Abstract
Our previous studies demonstrated that lipopolysaccharide (LPS)-stimulated splenocytes, retrovirally transduced with a glutamate decarboxylate 65 (GAD) and immunoglobulin G (IgG) fusion construct, can protect non-obese diabetic (NOD) mice from diabetes by inducing GAD-specific tolerance, and also that there are increased numbers of CD4+ regulatory T cells (Tregs) in GAD-IgG-treated NOD mice. However, little is known about the role of CD8+ Tregs in GAD-IgG gene-transferred tolerance induction in NOD mice. Here, we found that GAD-IgG-transduced splenocytes induced an increase in the number of CD8+ Foxp3+ Tregs in vitro. Using a T-cell depletion assay, we found that, compared with undepleted groups, NOD recipients transfused with CD8− or CD8− CD25− GAD-IgG-transduced splenocytes showed a decrease in the percentage of CD8+ Foxp3+ T cells, a high incidence of diabetes, serious insulitis, GAD-specific hyperresponsiveness at both the cellular and humoral levels, and changes in cytokine expression. These results indicate that CD8+ Tregs, which were induced in vitro by GAD-IgG-transduced splenocytes, were also responsible for GAD-IgG gene-transferred tolerance induction in NOD mice.
Keywords: CD8+ regulatory T cells, diabetes, gene therapy, NOD mice, tolerance
Introduction
Insulin-dependent diabetes mellitus (IDDM) results from T cell-mediated destruction of insulin-producing pancreatic β cells.1 The non-obese diabetic (NOD) mouse is a widely used animal model of IDDM that shares major disease characteristics with the human disease.2 Because CD4+ and CD8+ T-cell responses and autoantibodies against glutamate decarboxylate 65 (GAD) are among the first to be detected in NOD mice3,4 and humans,5 it was suggested that GAD may be a major autoantigen in the initiation of the immune response against β cells.
Previous studies have demonstrated the induction of tolerance using lipopolysaccharide (LPS)-activated B cells retrovirally transduced with an immunoglobulin-antigen fusion construct.6–9 This protocol leads to epitope-specific protection not only in naïve but also in already primed recipients.6,10,11 Retroviral gene therapy with an immunoglobulin-antigen fusion construct has been extended to the treatment of autoimmune diseases, such as experimental autoimmune uveitis,7 experimental allergic encephalomyelitis (EAE),10 diabetes in NOD mice,10,11 and haemophilia A.12
This gene therapy system is based on the tolerogenic properties of immunoglobulin G (IgG) carriers combined with the efficacy of B-cell antigen presentation for unresponsiveness. B cells are known to be important in peptide-IgG gene-transferred tolerance induction because transduced B cell-knockout bone marrow fails to induce tolerance. Furthermore, major histocompatibility complex (MHC) class II expression on the transduced cells is required for tolerance induction, indicating that peptide-IgG fusion proteins are directly processed and presented by the transduced B cells.9
We and others previously utilized this approach to carry out immunotherapy of spontaneous diabetes in NOD mice, and found that LPS-stimulated splenocytes, retrovirally transduced with a GAD-IgG fusion construct, induced a significant GAD-specific hyporesponsiveness at both the cellular and the humoral levels and reduced the incidence of diabetes in female NOD mice.10,11 Subsequently, studies demonstrated that CD4+ CD25+ Foxp3+ regulatory T cells were required for the induction as well as the maintenance of tolerance in this gene therapy model.11,13,14
Tolerance to self is achieved through multiple and diverse mechanisms. Chief among these processes is suppression of immune responses by regulatory T cells (Tregs).15,16 A growing body of evidence suggests that CD4+ CD25+ Foxp3+ Tregs are critical for controlling a wide variety of immune responses, including those that cause many types of autoimmune disease.17–19 Suppressive T cells are not strictly confined to the CD4+ T-cell compartment and CD8+ Tregs have been characterized in both clinical and experimental conditions.20–26 Recent papers have shown that highly suppressive adaptive CD8+ CD25+ Foxp3+ Tregs can be generated in vitro and in vivo.27–36
Here, we analysed the role of CD8+ CD25+ Foxp3+ Tregs in GAD-IgG gene-transferred tolerance induction in NOD mice.
Materials and methods
Mice
NOD mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in our animal facilities under specific pathogen-free conditions. In females, spontaneous diabetes begins to appear by 12 weeks of age (increasing to an incidence of 80–90% at 30 weeks of age). Mice were screened for glucose levels every 1–2 weeks and considered diabetic when glucose levels were ≥ 250 mg/dl on two consecutive measurements. The onset of diabetes was dated from the first of these sequential measurements. Donor and recipient female NOD mice in this study were aged 5–10 and 6·5–7 weeks old, respectively. This study was approved by the Animal Care and Use Committee of the Beijing Institute of Basic Medical Science.
Antigen preparation
Antigen preparation has been described previously.11 Briefly, the recombinant peptide GAD500-585, representing the corresponding amino acids of GAD, was produced from Escherichia coli BL-21 containing the bacterial expression vector pET28a+. Recombinant GAD500-585 was purified using Co2+/TALONIMAC resin (Clontech, Mountain View, CA) according to the manufacturer's instructions. Eluted GAD500-585 fractions were dialysed against phosphate-buffered saline (PBS) (pH7·2).
