Abstract
The development of fluorochrome-conjugated peptide–major histocompatibility complex (pMHC) multimers in conjunction with continuing advances in flow cytometry has transformed the study of antigen-specific T cells by enabling their visualization, enumeration, phenotypic characterization and isolation from ex vivo samples. Here, we bring together and discuss some of the ‘tricks’ that can be used to get the most out of pMHC multimers. These include: (1) simple procedures that can substantially enhance the staining intensity of cognate T cells with pMHC multimers; (2) the use of pMHC multimers to stain T cells with very-low-affinity T-cell receptor (TCR)/pMHC interactions, such as those that typically predominate in tumour-specific responses; and (3) the physical grading and clonotypic dissection of antigen-specific T cells based on the affinity of their cognate TCR using mutant pMHC multimers in conjunction with new approaches to the molecular analysis of TCR gene expression. We also examine how soluble pMHC can be used to examine T-cell activation, manipulate T-cell responses and study allogeneic and superantigen interactions with TCRs. Finally, we discuss the problems that arise with pMHC class II (pMHCII) multimers because of the low affinity of TCR/pMHCII interactions and lack of ‘coreceptor help’.
Keywords: antigen, flow cytometry, peptide-MHC, T-cell, TCR, tetramer
Introduction
T cells detect antigens in the form of peptides bound to self major histocompatibility complex (MHC) molecules at the cell surface. T-cell specificity is determined by the T-cell receptor (TCR), which engages the peptide presentation platform of the peptide–MHC (pMHC) via its highly variable complementarity-determining regions (CDRs). The TCR/pMHC interaction is very weak and classically lasts for no longer than a few seconds at physiological temperatures. Advances over the past decade, however, have produced multimeric forms of soluble pMHC molecules that can be utilized to visualize T cells that bear cognate TCRs. Our laboratory has been involved in refining the utility of these reagents. This process has taught us a number of tricks that have enabled us to optimize pMHC multimers for particular uses. Here we describe some of these techniques, both published and unpublished, and discuss what multimeric pMHC molecules can tell us about certain aspects of T-cell immunity.
The avidity ‘bonus’
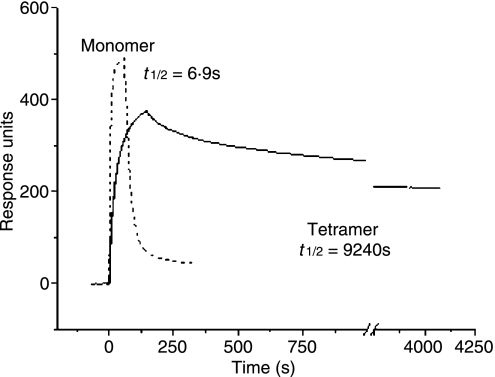
In biology, the term avidity is used to describe the combined ‘strength’ of binding of a molecule with multiple binding sites, whereas the term affinity is used to describe simple interactions with 1 : 1 stoichiometry such as classic receptor–ligand interactions. The binding of multivalent molecules, such as antibodies, leads to a considerable ‘bonus effect’ as the result of cooperative interactivity. This effect arises because the probability that all monomeric interactions will dissociate simultaneously is exceedingly small. If one interaction is dissociated, the others remain associated and enhance the probability that the dissociated interaction(s) will reassociate. This ‘bonus effect’ ensures that the avidity far exceeds the sum of the contributing affinities. We have demonstrated the avidity effect for soluble TCR/pMHC interactions by surface plasmon resonance (SPR).1 For example, the human leucocyte antigen (HLA) A2-restricted A6 TCR, which is specific for human T-cell leukaemia virus 1 Tax11–19, binds to its cognate ligand with a half-life of ∼ 7 seconds in SPR experiments performed at 25°. Tetramerized TCRs, believed to bind three cognate pMHC class I (pMHCI) molecules, bind to the surface of the same BIAcore™ chip (GE Healthcare Ltd, Buckinghamshire, UK) with a half-life of > 2·5 hr (Fig. 1). Thus, the avidity effect of magnifying the monomeric affinities is sufficient to bring even short interactions into a duration range that enables their use for cell surface staining in a variety of applications.
Figure 1.
Multivalent T-cell receptor/peptide–major histocompatibility complex (TCR/pMHC) binding leads to a considerable avidity ‘bonus effect’ as the result of cooperate interactivity and extends the interaction half-life. Streptavidin was linked to a BIAcore™ (GE Healthcare Ltd) CM-5 chip by amine coupling, biotin-tagged pMHCI was loaded onto each flow cell, and data were collected at 25° with a flow rate of 5 μl/min. Five microlitres of biotinylated TCR monomer at 1 mg/ml and 25 μl of TCR tetramer at 50 μg/ml were flowed over all flow cells. Negligible responses were observed to non-cognate pMHCI for both TCR monomers and tetramers. To facilitate visual comparison of monomer and tetramer binding events, the much larger monomer response values were normalized to the peak values for the tetramers. Kinetic binding parameters for the tetramers were estimated using BIAEvaluation™ software as described in ref [1]. There are two apparent off-rates for the TCR tetramers: (1) a minority fast off-rate thought to correspond to those tetramers binding less than three antigens; and, (2) a slow (true) off-rate for those tetramers probably binding three pMHCI molecules. The latter half-life is shown. Some irreversible binding of biotinylated TCR monomers is observed owing to incomplete blocking of the streptavidin-coated chip surface with soluble biotin. Representative data are shown. Curves are the best fit of the model described in.1 Data reproduced from1 with permission.
Cell staining with pMHC
The avidity effect of pMHC multimerization was first used for staining T cells in a groundbreaking study by Altman et al. in 1996.2 Avidin–biotin-based pMHC ‘tetramers’, still the most common format for pMHC multimers, were used to identify specific T-cell populations directly ex vivo using flow cytometry. Over the ensuing decade, there have been many hundreds of papers describing the use of multimeric pMHC for direct visualization, enumeration, phenotypic characterization, isolation and cloning of antigen-specific T cells; indeed, pMHC multimers have become a regular feature in the immunologist’s tool box.
pMHCI versus pMHCII
pMHC multimers have been successfully employed to stain both CD8+ and CD4+ T cells. The use of pMHCI multimers, which stain CD8+ T cells, is more widespread than the use of pMHCII multimers. Accordingly, this review will focus primarily on the use of pMHCI multimers. The use of pMHCII multimers has generally proven to be more problematic; possible reasons for these issues, as well as means to circumvent them, are discussed at the end of this review.
Multimeric scaffolds
pMHC tetramers
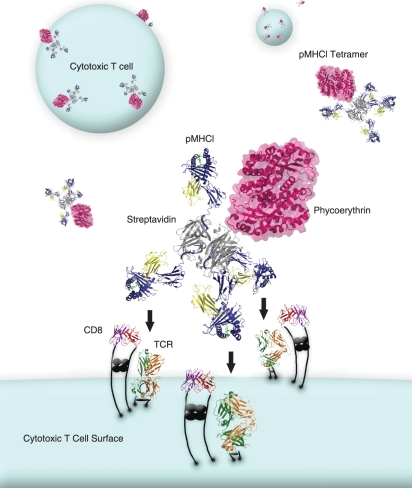
The original avidin–biotin-based ‘tetramer’ platform for pMHC multimers is still the most common in use. These reagents are now easily manufactured in the laboratory or can be purchased from a number of commercial sources. Avidin cooperatively binds four biotin molecules; the resultant tetrahedral nature of the complex makes it unlikely that all four pMHC molecules will engage cell-surface TCR simultaneously, while three of them will readily engage TCRs at the same time (Fig. 2). We use our tetramers for biophysical analysis at the cell surface3 and have therefore been careful to ensure that the reagents we produce are > 99% tetrameric by gel filtration chromatography. For most applications, however, such rigour is not required. Many commercially available preparations of fluorochrome-conjugated (strept)avidin contain oligomers and so produce molecules with higher order valencies than tetramers.4 These higher order valencies potentially enhance T-cell staining and may therefore be beneficial in some settings, although concomitant background increases can be problematic.
Figure 2.
Tetrahedral avidin–biotin-based peptide–major histocompatibility complex class I (pMHCI) tetramers engage three T-cell receptors (TCRs) and three CD8 molecules at the cell surface.
Dimers, pentamers, octamers, dextramers and polymers
Various other means of pMHC multimerization have been devised. These have been reviewed elsewhere by Bakker and Schumacher.5 Here, we confine our discussion to the most commonly used pMHC multimer format, the avidin–biotin-based tetramer. The issues discussed are likely to be relevant for all multimeric formats.
The key to cell surface staining
Regardless of the scaffold used, or valency, the ability of pMHC multimers to stain cognate T cells is likely to hinge on the half-life of the corresponding monomeric TCR/pMHC interactions. For the avidity ‘bonus effect’ described above to come into play, the mean duration of engagement of the first TCR/pMHC interaction must be long enough to allow another pMHC in the multimer to bind a second TCR. The avidity effect of this dimeric binding will then probably be sufficient to allow other pMHCs in the multimer to bind. The main factor that determines whether a pMHC multimer exhibits stable binding is therefore likely to be the duration of the primary, monomeric interaction with TCR. The on-rate of the interaction at the cell surface will also be crucial to the avidity effect by determining whether a second TCR is engaged during the time that the first TCR remains bound. In the environment of the T-cell surface, this on-rate is likely to be highly dependent on whether another TCR is available for binding to a second pMHC molecule. The availability of further TCRs is, in turn, likely to be dependent on both the amount of TCR on the T-cell surface (which can vary by over an order of magnitude) and its ability to diffuse within the lipid bilayer. Recent reports have suggested that some TCRs on the T-cell surface may exist as preformed multimers.6 If present, such TCR clusters may be expected to be considerably better at binding pMHC multimers than dispersed monomeric TCRs, with potentially dramatic effects on the resulting staining patterns.
