Abstract
We previously reported that activation-induced deaminase (AID) heterozygous MRL/lpr mice have substantially lower levels of serum anti-dsDNA autoantibodies than AID wild-type littermates. Given the known functions of AID, here we examined whether this decrease in pathogenic autoantibodies in the heterozygotes was the result of a defect in class switch recombination, somatic hypermutation, or both. We report significant impairment of switch recombination to most isotypes except immunoglobulin G3 (IgG3) in vitro. However, serum levels of IgG were similar to AID wild-type levels even in very young mice. Mutation accumulation in the B cells from Peyer's patches also revealed reduced somatic hypermutation in the heterozygotes. Unlike the switch defect, the hypermutation defect probably resulted in an in vivo effect because the serum IgG antibodies from the heterozygotes were of strikingly lower affinity to dsDNA than serum IgG antibodies from wild-type littermates. This suggests that the somatic hypermutation defect resulted in impaired affinity maturation of autoantibodies in these mice and explains the low levels of specific anti-dsDNA antibodies in the heterozygotes. This correlated with a delay in the development of kidney damage. These results imply that AID levels impact the class switch recombination and somatic hypermutation mechanisms and directly implicate affinity maturation of autoantibodies in autoimmunity.
Keywords: affinity maturation, autoantibodies, autoimmunity, B cells, immunogenetics
Introduction
The MRL/lpr mouse is a good model of systemic lupus erythematosus (SLE) because it shares many characteristics with SLE such as the involvement of multiple loci, the presence of high levels of autoantibodies, particularly to double-stranded (ds) DNA, and the development of lupus nephritis characterized by glomerulonephritis, mononuclear cell infiltration and immune complex deposition.1–4 Multiple factors have been implicated in the development of this disease such as breakdown in lymphocyte tolerance, complement defects and defective apoptosis.5–20 Early work delineated the characteristics of autoantibodies in MRL/lpr mice21–24 but it was not clear whether autoantibodies were correlated with the syndrome in these mice or were actively contributing to disease development. Several pieces of evidence conclusively demonstrated an active role for autoantibodies and B cells in the lupus-like syndrome. These included the development of transgenic mice rendered autoimmune by the expression of autoreactive B-cell specificities, the identification of defects associated specifically with B-cell tolerance, and studies demonstrating that B cells play multiple (key) roles in the development of the lupus syndrome associated with MRL/lpr mice, both as secretors of autoantibodies and as cells that can stimulate autoreactive T lymphoytes.25–28 Additional studies have strongly implicated defects in several B-cell tolerance checkpoints such as in early B-cell development and in peripheral tissues.29–33 That immunoglobulin G (IgG) autoantibodies are required for kidney damage is suggested by the reduction in glomerular injury in mice that are deficient in FcRγ and FcγRIII.34,35 Furthermore, we recently demonstrated that the kidney pathology associated with MRL/lpr mice is critically dependent on the presence of the activation-induced deaminase (AID) protein that triggers the generation of somatically mutated, high-affinity, isotype-switched autoantibodies.36
The protein AID is a deaminase that triggers class switch recombination (CSR) and somatic hypermutation (SHM) during B-cell immune responses via the deamination of cytosines in the DNA encoding the variable (V) regions of immunoglobulin loci in SHM or the switch regions in CSR.37–40 During an immune response both CSR and SHM are activated in B cells in the germinal centres that are formed in secondary lymphoid tissues such as the spleen and lymph nodes.41,42 The germinal centres are transient structures wherein the coordination of SHM with a cellular mechanism that selects B cells that have acquired mutations enhancing their affinity to foreign antigen leads to the formation of B cells that secrete high-affinity antibodies.43,44 In systemic autoimmune disorders such as SLE and in the lupus syndrome of MRL/lpr mice, most autoantibodies are secreted by B cells that have undergone hypermutation. However, given that SHM can occur outside the germinal centres in autoimmunity,45 it is unclear whether these antibodies are similar in affinity to those that might be obtained from the germinal centre environment where hypermutation and affinity maturation may be optimally coordinated. Moreover, because SHM and CSR tend to occur under the same circumstances (in germinal centres, during T-cell-dependent immune responses) and both depend on AID, it has been difficult to distinguish the role of SHM and affinity maturation from that of CSR in the development of pathogenic autoantibodies. In our recently described novel mouse model of attenuated lupus nephritis wherein inactivation of the AID gene in MRL/lpr mice resulted in a dramatic increase in survival, we reported a lag in the formation of anti-dsDNA antibodies in the AID heterozygous MRL/lpr mice.36 Given the known functions of AID in SHM and CSR, we speculated that it was a defect in either or both of these mechanisms that correlated with the decrease in the levels of autoantibodies in young AID heterozygous MRL/lpr mice. In this study, we carefully examined SHM rates in Peyer's patch-derived B cells and CSR both in vitro and in vivo, as well as the affinity of serum autoantibodies in AID heterozygous MRL/lpr mice. We present conclusive evidence demonstrating defects in both CSR and SHM. However, in vivo analysis suggests that the decreased autoimmunity correlated best with a striking decrease in the affinity of IgG antibodies against dsDNA in the heterozygotes that may result from the decrease in the rate of SHM.
Methods and materials
Mice
C57BL/6J and MRL/lpr mice were purchased from the Jackson Laboratory (Bar Harbor, ME). AID−/− B6 mice were kindly provided by Tasuku Honjo and AID−/− MRL/lpr mice were generated in this laboratory by backcrossing AID−/− B6 mice with MRL/lpr mice as previously reported.36 Except when specified, the mice used in this study were the ninth or higher generations of the backcross. The AID heterozygous MRL/lpr mice were inbred to generate the AID wild-type, heterozygous and homozygous mutant siblings used in this study. AID+/− B6 and AID−/− B6 mice were also used for comparisons. All the mice were housed in the animal facility at the National Institutes of Health/National Institute of Environmental Health Sciences under specific pathogen-free conditions. Sample sizes per group and the number of times a given experiment was repeated are described in the figure legends.