Retroviral constructs and virus-producer cell lines
Retroviral constructs and virus production have been described previously.11 Briefly, the moloney leukemia retroviral vector (MBAE) construct containing full-length GAD fused in-frame to the N terminus of the murine IgG1 heavy chain (GAD-IgG/MBAE), the control construct (IgG/MBAE) and the viral producer cell line (GPE-86) were prepared and provided by Scott's Laboratory at the American Red Cross (Rockville, MD). Virus-producer cell lines were prepared by transduction of GPE-86 packaging cell lines with GAD-IgG/MBAE and IgG/MBAE constructs, respectively. By selection under neomycin (G418, 0·5 mg/ml), high-titre clones [105–106 neomycin-resistant NIH3T3 colony-forming units (CFU)/ml] were obtained and stored in liquid nitrogen, and freshly thawed for each experiment. GPE-86 parental cells were used for mock transfection. The J558L myeloma cell line was produced by culture of J558L cells in the presence of the supernatants of the virus-producer cell line.
Preparation of cells
Lymphocytes from donor (aged 5–10 weeks) or recipient (aged 6·5–7 weeks) female NOD mice in this study were collected under sterile conditions. A lymphocyte suspension from the spleen was obtained with mouse Ficoll separate liquid. Lymphocyte suspensions were washed two times in incomplete RPMI medium and then re-suspended at 5 × 106 cells/ml in 10% fetal calf serum (Atlanta Biologicals, Atlanta, GA) and RPMI-1640 medium containing 1 mm non-essential amino acids (ICN Pharmaceuticals, Costa Mesa, CA), 2 mm L-glutamine (ICN Pharmaceuticals) and 50 mm 2-mercaptoethanol (ICN Pharmaceuticals).
Retroviral-mediated gene transfer into LPS-stimulated splenocytes
Retroviral-mediated gene transfer into LPS (Sigma, St Louis, MO)-stimulated splenocytes has been described previously.11 Briefly, cells (3 × 106cells/ml) from donor female NOD mice were first stimulated with LPS (50 μg/ml) for 24 hr and then cultured in the presence of the supernatants of GAD-IgG or IgG virus-producer cell lines together with polybrene (6 μg/ml) and LPS (50 μg/ml) for another 24 hr. The transduced cells were then injected intravenously (i.v.) (1 × 107 cells/mouse) into recipient female NOD mice at 6·5–7 weeks of age.
Flow cytometry
Cells (1 × 105 cells/sample) were washed with fluorescence-activated cell sorter (FACS) staining buffer [PBS, 2% fetal bovine serum (FBS) or 1% bovine serum (BSA), and 0·1% sodium azide] and stained for 30 min at 4° with fluorochrome-conjugated monoclonal antibodies (mAbs) at the concentration recommended by the manufacturer. Cells were washed and fixed with 1% paraformaldehyde prior to analysis in a FACSCalibur flow cytometer using cellquest version 3·3 software (BD Biosciences, San Jose, CA). Lymphocytes were collected 3–5 weeks after therapy, stained for surface antigens and analysed by flow cytometry using standard methods. Phycoerythrin (PE)-conjugated anti-mouse CD8 monoclonal antibody was purchased from Pharmingen (San Jose, CA). For intracellular staining to visualize FoxP3 expression, fixed cells were permeabilized with Perm Buffer (BD Biosciences) for 15 min at room temperature. Cells were incubated for 30 min at 4° with fluorochrome-conjugated mAbs, washed, and analysed in a flow cytometer.
Cell depletion
Depletion of CD8+ or CD8+ CD25+ T cells was performed using magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. GAD-IgG-transduced cells were stained with CD8(Ly-2) MicroBeads (Miltenyi Biotec) for depletion of CD8+ cells. For depletion of CD8+ CD25+ T cells, GAD-IgG-transduced cells were stained with bio-CD4 (L3T4), bio-CD45R (B220), bio-CD49b (DX5), bio-CD11b (Mac-1) and bio-Ter-119 antibodies and depleted non-CD8+ T cells with anti-Bio MicroBeads, and then sorted CD8+ T cells were stained with CD25-PE+ anti-PE MicroBeads (Miltenyi Biotec) for depletion of CD25+ cells, and finally all cells were collected except for CD8+ CD25+ T cells. The depletion efficiency of CD8+ or CD8+ CD25+ T cells was > 90%. The cells were divided into four groups: the IgG group (LPS-stimulated splenocytes transduced with the IgG construct), the GAD-IgG group (LPS-stimulated splenocytes transduced with the GAD-IgG fusion construct), the GAD-IgG/CD8− group (LPS-stimulated splenocytes depleted of CD8+ T cells and transduced with the GAD-IgG fusion construct), and the GAD-IgG/CD8− CD25− group (LPS-stimulated splenocytes depleted of CD8+ CD25+ T cells and transduced with the GAD-IgG fusion construct). Each group of cells was then injected i.v. (1 × 107 cells/mouse) into recipient female NOD mice at 6·5–7 weeks of age.