Three pMHCs are more than enough
Higher order multimers (pentamers, octamers, dextramers and polymers) will generally have longer interaction half-lives at the cell surface than tetramers. However, when staining is performed at physiological temperatures, pMHC multimers are rapidly internalized.7 Under these conditions, the potential advantage of longer multimer dwell times is largely irrelevant to the amount of pMHC multimer captured from solution. Tetramers, which are thought to engage three different TCRs,3 are therefore more than sufficient for most staining applications; indeed, in many cases, a simple pMHC dimer is effective.8,9
What do tetramers stain?
Despite extensive work with pMHC multimers, there has been no systematic attempt to define the exact characteristics of the TCR/pMHC interaction that render a cell amenable to multimer staining. We have recently described a range of altered peptide ligands for a monoclonal T-cell that recognizes the HLA A2-restricted peptide ILAKFLHWL derived from human telomerase reverse transcriptase (hTERT).10,11 These ligands exhibit a spectrum of activation profiles from superagonism through to weak agonism and bind to the cognate TCR with a wide range of affinities (KD from 3 to > 250 μm); this range is further extended by the weak agonist 8E variant (ILAKFLHEL), which exhibits a remarkably low affinity that lies on the threshold of detection by SPR (KD∼ 2 mm). These agonist ligands for the same TCR therefore vary in their binding affinity by well over 100-fold and this has enabled us to quantify the requirements for tetramer staining and T-cell activation in this system.10
Tetramer staining is dependent on TCR/pMHC affinity
As described above, the key to whether a T-cell stains with a particular pMHC multimer is likely to reside in whether the duration of the interaction of the first engaged TCR is sufficient to enable the engagement of a second TCR and allow the ‘avidity effect’ to come into play. The probability that binding of a second TCR occurs before the first one dissociates equals [TCR]/(σKD + [TCR]) where [TCR] is the cell surface density of TCRs, σ is a steric factor, and KD = koff/kon and is the dissociation constant. Affinity (KD) is a key determinant of staining intensity. In fact, at relatively low TCR densities, we have [TCR]/(σKD + [TCR]) ≈ [TCR]/σKD; i.e. the probability is directly proportional to both affinity and TCR density. We have used the hTERT system described above to determine that, under ‘normal’ conditions (staining with 10 μg/ml tetrameric pMHCI at 37° for ∼ 30 min), a TCR/pMHCI interaction of KD ≤ 40 μm is required to observe good tetramer staining (Fig. 3). This direct comparison of the requirements for tetramer staining and for T-cell activation10 may help to explain some of the anomalies in the literature described below.
Figure 3.
The T-cell receptor/peptide–major histocompatibility complex (TCR/pMHC) interaction threshold for tetramer staining varies with CD8 engagement and temperature. Staining of cytotoxic T-lymphocyte (CTL) clone ILA1 with seven different human telomerase reverse transcriptase (hTERT540–548) variants, as indicated, refolded with wild-type human leucocyte antigen (HLA) A2 (a) or CD8-null HLA A2 D227K/T228A (b) at 37°. The mean fluorescence intensity (MFI) values observed with pMHCI tetramer staining are plotted against the TCR/pMHCI interaction KD values for experiments conducted at 37° (c) and 4° (d) with wild-type HLA A2 and CD8-null HLA A2 molecules for each variant added at a final concentration of 220 nm (10 μg/ml); colour codes correspond to those shown in (a) and (b). Staining with the set of APLs refolded with each type of heavy chain was performed at least three times. Representative data are shown. Curves are the best fit of the model described in Laugel et al.10 Data reproduced from10 with permission.
Poor tetramer staining of anti-tumour and autoimmune T cells
Our recent biophysical analysis of the binding properties of 14 different human TCR/pMHC interactions12 indicated that TCRs directed against self antigens (ubiquitous tumour-derived or autoimmune epitopes) bind with a lower affinity than those directed against pathogen-derived (non-self) epitopes. This distinction was not wholly unexpected because it is known that the process of negative selection in the thymus culls T cells that exhibit strong interactions with self antigens. The apparent correlation between tetramer staining and monomeric TCR/pMHC interaction affinity10 suggests that, in general, it will be more difficult to stain T cells specific for self antigens compared to T cells directed against non-self antigens. This concept is supported by work in the murine system, which suggests that T-cell responses dominated by relatively low-affinity TCR interactions, such as those that target ubiquitous tumour-derived or autologous antigens, will be difficult to detect using standard tetramer-based techniques.13,14
Agonism without tetramer staining
Functional T cells that do not stain with cognate pMHC tetramer have also been described in the human system.15,16 Indeed, the TCR/pMHC interaction affinity that allows cell surface staining with tetrameric pMHC can be substantially higher than that required for T-cell activation.10 Normal staining procedures with wild-type tetramers do not necessarily detect all the T cells that can respond to a particular agonist; similarly, not all agonists for a particular T-cell can be identified physically with pMHC tetramers. These potential limitations of pMHC multimer staining have important implications for data interpretation.
The importance of staining conditions
Whether or not a particular T-cell stains with a given pMHC multimer, and thus what a tetramer stains, is strongly dependent on the particular staining conditions and the state of the cells being stained. We have recently discovered several ways of improving tetramer staining and have been able to stain cells with wild-type tetramers where the monomeric TCR/pMHC affinity is significantly lower than we had previously achieved (e.g. KD∼ 120 μm; Fig. 4). Several factors can be adjusted to improve tetramer staining.
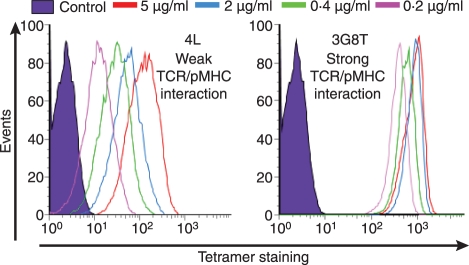
Figure 4.
Peptide–major histocompatibility complex (pMHC) multimer concentration differentially affects staining of cognate T cells. The ILA-1 cytotoxic T-lymphocyte (CTL) clone was stained with two human telomerase reverse transcriptase (hTERT540–548) peptide variant human leucocyte antigen (HLA) A*0201 tetramers at a number of different concentrations (5, 2, 0·4 and 0·2 μg/ml as indicated). Staining with the low-affinity 4L variant (KD = 117 μm) is shown in the left panel; staining with the high-affinity 3G8T variant (KD = 4·04 μm) is shown in the right panel. The data demonstrate that tetramer staining with low-affinity ligands is far more dependent on pMHC concentration; higher tetramer concentrations can therefore improve visualization of T cells that bind cognate ligand with low TCR/pMHCI affinities.
Concentration
The amount of a ligand that binds to a particular receptor is dependent on the concentration of both species. The concentration of pMHC multimer can be readily altered during tetramer staining. It is also possible to vary the TCR density at the T-cell surface.
pMHC concentration
The amount of pMHC tetramer used in staining can have dramatic effects. This is particularly true for weak TCR/pMHC interactions that may fall near or below the dissociation constant for tetramer binding (Fig. 4). In general, the use of high concentrations of pMHC multimer is preferable, although care must always be taken not to approach levels that impact significantly on background staining. However, the use of high tetramer concentrations adds to the expense and makes other forms of optimization described below preferable.
TCR concentration
Recent experiments in our laboratory have shown that it is possible to increase surface expression levels of both TCR and CD8 coreceptor by incubating cells with the protein tyrosine kinase inhibitor dasatinib17 for 3 hr. We have since shown that a short incubation period (∼ 30 seconds) with such inhibitors can double the staining intensity achieved with cognate pMHCI tetramers without increasing the staining of non-cognate T cells (Fig. 5).18 The major reason for such a substantial improvement in staining appears to be that dasatinib inhibits the downregulation of TCRs from the T-cell surface following TCR engagements that do not result in the capture of tetramer from solution, although experiments aimed at understanding this effect are still in progress. Regardless of the exact mechanism, these promising results instil confidence that this particular trick to enhance the effective TCR concentration at the T-cell surface will find widespread use in the physical detection of T cells with multimeric pMHC molecules.
Figure 5.
Incubation with the protein tyrosine kinase inhibitor dasatinib substantially improves the staining of cognate T cells with peptide–major histocompatibility complex class I (pMHCI) tetramers. (a) A cytotoxic T-lymphocyte (CTL) line specific for the melanoma-derived EAAGIGILTV epitope was stained with cognate human leucocyte antigen (HLA) A2 tetramer at a concentration of 10 μg/ml. (b) The same experiment performed after preincubation of the CTL line with 50 nm dasatinib for 15 min at 37°. As shown, dasatinib treatment substantially improves the staining of cognate T cells in the line without affecting the staining of non-cognate T cells; this enhancement varies from two to 50-fold and is greatest for the cognate T-cell populations that bind tetramer poorly. Similar results have been observed in a wide range of systems.18
Temperature
The temperature at which pMHC multimers are used can also have dramatic effects on staining;7 these effects are probably largely the result of whether or not the tetramers are efficiently internalized by cognate T cells. At physiological temperature, pMHC tetramers are rapidly internalized into early endosomes.7 Any tetramer that can associate with the T-cell surface for just a few minutes at 37° will be internalized and subsequently unable to dissociate from the cell during an experiment. In contrast, pMHC multimers are not efficiently internalized at low temperatures7 so at low temperatures staining intensity depends more critically on surface-bound, non-internalized pMHC multimers, and therefore on the ability of these multimers to remain associated for the duration of the experiment. In our HLA A2 telomerase system, pMHCI tetramer staining is more stringent and requires higher affinity TCR/pMHCI interactions at low temperatures (Fig. 3). We have, however, also described a system where a weak agonist ligand (of unknown affinity and kinetics) was able to stain cognate T cells at low temperature.7 Individual TCR/pMHC binding combinations can be either enthalpically or entropically driven, but it is not known how such differences manifest themselves in terms of temperature effects on pMHC multimer staining. In summary, it is not yet possible to predict in general how temperature will affect all systems and, therefore, it is advisable to assess the effects of temperature for each individual system.