Beginning at 2 months of age, mice were bled monthly by retro-orbital puncture. Sera were collected and stored at −20°. Urine samples were also collected to monitor kidney damage. Urine protein was tested with Multistix 10 SG (Bayer, Elkhart, IN) and scored from 0 to 5 as previously described.36
B-cell culture and stimulation
Spleens were collected from 2- to 3-month-old mice and single-cell suspensions were made from pooled spleens. Red blood cells were removed using ACK lysing buffer (0·15 m NH4Cl, 10·0 mm KHCO3, 0·1 mm Na2EDTA, pH 7·4). Resting/naïve B cells were purified by elimination of CD43+ cells with magnetic-activated cell sorting CD43 MicroBeads following the manufacturer's instructions (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). CD43− cells were collected, washed and resuspended at 1 × 106 cells/ml in complete B-cell culture medium (Dulbecco's modified Eagle's medium, supplemented with 10% heat-inactivated fetal bovine serum, 1% non-essential amino acids, 2 mm l-glutamine, 50 μm 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin sulphate, 10 mm HEPES, pH 7·4, all provided by Invitrogen, Carlsbad, CA). One millilitre of culture (1 × 106 cells) was placed in each well in 24-well plates. Cells were treated with lipopolysaccharide (LPS; 50 μg/ml), with or without the following cytokines for 15 hr, 24 hr, 48 hr and 72 hr: murine interferon-γ (mIFN-γ; 20 ng/ml), murine interleukin-4 (mIL-4; 20 ng/ml), and human transforming growth factor-β (hTGF-β) (2 ng/ml). The LPS was purchased from Sigma Co. (St Louis, MO), while the cytokines were obtained from R&D Systems (Minneapolis, MN). At the indicated time-points, culture medium was harvested to test for secreted antibodies, while the cells were either directly lysed in TRIzol (Invitrogen) or harvested for flow cytometric analysis.
Enzyme-linked immunosorbent assay for antibodies with different isotypes
Enzyme-linked immunosorbent assay (ELISA) kits for the detection of mouse IgM, total IgG, IgG3, IgG1, IgG2b, IgG2a and IgA were all purchased from Bethyl Laboratories (Montgomery, TX). Culture supernatants collected at different time-points were diluted for testing as follows: IgM (1 : 12·5), IgG3 (1 : 2), and IgG1 (1 : 2), or directly tested for IgG2b, IgG2a and IgA (no dilution). Mouse serum samples were diluted from 1 : 10 000 to 1 : 50 000 for testing different isotypes. All ELISAs were performed following the manufacturer's instructions.
Reverse transcription–polymerase chain reaction
RNA was prepared from TRIzol lysates of cultured splenic B cells as mentioned above, resuspended in 10 μl diethyl pyrocarbonate (DEPC) treated water, and quantified using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE). One microgram RNA was used as template for complementary DNA (cDNA) synthesis in the reverse transcriptase reaction by using a SuperScript III First-Strand Synthesis System for reverse transcription–polymerase chain reaction (RT-PCR; Invitrogen). Two microlitres cDNA and its dilutions at 1 : 4 and 1 : 16 were subjected to RT-PCR. Amplification of AID cDNA was modified from a previously described protocol.46 DNA fragments of 606 base pairs in size were amplified using Taq DNA polymerase (Invitrogen) and the AID F primer (5′-GGA GAC CGA TAT GGA CAG CCT TCT G-3′) and the AID R primer (5′-TCA AAA TCC CAA CAT ACG AAA TGC-3′). The PCR conditions were: 94° for 2 min; 28 cycles of 94° for 45 seconds, 55° for 30 seconds and 72° for 45 seconds; and 72° for 5 min. To detect γ1 circle transcripts, a hot-start RT-PCR was modified from the protocol of Kinoshita et al.47 AccuPrime™Pfx DNA polymerase (Invitrogen) was used to ensure the PCR yield and the PCR was performed at 95° for 2 min; 35 cycles of 95° for 20 seconds and 61° for 30 seconds; and 68° for 2 min. The primers were: KKIγ1F, 5′-GGC CCT TCC AGA TCT TTG AG-3′; KKCμR, 5′-AAT GGT GCT GGG CAG GAA GT-3′ and the PCR products were 408 base pairs. The RT-PCR amplification of γ1 germline transcripts was modified from the protocol of Dunnick et al.48 DNA fragments of 578 base pairs were amplified with WDIγ1 primer (5′-GACGGCTGCTTTCACAGCTT-3′) and WDCγ1 primer (5′-GCATGATGGGAAGTTCACTGACTG-3′) using the following conditions: 95° for 3 min; 32 cycles of 95° for 45 seconds, 58° for 1 min and 72° for 1 min; and 72° for 5 min. The RT-PCR of mouse GAPDH was performed with GAPDH F primer (5′-ACC ACA GTC CAT GCC ATC AC-3′) and GAPDH R primer (5′-TCC ACC ACC CTG TTG CTG TA-3′) and the PCR conditions used were: a total of 23 cycles of 94° for 30 seconds, 59° for 30 seconds, and 72° for 45 seconds.
Flow cytometry analysis
Cultured splenic B cells stimulated for 72 hr with LPS plus mouse IL-4 were harvested, incubated with rat anti-mouse CD16/CD32 (Fcγ III/II receptor), and stained with the following conjugated monoclonal antibodies (mAbs): phycoerythrin-Cy7-conjugated rat anti-mouse CD19 mAb, peridinin chlorophyll protein-Cy5.5-conjugated rat anti-mouse IgM mAb, and phycoerythrin-conjugated rat anti-mouse IgG1 mAb (all from BD Biosciences, San Jose, CA). Stained cells were run through a BD LSR II flow cytometer (BD Biosciences) and viable cells confirmed by propidium iodide staining were gated for analysis.