In vitro proliferation
For analysis of the cellular response, lymphocytes were collected 3–5 weeks after therapy and cultured (5 × 105 cells/well) in triplicate in 0·1 ml of RPMI-1640 medium supplemented with 1% horse serum and re-stimulated with 0, 3, 10 and 30 μg/ml GAD500-585. On day 3 (for the splenocytes) or day 5 (for the inguinal lymph node cells), cultures were pulsed with 1 μCi/well of [3H]thymidine for the last 16 hr, and the cells were harvested and counted by standard liquid scintillation. The results are expressed as the stimulation index [SI; counts per minute (c.p.m.) with antigen divided by c.p.m. with medium alone]. Data are for one antigen concentration (30 μg/ml); however, similar results were obtained with 3, 10 and 30 μg/ml.
Determination of cytokine production
The splenocytes were collected 3–5 weeks after therapy and cultured as for the lymphocyte proliferation assay in the presence of GAD500-585 (30 μg/ml). Supernatants of the cells were harvested 24 hr later for interleukin (IL)-2 and 48 hr later for interferon (IFN)-γ, IL-4, IL-10 and transforming growth factor (TGF)-β1 assays using a sandwich enzyme-linked immunosorbent assay (ELISA). The ELISA kits used in this study were purchased from Biosource (Worcester, MA) (IL-2), R&D (Minneapolis, MN) (TGF-β1) and BD Pharmingen (IFN-γ, IL-4 and IL-10). Serum IL-10 and TGF-β1 levels in the treated NOD mice were determined following the manufacturers' instructions.
Assay of Abs against GAD500-585
Sera were collected from NOD mice when they were killed. Peptide rGAD500-585 (5 μg/ml, in 0·1 mol/l NaHCO3, pH8·5) was coated onto a 96-well microplate at 4° for 20–22 hr. The plate was then blocked with 10% FCS for 2–3 hr at 37°. After washing, serially diluted sera were added to the plate for 1 hr at 37°. Unbound Ab was washed away. Then 2 μg/ml goat anti-mouse IgG was added and the mixture was incubated for 1 hr at 37°. After extensive washing, peroxidase-conjugated rabbit anti-goat Ab (Sigma-Aldrich, St Louis, MO; diluted 1 : 3000) was added and the mixture was incubated for another 1 hr at 37°. Finally, the colour was developed by incubation with o-phenylenediamine. The optical density (OD) was read at 492 nm with an ELISA reader (Bio-Rad, Hercules, CA). Ab titres were calculated as the dilution of test serum needed to reduce the signal level threefold relative to the signal from the serum of BALB/c mice with same dilution.
Histopathology
NOD mice that had been injected with IgG-transduced splenocytes, GAD-IgG-transduced splenocytes, CD8− GAD-IgG-transduced splenocytes or CD8− CD25− GAD-IgG-transduced splenocytes were killed at 12 weeks of age, and the pancreas was removed. Each pancreas was fixed with 10% buffered formalin, embedded in paraffin, sectioned at 4·5 μm, and stained with haematoxylin and eosin to detect mononuclear cell infiltration. The insulitis grade was determined as follows: 0, normal islets; 1, mononuclear infiltration, largely in the periphery, in < 25% of the islets; 2, 25–50% of the islets showing mononuclear infiltration; 3, > 50% of the islets showing mononuclear infiltration; and 4, small, retracted islets with few mononuclear cells.
Statistical analysis
Differences in the incidence of diabetes were assessed by Kaplan–Meier analysis. Student's t-test was used to evaluate the significance of the results of the other immunological experiments. Values of P< 0·05 were considered significant.
Results
GAD-IgG-transduced splenocytes in vitro induced CD8+ Foxp3+ Tregs
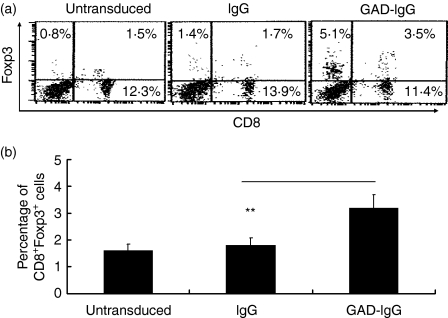
We and others previously demonstrated that LPS-stimulated splenocytes, transduced with the GAD-IgG fusion construct, protected NOD mice against diabetes through induction of CD4+ Tregs.11,13,14 Recent data have shown that Foxp3+ CD8+ CD25+ T cells are also Tregs.27–36 Therefore, we examined the role of CD8+ Tregs in GAD-IgG gene transfer-induced tolerance. First we determined whether the percentage of CD8+ Tregs increased in GAD-IgG-transduced splenocytes in vitro by analysing Foxp3+ CD8+ T cells, because Foxp3 programmes the development and function of Tregs.17–19 The results showed that the percentage of CD8+ Foxp3+ Tregs in untransduced splenocytes, IgG-transduced splenocytes and GAD-IgG-transduced splenocytes was 1·52, 1·76 and 3·51%, respectively, while the percentage of CD8+ Foxp3− populations in control, mock-transfected and GAD-IgG-transfected splenocytes did not vary appreciably (Fig. 1a). The statistical analysis suggested that GAD-IgG-transduced splenocytes induced CD8+ Foxp3+ Tregs in vitro (Fig. 1b). In addition, we found that the percentage of CD8− Foxp3+ T cells, which may be the CD4+ Foxp3+ Tregs described by us previously,14 had increased even more than that of CD8+ Foxp3+ Tregs (Fig. 1a). These results suggest that, in addition to CD4+ Foxp3+ Tregs, CD8+ Foxp3+ Tregs also increase in GAD-IgG-transduced splenocytes.