Anti-coreceptor antibody
Tetramers are most often used to identify T-cell populations directly ex vivo in conjunction with the relevant anti-coreceptor antibody. Both anti-CD8 and anti-CD4 antibodies can affect tetramer staining.19,20 The effects of human anti-CD8 antibodies occur whether or not the particular pMHC used for staining is able to engage CD8.19 For the most part, the effects of anti-coreceptor antibodies are disruptive and it is recommended that cells are stained with pMHC multimer before they are stained with anti-coreceptor antibody.7,19,20 In the murine system, an antibody has been described that enhances tetramer binding.21 We have observed increases in pMHCI tetramer staining of some human cytotoxic T-lymphocyte (CTL) clones with some anti-CD8 antibodies.19 However, such effects are minor, rare and clone-specific, and we are unaware of a reliable, broadly applicable protocol that utilizes anti-CD8 antibodies to improve tetramer binding in the human system.
Applications of pMHC tetramers
The list of ingenious ways in which researchers have exploited the properties of wild-type and modified pMHC multimers is ever increasing. By far the most popular applications relate to the visualization, enumeration and phenotypic characterization of antigen-specific T-cell populations by flow cytometry.2 Technology in this field continues to develop apace, expanding the capabilities of polychromatic flow cytometry and so the potential of pMHC multimer-based approaches in multiplex applications.22 It should be noted that antigen-specific T-cell populations can also be detected by flow cytometry based on cell surface and/or intracellular functional and/or activation markers. By definition, these activation-based methodologies require that T cells respond biologically to antigen before they can be detected. For some applications, the physical detection of T cells using pMHC multimers offers advantages over activation-based strategies. First, pMHC multimer detection allows the identification of T cells that bear cognate TCRs but that do not respond to antigen; this may be particularly useful when T-cell populations become exhausted, for example during persistent viral infections.23,24 Second, physical detection with pMHC multimers enables the characterization of T-cell phenotype in direct ex vivo samples without the requirement to alter that phenotype by antigen-based activation. Overall, the ‘original’ use of pMHC multimers for the detection of antigen-specific T cells has been amply described elsewhere and will not be considered further here. Other, more recent, applications for pMHC multimers and mutant pMHC multimers are described below; by necessity, this discussion is selective.
Tetramers for ‘grading’ T cells
T cells differ in their functional sensitivity
T cells that recognize the same pMHC antigen are known to differ in their sensitivity to this antigen by several orders of magnitude.25,26 A landmark study in 199626 demonstrated the importance of this phenomenon by showing that CTL grown under conditions of limiting antigen were more sensitive than CTL expanded in the presence of high antigen densities. These highly sensitive CTL were almost 1000-fold more effective in adoptive transfer experiments at mediating viral clearance in a murine model compared to CTL exhibiting low antigen sensitivity. Numerous studies have subsequently demonstrated that T cells with an inherent ability to recognize very low antigen densities are the most effective at eliminating tumours and viruses in vivo26–33 and it is becoming increasingly accepted that the quality of a T-cell response may be just as important as its quantity. Tetramers can be invaluable for ‘grading’ the quality of an antigen-specific T-cell population. To outline these various applications, however, we first need to review some key aspects of the molecular basis of T-cell activation.
Molecular control of T-cell sensitivity
There are several factors that might control the sensitivity of T cells at the molecular level and so explain why T cells with the same specificity require different densities of antigen to trigger activation. In the most obvious explanation, the bona fide‘avidity’ model, TCRs from highly sensitive T cells bind to antigen with higher affinity than the TCRs from T cells that require higher antigen densities to activate. Higher affinity, longer lasting TCR/pMHC interactions are known to result in a greater sensitivity to antigen.10,34 However, it is becoming apparent that TCR affinity is not the sole factor that determines the sensitivity of T-cell activation. Indeed, it has recently been shown that an individual T-cell clone can generate effector cells that exhibit both high and low antigen sensitivities.35
There are several potential mechanisms by which T cells with the same TCR might differentially regulate their sensitivity to antigen. First, it is known that the level of TCR on the T-cell surface can vary by > 10-fold. It is not difficult to envisage how a T-cell with a greater TCR density might be better equipped to recognize low levels of antigen. Second, if multivalent forms of the TCR exist, then these TCRs are likely to enhance the avidity of antigen binding because of cooperative effects as described above; so a T-cell with enhanced levels of multivalent TCR might be expected to exhibit a greater sensitivity for antigen.6 Third, it is known that the location of the TCR on the cell surface is important. Certain cholesterol-rich regions of the plasma membrane (so-called lipid rafts) are known to act as privileged sites for TCR-mediated signal transduction and it is possible that differences in the distribution of TCRs within these regions may also provide an explanation for differences in T-cell sensitivity.36,37 A fourth mechanism postulates that different T cells require different thresholds of TCR triggering to activate. The fact that T cells exhibit differential dose responses to anti-CD3 antibody-mediated cross-linking in addition to antigen levels offers some support for such suggestions.38,39 The above potential TCR-dependent mechanisms for tuning T-cell sensitivity are not mutually exclusive and could combine with TCR–antigen affinity to regulate the amount of antigen required for T-cell activation.
Uniquely, normal recognition of pMHC antigen by T cells requires the engagement of another, distinct receptor to the same pMHC ligand. The CD8 and CD4 membrane glycoproteins bind to MHCI and MHCII, respectively, at sites distinct from the TCR docking platform40,41 and are known to boost antigen recognition.42 CD8 and CD4 play a very different role from other accessory molecules as they ‘coreceive’ the pMHC ligand; because of this distinctive role, these receptors are termed T-cell coreceptors.43 It is well established that the CD8 and CD4 coreceptors can enhance sensitivity to exogenous antigen by up to a million-fold via the mechanisms described below.42 Recent data suggest that the CD8 ‘booster effect’ exerts differential effects on the deployment of CTL effector functions44 and can be varied by T cells to adjust their sensitivity.45 CD4 may act in a similar fashion.
The CD8 and CD4 coreceptors are known to play a key role in TCR-mediated signal transduction through several mechanisms. These mechanisms include: the recruitment of key protein tyrosine kinases and adaptor molecules involved in early TCR-mediated signal transduction to the cytoplasmic side of the TCR/CD3/ζ complex;46–49 stabilization of the TCR/pMHC interaction at the cell surface by ∼ two-fold;3 enhancement of TCR/pMHC on-rate10,50 and the delivery of the TCR to lipid rafts.36 There is also a body of evidence to suggest that the TCR and coreceptor may coexist on the cell surface.19,20,36,51–56 Pre-existing TCR–CD8 or TCR–CD4 adducts on the T-cell surface would act as one receptor with two binding sites and, a priori, would be substantially better at engaging antigens in productive TCR/pMHC/coreceptor tripartite interactions compared to the scenario in which TCR and coreceptor were spatially distinct on the cell surface before ligand engagement. Indeed, the existence of TCR–coreceptor adducts on the TCR surface might provide an explanation for how CD8 can increase the TCR on-rate and reduce its off-rate,3,10 given that the soluble extracellular domains of the TCR and coreceptor do not exhibit cooperative binding to pMHC.36,57,58 If TCR–coreceptor adducts exist, then their differential expression could explain differences in sensitivity to both antigen and CD3 cross-linking.
Early concepts that the role of the coreceptor in determining antigen sensitivity is static have recently been brought into question and it now looks increasingly likely that various mechanisms act to tune the contribution of the coreceptor and allow a T cell to ‘focus’ on a particular agonist ligand within the range of peptide ligands that it is able to recognize.59 Further topical work has demonstrated that signals from interleukin-7 and other common γ-chain cytokines increase CD8 expression and suggests that interplay between this cytokine and TCR signalling modulates CD8 expression to ensure that it is inversely proportional to the intensity of TCR signals induced by self antigens. This dynamic feedback loop has been termed ‘coreceptor tuning’ and is thought to enhance CD8+ T-cell survival and sensitivity within the limits permitted by self-tolerance.45,60,61
The sensitivity of T cells could be further modulated by varied expression of molecules other than those involved in the act of antigen engagement. Accessory and/or costimulatory molecules such as CD28, LFA-1, ICAM-1, OX40, CD80 and TNF family members like 4-1BBL all have the potential to enhance T-cell sensitivity.62 It is also possible that upregulated expression of key early signal transduction molecules such as p56lck and ZAP 70 could lower the threshold for T-cell triggering. On the flip side, molecules such as CD45 or SHP-1 that can negatively regulate signal transduction63 could also act to alter the level of antigen density required for full T-cell activation.64,65
In summary, it is becoming increasingly apparent that T-cell sensitivity is not only extremely important for a successful immune response, but, moreover, that this sensitivity is dynamically adjusted by several different mechanisms, which may continually act to fine-tune the effectiveness of antigen-specific T cells throughout the course of a normal immune response. The terms high avidity and low avidity have become entrenched in the literature since their first use to describe T cells with a low or high requirement, respectively, for antigen.26 The idea underlying functional avidity in the sense of Alexander-Miller et al.26 is that the signal registered by the T-cell is an increasing function of both the antigen dose and a quality parameter (‘functional avidity’), such that a high antigen dose can compensate to some extent for a relatively low value of the quality parameter. Differences in TCR expression, TCR affinity, TCR valency, CD8 expression, TCR–coreceptor adduct levels and the expression of accessory molecules are all likely to modulate this quality parameter. However, it remains possible that other mechanisms that do not involve ‘avidity’ in the biological sense defined above also act to regulate this parameter. We therefore prefer to use the term ‘functional sensitivity’ rather than ‘functional avidity’ to describe the quality parameter (i.e. how well T cells respond to different antigen densities) as it is almost always the former property that has been measured.