Somatic hypermutation in Peyer's patch B cells
Peyer's patches were collected from mice at ages of 8, 12 and 16–18 weeks. Single-cell suspensions were made by squashing Peyer's patches between two frosted slides. After washing, cells were stained with conjugated antibodies: phycoerythrin-conjugated anti-B220, phycoerythrin-Cy7-conjugated anti-CD19 and fluorescein isothiocyanate-conjugated anti-GL7 (all from BD Biosciences). B220+ CD19+ GL7+ cells were sorted using a Becton Dickinson FACSVantage SE Flow Cytometer (Franklin Lakes, NJ). Cell DNA was prepared with a DNeasy® Blood and Tissue Kit (Qiagen, Valencia, CA). The IgH variable region was amplified by PCR as previously described.49 Briefly, for the IgH locus, a 1·2-kilobase (kb) fragment from the intronic region 3′ of the rearranged endogenous VH genes was amplified using a primer complementary to a sequence common to FR3 of most VHJ558 family members (GCCTGACATCTGAGGACTCTGC) along with a primer specific to a sequence located at the 3′-end of the IgH intronic enhancer (CCTCTCCAGTTTCGGCTGAATCC) with Phusion DNA polymerase (New England BioLab, Ipswich, MA). The PCR was performed at 98° for 3 min; 35 cycles of 98° for 10 seconds, 70° for 30 seconds and 72° for 40 seconds; and 72° for 6 min. The PCR products were electrophoresed through 1% agarose gel (SeaKem® LE Agarose, Lonza Rockland, Rockland, ME). A bright 1·2-kb band was cut and purified using a QIAquick® Gel Extraction Kit (Qiagen). The DNA fragments were treated with Taq DNA polymerase to add A overhangs to their blunt ends. Then, the 1·2-kb DNA fragments were cloned using a TOPO TA Cloning®, pCR®2.1-TOPO® Kit (Invitrogen) by following the manufacturer's instructions. MAX Efficiency® DH5™ Competent Cells (Invitrogen) were transformed with the TOPO vector, spread on Luria broth (LB)-agar (Amp) plates which had been spread with 40 μl X-gal (40 mg/ml in dimethylformamide), incubated at 37°C for 15 hr. Plasmid DNA was prepared from positive clones and the inserted VH 1·2-kb PCR fragments were sequenced with a primer specific to the JH4 region (5′-TAT GCT ATG GAC TAC TGG-3′) using BigDye Terminator Ready Reaction Mix (Applied Biosystems, Foster City, CA).
Detection of serum anti-dsDNA antibodies and apparent affinity assay
Mouse serum anti-dsDNA IgG was measured as previously reported.36 Briefly, 96-well ELISA plates (Costar® High Binding 96-well EIA/RIA Plate; Corning Incorporated, Corning, NY) were coated with streptavidin [Sigma, 1 μg/ml in phosphate-buffered saline (PBS)] at 100 μl/well at 4° overnight, followed by another overnight incubation with biotinylated dsDNA (biotin-dsDNA) at 400 ng/ml in PBS. The plates were treated with blocking buffer (PBS, pH 7·4 plus 1% bovine serum albumin) at room temperature for 2 hr. Mouse sera at 1 : 1000 dilution in a sample diluent (blocking buffer plus 0·05% Tween-20) were added to the plates at 100 μl/well and incubated for 1 hr at room temperature. Pooled sera from four sick MRL/lpr mice were serially diluted with the sample diluent from 1 : 400 to 1 : 102 400 to be used as standards. After 1 hr of incubation with horseradish peroxidase-conjugated goat anti-mouse IgG (Bethyl Laboratories, at 1 : 10 000 dilution), tetramethylbenzidine enzyme substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added to the plates and incubated for 30 min at room temperature. The reaction was stopped by adding 100 μl 1 m H2SO4. The absorbance at 450 nm was measured in a Multiskan Ascent microplate reader (Thermo Labsystems, Waltham, MA). The amount of anti-dsDNA IgM and IgG in the wells was calculated according to the standard curve in which the pooled standard sera were defined as a value of 1.
Anti-dsDNA IgG affinity assay was developed from our anti-dsDNA IgG ELISA protocol mentioned above according to the principles described by Hetherington50 Briefly, streptavidin (Sigma, 0·5 μg/ml in PBS) was added to 96-well ELISA plates (Costar® High Binding 96 Well EIA/RIA Plate) at 100 μl/well and incubated at room temperature for 4 hr. After washing, 100 μl biotinylated dsDNA (400 ng/ml in PBS) was added to each well and incubated at 4° overnight. The plates were washed and blocked with blocking buffer (PBS, pH 7·4, plus 6% fetal bovine serum) at room temperature for 2 hr. One requirement for the method was that the concentration of antibody tested be small compared to both solid-phase and fluid-phase antigen. It was not known exactly how many anti-dsDNA IgG molecules existed in the serum so all serum samples were diluted with sample diluent (blocking buffer, plus 0·05% Tween-20) so that their ELISA optical density (OD) readings would be around 0·8. Appropriately diluted serum was added to seven rows of the plates at 50 μl/well. The same serum was also further diluted twofold and added to one row at 100 μl/well. Meanwhile, seven different concentrations of inhibitive dsDNA were prepared by serial twofold dilution starting from 4 μg/ml to 0·0625 μg/ml and 50 μl of each was added to the seven rows which contained 50 μl serum per well. After 2 hr incubation at room temperature, the plates were washed and incubated with goat anti-mouse horseradish peroxidase-conjugated IgG (SouthernBiotech, 1 : 12000 dilution in sample diluent) at 100 μl/well at room temperature for 1 hr. 100 μl tetramethylbenzidine enzyme substrate was added to each well and incubated at room temperature for 30 min in dim light. After stopping the reaction by adding 50 μl 2m H2SO4 to each well, absorbance at 450 nm was measured in the Multiskan Ascent microplate reader. An inhibitive curve was drawn by using inhibitive dsDNA concentrations against the OD values of each serum. A corresponding concentration of inhibitive dsDNA on the inhibitive curve was obtained by using the OD value at one-half of the serum concentration (from wells containing 100 μl serum). The reciprocal of the concentration was the apparent affinity constant for the serum anti-dsDNA antibodies.50,51 A large apparent affinity constant represents a high affinity.
Results
The level of AID messenger RNA transcripts in activated B cells of AID heterozygous MRL/lpr mice is reduced
To examine the levels of messenger RNA transcripts in AID heterozygotes and wild-type MRL/lpr mice, splenic B cells were activated with LPS alone, LPS plus IL-4, and LPS plus TGF-β, RNA was isolated at 72 hr following activation and cDNA was made. The RT-PCR analysis at various dilutions demonstrated a reduction in AID transcripts in the AID heterozygous MRL/lpr mice compared to their wild-type littermates, and this difference was consistently seen for all treatments (Fig. 1).
Figure 1.