Figure 1.
CD8+ Foxp3+ regulatory T cells (Tregs) were up-regulated in glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-transduced splenocytes. Splenocytes (3 × 106 cells/ml) from donor female non-obese diabetic (NOD) mice were first stimulated with lipopolysaccharide (LPS; 50 μg/ml) for 24 hr and then cultured in the presence of the supernatants of GAD-IgG or IgG virus-producer cell lines together with polybrene (6 μg/ml) and LPS (50 μg/ml) for another 24 hr. The transduced cells were then collected. Untransduced splenocytes and IgG- and GAD-IgG-transduced splenocytes were stained for CD8 and Foxp3 and analysed by flow cytometry. The percentages of CD8+ Foxp3+ Tregs, CD8+ Foxp3− Tregs and CD8− Foxp3+ Tregs (a) and the statistical analysis of the percentage of CD8+ Foxp3+ Tregs (b) in total splenocytes are shown (**P< 0·01).
Effect of exogenous CD8+ Tregs on CD8+ Tregs in GAD-IgG-treated NOD mice
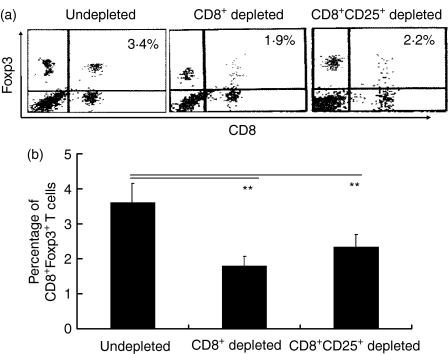
Next, we examined the role of exogenous CD8+ Tregs in an increasing number of CD8+ Tregs in GAD-IgG-treated NOD mice (data for control not shown). Flow cytometry analysis showed that the percentage of CD8+ Foxp3+ Tregs in GAD-IgG-transduced splenocytes and CD8− and CD8− CD25− GAD-IgG-transduced splenocytes was 3·42, 1·97 and 2·25%, respectively (Fig. 2a). The statistical analysis suggested that CD8+ Tregs, induced in vitro by GAD-IgG-transduced splenocytes, were responsible for an increase in the number of CD8+ Foxp3+ Tregs in GAD-IgG-treated NOD mice (Fig. 2b).
Figure 2.
The role of exogenous CD8+ regulatory T cells (Tregs) in producing an increase in the number of CD8+ Foxp3+ Tregs in glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-treated non-obese diabetic (NOD) mice. Splenocytes were collected from NOD mice intravenously injected with 1 × 107 GAD-IgG-transduced splenocytes, CD8− GAD-IgG-transduced splenocytes, or CD8− CD25− GAD-IgG-transduced splenocytes (containing all cells, including CD8+ CD25− cells, except for CD8+ CD25+ T cells), stained for CD8 and Foxp3, and analysed by flow cytometry. The percentage of CD8+ Foxp3+ Tregs (a) and the statistical analysis (b) are shown (**P< 0·01). The results are representative of four separate experiments with three mice per group per experiment.
CD8+ Tregs were responsible for GAD-IgG gene-transferred prevention of diabetes in NOD mice
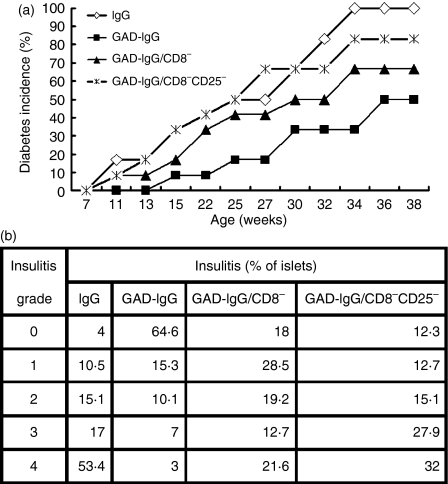
Subsequently, we tested whether CD8+ Tregs, induced in vitro in GAD-IgG-transduced splenocytes, were responsible for GAD-IgG gene-transferred prevention of diabetes in NOD mice. CD8− or CD8− CD25− GAD-IgG-transduced splenocytes were transfused into 7-week-old NOD mice. A group of NOD mice treated with IgG- or GAD-IgG-transduced splenocytes served as a control. Mice were examined for the development of overt diabetes. Our previous studies showed that mice injected with GAD-IgG-transduced splenocytes had reduced diabetes compared with those that were IgG-transduced and there was a significant reduction of the protective effect if the transduced splenocytes were depleted of CD4+ or CD4+ Foxp3+ T cells.11,14 Here, by Kaplan–Meier analysis, we found that depletion of CD8+ cells or CD8+ CD25+ cells could also significantly reduce the protective effect (Fig. 3a), although the reduction of the protective effect was lower in the CD8+ Treg-depleted group than in the CD4+ Treg-depleted group. In addition, at least eight (66·67%) of the 12 mice that received GAD-IgG-transduced splenocytes depleted of CD8+ cells or CD8+ CD25+ cells developed diabetes, whereas only six (50%) of the 12 mice that received undepleted transduced splenocytes did so (Fig. 3a). These data indicate that CD8+ Tregs, induced in vitro in GAD-IgG-transduced splenocytes, significantly decreased the incidence of diabetes in NOD mice.