The need for variations in T-cell sensitivity
There is an increasing body of literature suggesting that T cells can exhibit a range of functional sensitivities for the same antigen. This sensitivity is clearly regulated, because early in an immune response a single T-cell has the capacity to produce daughter cells with high and low functional sensitivities.35 T cells with high functional sensitivity are known to kill target cells more rapidly and at earlier times postinfection than cells with low sensitivity.66 As a result, highly antigen-sensitive T cells are better equipped to limit disease spread and must generally be the cells of choice for a robust immune response. Indeed, as discussed above, such T cells have been shown to be the optimal effectors in a variety of systems.26–33 However, there are likely to be circumstances in which the presence of T cells with low antigen sensitivity or a spread of sensitivities will be beneficial. In vitro stimulation of highly sensitive T cells with supraoptimal peptide levels is known to result in apoptosis, while cells with low antigen sensitivities die by neglect if only stimulated with low levels of antigen.67 This suggests that T cells with lower antigen sensitivity may be better suited to cope in the presence of sustained high levels of antigen in vivo. Indeed, many studies appear to support a model where highly sensitive T cells become overwhelmed in vivo by persistent viral infections.68–75 The existence of T cells with a low sensitivity for antigen and/or T cells that encompass the ability to detune their sensitivity might be beneficial to the host under conditions of sustained high antigen densities. The ability of T cells to regulate their own sensitivity may also be important during T-cell development as an early thymocyte is likely to benefit if it is ‘tuned up’ to receive signals from self pMHC. Continued tuning of T-cell sensitivity throughout the lifetime of a T-cell would allow the secondary benefit of regulating maximum sensitivity without autoreactivity.45
Tetramers for the physical grading of T cells based on bona fide avidity
The discovery that the functional sensitivity of a T-cell response is of critical importance to the outcome of immunity has generated huge interest in strategies to induce and detect T cells with high levels of antigen sensitivity. At the other end of the spectrum, it is now clear that the affinity threshold for T-cell activation can extend to very-low-affinity TCR/pMHC interactions that cannot be detected physically with standard tetramer-based approaches.10 We next discuss our recent extensions of pMHCI tetramer technology to address both of these issues.
CD8-null tetramers selectively stain CTL with high-affinity TCRs
As described above, the pMHCI/CD8 interaction acts to enhance TCR/pMHCI on-rate10,50 and decrease TCR/pMHCI off-rate3 at the cell surface. Both of these effects appear modest for monomeric interactions (∼ twofold) but can have dramatic effects when it comes to both T-cell activation and tetramer binding. The duration of the TCR/pMHC interaction is critical for TCR triggering and T-cell activation34 and the role of CD8 in extending such interactions is likely to be important in its own right.3,76 These effects add to the other important roles of CD8 binding, most notably in signal transduction, to ensure that ‘CD8-null’ pMHCI molecules are extremely poor T-cell activators.47,77–79 We predicted that the effects of CD8 engagement on TCR/pMHCI interactions would act to extend the range of TCR/pMHCI interactions that are permissive for tetramer staining. Our recent experiments with a series of altered peptide ligands in an HLA A2-restricted hTERT system have allowed us to define the importance of TCR/pMHCI affinity for tetramer binding, as described in Fig. 3, and to examine the role of CD8 in tetramer binding. The seemingly modest, although important, effects of CD8 engagement on TCR/pMHCI interactions are multiplied in the binding of multimeric forms of pMHCI. A pMHCI tetramer is believed to engage three TCRs and three CD8 molecules at the cell surface (Fig. 2). This ensures that CD8 binding affects just the off-rate of tetramer alone by > 10-fold.3 The net result of these avidity effects is to ensure that CD8-null tetramers act as more stringent tools for the analysis of T-cell populations because they only bind to the cell surface if the monomeric TCR/pMHC interaction exceeds a certain affinity threshold (Fig. 3 and ref. 10). This effect then allows the specific discrimination of T cells that have a high-affinity TCR interaction with the multimerized antigen.76,80
CD8-enhanced tetramers can identify CTL with low-affinity TCRs
We have also succeeded in enhancing pMHCI/CD8 interactions without affecting TCR/pMHCI engagement by mutating the CD8-binding domain.3,81 Substantial alterations in the pMHCI/CD8 interaction (> 10-fold) bring this interaction into the affinity range of strong TCR/pMHCI interactions. As a result, pMHCI tetramers incorporating such high-affinity CD8 binding efficiently adhere to most CD3+CD8+ cells 3 and are therefore of limited use for identifying antigen-specific T-cell populations. In contrast, point mutations in the pMHCI/CD8 binding domain such as a Q115E substitution, predicted to add just one further hydrogen bond to the interaction,3,77 enhance CD8 binding by ∼ 50% and have far more subtle effects. CD8-enhanced (Q115E) tetramers retain specificity but allow the staining of T cells with substantially weaker TCR/pMHCI interactions.82 As a result, CD8-enhanced tetramers can be useful when examining T cells with weak TCR/pMHCI interactions such as cross-reactive T cells83 and tumour-specific T cells (J. Melenhorst, unpublished data). Due to their higher overall avidity Q115E tetramers can also be used at lower concentrations than wild-type reagents.82
Physical grading of TCR/pMHC interactions
CD8-null, wild-type and CD8-enhanced tetramers stain T cells with ever decreasing TCR/pMHCI affinities and so afford a method of grading these interactions within a population of T cells (Fig. 6). Under ‘normal’ staining conditions (10 μg/ml tetrameric pMHCI at 37° for ∼ 30 min), these three reagents exhibit a threshold of monomeric TCR/pMHCI interactions that enable staining with approximate dissociation constants of < 30 μm, < 80 μm and > 250 μm, respectively. However, these thresholds are not static because they alter with staining conditions and are continually being improved by application of some of the ‘tricks’ described above. In general, the most stringent staining conditions involve the use of CD8-null tetramers at low concentration and at low temperature whereas the least stringent stains use CD8-enhanced tetramers at high concentration in the presence of certain protein kinase inhibitors. As described above, several factors in addition to TCR/pMHC affinity are likely to control the sensitivity of T cells to antigen density on the target cell surface.18 Many of these factors, such as TCR expression levels, TCR valency, CD8 expression and TCR–coreceptor adduct levels are likely to affect both the sensitivity to antigen and tetramer staining in a similar direction. To date, our studies of more than 30 TCR/pMHC interactions have confirmed that the most sensitive T cells bear the highest affinity receptors and are the most independent of CD8 engagement for T-cell activation and tetramer staining. Nevertheless, exceptions to the rule remain possible, which would allow a T-cell with a weak TCR/pMHC interaction to be highly sensitive to antigen.
Figure 6.
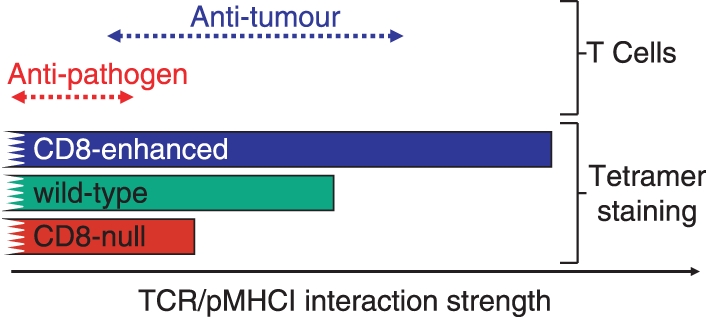
CD8-null, wild-type and CD8-enhanced peptide–major histocompatibility complex class I (pMHCI) tetramers enable the staining of T cells with increasingly weak T-cell receptor (TCR)/pMHCI interactions. CD8-null tetramers require a high TCR/pMHC affinity to stain cognate T cells. Wild-type tetramers can stain cells with weaker affinity interactions.10 The extra help afforded by CD8-enhanced tetramers allows them to stain cells when the TCR/pMHCI interaction is even weaker.82 The use of these three types of reagent enables the grading of T cells based on the strength of their interaction with the soluble pMHC ligand. Most natural human anti-pathogen TCR/pMHCI interactions fall within a range that allows staining with CD8-null tetramers. Many anti-tumour T cells bear TCRs that interact weakly with pMHCI and thus require CD8 help or enhanced CD8 help to be visualized successfully with tetramers.
Clonotypic characterization of antigen-specific T-cell responses
One of the most exciting recent developments in the field of adaptive T-cell immunity has been the combination of tetramer technology with TCR repertoire studies to examine the clonotypic architecture of antigen-specific T-cell populations. To conduct reliable studies of TCR usage, the T-cell population of interest must be identified accurately with negligible non-specific background noise and isolated without contaminant events to an extremely high degree of purity; in addition, for RNA-based approaches to clonotypic characterization, the relevant cells must be intact at the time of isolation (i.e. such methods are incompatible with cell fixation/permeabilization techniques). Tetramers are the perfect tools to fulfil these requirements, enabling rapid sorting of antigen-specific T-cell populations by high-resolution flow cytometry to > 99% purity with minimal adverse effects on cellular integrity in the short-term. Furthermore, in conjunction with optimized techniques for the comprehensive and unbiased molecular analysis of TCR gene expression,84–86 the development of point-mutated pMHCI tetramers with altered CD8-binding properties has enabled the direct ex vivo characterization of constituent clonotypes within populations of cognate T cells based on their intrinsic avidity for antigen.87 Combined with polychromatic flow cytometric analyses of phenotype and function at the single cell level, it is now possible to begin deconstructing antigen-specific T-cell repertoires with the aim of understanding the principles of clonal selection in the periphery; recent advances in this field are beyond the scope of the present discussion, but are well reviewed elsewhere for the interested reader.88,89
Tetramers for cloning T cells
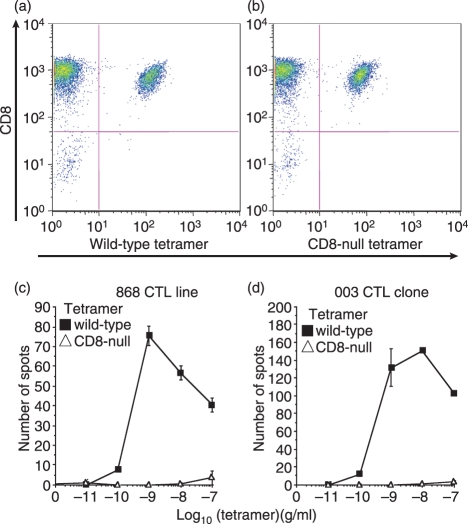
The pMHCI multimers can also be used to sort-clone antigen-specific T cells (e.g. ref. 90). However, pMHCI multimers are known to kill cognate CTL by both activation-dependent, mitochondrial dysfunction and Fas-mediated mechanisms.91,92 Our own unpublished observations that highly antigen-sensitive CTL are rapidly killed by pMHCI tetramer staining are supported by the studies of Luescher and colleagues showing the selective loss of such cells in Melan A-specific T-cell populations exposed to regular biotin-based tetramers.93 This study made ingenious use of desthiobiotin (DTB) tetramers. While stable at low temperature, DTB tetramers rapidly disintegrate into their constituent monomers at physiological temperatures in the presence of free biotin.93 Thermolabile DTB ‘reversible’ tetramers exhibit a greatly reduced capacity to activate and damage CTL and enable the cloning of CTL with an overall higher functional sensitivity and greater susceptibility to apoptosis compared to those that can be cloned with wild-type tetramers.93 Our recent demonstration that the TCRs from anti-pathogen CTL bind with significantly higher affinity than those of anti-tumour CTLs12 suggests that anti-pathogen CTLs may be more susceptible to tetramer-induced cell death than the Melan A-specific populations studied by Guillaume et al.93 Standard pMHCI tetramers may be suboptimal for cloning T cells with high levels of antigen sensitivity and so other detection methods, such as antigen-induced activation markers consistent with cell viability94 or ‘reversible’ tetramers,93 are preferable for sorting these highly desirable cells.
Tetramers to examine superantigens and alloreactive T cells
The utility of pMHC tetramers will extend to any TCR/pMHC interactions of sufficiently high affinity. As such, these reagents can be used to identify and study allogeneic T cells. Allogeneic interactions have the potential to be even stronger than syngeneic interactions as they have not been subjected to negative selection in the thymus. Several studies have used pMHC multimers to study allorecognition31,95,96 and pMHC tetramers were used successfully to deplete allogeneic T cells and significantly reduce morbidity and mortality from graft-versus-host disease in a murine transplantation model.97 Similarly, it is also possible that tetramer staining might prove useful for removing specific T-cell malignancies from patient samples before reinfusion.
Bacterially expressed molecules called superantigens are known to cross-link the β chain variable region of some TCRs to MHCII. We have recently demonstrated that such cross-linking is strong enough to enable its investigation using pMHCII tetramer staining of samples directly ex vivo.98 This technique allows the rapid identification of TCRs that bind and respond to putative superantigens.
Tetramers for T-cell activation
The confounding issue of peptide representation by endogenous MHC molecules
Soluble multimeric pMHC molecules have been used to examine the activation requirements of T cells. However, the possibility of peptide transfer from soluble pMHC molecules to MHC molecules on the surface of the T cells themselves, or other cells in the test sample, was widely ignored in early experiments. As a result, activation of cognate T cells by pMHC monomers was interpreted to mean that monovalent engagement of TCR by soluble pMHCI was sufficient for T-cell activation.99 Several independent lines of evidence have since brought this interpretation into serious doubt by demonstrating the confounding effect of peptide transfer from soluble pMHC molecules. An examination of the activation of CD8+ T cells expressing the 2C TCR by Stern and colleagues100 took advantage of the fact that this TCR is known to recognize a variety of different pMHCI complexes including the strong agonist ligand H2-Kb-SIYRYYGL and the potent allogen H2-Ld-QLSPFPFDL. 2C T cells, which express H2-Kb but not H2-Ld, were activated by H2-Kb-SIYRYYGL monomer but not by the stronger binding allogeneic ligand, suggesting that the SIYRYYGL peptide might be being transferred to endogenous H2-Kb molecules on the surface of 2C T cells.100 This explanation was further supported by experiments demonstrating that while the activation of 2C T cells that lacked H2-Kb expression by H2-Kb expressing antigen-presenting cells (APCs) was normal, these T cells could not be activated by monomeric H2-Kb-SIYRYYGL.100 Schott et al. have confirmed the confounding role of peptide transfer using the OT-1 system.78 OT-1 T cells that lacked H2-Kb molecules could not be activated by soluble monomeric pMHCI. In contrast, H2-Kb tetramers, which cross-link TCRs on the T-cell surface, were capable of activating OT-1 T cells that lacked expression of H2-Kb molecules.78 In summary, it appears that T cells can be activated by soluble multimeric pMHC but not by soluble pMHC monomer. However, it is likely that peptide representation from pMHCI multimer will account for a proportion of the activation induced by these molecules and researchers must be aware of, and control for, such effects when using soluble pMHC to activate T cells.
Tetramer-induced activation requires only TCR and coreceptor binding
Original versions of the kinetic segregation model of T-cell activation incorporated a requirement for T-cell–target cell contact to exclude large cell surface glycoproteins.101 Some of these large molecules, especially CD43, CD45 and CD148, inhibit T-cell activation and models of T-cell activation that involve segregation of these proteins away from TCR and related signal transduction machinery remain popular.102 Moreover, it is often thought that secondary costimulation signals from other molecules such as CD28 are an obligatory requirement for T-cell activation. Accordingly, many immunobiologists appear to be surprised by the fact that soluble pMHC tetramers can activate T cells. We have demonstrated that cross-linking cell surface TCR and CD8 with soluble pMHCI tetrameric complexes produces a pattern of early tyrosine phosphorylation (within 2 min) that is almost identical to that induced by antigen-pulsed presenting cells.47 Cross-linking with CD8-null tetramers, or anti-CD3, produces a different intracellular signalling pattern and is substantially less effective at activating T cells, even when CD8 is not required for tetramer binding. Experiments in mice have confirmed the requirement for CD8 binding for tetramer-induced activation.79
Tetramer activation is very sensitive and can induce a full range of effector functions
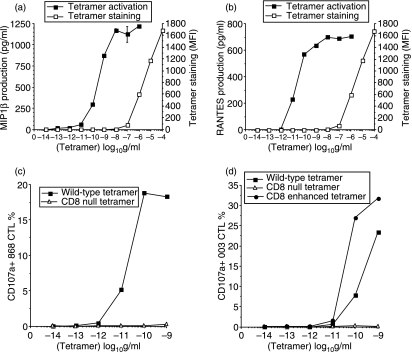
Activation of effector CTL by pMHCI tetramers can be so potent that cells activate at over 1000-fold lower concentrations of tetramer than is required to visualize even small shifts in mean fluorescence intensity in staining experiments (Fig. 7a,b). Such low amounts of tetramer staining are thought to correspond to just a few engaged tetramers per T-cell1 and demonstrate the remarkable sensitivity of pMHC multimer-induced T-cell activation. CD8-null tetramers bind to 868 and 003 T cells (Fig. 8) but do not induce activation (Fig. 7c,d). CD8-null reagents are actually less stable than wild-type tetramers77 and can be used to control for peptide representation.78 In addition to inducing a normal pattern of T-cell signalling,47 tetramer activation results in lytic granule release, a full profile of cytokine and chemokine release and the production of a wide range of cell surface activation markers.81Figure 7 shows examples of tetramer-induced chemokine production and cellular degranulation; Fig. 8 shows that tetramers can also induce the release of cytokines such as interferon-γ.
Figure 7.
T-cell activation by soluble peptide–major histocompatibility complex class I (pMHCI) tetramers is very sensitive. Tetramer staining (□) and tetramer-induced MIP1β (a) and RANTES (b) production (▪) by the 003 cytotoxic T-lymphocyte (CTL) clone specific for the human immunodeficiency virus-derived human leucocyte antigen (HLA) A2-restricted Gag epitope SLYNTVATL (residues 77–85). In each case, staining is only just visible by flow cytometry at a tetramer concentration of 100 ng/ml, whereas tetramer-induced activation occurs at < 100 pg/ml. The 868 T-cell receptor (TCR), which recognizes the same antigen, binds cognate pMHCI with a KD of < 150 nm by surface plasmon resonance,115 thereby making it the strongest TCR/pMHCI interaction measured to date. Tetramer decay experiments indicate that the 003 TCR binds with even higher affinity.3 These high affinities ensure that tetramer staining of these CTL is virtually independent of CD8 binding. Despite binding well to cells and cross-linking similar amounts of TCR, CD8-null tetramers are unable to induce degranulation of either cell (c,d). CD8-enhanced (Q115E) tetramers are more antigenic that wild-type tetramers (d).
Figure 8.
Peptide–major histocompatibility complex (pMHCI) tetramer-induced activation does not require cell–cell contact. The 868 cytotoxic T-lymphocyte (CTL) line specific for the human immunodeficiency virus-derived human leucocyte antigen (HLA) A2-restricted Gag epitope SLYNTVATL (residues 77–85) stains well with both wild-type and CD8-null (D227K/T228A) cognate tetramers at 5 μg/ml (a,b). Staining intensity with CD8-null tetramer is slightly reduced when compared to wild-type tetramer. The 003 CTL clone exhibits less of a difference in staining with the two types of reagent than the 868 CTL line; indeed, staining of 003 CTL with the CD8-null tetramer is only ∼ 50% lower than that seen with the corresponding wild-type tetramer even when used at 10−8 g/ml, the lowest concentration at which we have been able to visualize staining by flow cytometry.47 CD8-null tetramers stain, but generally fail to activate, CTL.47,79 Tetramer-induced activation results in a wide range of effector functions including interferon-γ (IFN-γ) release. In the activation experiments shown, 1000 cells from the 868 CTL line (c) or the 003 CTL clone (d)116 were used in an IFN-γ ELISpot without antigen-presenting cells. These conditions minimize cell–cell contact and largely prevent T cells from representing peptide antigen to each other.117 Even under these conditions, wild-type but not CD8-null tetramers are able to activate cells and induce IFN-γ production; the CD8-null tetramers in these experiments serve as an additional control for peptide representation. Higher concentrations of tetramer are known to induce rapid apoptosis of these CTL.92 This cell death probably accounts for the decrease in spot-forming cells apparent at tetramer concentrations of > 100 pg/ml.
Learning from tetramer-induced T-cell activation
The activation of T cells by soluble multimeric pMHC molecules has already revealed important information about the requirements for T-cell activation by showing that, at a minimum, activation by antigen only requires multivalent engagement of TCR and coreceptor. In addition, pMHC multimer-induced activation does not require any cell–cell contact as shown by the fact that tetramers efficiently activate CTL under conditions where they are unlikely to contact each other (Fig. 8). Tetramers have already taught us that effector human CTL can be activated in the absence of molecular segregation between two adjacent lipid bilayers, costimulatory signals or classic immune synapse formation; while we do not know if an analogous minimum-requirement activation process occurs physiologically, our findings do raise this possibility and so shift the burden of proof toward models of T-cell activation based on an obligatory requirement for such additional processes. Tetramer-induced activation of T cells is also likely to teach us more about the activation process in the future because it enables the study of TCR/pMHC and pMHC/coreceptor interactions in isolation without the problem of altering the kinetics of these interactions that is inherent to studies using antibodies against these components for cell triggering.
Tetramers to manipulate T-cell responses in vivo
It is possible to envisage several ways in which soluble pMHC multimers might be useful for suppressing unwanted T-cell responses or for boosting desirable responses in vivo. First, such reagents might be used for the ex vivo removal of unwanted T cells, such as alloreactive T cells, before transplantation.97 Second, pMHC multimers might be used to delete autoimmune T cells. Several studies have indicated that multimerized pMHC can be used to inactivate autoimmune T cells and prevent diseases such as type I diabetes103,104 and arthritis.105 Third, soluble pMHCI reagents administered in vivo can activate antigen-specific CTL106 and prime CTL for a more proliferative response on subsequent exposure to antigen.107 More recent developments have produced ‘suicide’ pMHC multimers by coupling tetramers to alpha particle emitting225 Actinium108 or biological poisons such as the ribosome-inhibiting protein saporin.109 However, it is important to realize that the use of pMHCI multimers in vivo has several limitations as the effect may vary with the magnitude and number of doses administered;107 for example, pMHCI multimers can result in significant T-cell apoptosis4,47,92 and repeated use of these reagents might induce an immune response against the multimerization scaffold itself. The situation is further complicated by the fact that peptide from soluble pMHC can be represented at the T-cell surface in the context of endogenous MHC molecules and result in further activation.78,100 It is therefore important that these issues are addressed before pMHCI reagents can be routinely used for therapeutic applications in vivo.
pMHCII tetramers
MHCII-restricted TCRs are weaker than MHCI-restricted TCRs
A study of 14 different TCR/pMHC interactions showed that TCR/pMHCI interactions were of significantly higher affinity than TCR interactions with MHCII-restricted peptides.12 Our recent studies of other TCRs have confirmed this difference and highlighted significant differences in the thermodynamics of binding between MHCI-restricted TCRs and MHCII-restricted TCRs (D. Cole, unpublished data). As TCR/pMHC affinity plays such an important role in the binding of pMHC to the T-cell surface, these observations suggest that, in general, it may be more difficult to stain MHCII-restricted T cells with pMHC multimers compared to MHCI-restricted T cells.
The CD4 coreceptor does not stabilize the TCR/pMHCII interaction
The pMHCI/CD8 interaction is known to enhance the on-rate and slow the off-rate of TCR/pMHCI interactions. The combined avidity of these effects can substantially impact on tetramer staining (refs 10,76,80 and Fig. 6). The pMHCII/CD4 interaction may have similar potential. However, the human pMHCII/CD4 interaction remains below the limits of those reliably detected by techniques such as SPR (A. Sewell, unpublished observations) and reports to date have concluded that the pMHCII/CD4 interaction does not stabilize TCR/pMHCII interactions and plays no role in the binding of pMHCII tetramers.110–112
Potential problems for pMHCII tetramers
There is no doubt that the strength of TCR/pMHCI interactions plays a pivotal determinative role in pMHCI multimer binding at the T-cell surface.10 The CD8 interaction is able to aid the TCR/pMHC interaction and reduce the TCR/pMHCI affinity threshold required for tetramer staining three-fold.10 As described above, TCR/pMHCII interactions appear to be significantly weaker than TCR/pMHCI interactions12 and CD4 does not help to stabilize tetramer binding in the same manner as CD8. If the rules that govern the binding of pMHCII multimers are similar to those that we have observed for pMHCI multimers, then the combination of lower affinity TCR/pMHC interactions and a lack of coreceptor help may conspire to ensure that only MHCII-restricted T cells with the very strongest TCR affinities lend themselves to tetramer staining. We regularly use pMHCII tetramers to stain anti-pathogen T cells (refs20,113 and unpublished results), but have had limited success with the use of these reagents to stain anti-tumour and anti-self (autoimmune) T-cell populations (unpublished) that have lower affinity TCR/pMHC interactions.12 Such results are less likely to find their way into the literature and it remains possible that problems with pMHCII multimer staining of all but the T cells with the strongest TCR affinities are widespread. Ongoing work in our laboratory aims to understand the differences between pMHCI and pMHCII multimer technologies and to close the gap between them; it is hoped that by enabling pMHCII tetramers to stain T cells with lower affinity TCR interactions, we can help these reagents to reach their full potential. Valler and Stern have provided recent discussion on pMHCII tetramers for interested readers.114
Conclusions
In the decade since their initial inception, pMHC multimers have become a regular tool in the immunologist’s repertoire and have spawned the formation of several commercial companies. Some of the next generation of pMHC multimers described above have extended the use of these reagents beyond that of simply looking to see whether a particular antigen-specific T-cell population is present in a sample. It is now possible to use pMHC multimers to grade T cells based on TCR affinity for antigen, dissect the properties of antigen-specific T-cell clonotypes directly ex vivo, isolate and propagate individual T-cell clones and kill specific T cells. The future of pMHC tetramers continues to look bright and we anticipate that further developments may allow the therapeutic use of pMHC multimers to enrich or boost desirable T-cell populations and remove or inhibit undesirable T-cell populations in clinical settings.
References
- 1.Laugel B, Boulter JM, Lissin N, et al. Design of soluble recombinant T cell receptors for antigen targeting and T cell inhibition. J Biol Chem. 2005;280:1882–92. doi: 10.1074/jbc.M409427200. [DOI] [PubMed] [Google Scholar]
- 2.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 3.Wooldridge L, van den Berg HA, Glick M, et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor–antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillaume P, Legler DF, Boucheron N, Doucey MA, Cerottini JC, Luescher IF. Soluble major histocompatibility complex–peptide octamers with impaired CD8 binding selectively induce Fas-dependent apoptosis. J Biol Chem. 2003;278:4500–9. doi: 10.1074/jbc.M208863200. [DOI] [PubMed] [Google Scholar]
- 5.Bakker AH, Schumacher TN. MHC multimer technology: current status and future prospects. Curr Opin Immunol. 2005;17:428–33. doi: 10.1016/j.coi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Schamel WW, Arechaga I, Risueno RM, van Santen HM, Cabezas P, Risco C, Valpuesta JM, Alarcon B. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelan JA, Dunbar PR, Price DA, et al. Specificity of CTL interactions with peptide–MHC class I tetrameric complexes is temperature dependent. J Immunol. 1999;163:4342–8. [PubMed] [Google Scholar]
- 8.Dal Porto J, Johansen TE, Catipovic B, et al. A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc Natl Acad Sci U S A. 1993;90:6671–5. doi: 10.1073/pnas.90.14.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greten TF, Slansky JE, Kubota R, et al. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19- specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA. 1998;95:7568–73. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laugel B, van den Berg HA, Gostick E, et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282:23799–810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 11.Purbhoo MA, Li Y, Sutton DH, et al. The HLA A*0201-restricted hTERT(540-548) peptide is not detected on tumor cells by a CTL clone or a high-affinity T-cell receptor. Mol Cancer Ther. 2007;6:2081–91. doi: 10.1158/1535-7163.MCT-07-0092. [DOI] [PubMed] [Google Scholar]
- 12.Cole DK, Pumphrey NJ, Boulter JM, et al. Human TCR-binding affinity is governed by MHC class restriction. J Immunol. 2007;178:5727–34. doi: 10.4049/jimmunol.178.9.5727. [DOI] [PubMed] [Google Scholar]
- 13.Falta MT, Fontenot AP, Rosloniec EF, et al. Class II major histocompatibility complex-peptide tetramer staining in relation to functional avidity and T cell receptor diversity in the mouse CD4(+) T cell response to a rheumatoid arthritis-associated antigen. Arthritis Rheum. 2005;52:1885–96. doi: 10.1002/art.21098. [DOI] [PubMed] [Google Scholar]
- 14.Gebe JA, Falk BA, Rock KA, Kochik SA, Heninger AK, Reijonen H, Kwok WW, Nepom GT. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur J Immunol. 2003;33:1409–17. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 15.Rubio-Godoy V, Dutoit V, Rimoldi D, et al. Discrepancy between ELISPOT IFN-γ secretion and binding of A2/peptide multimers to TCR reveals interclonal dissociation of CTL effector function from TCR–peptide/MHC complexes half-life. Proc Natl Acad Sci USA. 2001;98:10302–7. doi: 10.1073/pnas.181348898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons GE, Roszkowski JJ, Man S, Kast WM, Nishimura MI. T-cell receptor tetramer binding or the lack there of does not necessitate antigen reactivity in T-cell receptor transduced T cells. Cancer Immunol Immunother. 2006;55:1142–50. doi: 10.1007/s00262-005-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weichsel R, Dix C, Wooldridge L, et al. Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res. 2008;14:2484–91. doi: 10.1158/1078-0432.CCR-07-4393. [DOI] [PubMed] [Google Scholar]
- 18.Lissina A, Ladell K, Skowera A, et al. Protein kinase inhibitors substantially improve the physical detection of T cells with peptide-MHC tetramers. J Immunol Methods. 2008 doi: 10.1016/j.jim.2008.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wooldridge L, Hutchinson SL, Choi EM, et al. Anti-CD8 antibodies can inhibit or enhance peptide–MHC class I (pMHCI) multimer binding: this is paralleled by their effects on CTL activation and occurs in the absence of an interaction between pMHCI and CD8 on the cell surface. J Immunol. 2003;171:6650–60. doi: 10.4049/jimmunol.171.12.6650. [DOI] [PubMed] [Google Scholar]
- 20.Wooldridge L, Scriba TJ, Milicic A, Laugel B, Gostick E, Price DA, Phillips RE, Sewell AK. Anti-coreceptor antibodies profoundly affect staining with peptide–MHC class I and class II tetramers. Eur J Immunol. 2006;36:1847–55. doi: 10.1002/eji.200635886. [DOI] [PubMed] [Google Scholar]
- 21.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–46. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chattopadhyay PK, Price DA, Harper TF, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12:972–7. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 23.Barnes E, Ward SM, Kasprowicz VO, Dusheiko G, Klenerman P, Lucas M. Ultra-sensitive class I tetramer analysis reveals previously undetectable populations of antiviral CD8+ T cells. Eur J Immunol. 2004;34:1570–7. doi: 10.1002/eji.200424898. [DOI] [PubMed] [Google Scholar]
- 24.Oxenius A, Sewell AK, Dawson SJ, et al. Functional discrepancies in HIV-specific CD8+ T-lymphocyte populations are related to plasma virus load. J Clin Immunol. 2002;22:363–74. doi: 10.1023/a:1020656300027. [DOI] [PubMed] [Google Scholar]
- 25.Alexander-Miller MA. Differential expansion and survival of high and low avidity cytotoxic T cell populations during the immune response to a viral infection. Cell Immunol. 2000;201:58–62. doi: 10.1006/cimm.1999.1632. [DOI] [PubMed] [Google Scholar]
- 26.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–7. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutoit V, Rubio-Godoy V, Doucey MA, et al. Functional avidity of tumor antigen-specific CTL recognition directly correlates with the stability of MHC/peptide multimer binding to TCR. J Immunol. 2002;168:1167–71. doi: 10.4049/jimmunol.168.3.1167. [DOI] [PubMed] [Google Scholar]
- 28.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J Exp Med. 1998;187:1647–57. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between MHC polymorphism, T-cell avidity, and diversity in immune defense. Science. 2002;298:1797–800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 30.Sedlik C, Dadaglio G, Saron MF, Deriaud E, Rojas M, Casal SI, Leclerc C. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J Virol. 2000;74:5769–75. doi: 10.1128/jvi.74.13.5769-5775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide–MHC tetramers. J Immunol. 1999;162:2227–34. [PubMed] [Google Scholar]
- 32.Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, Wang C, Davis MM. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111:639–47. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162:989–94. [PubMed] [Google Scholar]
- 34.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A. 1995;92:5042–6. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroger CJ, Alexander-Miller MA. Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179:748–51. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 36.Arcaro A, Gregoire C, Bakker TR, et al. CD8β endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med. 2001;194:1485–95. doi: 10.1084/jem.194.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang J, Edwards LJ, Evavold BD, Zhu C. Kinetics of MHC–CD8 interaction at the T cell membrane. J Immunol. 2007;179:7653–62. doi: 10.4049/jimmunol.179.11.7653. [DOI] [PubMed] [Google Scholar]
- 38.Kwan-Lim GE, Ong T, Aosai F, Stauss H, Zamoyska R. Is CD8 dependence a true reflection of TCR affinity for antigen? Int Immunol. 1993;5:1219–28. doi: 10.1093/intimm/5.10.1219. [DOI] [PubMed] [Google Scholar]
- 39.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: correlation with CD8αβ versus CD8αα expression. J Immunol. 2001;167:2577–84. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 40.Gao GF, Tormo J, Gerth UC, et al. Crystal structure of the complex between human CD8α(α) and HLA-A2. Nature. 1997;387:630–4. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- 41.Wang JH, Meijers R, Xiong Y, Liu JH, Sakihama T, Zhang R, Joachimiak A, Reinherz EL. Crystal structure of the human CD4 N-terminal two-domain fragment complexed to a class II MHC molecule. Proc Natl Acad Sci U S A. 2001;98:10799–804. doi: 10.1073/pnas.191124098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–64. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 43.Janeway CA., Jr The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992;10:645–74. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 44.Laugel B, Price DA, Milicic A, Sewell AK. CD8 exerts differential effects on the deployment of cytotoxic T lymphocyte effector functions. Eur J Immunol. 2007;37:905–13. doi: 10.1002/eji.200636718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park JH, Adoro S, Lucas PJ, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–59. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 46.Glaichenhaus N, Shastri N, Littman DR, Turner JM. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991;64:511–20. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- 47.Purbhoo MA, Boulter JM, Price DA, et al. The human CD8 coreceptor effects cytotoxic T cell activation and antigen sensitivity primarily by mediating complete phosphorylation of the T cell receptor zeta chain. J Biol Chem. 2001;276:32786–92. doi: 10.1074/jbc.M102498200. [DOI] [PubMed] [Google Scholar]
- 48.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 49.Bosselut R, Zhang W, Ashe JM, Kopacz JL, Samelson LE, Singer A. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T-cell receptor signal transduction. J Exp Med. 1999;190:1517–26. doi: 10.1084/jem.190.10.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gakamsky DM, Luescher IF, Pramanik A, Kopito RB, Lemonnier F, Vogel H, Rigler R, Pecht I. CD8 kinetically promotes ligand binding to the T-cell antigen receptor. Biophys J. 2005;89:2121–33. doi: 10.1529/biophysj.105.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dianzani U, Shaw A, al-Ramadi BK, Kubo RT, Janeway CA., Jr Physical association of CD4 with the T cell receptor. J Immunol. 1992;148:678–88. [PubMed] [Google Scholar]
- 52.Doucey MA, Goffin L, Naeher D, Michielin O, Baumgartner P, Guillaume P, Palmer E, Luescher IF. CD3 delta establishes a functional link between the T cell receptor and CD8. J Biol Chem. 2003;278:3257–64. doi: 10.1074/jbc.M208119200. [DOI] [PubMed] [Google Scholar]
- 53.Kwan Lim GE, McNeill L, Whitley K, Becker DL, Zamoyska R. Co-capping studies reveal CD8/TCR interactions after capping CD8 beta polypeptides and intracellular associations of CD8 with p56(lck) Eur J Immunol. 1998;28:745–54. doi: 10.1002/(SICI)1521-4141(199802)28:02<745::AID-IMMU745>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Naeher D, Luescher IF, Palmer E. A role for the alpha-chain connecting peptide motif in mediating TCR–CD8 cooperation. J Immunol. 2002;169:2964–70. doi: 10.4049/jimmunol.169.6.2964. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki S, Kupsch J, Eichmann K, Saizawa MK. Biochemical evidence of the physical association of the majority of CD3 delta chains with the accessory/co-receptor molecules CD4 and CD8 on nonactivated T lymphocytes. Eur J Immunol. 1992;22:2475–9. doi: 10.1002/eji.1830221002. [DOI] [PubMed] [Google Scholar]
- 56.Demotte N, Stroobant V, Courtoy PJ, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–24. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Wyer JR, Willcox BE, Gao GF, Gerth UC, Davis SJ, Bell JI, van der Merwe PA, Jakobsen BK. T cell receptor and coreceptor CD8 αα bind peptide–MHC independently and with distinct kinetics. Immunity. 1999;10:219–25. doi: 10.1016/s1074-7613(00)80022-9. [DOI] [PubMed] [Google Scholar]
- 58.Cole DK, Dunn SM, Sami M, Boulter JM, Sewell AK, Jakobsen BK. Co-engagement of the CD8αα co-receptor to peptide–major histocompatibility complexes: the effect of T-cell receptor docking on CD8 binding. Mol Immunol. 2008;45:2700–9. doi: 10.1016/j.molimm.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 59.van den Berg HA, Wooldridge L, Laugel B, Sewell AK. Coreceptor CD8-driven modulation of T cell antigen receptor specificity. J Theor Biol. 2007;249:395–408. doi: 10.1016/j.jtbi.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maile R, Siler CA, Kerry SE, Midkiff KE, Collins EJ, Frelinger JA. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–27. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 61.Kroger CJ, Alexander-Miller MA. Dose-dependent modulation of CD8 and functional avidity as a result of peptide encounter. Immunology. 2007;122:167–78. doi: 10.1111/j.1365-2567.2007.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, Sadelain M. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med. 2007;13:1440–9. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 63.Plas DR, Thomas ML. Negative regulation of antigen receptor signaling in lymphocytes. J Mol Med. 1998;76:589–95. doi: 10.1007/s001090050254. [DOI] [PubMed] [Google Scholar]
- 64.Johnson KG, LeRoy FG, Borysiewicz LK, Matthews RJ. TCR signaling thresholds regulating T cell development and activation are dependent upon SHP-1. J Immunol. 1999;162:3802–13. [PubMed] [Google Scholar]
- 65.Sathish JG, Dolton G, Leroy FG, Matthews RJ. Loss of Src homology region 2 domain-containing protein tyrosine phosphatase-1 increases CD8+ T cell–APC conjugate formation and is associated with enhanced in vivo CTL function. J Immunol. 2007;178:330–7. doi: 10.4049/jimmunol.178.1.330. [DOI] [PubMed] [Google Scholar]
- 66.Derby M, Alexander-Miller M, Tse R, Berzofsky J. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J Immunol. 2001;166:1690–7. doi: 10.4049/jimmunol.166.3.1690. [DOI] [PubMed] [Google Scholar]
- 67.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J Exp Med. 1996;184:485–92. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuller MJ, Khanolkar A, Tebo AE, Zajac AJ. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J Immunol. 2004;172:4204–14. doi: 10.4049/jimmunol.172.7.4204. [DOI] [PubMed] [Google Scholar]
- 69.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–86. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 70.Gallimore A, Glithero A, Godkin A, Tissot AC, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I–peptide complexes. J Exp Med. 1998;187:1383–93. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moskophidis D, Laine E, Zinkernagel RM. Peripheral clonal deletion of antiviral memory CD8+ T cells. Eur J Immunol. 1993;23:3306–11. doi: 10.1002/eji.1830231237. [DOI] [PubMed] [Google Scholar]
- 73.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–61. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 74.Xiong Y, Luscher MA, Altman JD, Hulsey M, Robinson HL, Ostrowski M, Barber BH, MacDonald KS. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J Virol. 2001;75:3028–33. doi: 10.1128/JVI.75.6.3028-3033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–13. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choi EM, Chen JL, Wooldridge L, et al. High avidity antigen-specific CTL identified by CD8-independent tetramer staining. J Immunol. 2003;171:5116–23. doi: 10.4049/jimmunol.171.10.5116. [DOI] [PubMed] [Google Scholar]
- 77.Hutchinson SL, Wooldridge L, Tafuro S, et al. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J Biol Chem. 2003;278:24285–93. doi: 10.1074/jbc.M300633200. [DOI] [PubMed] [Google Scholar]
- 78.Schott E, Bertho N, Ge Q, Maurice MM, Ploegh HL. Class I negative CD8 T cells reveal the confounding role of peptide-transfer onto CD8 T cells stimulated with soluble H2-Kb molecules. Proc Natl Acad Sci USA. 2002;99:13735–40. doi: 10.1073/pnas.212515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schott E, Ploegh HL. Mouse MHC class I tetramers that are unable to bind to CD8 reveal the need for CD8 engagement in order to activate naive CD8 T cells. Eur J Immunol. 2002;32:3425–34. doi: 10.1002/1521-4141(200212)32:12<3425::AID-IMMU3425>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 80.Pittet MJ, Rubio-Godoy V, Bioley G, et al. Alpha 3 domain mutants of peptide/MHC class I multimers allow the selective isolation of high avidity tumor-reactive CD8 T cells. J Immunol. 2003;171:1844–9. doi: 10.4049/jimmunol.171.4.1844. [DOI] [PubMed] [Google Scholar]
- 81.Wooldridge L, Lissina A, Vernazza J, et al. Enhanced immunogenicity of CTL antigens through mutation of the CD8 binding MHC class I invariant region. Eur J Immunol. 2007;37:1323–33. doi: 10.1002/eji.200636765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Melenhorst JJ, Scheinberg P, Lissina A, et al. Detection of low avidity CD8+ T cells with coreceptor-enhanced peptide-MHC class I tetramers. J Immunol Methods. 2008;338:3390–402. doi: 10.1016/j.jim.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasprowicz V, Ward SM, Turner A, et al. Defining the directionality and quality of influenza virus-specific CD8+ T cell cross-reactivity in individuals infected with hepatitis C virus. J Clin Invest. 2008;118:1143–53. doi: 10.1172/JCI33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douek DC, Betts MR, Brenchley JM, et al. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J Immunol. 2002;168:3099–104. doi: 10.4049/jimmunol.168.6.3099. [DOI] [PubMed] [Google Scholar]
- 85.Price DA, Sewell AK. Quantitative clonotypic analysis of antigen-specific T-cell responses. Trends Immunol. 2002;23:336–7. [Google Scholar]
- 86.Price DA, West SM, Betts MR, et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity. 2004;21:793–803. doi: 10.1016/j.immuni.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 87.Price DA, Brenchley JM, Ruff LE, et al. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J Exp Med. 2005;202:1349–61. doi: 10.1084/jem.20051357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davenport MP, Price DA, McMichael AJ. The T cell repertoire in infection and vaccination: implications for control of persistent viruses. Curr Opin Immunol. 2007;19:294–300. doi: 10.1016/j.coi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Gras S, Kjer-Nielsen L, Burrows SR, McCluskey J, Rossjohn J. T-cell receptor bias and immunity. Curr Opin Immunol. 2008;20:119–25. doi: 10.1016/j.coi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Dunbar PR, Chen JL, Chao D, et al. Cutting edge: rapid cloning of tumor-specific CTL suitable for adoptive immunotherapy of melanoma. J Immunol. 1999;162:6959–62. [PubMed] [Google Scholar]
- 91.Cebecauer M, Guillaume P, Hozak P, Mark S, Everett H, Schneider P, Luescher IF. Soluble MHC–peptide complexes induce rapid death of CD8+ CTL. J Immunol. 2005;174:6809–19. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 92.Xu XN, Purbhoo MA, Chen N, et al. A novel approach to antigen-specific deletion of CTL with minimal cellular activation using alpha3 domain mutants of MHC class I/peptide complex. Immunity. 2001;14:591–602. doi: 10.1016/s1074-7613(01)00133-9. [DOI] [PubMed] [Google Scholar]
- 93.Guillaume P, Baumgaertner P, Angelov GS, Speiser D, Luescher IF. Fluorescence-activated cell sorting and cloning of bona fide CD8+ CTL with reversible MHC–peptide and antibody Fab′ conjugates. J Immunol. 2006;177:3903–12. doi: 10.4049/jimmunol.177.6.3903. [DOI] [PubMed] [Google Scholar]
- 94.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 95.Moris A, Teichgraber V, Gauthier L, Buhring HJ, Rammensee HG. Cutting edge: characterization of allorestricted and peptide-selective alloreactive T cells using HLA-tetramer selection. J Immunol. 2001;166:4818–21. doi: 10.4049/jimmunol.166.8.4818. [DOI] [PubMed] [Google Scholar]
- 96.Whitelegg AM, Oosten LE, Jordan S, Kester M, van Halteren AG, Madrigal JA, Goulmy E, Barber LD. Investigation of peptide involvement in T cell allorecognition using recombinant HLA class I multimers. J Immunol. 2005;175:1706–14. doi: 10.4049/jimmunol.175.3.1706. [DOI] [PubMed] [Google Scholar]
- 97.Kappel BJ, Pinilla-Ibarz J, Kochman AA, et al. Remodeling specific immunity by use of MHC tetramers: demonstration in a graft-versus-host disease model. Blood. 2006;107:2045–51. doi: 10.1182/blood-2005-07-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Massey RC, Scriba TJ, Brown EL, Phillips RE, Sewell AK. Use of peptide–major histocompatibility complex tetramer technology to study interactions between Staphylococcus aureus proteins and human cells. Infect Immun. 2007;75:5711–5. doi: 10.1128/IAI.00875-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delon J, Gregoire C, Malissen B, Darche S, Lemaitre F, Kourilsky P, Abastado JP, Trautmann A. CD8 expression allows T cell signaling by monomeric peptide–MHC complexes. Immunity. 1998;9:467–73. doi: 10.1016/s1074-7613(00)80630-5. [DOI] [PubMed] [Google Scholar]
- 100.Ge Q, Stone JD, Thompson MT, Cochran JR, Rushe M, Eisen HN, Chen J, Stern LJ. Soluble peptide–MHC monomers cause activation of CD8+ T cells through transfer of the peptide to T cell MHC molecules. Proc Natl Acad Sci USA. 2002;99:13729–34. doi: 10.1073/pnas.212515299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Davis SJ, van der Merwe PA. The structure and ligand interactions of CD2: implications for T-cell function. Immunol Today. 1996;17:177–87. doi: 10.1016/0167-5699(96)80617-7. [DOI] [PubMed] [Google Scholar]
- 102.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide–MHC ligand. Nature. 2005;436:578–82. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 103.Casares S, Hurtado A, McEvoy RC, Sarukhan A, von Boehmer H, Brumeanu TD. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide–MHC class II chimera. Nat Immunol. 2002;3:383–91. doi: 10.1038/ni770. [DOI] [PubMed] [Google Scholar]
- 104.Masteller EL, Warner MR, Ferlin W, Judkowski V, Wilson D, Glaichenhaus N, Bluestone JA. Peptide–MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol. 2003;171:5587–95. doi: 10.4049/jimmunol.171.10.5587. [DOI] [PubMed] [Google Scholar]
- 105.Zuo L, Cullen CM, DeLay ML, Thornton S, Myers LK, Rosloniec EF, Boivin GP, Hirsch R. A single-chain class II MHC–IgG3 fusion protein inhibits autoimmune arthritis by induction of antigen-specific hyporesponsiveness. J Immunol. 2002;168:2554–9. doi: 10.4049/jimmunol.168.5.2554. [DOI] [PubMed] [Google Scholar]
- 106.Sakita I, Horig H, Sun R, Wang F, Nathenson SG. In vivo CTL immunity can be elicited by in vitro reconstituted MHC/peptide complex. J Immunol Methods. 1996;192:105–15. doi: 10.1016/0022-1759(96)00027-0. [DOI] [PubMed] [Google Scholar]
- 107.Maile R, Wang B, Schooler W, Meyer A, Collins EJ, Frelinger JA. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J Immunol. 2001;167:3708–14. doi: 10.4049/jimmunol.167.7.3708. [DOI] [PubMed] [Google Scholar]
- 108.Yuan RR, Wong P, McDevitt MR, et al. Targeted deletion of T-cell clones using alpha-emitting suicide MHC tetramers. Blood. 2004;104:2397–402. doi: 10.1182/blood-2004-01-0324. [DOI] [PubMed] [Google Scholar]
- 109.Hess PR, Barnes C, Woolard MD, Johnson MD, Cullen JM, Collins EJ, Frelinger JA. Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood. 2007;109:3300–7. doi: 10.1182/blood-2006-06-028001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boniface JJ, Rabinowitz JD, Wulfing C, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected] Immunity. 1998;9:459–66. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 111.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–82. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 112.Hamad AR, O’Herrin SM, Lebowitz MS, Srikrishnan A, Bieler J, Schneck J, Pardoll D. Potent T cell activation with dimeric peptide–major histocompatibility complex class II ligand: the role of CD4 coreceptor. J Exp Med. 1998;188:1633–40. doi: 10.1084/jem.188.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scriba TJ, Purbhoo M, Day CL, et al. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J Immunol. 2005;175:6334–43. doi: 10.4049/jimmunol.175.10.6334. [DOI] [PubMed] [Google Scholar]
- 114.Vollers SS, Stern LJ. Class II major histocompatibility complex tetramer staining: progress, problems and prospects. Immunology. 2008;123:305–13. doi: 10.1111/j.1365-2567.2007.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008 doi: 10.1038/nm.1779. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sewell AK, Harcourt GC, Goulder PJ, Price DA, Phillips RE. Antagonism of cytotoxic T lymphocyte-mediated lysis by natural HIV-1 altered peptide ligands requires simultaneous presentation of agonist and antagonist peptides. Eur J Immunol. 1997;27:2323–9. doi: 10.1002/eji.1830270929. [DOI] [PubMed] [Google Scholar]
- 117.Green AE, Lissina A, Hutchinson SL, et al. Recognition of nonpeptide antigens by human Vγ9Vδ2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004;136:472–82. doi: 10.1111/j.1365-2249.2004.02472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]