Reduction of activation-induced deaminase (AID) messenger RNA transcripts in AID-heterozygous MRL/lpr mice. Resting/naïve B cells were prepared from spleens of AID-wild-type (WT; n= 2) and heterozygous (Het; n= 3) MRL/lpr mice at 12 weeks of age as described in the text. After treatment with lipopolysaccharide (LPS) alone or plus interleukin-4 (IL-4) or transforming growth factor-β (TGF-β) as indicated for 72 hr, cells were lysed and complementary DNA (cDNA) was prepared. One-tenth of the cDNA reaction and its dilutions at 1 : 5 and 1 : 25 were used for amplification of AID fragments by polymerase chain reaction (PCR). The reverse transcription–PCR products were visualized in agarose gel and quantified with Image Quant software. Error bars represent the standard error of two experiments for each of the treatments.
Kidney pathology in young AID heterozygous MRL/lpr mice is diminished compared to AID wild-type MRL/lpr littermates
Proteinuria was measured over time for AID wild-type and heterozygous MRL/lpr mice and a significant decrease was detected among heterozygotes for both the F5 (at 12–15 weeks of age) and F6 (at 17–19 weeks of age) backcrossed generations (Fig. 2a,b; Wilcoxon signed-ranks test: P< 0·05). For the F5 backcrossed mice, it appears that by 16–18 weeks of age, the heterozygotes began to catch up with the wild-type mice, although this was not the case in F6 mice, where the reduction could still be seen at 17–19 weeks of age. However, the survival rates after 12 months were only slightly better in AID heterozygous MRL/lpr mice compared to wild-type MRL/lpr mice, confirming that the decrease in nephritis is temporary.36 Other parameters of nephritis also revealed a delay in kidney damage in the heterozygotes, such as decreased deposition of C3 and decreased kidney weight (Fig. 2c; Mann–Whitney: P< 0·05).
Figure 2.
Decreased kidney damage in activation-induced deaminase (AID) heterozygous MRL/lpr mice. A. urine protein was tested in mice at different ages as indicated. Results for the F5 backcrossed mice are depicted in (a) (19 AID wild-type and 18 heterozygous MRL/lpr mice were used). (b) Proteinuria results for F6 generation (15 AID wild-type and 15 heterozygous MRL/lpr mice were used). (c) The proportion of mice with high levels of C3 deposition measured as previously reported36 is decreased in the heterozygotes and so are their kidney weights in the 16- to 18-week-old F5 mice. Values from heterozygotes were compared to those in wild-type mice which were set at 1. (d) Electron microscopy of the kidney of AID heterozygous MRL/lpr mice reveal intact podocyte foot processes (black arrow) but macrophage infiltration (white arrows). (e) Anti-dsDNA immunoglobulin G in serum of 19 AID wild-type and 18 heterozygous MRL/lpr mice. The serum anti-dsDNA IgG data are representative of data presented previously.36 All error bars depict standard errors.
Electron microscopy analysis revealed an intermediate phenotype in the kidneys of 16- to 18-week-old AID heterozygous MRL/lpr mice. The podocyte's foot processes were normal in morphology in the heterozygotes (Fig. 2d), which is similar to previous observations in AID-knockout homozygous MRL/lpr mice and contrasts with AID wild-type MRL/lpr mice.36 However, infiltration of macrophages was evident in the blood vessels (Fig. 2d). The decrease in kidney damage observed here correlated with an over 60% reduction in the levels of anti-dsDNA IgG antibodies (Fig. 2e) reported for the heterozygotes.36
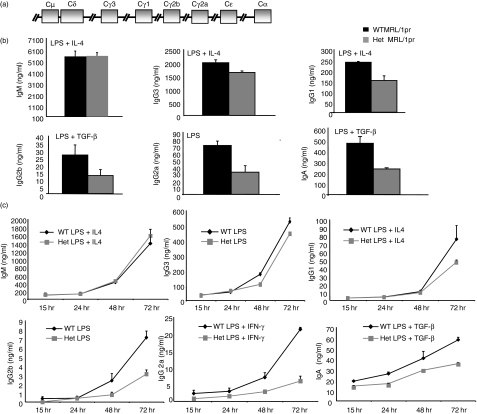
In vitro CSR is affected in AID heterozygous MRL/lpr mice but the impact varies with isotype
To examine if the lag in kidney pathology in AID heterozygous MRL/lpr mice was the result of a defect in CSR, splenic B cells from AID heterozygous and wild-type MRL/lpr mice were stimulated with cytokine mixtures known to induce CSR to particular isotypes. Strikingly, splenic B cells derived from AID heterozygous mice displayed a defect in the generation of all switched antibodies except IgG3, the isotype encoded by the segment closest to IgM (Fig. 3a,b). To examine the kinetics of this defect, supernatants were taken at various time-points in a different set of experiments, and again there was a significant reduction in the generation of all switched antibodies except IgG3 (Fig. 3c). Interestingly, there was a trend for the defect to worsen and to appear at earlier time-points with distance from the IgM switch region (Fig. 3). This decrease in the levels of switched antibodies consisting of isotypes downstream of IgG3, can be seen in the levels of surface IgG1 in activated B cells, even in AID heterozygotes in the C57BL/6 background – AID heterozygous MRL/lpr mice had 63% IgG1-positive cells following activation with LPS and IL-4 compared to 77% in wild-type littermates, while AID heterozygotes in the C57BL/6 background had a 70% reduction in IgG1-positive cells compared to their wild-type counterparts. This defect in CSR in AID heterozygous MRL/lpr mice was not the result of a defect in proliferation or of increased apoptosis following activation, as the number of live B cells was similar among genotypes for all isotypes following 72 hr of stimulation (data not shown).
Figure 3.
Impact of decreased activation-induced deaminase (AID) levels in heterozygotes on isotype switching. (a) Diagram depicts the order of switch regions in the murine immunoglobulin locus. (b) Resting/naïve B cells were pooled from two or three AID wild-type (WT) and three or four heterozygous (Het) MRL/lpr mice at 10–12 weeks of age and activated with indicated cytokines and supernatants collected at 72 hr: IL-4, interleukin-4; TGF-β, transforming growth factor-β; IFN-γ, interferon-γ. The experiment was repeated three times. (c) Cells were pooled and treated with lipopolysaccharide (LPS) alone or plus cytokines for different times as indicated for four AID wild-type and four AID heterozygous MRL/lpr mice at 10–12 weeks of age. Supernatants were collected at multiple time-points as depicted and each treatment was repeated twice. Error bars represent standard errors.