Figure 3.
CD8+ regulatory T cells (Tregs) were responsible for the prevention of diabetes by glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-transduced splenocytes in non-obese diabetic (NOD) mice. Seven-week-old female NOD mice were injected with 1 × 107 IgG-transduced splenocytes (⋄, n= 12), GAD-IgG-transduced splenocytes (▪, n= 12), CD8− GAD-IgG-transduced splenocytes (△, n= 12) or CD8− CD25− GAD-IgG-transduced splenocytes (×, n= 12). (a) The development of diabetes in recipient NOD mice from all groups was monitored until 38 weeks of age by observing the onset of hyperglycaemia. Kaplan–Meier analysis showed that the incidence of diabetes was significantly different between the group injected with GAD-IgG-transduced splenocytes and the group injected with CD8− GAD-IgG-transduced splenocytes or CD8− CD25− GAD-IgG-transduced splenocytes. (b) Non-diabetic animals from all groups were killed at 12 weeks of age, and histological examination of pancreatic islets (at least 60 islets for each mouse) was performed. The insulitis score was determined, as described in the Materials and methods.
Meanwhile, we also tested whether CD8+ Tregs, induced in vitro in GAD-IgG-transduced splenocytes, could prevent insulitis in recipient NOD mice. We found that a total of 80% of the examined islets from mice that received GAD-IgG-transduced splenocytes were intact, whereas > 30% of the islets from mice that received CD8− or CD8− CD25− GAD-IgG-transduced splenocytes showed moderate to severe insulitis at 12 weeks of age (Fig. 3b). The results show that CD8+ Tregs, induced in vitro in GAD-IgG-transduced splenocytes, efficiently prevented the progression of insulitis in NOD mice.
CD8+ Tregs were responsible for antigen-specific cellular and humoral suppression in GAD-IgG-treated NOD mice
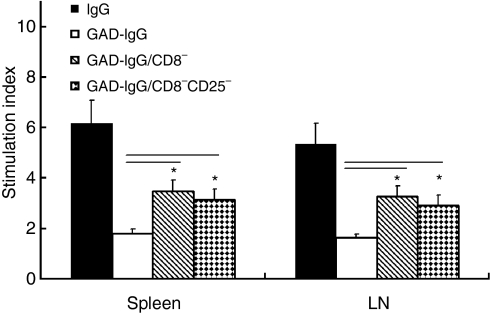
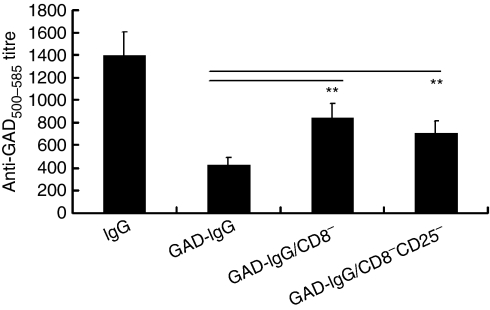
The GAD-specific T-cell response was also evaluated using in vitro proliferation of splenocytes and stimulation of lymph node cells by a major GAD antigen peptide, GAD500-585. Significant hyperresponsiveness of splenocytes in response to GAD500-585 was observed in NOD mice receiving CD8− or CD8− CD25− GAD-IgG-transduced splenocytes (P< 0·05), compared with NOD mice receiving GAD-IgG-transduced splenocytes (Fig. 4). For measurement of antigen-specific humoral responsiveness in NOD mice, anti-GAD500-585 antibody titres were determined in the serum of the recipient mice. The antibody responses to GAD500–585 were up-regulated significantly in NOD mice receiving CD8− or CD8− CD25− GAD-IgG-transduced splenocytes (P< 0·01), compared with NOD mice receiving GAD-IgG-transduced splenocytes (Fig. 5). Collectively, these results indicated that CD8+ Tregs were responsible for antigen-specific cellular and humoral suppression in NOD mice receiving GAD-IgG-transduced splenocytes.
Figure 4.
CD8+ regulatory T cells (Tregs) were responsible for antigen-specific cellular suppression in glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-treated non-obese diabetic (NOD) mice. Splenocytes or inguinal lymph node cells were collected from NOD mice injected with IgG-transduced splenocytes (IgG), GAD-IgG-transduced splenocytes (GAD-IgG), CD8− GAD-IgG-transduced splenocytes (GAD-IgG/CD8−), or CD8− CD25− GAD-IgG-transduced splenocytes (GAD-IgG/CD8−CD25−), and re-stimulated with 30 μg/ml GAD500-585 for 3 days. Cell proliferation was measured by incorporation of [3H]thymidine during the final 16 hr of culture. Data are presented as stimulation index [the ratio of counts per minute (c.p.m.) with antigen to c.p.m. with medium alone] with a background of 1100–3500 c.p.m. (splenocytes) or 1400–4200 c.p.m. (lymph node cells). A significant hyperresponsiveness of splenocytes in response to GAD500-585 was observed in GAD-IgG-transduced splenocytes depleted of CD8+ cells or CD8+ CD25+ cells (*P< 0·05), compared with GAD-IgG-transduced splenocytes. The results are representative of three separate experiments.
Figure 5.
CD8+ regulatory T cells (Tregs) were responsible for humoral suppression in glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-treated non-obese diabetic (NOD) mice. Sera were collected from NOD mice injected with IgG-transduced splenocytes (IgG), GAD-IgG-transduced splenocytes (GAD-IgG), CD8− GAD-IgG-transduced splenocytes (GAD-IgG/CD8−), or CD8− CD25− GAD-IgG-transduced splenocytes (GAD-IgG/CD8− CD25−), and anti-GAD500-585 antibody titres were determined by enzyme-linked immunosorbent assay (ELISA). Antibody titres were calculated as the dilution of test serum needed to reduce the signal level threefold relative to the signal from the serum of BALB/c mice with same dilutions. The antibody responses to GAD500-585 were increased significantly in NOD mice with CD8− or CD8− CD25− GAD-IgG-transduced splenocytes (**P< 0·01), compared with GAD-IgG-treated NOD mice. One set of five repeated experiments is shown, with four mice per group.
Effect of CD8+ Tregs on T helper type 1 (Th1)/Th2 cytokine production in GAD-IgG-treated NOD mice
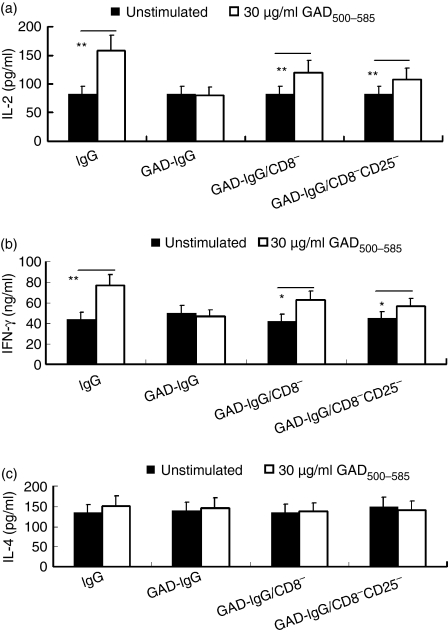
Th1 and Th2 cytokines can affect the immune balance. The production of both Th1 and Th2 cytokines in response to the recall antigen GAD was determined in in vitro culture supernatants. GAD500-585 significantly stimulated splenic IL-2 (P< 0·01) and IFN-γ (P< 0·05) production in NOD mice receiving CD8− or CD8− CD25− GAD-IgG-transduced splenocytes compared with NOD mice receiving GAD-IgG-transduced splenocytes. However, splenic production of IL-4 in response to GAD500-585 was not significantly different among the groups (Fig. 6). The results indicate that CD8+ Tregs suppressed the increase in Th1 cytokines in GAD-IgG-treated NOD mice.
Figure 6.
CD8+ regulatory T cells (Tregs) suppressed the increase in Th1 cytokines in glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-treated non-obese diabetic (NOD) mice. Splenocytes were collected from NOD mice injected with IgG-transduced splenocytes (IgG), GAD-IgG-transduced splenocytes (GAD-IgG), CD8− GAD-IgG-transduced splenocytes (GAD-IgG/CD8−), or CD8− CD25− GAD-IgG-transduced splenocytes (GAD-IgG/CD8− CD25−), and stimulated with GAD500-585 (30 μg/ml), and the production of interleukin (IL)-2, interferon (IFN)-γ and IL-4 was determined by sandwich enzyme-linked immunosorbent assay (ELISA) 24 hr (for IL-2) or 48 hr (for IFN-γ and IL-4) later in the culture supernatants of the splenocytes. The results are representative of three separate experiments, with four mice per group (**P< 0·01 and *P< 0·05).
Effect of CD8+ Tregs on IL-10 and TGF-β1 cytokine production in GAD-IgG-treated NOD mice
IL-10 and TGF-β are immunoregulatory cytokines that are involved in the induction and maintenance of peripheral tolerance through effects on the autoreactive T-cell-mediated response.37–39 The regulatory effects of these two cytokines on the onset of diabetes in NOD mice have been demonstrated previously.19 The levels of IL-10 and TGF-β1 in recipient NOD mice were determined by ELISA. The levels of splenic and serum TGF-β1 and IL-10 significantly decreased in NOD mice receiving CD8− or CD8− CD25− GAD-IgG-transduced splenocytes, compared with GAD-IgG-treated NOD mice (Fig. 7). These results indicate that CD8+ Tregs, induced in vitro by GAD-IgG-transduced splenocytes, were responsible for an increase in the levels of TGF-β1 and IL-10 in GAD-IgG-treated NOD mice.
Figure 7.
The effect of CD8+ regulatory T cells (Tregs) on the levels of interleukin (IL)-10 and transforming growth factor (TGF)-β1 in glutamate decarboxylate 65 (GAD)-immunoglobulin G (IgG)-treated non-obese diabetic (NOD) mice. NOD mice were injected with IgG-transduced splenocytes (IgG), GAD-IgG-transduced splenocytes (GAD-IgG), CD8− GAD-IgG-transduced splenocytes (GAD-IgG/CD8−), or CD8− CD25− GAD-IgG-transduced splenocytes (GAD-IgG/CD8− CD25−). The serum levels of (a) IL-10 and (b) TGF-β1 and the production of (c) IL-10 and (d) TGF-β1 in the supernatant of splenocytes re-stimulated with GAD500-585 for 48 hr were measured by sandwich enzyme-linked immunosorbent assay (ELISA). One set of three repeated experiments is shown, with four mice per group (**P< 0·01 and *P< 0·05).
Discussion
Our previous studies demonstrated that LPS-stimulated splenocytes, retrovirally transduced with the GAD and IgG fusion construct, can protect NOD mice against diabetes by inducing GAD-specific tolerance,11 and that CD4+ Foxp3+ Tregs induced in vitro by GAD-IgG are responsible for this tolerance.14 Here, we have shown that CD8+ CD25+ Foxp3+ Tregs induced in vitro by GAD-IgG are also responsible for this tolerance.
CD8+ Tregs share phenotypic features with CD4+ Tregs and, like CD4+ Tregs, can be generated both in the thymus and in the periphery.27–36 We found that GAD-IgG-transduced splenocytes induced CD8+ T cells in vitro to up-regulate Foxp3 and CD25. In parallel with this, the percentage of CD8+ CD25+ Foxp3+ T cells increased in GAD-IgG-transduced splenocytes, compared with IgG-transduced splenocytes (Fig. 1). Previous studies have shown that continuous antigen stimulation of CD8+ T cells leads to generation of adaptive CD8+ CD25+ Foxp3+ T cells.27,28,30–32
Depletion of CD8+ or CD8+ CD25+ T cells from GAD-IgG-transduced splenocytes before transfer significantly reduced the percentage of CD8+ Foxp3+ T cells in recipient NOD mice (Fig. 2). CD8+ CD25+ Foxp3+ T cells, induced in vitro by GAD-IgG-transduced splenocytes, were critical for the up-regulation of CD8+ CD25+ Foxp3+ T cells in NOD mice receiving GAD-IgG-transduced splenocytes. Similarly, CD4+ CD25+ Foxp3+ T cells, induced in vitro by GAD-IgG-transduced splenocytes, were critical for the up-regulation of CD4+ CD25+ Foxp3+ T cells in NOD mice receiving GAD-IgG-transduced splenocytes.14 Although there are several studies showing that antigen stimulation of CD8+ T cells leads to the generation of adaptive CD8+ CD25+ Foxp3+ T cells,27,28,30–32 the mechanisms by which GAD-IgG-transduced splenocytes induce CD8+ Tregs in vitro are largely unknown. We speculate that (1) antigen constructed in this way might be presented in a different pattern, (2) LPS-activated B cells, transduced with IgG fusion proteins, are highly tolerogenic antigen-presenting cells, and (3) cytokines facilitated the in vitro induction of Tregs.
Next, to examine the role of CD8+ CD25+ Foxp3+ T cells in tolerance induction in NOD mice, we depleted CD8+ or CD8+ CD25+ T cells from GAD-IgG-transduced splenocytes before transfer. Compared with the undepleted group, CD8+ or CD8+ CD25+ T cell depletion resulted in a high incidence of diabetes (Fig. 3a), severe insulitis (Fig. 3b), GAD-specific hyperresponsiveness at the cellular and humoral levels (Fig. 4, 5), increases in the levels of Th1 cytokines (Fig. 6), and significant decreases in the levels of inhibitory cytokines IL-10 and TGF-β (Fig. 7). These results suggest that CD8+ CD25+ Foxp3+ T cells, induced in vitro by GAD-IgG-transduced splenocytes, were CD8+ Tregs. Other studies also indicated that CD8+ CD25+ Foxp3+ Tregs affect specific immunosuppression by depleting or suppressing potentially pathogenic self-reactive T-cell clones in the periphery. CD8+ Tregs participate in resistance to the re-induction of EAE,28 suppression of autoimmunity in a murine lupus model33 and blockade of transplant rejection.35,36
TGF-β plays an important role in the generation and function of Tregs. CD8+ CD25+ Foxp3+ Tregs inhibit T-cell immune responses by secretion of humoral factors, in contrast to adaptive CD4+ Tregs. Although the adaptive CD8+ Tregs express IL-10 and TGF-β (Fig. 7), the suppressive mechanism appears to be cell contact-dependent. However, TGF-β can induce CD8+ T cells to express Foxp3.36 In addition, antigen-specific diabetogenic CD4+ and CD8+ T cells can be converted to TGF-β-producing non-pathogenic regulatory cells following Foxp3 transduction.34
Our previous studies showed that CD4+ Foxp3+ Tregs are critical for tolerance in mice injected with GAD-IgG-transduced splenocytes and reduce the incidence of diabetes.14 Here, we found that regulatory CD8+ T cells were also responsible for the protective role of GAD-IgG-transduced splenocytes in diabetes. These results suggest that regulatory CD4+ and CD8+ T cells, induced by GAD-IgG-transduced splenocytes, are important in protecting NOD mice against diabetes.
In summary, our data suggest that Tregs, including CD8+ CD25+ Foxp3+ Tregs, induced in vitro by GAD-IgG-transduced splenocytes, were responsible for tolerance induction and prevention of diabetes by GAD-IgG-transduced splenocytes.
Acknowledgments
We gratefully acknowledge Dr Jiannan Feng for useful advice. This work was supported by National Nature and Science Funds (30571732) and National ‘973’ Fund Grant 2007CB512406.
Abbreviations
- GAD
glutamate decarboxylate 65
- NOD
non-obese diabetic
- Treg
regulatory T cell
References
- 1.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–4. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DL, Clare-Salzler M, Tian J, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisch R, Yang X, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–5. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson MA, Kaufman DL, Campbell L, et al. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992;339:458–9. doi: 10.1016/0140-6736(92)91061-c. [DOI] [PubMed] [Google Scholar]
- 6.Zambidis ET, Kurup A, Scott DW. Genetically transferred central and peripheral immune tolerance via retroviral-mediated expression of immunogenic epitopes in hematopoietic progenitors or peripheral B lymphocytes. Mol Med. 1997;3:212–24. [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal RK, Kang Y, Zambidis E, Scott DW, Chan CC, Caspi R. Retrovival gene transfer of an immunoglobulin-antigen fusion contruct protects from experimental autoimmune uveitis. J Clin Invest. 2000;106:245–52. doi: 10.1172/JCI9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang Y, Melo M, Deng E, Tisch R, EL-Amine M, Scott DW. Induction of hyporesponsiveness to intact foreign protein via retroviral-mediated gene expression: the IgG scaffold is important for induction and maintenance of immune hyporesponsiveness. Proc Natl Acad Sci USA. 1999;96:8609–14. doi: 10.1073/pnas.96.15.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EL-Amine M, Melo M, Kang Y, Nguyen H, Qian J, Scott DW. Mechanisms of tolerance induction by a gene transferred peptide-IgG fusion protein expression in B-lineage cells. J Immunol. 2000;165:5631–6. doi: 10.4049/jimmunol.165.10.5631. [DOI] [PubMed] [Google Scholar]
- 10.Melo M, Qian JH, EL-Amine M, Agarwal RK, Soukhareva N, Kang Y, Scott DW. Gene transfer of Ig-fusion proteins into B cells prevents and treats autoimmune diseases. J Immunol. 2002;168:4788–95. doi: 10.4049/jimmunol.168.9.4788. [DOI] [PubMed] [Google Scholar]
- 11.Song L, Wang J, Wang R, et al. Retroviral delivery of GAD-IgG fusion construct induces tolerance and modulates diabetes: a role for CD4+ regulatory T cells and TGF-β? Gene Ther. 2004;11:1487–96. doi: 10.1038/sj.gt.3302327. [DOI] [PubMed] [Google Scholar]
- 12.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105:4865–70. doi: 10.1182/blood-2004-11-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soukhareva N, Jiang Y, Scott DW. Treatment of diabetes in NOD mice by gene transfer of Ig-fusion proteins into B cells: role of T regulatory cells. Cell Immunol. 2006;240:41–6. doi: 10.1016/j.cellimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang R, Song L, Han G, et al. Mechanisms of regulatory T-cell induction by antigen-IgG-transduced splenocytes. Scand J Immunol. 2007;66:515–22. doi: 10.1111/j.1365-3083.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- 15.Chatenoud L, Salomon B, Bluestone JA. Suppressor T cells – they're back and critical for regulation of autoimmunity. Immunol Rev. 2001;182:149–63. doi: 10.1034/j.1600-065x.2001.1820112.x. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 17.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 18.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+ CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Rifa'I M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+ CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–34. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xystrakis E, Dejean AS, Bernard I, Druet P, Liblau R, Gonzalez-Dunia D, Saoudi A. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294–301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 22.Zheng SG, Wang JH, Koss MN, Quismorio FJr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–9. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 23.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 24.Jarvis LB, Matyszak MK, Duggleby RC, Goodall JC, