IFN-α.18 Monomeric CpG-B ODNs, on the other hand, primarily translocate to endosomal vesicles expressing a ‘late’ phenotype (as characterized by accumulation of lysotracker or presence of the specific late endosomal/lysosomal marker LAMP-1 in the membrane).18,19 The TLR-9 activation in late endosomes induces full maturation and activation of pDCs, based on specific recruitment of the MyD88/TRAF6/IRAK1/4 signalling complex and, therefore, predominant nuclear factor-κB activation.19,31 In support, manipulation of the endosomal routing of monomeric TLR-9 ligands by complex formation with cationic lipids or nanoparticles leads to early endosomal compartmentation and converts CpG-B ODNs into potent IFN-α inducers.18–21 On the other hand, CpG-A ODNs, rendered single-stranded and so functioning as simple monomers, locate to late endosomes, lose their IFN-α induction capacity but induce pDC maturation and activation instead.19 These data suggest that the tertiary structure (monomeric versus multimeric) of CpG ligands determines the endosomal compartmentation and so the mode of pDC activation.
In exploring the rules governing TLR-9 activation by natural PD ODNs versus PS-modified ODNs, we recently observed that base-free PD 2-deoxyribose homopolymers, which constitute the regular sugar backbones of natural PD ODNs, act as basal TLR-9 agonists upon enhanced endosomal translocation. The presence of DNA bases, even when short of CpG motifs, enhances the TLR-9-stimulatory effect. The PS 2-deoxyribose homopolymers, in contrast, act as TLR-9 and TLR-7 antagonists. Alignment of random bases does not alter such antagonist activity, but DNA bases containing a CpG-motif transform TLR-9-inhibitory into robust TLR-9-stimulatory activity. These data showed that TLR-9 activation by PS-modified ODNs is strictly CpG-motif-dependent, whereas natural PD ODNs drive the TLR-9 activation sequence independently.7,21 In these experiments, enhanced endosomal uptake of natural PD ODNs was achieved either by complex formation with DOTAP or by 3′ extension with poly-G tails. Surprisingly, multimerized PD ODNs, even lacking CpG motifs, not only led to DC activation and proinflammatory cytokine induction in Flt3L-DCs but also caused TLR-9-dependent IFN-α production in pDCs.7,21 Here, we analysed the conditions of IFN-α induction in pDCs by natural PD ODNs in detail. We show that 3′ poly-G extension of PD ODNs led to efficient multimer formation (comparable to CpG-A ODN), paralleled by improved cellular uptake and CpG-independent IFN-α induction. Notably, 3′ poly-G extension redirected endosomal routing of PD ODNs upon uptake into pDCs, in that monomeric PD ODNs predominantly located to endosomal vesicles with a late phenotype (LAMP-1-positive), whereas poly-G multimerized PD ODNs colocalized with CpG-A ODN in LAMP-1-negative vesicles. We were unable to further identify these CpG-A-positive vesicles using conventional, commercially available early endosomal markers because the variance between these markers was considerable and did not allow for a confident phenotypical identification of a specific and overlapping vesicle population. Colocalization with CpG-A in LAMP-1-negative compartments was therefore the most reliable marker for this distinct, presumably ‘early’ endosomal compartment in our hands. This endosomal redirection of multimeric PD ODN ligands to CpG-A-positive compartments correlated with the induction of IFN-α. Specific endosomal compartmentation and IFN-α induction were noted with 3′ poly-G-extended PD CpG-B ODNs and with PD non-CpG ODNs (PD AP-1 ODN and PD pTC ODN). These data indicate that the propensity to adopt a multimeric spatial organization, rather than the DNA sequence itself, determines IFN-α production in pDCs upon TLR-9 activation by natural PD ODN ligands.
In essence, IFN-α induction in pDCs by poly-G-extended, multimeric natural PD ODNs appears to be determined by three different mechanisms. First, poly-G-mediated multimerization protects PD ODNs from extracellular and presumably also endolysosomal DNase degradation. Second, multimerized PD ODNs display enhanced uptake into DCs, leading to increased endosomal ligand concentrations. While the latter appears to be responsible for CpG-independent TLR-9 activation (balancing lower TLR-9 affinity of PD non-CpG ODNs compared to PD CpG ODNs), it is not sufficient for IFN-α induction. In addition, third, a multimeric ligand structure, as conferred by 3′ poly-G extension or complex formation with DOTAP, is required, which determines differential, early endosomal routing and TLR-9 activation. Interestingly, complex formation of ODNs with PMXB also results in nanoparticular ODN structures and early endosomal routing. However, ODN–PMXB complexes do not display significantly enhanced cellular uptake compared to ODN alone and therefore do not benefit from increased endosomal concentrations. As a consequence, only PMXB complexes of PD CpG-B ODNs, shown to display higher affinity to TLR-9 than PD non-CpG ODNs,7,26 induce IFN-α production, whereas PMXB complexes of PD non-CpG ODNs do not. Obviously, the endosomal concentration of the latter is too low to induce CpG-independent IFN-α production. This contrasted with poly-G-mediated or DOTAP-mediated complex formation, which efficiently increased endosomal ligand concentrations and so allowed for CpG-independent TLR-9 stimulation even by less affine PD non-CpG ODNs. As such, these data imply that the nature of the immune response, coordinated by pDCs upon contact with natural PD DNA, is determined by both endosomal concentration and endosomal routing.
The finding that efficient early endosomal translocation of PD ODNs lacking canonical CpG motifs (usually considered a ‘foreign’ signature) triggers TLR-9-dependent IFN-α production has important implications for certain autoimmune diseases, where the barrier of self-tolerance is breached. Endogenous self-DNA or chromatin, poor in unmethylated CpG motifs, may therefore, upon early endosomal translocation and partial DNase digestion, provide a source of TLR-9 ligands that drive IFN-α production in pDCs. In the case of psoriasis, efficient complex formation and endosomal translocation of self-DNA is brought about by the antimicrobial peptide LL37,28 whereas in systemic lupus erythematosus Fc receptors mediate robust endosomal translocation of anti-DNA antibody/self-DNA immune complexes.32 Based on our data, we propose that adequate concentrations of host-derived DNA within early endosomes trigger TLR-9-dependent IFN-α induction in pDCs.
Acknowledgments
We thank H. Drexler for excellent technical assistance, M. Schiemann for FACS-sorting of pDCs, A. Krug for providing anti-mouse 120G8 antibodies, T. Sparwasser and the Walter and Eliza Hall Institute of Medical Research (Australia) for providing the Chinese hamster ovary cell line expressing FLAG-tagged murine Flt3-ligand. We further wish to thank S. Akira for generously providing TLR9−/− mice. This work was supported by Sonderforschungsbereich (SFB) 456 and the Nationales Genomforschungsnetz (NGFN) 2, all grants to H.W.
Abbreviations
- IRF
interferon regulatory factor
- mDC
myeloid dendritic cell
- ODN
oligodeoxynucleotide
- PD
phosphodiester
- pDC
plasmacytoid dendritic cell
- poly-G
poly-guanosine
- PS
phosphorothioate
Conflict of interest disclosure
The authors declare having no financial or commercial conflicts of interest.
References
- 1.Hornung V, Ellegast J, Kim S, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–7. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 2.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 3.Ishii KJ, Coban C, Kato H, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–8. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–68. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Haas T, Metzger J, Schmitz F, Heit A, Müller T, Latz E, Wagner H. The DNA sugar backbone 2′ deoxyribose determines toll-like receptor 9 activation. Immunity. 2008;28:315–23. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Diebold SS, Kaisho T, Hemmi H, Akira S, Reise Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–31. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 9.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 11.Honda K, Yanai H, Negishi H, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–7. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 12.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–26. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 13.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–51. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 14.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–13. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 16.Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J Immunol. 2000;165:4165–73. doi: 10.4049/jimmunol.165.8.4165. [DOI] [PubMed] [Google Scholar]
- 17.Latz E, Schoenemeyer A, Visintin A, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–8. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 18.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–40. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 19.Guiducci C, Ott G, Chan JH, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerkmann M, Costa LT, Richter C, et al. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem. 2005;280:8086–93. doi: 10.1074/jbc.M410868200. [DOI] [PubMed] [Google Scholar]
- 21.Yasuda K, Yu P, Kirschning CJ, et al. Endosomal translocation of vertebrate DNA activates dendritic cells via TLR9-dependent and -independent pathways. J Immunol. 2005;174:6129–36. doi: 10.4049/jimmunol.174.10.6129. [DOI] [PubMed] [Google Scholar]
- 22.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol. 2003;171:6466–77. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 23.Roberts TL, Dunn JA, Terry TD, Jennings MP, Hume DA, Sweet MJ, Stacey KJ. Differences in macrophage activation by bacterial DNA and CpG-containing oligonucleotides. J Immunol. 2005;175:3569–76. doi: 10.4049/jimmunol.175.6.3569. [DOI] [PubMed] [Google Scholar]
- 24.Bishop JS, Guy-Caffey JK, Ojwang JO, Smith SR, Hogan ME, Cossum PA, Rando RF, Chaudhary N. Intramolecular G-quartet motifs confer nuclease resistance to a potent anti-HIV oligonucleotide. J Biol Chem. 1996;271:5698–703. doi: 10.1074/jbc.271.10.5698. [DOI] [PubMed] [Google Scholar]
- 25.Dalpke AH, Zimmermann S, Albrecht I, Heeg K. Phosphodiester CpG oligonucleotides as adjuvants: polyguanosine runs enhance cellular uptake and improve immunostimulative activity of phosphodiester CpG oligonucleotides in vitro and in vivo. Immunology. 2002;106:102–12. doi: 10.1046/j.1365-2567.2002.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yasuda K, Rutz M, Schlatter B, et al. CpG motif-independent activation of TLR9 upon endosomal translocation of “natural” phosphodiester DNA. Eur J Immunol. 2006;36:431–6. doi: 10.1002/eji.200535210. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JD, Higgins D, Abbate C, et al. Polymyxin B enhances ISS-mediated immune responses across multiple species. Cell Immunol. 2004;229:93–105. doi: 10.1016/j.cellimm.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 29.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 30.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–32. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 31.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 32.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]