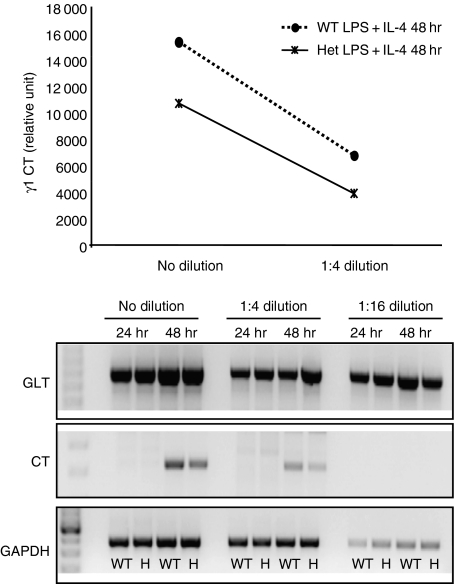
Given the role of AID in CSR as the inducer of non-homologous end-joining via the generation of abasic sites followed by DNA breaks, we speculated that decreased CSR in the heterozygotes would reveal a decrease in the generation of deletion (circle) transcripts but not in the generation of germline transcripts. Indeed, we found that while the levels of circle transcripts were reduced in the heterozygotes, germline transcript levels appeared unaltered (Fig. 4). These results are consistent with a defect in a CSR step downstream from the generation of germline transcripts indicative of locus accessibility52 and will be discussed further below.
Figure 4.
Reduced levels of γ1 circle transcripts (CT) but unaltered levels of γ1 germline transcripts (GLT) in activation-induced deaminase (AID) heterozygotes indicate that the defect in heterozygotes is downstream of transcription of switch regions. Resting/naïve B cells were prepared from AID wild-type mice (n= 3; WT) and heterozygotes (n= 5; Het) on the MRL/lpr background. B cells with the same genotypes were pooled and treated with lipopolysaccharide plus interleukin-4 (LPS + IL-4) for 24 hr and 48 hr. Cells were lysed in TRIzol for RNA preparation. Complementary DNA was synthesized and used at different dilutions to determine γ1 circle transcripts and germline transcripts by reverse transcription–polymerase chain reaction (PCR). The PCR products were visualized in agarose gel and quantified by imagequant software. The reduction in circle transcripts was observed in at least two separate experiments. WT, wild-type; H, heterozygous.
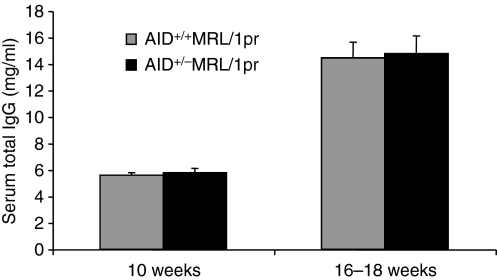
In spite of the intrinsic defect in CSR with AID heterozygosity, the serum levels of IgG in AID heterozygous MRL/lpr mice were comparable to AID wild-type MRL/lpr mice in all ages examined, including 10-week-old mice which have yet to demonstrate any signs of the syndrome (Fig. 5). No significant differences were detected in IgA serum levels among AID heterozygotes and wild-type MRL/lpr mice either (data not shown). This is not entirely surprising because it was previously shown that defects in CSR can sometimes only be revealed by a careful in vitro analysis as performed here, mostly because switched antibodies accumulate in the blood masking CSR defects.39 In addition, even though we found a defect in CSR with decreased AID levels in the heterozygotes, switching to IgG3 was unaffected; IgG3 anti-dsDNA autoantibodies are typically highly nephritic.51,53–55 Therefore, in spite of the reduced efficiency of CSR revealed in the in vitro experiments, but only in isotypes downstream of IgG3, the near normal levels of serum IgG even in very young mice (10 weeks old) makes the CSR defect with AID heterozygosity an unlikely explanation for the lag in kidney lesions in AID heterozygous MRL/lpr mice.
Figure 5.
Comparable levels of serum total immunoglobulin G (IgG) between activation-induced deaminase (AID) wild-type and heterozygous MRL/lpr mice. Sera were prepared from AID wild-type mice (n= 19) and heterozygotes (n= 18) at 16–18 weeks (n= 19 and n= 20 for 10-week-old AID wild-type and heterozygous MRL/lpr mice, respectively) and tested for total IgG using commercial enzyme-linked immunosorbent assay kits. Error bars represent standard errors. No statistical differences were found between AID wild-type and heterozygous mice for any of the age groups (P> 0·05, Wilcoxon rank sums test). Similar results were obtained for serum levels of IgA (data not shown).
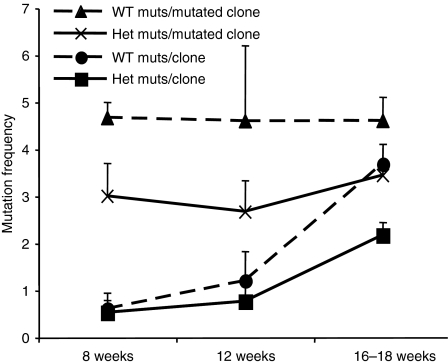
AID heterozygous MRL/lpr mice exhibit a decrease in the accumulation of mutations over time in Peyer's patches
A strategy for detecting differences in the rate of accumulation of mutations as the result of SHM in the absence of selection is by estimating the frequency of mutation in the intronic region immediately downstream of the rearranged variable gene in chronically activated Peyer's patch B cells of mice with increasing age.56 To examine if AID heterozygous MRL/lpr mice experienced a decrease in the rate of hypermutation, we measured mutation frequencies in AID heterozygous and wild-type MRL/lpr mice at ages ranging from 8 to 18 weeks. The mutation frequency in germinal centre B cells derived from ileal Peyer's patches increased over time for both heterozygous and wild-type MRL/lpr mice (Wilcoxon ranks sum test, P< 0·05) but heterozygotes lagged behind wild-type MRL/lpr mice (Fig. 6). While all mutation frequency comparisons hovered around P-values of 0·06, the reduction in the heterozygote overall frequency, as defined by mutation among clones that were mutated at least once, was significant at the 0·05 level (t-test, P< 0·05; Fig. 6). Given the role of SHM in affinity maturation of B cells to specific antigen, we then examined the affinity of serum IgG antibodies to dsDNA, a common autoantibody in the lupus syndrome of MRL/lpr mice that has been directly implicated in glomerulonephritis.
Figure 6.
Reduced somatic hypermutation in activation-induced deaminase (AID) heterozygotes in the absence of selection. Peyer's patches were collected from AID wild-type (WT) and heterozygous (Het) MRL/lpr mice at 8, 12 and 16–18 weeks of age (n= 6, n= 6 and n= 18, respectively). Peyer's patches from mice with the same genotypes and ages were pooled for the 8- and 12-week-old age groups but not the 16- to 18-week-old mice, in which mice were analysed individually. B220+ CD19+ GL7+ cells were sorted and lysed for DNA preparation. A 1·2-kb fragment from the intronic region 3′ of the rearranged endogenous VH genes was amplified by polymerase chain reaction and cloned for DNA sequencing. Totals of 46 (8 weeks), 40 (12 weeks), and 179 (16–18 weeks) sequences were analysed with approximately the same number of clones for heterozygotes and wild-type mice. The mutation frequency was calculated among all clones (mutations per clone) and among only those clones that were mutated at least once (mutations per mutated clone). Error bars represents standard errors.
Apparent affinity of anti-dsDNA antibodies in the serum of AID heterozygous MRL/lpr mice is significantly lower than in wild-type littermates
B-cell receptors and the antibodies they secrete acquire higher affinity to antigens via a two-step process: SHM and selection for high-affinity variants. Since the observed lag in the accumulation of mutations in the Peyer's patches may reflect an intrinsic defect in the rate of SHM with decreased AID levels, there may be a partial impairment or a lag in the accumulation of high-affinity autoantibodies. Strikingly, serum anti-dsDNA antibodies from the heterozygotes displayed substantially lower affinities than similarly measured anti-dsDNA antibodies from wild-type littermates and this difference was highly significant (Wilcoxon rank sums, P< 0·01; Fig. 7) strongly implicating decreased affinity of autoantibodies in the delay of pathogenesis of the kidneys of AID heterozygous MRL/lpr mice.
Figure 7.

Serum anti-dsDNA immunoglobulin G (IgG) in activation-induced deaminase (AID) heterozygous MRL/lpr mice (Het) are of significantly lower affinity than those from AID wild-type MRL/lpr littermates (WT). Sera were prepared from AID wild-type and heterozygous MRL/lpr mice at 16–18 weeks of age and tested for apparent affinity of anti-dsDNA IgG by enzyme-linked immunosorbent assay. Each dot represents a mouse serum sample.
Discussion
We have previously shown that AID heterozygous MRL/lpr mice experience a delay in the accumulation of anti-dsDNA antibodies.36 Herein we show that this lag in the heterozygotes is accompanied by diminished pathology as revealed by several parameters of lupus nephritis. To determine whether the delay was caused by a defect in any of the known functions of AID, we examined the frequency of SHM in the absence of selection in AID+/− MRL/lpr mice, and the intrinsic rate of CSR to most isotypes in vitro by activation of splenic B cells. The results revealed that AID heterozygosity results in a decrease in hypermutation frequency and CSR efficiency to all isotypes except IgG3, an exception that will be discussed further below.
The SHM defect with AID heterozygosity was characterized by a decrease in the accumulation of mutations (both at G : C and A : T base pairs), implying that with reduced AID levels, fewer mutations occurred. This is surprising because only a small fraction of AID is found in the nucleus of activated B cells as the result of a chromosome region maintenance/exportin-1 (CRM-1) mediated nuclear export.57–59 On the other hand, because artificially increased levels of AID via ectopic expression result in non-targeted mutagenesis of non-immunoglobulin loci, it may be that the nuclear levels of AID are very close to the threshold of what is needed for a high rate of hypermutation and even moderate decreases in AID levels can impact the rate of mutation accumulation. Over time a SHM defect with AID heterozygosity may be masked by multiple rounds of SHM, and high-affinity antibody production will only be delayed but will form eventually. However, such a delay in the rate of hypermutation might be revealed in disease processes such as autoimmunity, where the gradual accumulation of high-affinity pathogenic autoantibodies correlates with tissue damage.
In vitro CSR in AID heterozygous MRL/lpr mice was markedly affected for all isotypes except IgG3. This was detected at various levels including secreted antibody at various time-points, surface immunoglobulin levels, and the abundance of deletion circles at various time-points. The defect is not unique to MRL/lpr mice; we found a profound decrease in switching to IgG1 in splenic B cells isolated from AID heterozygous C57BL/6 mice following activation with large doses of LPS and IL-4 (AID haplotype insufficiency in a different strain was also recently recorded by others: personal communication Rafael Casellas). The levels of germline transcripts were unaffected in the heterozygotes, strongly implicating that the defect is downstream of locus accessibility. Given the proposed role of AID in CSR, namely as a deaminator of cytosines in the switch regions to induce the formation of abasic sites that remain unresolved resulting in DNA breaks, we expected the defect to be downstream of germline transcript generation, so this result was not surprising. However, oddly, it appears that there was a distance effect because isotypes further downstream from the IgM switch region than IgG3 seemed to be more susceptible to AID levels. These results suggest that isotypes downstream of IgG3 require greater levels of AID activity and perhaps DNA breaks to induce productive CSR, at least in the MRL/lpr background. Interestingly, a distance effect for CSR efficiency that is influenced by the number of DNA breaks has been reported.60 Alternatively, other roles of AID in CSR that would be downstream of germline transcript generation could also explain this effect, such as stabilization of synaptic complexes during CSR,61 or even help in recruiting the proteins required for resolution of DNA breaks.
The role of isotype-switched, somatically hypermutated autoantibodies in the various manifestations of the lupus syndrome in MRL/lpr mice, such as lupus nephritis, is well established, but little is known about the differential contribution of SHM, affinity maturation and CSR to autoimmunity. This is mostly because no molecule uniquely required for one mechanism but not the other has been discovered that could be used in the generation of autoimmune mice deficient in such a molecule. Also, even though they are independent from each other – as revealed by in vitro activation of CSR without SHM62 and Burkitt lymphoma cell lines that hypermutate constitutively but do not undergo CSR63– both CSR and SHM are activated by similar signals and so occur at similar times (following T-cell and foreign antigen activation) or in similar locations under normal conditions (germinal centres). We first reported an AID heterozygosity effect in MRL/lpr mice in a recent study in which there was a striking reduction in the levels of autoantibodies in young but not old heterozygotes.36 Given the roles of AID in CSR and SHM, and not knowing the cause of the heterozygote lag in the production of autoantibodies, we speculated that these mice could provide novel insights as to the differential contribution of SHM and CSR to autoimmunity, especially if the defect in the heterozygotes was unique to either of the mechanisms. While we detected a defect in both CSR and SHM in this study, the CSR defect was corrected in vivo, as serum levels of switched antibodies were not different from those in wild-type littermates, even in 10-week-old mice. Also, switching to the typically nephritic IgG3 isotype in vitro was not impaired in the heterozygotes. Therefore, it is very unlikely that the lag in autoimmunity in the heterozygotes was caused by the defect in CSR. A defect in SHM, on the other hand, could explain the most obvious deficiency in the AID heterozygous MRL/lpr mice in terms of autoimmunity: a dramatic decrease in the affinity of anti-dsDNA IgG antibodies. These results implicate affinity maturation of autoantibodies in the generation of highly nephritic antibodies in the lupus syndrome of MRL/lpr mice through a process of SHM and cellular selection and help delineate the contribution of SHM to autoimmunity.
Acknowledgments
We are grateful to John W. Drake, Laurent Verkoczy and Tom Kunkel for editing and commenting on the manuscript. We also thank Ron Herbert, Natasha Clayton and Warren Lieuallen for histological and electron microscopy analyses and Carl Bortner and Maria Sifre for assistance with flow cytometry. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
References
- 1.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 2.Andrews BS, Eisenberg RA, Theofilopoulos AN, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morse HC, 3rd, Davidson WF, Yetter RA, Murphy ED, Roths JB, Coffman RL. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982;129:2612–5. [PubMed] [Google Scholar]
- 4.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 5.Culton DA, O'Conner BP, Conway KL, Diz R, Rutan J, Vilen BJ, Clarke SH. Early preplasma cells define a tolerance checkpoint for autoreactive B cells. J Immunol. 2006;176:790–802. doi: 10.4049/jimmunol.176.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Li H, Ni D, Weigert M. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196:1543–52. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandik-Nayak L, Seo SJ, Sokol C, Potts KM, Bui A, Erikson J. MRL-lpr/lpr mice exhibit a defect in maintaining developmental arrest and follicular exclusion of anti-double-stranded DNA B cells. J Exp Med. 1999;189:1799–814. doi: 10.1084/jem.189.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pisetsky DS, Caster SA, Roths JB, Murphy ED. Ipr gene control of the anti-DNA antibody response. J Immunol. 1982;128:2322–5. [PubMed] [Google Scholar]
- 9.Peng SL, Craft J. T cells in murine lupus: propagation and regulation of disease. Mol Biol Rep. 1996;23:247–51. doi: 10.1007/BF00351176. [DOI] [PubMed] [Google Scholar]
- 10.Bao L, Osawe I, Haas M, Quigg RJ. Signaling through up-regulated C3a receptor is key to the development of experimental lupus nephritis. J Immunol. 2005;175:1947–55. doi: 10.4049/jimmunol.175.3.1947. [DOI] [PubMed] [Google Scholar]
- 11.Trouw LA, Seelen MA, Duijs JM, Benediktsson H, Van Kooten C, Daha MR. Glomerular deposition of C1q and anti-C1q antibodies in mice following injection of antimouse C1q antibodies. Clin Exp Immunol. 2003;132:32–9. doi: 10.1046/j.1365-2249.2003.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–32. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 13.Bao L, Haas M, Boackle SA, et al. Transgenic expression of a soluble complement inhibitor protects against renal disease and promotes survival in MRL/lpr mice. J Immunol. 2002;168:3601–7. doi: 10.4049/jimmunol.168.7.3601. [DOI] [PubMed] [Google Scholar]
- 14.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Garnier G, Circolo A, et al. Modulation of renal disease in MRL/lpr mice genetically deficient in the alternative complement pathway factor B. J Immunol. 2000;164:786–94. doi: 10.4049/jimmunol.164.2.786. [DOI] [PubMed] [Google Scholar]
- 16.Ghebrehiwet B, Peerschke EI. Role of C1q and C1q receptors in the pathogenesis of systemic lupus erythematosus. Curr Dir Autoimmun. 2004;7:87–97. doi: 10.1159/000075688. [DOI] [PubMed] [Google Scholar]
- 17.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 18.Pascual V, Banchereau J, Palucka A K. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003;15:548–56. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Theofilopoulos AN, Lawson BR. Tumour necrosis factor and other cytokines in murine lupus. Ann Rheum Dis. 1999;1(Suppl. 58):I49–55. doi: 10.1136/ard.58.2008.i49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickey MJ. Alterations in leucocyte trafficking in lupus-prone mice: an examination of the MRL/faslpr mouse. Immunol Cell Biol. 2003;81:390–6. doi: 10.1046/j.1440-1711.2003.01186.x. [DOI] [PubMed] [Google Scholar]
- 21.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A. 1987;84:9150–4. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radic M Z, Mascelli MA, Erikson J, Shan H, Shlomchik M, Weigert M. Structural patterns in anti-DNA antibodies from MRL/lpr mice. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 2):933–46. doi: 10.1101/sqb.1989.054.01.108. [DOI] [PubMed] [Google Scholar]
- 23.Shlomchik MJ, Nemazee DA, Sato VL, Van Snick J, Carson DA, Weigert MG. Variable region sequences of murine IgM anti-IgG monoclonal autoantibodies (rheumatoid factors). A structural explanation for the high frequency of IgM anti-IgG B cells. J Exp Med. 1986;164:407–27. doi: 10.1084/jem.164.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shlomchik M, Mascelli M, Shan H, Radic MZ, Pisetsky D, Marshak-Rothstein A, Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–92. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Li L, Kuma KR, et al. Lupus susceptibility genes may breach tolerance to DNA by impairing receptor editing of nuclear antigen-reactive B cells. J Immunol. 2007;179:1340–52. doi: 10.4049/jimmunol.179.2.1340. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Li H, Tian Q, Beardall M, Xu Y, Casanova N, Weigert M. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J Immunol. 2006;176:5183–90. doi: 10.4049/jimmunol.176.9.5183. [DOI] [PubMed] [Google Scholar]
- 27.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan C, Morel L, Yang P, Wakeland EK. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 1998;41:1652–62. doi: 10.1002/1529-0131(199809)41:9<1652::AID-ART17>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 30.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362–72. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: Sle2 on murine chromosome 4 leads to B cell hyperactivity. J Immunol. 1997;159:454–65. [PubMed] [Google Scholar]
- 32.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J Immunol. 1999;162:2415–21. [PubMed] [Google Scholar]
- 33.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002;169:2694–700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 34.Hazenbos WL, Gessner JE, Hofhuis FM, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII CD16 deficient mice. Immunity. 1996;5:181–8. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 35.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–90. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 36.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, Diaz M. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–31. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 38.Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase AID deficiency causes the autosomal recessive form of the Hyper-IgM syndrome HIGM2. Cell. 2000;102:565–75. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 39.Rada C, Williams GT, Nilsen H, Barnes DE, Lindahl T, Neuberger MS. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–55. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 40.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 41.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–9. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 42.MacLennan IC, Liu YJ, Johnson GD. Maturation and dispersal of B-cell clones during T cell-dependent antibody responses. Immunol Rev. 1992;126:143–61. doi: 10.1111/j.1600-065x.1992.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to 4-hydroxy-3-nitrophenylacetyl. V. Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–95. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke SH, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985;161:687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–70. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 46.Frasca D, Van der Put E, Riley RL, Blomberg BB. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–62. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 47.Kinoshita K, Harigai M, Fagarasan S, Muramatsu M, Honjo T. A hallmark of active class switch recombination: transcripts directed by I promoters on looped-out circular DNAs. Proc Natl Acad Sci U S A. 2001;98:12620–3. doi: 10.1073/pnas.221454398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunnick WA, Shi J, Graves KA, Collins JT. The 3′ end of the heavy chain constant region locus enhances germline transcription and switch recombination of the four gamma genes. J Exp Med. 2005;201:1459–66. doi: 10.1084/jem.20041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unniraman S, Schatz DG. Strand-biased spreading of mutations during somatic hypermutation. Science. 2007;317:1227–30. doi: 10.1126/science.1145065. [DOI] [PubMed] [Google Scholar]
- 50.Hetherington S. Solid phase disruption of fluid phase equilibrium in affinity assays with ELISA. J Immunol Methods. 1990;131:195–202. doi: 10.1016/0022-1759(90)90190-7. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi S, Nose M, Sasaki J, Yamamoto T, Kyogoku M. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol. 1991;147:515–9. [PubMed] [Google Scholar]
- 52.Manis JP, van der Stoep N, Tian M, Ferrini R, Davidson L, Bottaro A, Alt FW. Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. J Exp Med. 1998;188:1421–31. doi: 10.1084/jem.188.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gyotoku Y, Abdelmoula M, Spertini F, Izui S, Lambert PH. Cryoglobulinemia induced by monoclonal immunoglobulin G rheumatoid factors derived from autoimmune MRL/MpJ-lpr/lpr mice. J Immunol. 1987;138:3785–92. [PubMed] [Google Scholar]
- 54.Berney T, Fulpius T, Shibata T, et al. Selective pathogenicity of murine rheumatoid factors of the cryoprecipitable IgG3 subclass. Int Immunol. 1992;4:93–9. doi: 10.1093/intimm/4.1.93. [DOI] [PubMed] [Google Scholar]
- 55.Amoura Z, Koutouzov S, Chabre H, Cacoub P, Amoura I, Musset L, Bach JF, Piette JC. Presence of antinucleosome autoantibodies in a restricted set of connective tissue diseases: antinucleosome antibodies of the IgG3 subclass are markers of renal pathogenicity in systemic lupus erythematosus. Arthritis Rheum. 2000;43:76–84. doi: 10.1002/1529-0131(200001)43:1<76::AID-ANR10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 56.González-Fernández A, Gupta SK, Pannell R, Neuberger MS, Milstein C. Somatic mutation of immunoglobulin lambda chains: a segment of the major intron hypermutates as much as the complementarity-determining regions. Proc Natl Acad Sci USA. 1994;91:12614–8. doi: 10.1073/pnas.91.26.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brar SS, Watson M, Diaz M. Activation-induced cytosine deaminase (AID) is actively exported out of the nucleus but retained by the induction of DNA breaks. J Biol Chem. 2004;279:26395–401. doi: 10.1074/jbc.M403503200. [DOI] [PubMed] [Google Scholar]
- 58.Ito S, Nagaoka H, Shinkura R, Begum N, Muramatsu M, Nakata M, Honjo T. Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. Proc Natl Acad Sci USA. 2004;101:1975–80. doi: 10.1073/pnas.0307335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McBride KM, Barreto V, Ramiro AR, Stavropoulos P, Nussenzweig MC. Somatic hypermutation is limited by CRM1-dependent nuclear export of activation-induced deaminase. J Exp Med. 2004;199:1235–44. doi: 10.1084/jem.20040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zarrin AA, Del Vecchio C, Tseng E, Gleason M, Zarin P, Tian M, Alt FW. Antibody class switching mediated by yeast endonuclease-generated DNA breaks. Science. 2007;315:377–81. doi: 10.1126/science.1136386. [DOI] [PubMed] [Google Scholar]
- 61.Wuerffel R, Wang L, Grigera F, Manis J, Selsing E, Perlot T, Alt FW, Cogne M, Pinaud E, Kenter AL. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–22. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honjo T. Does AID need another aid. Nat Immunol. 2002;3:800–1. doi: 10.1038/ni0902-800. [DOI] [PubMed] [Google Scholar]
- 63.Sale JE, Neuberger MS. TdT-accessible breaks are scattered over the immunoglobulin V domain in a constitutively hypermutating B cell line. Immunity. 1998;9:859–69. doi: 10.1016/s1074-7613(00)80651-2. [DOI] [PubMed] [Google Scholar]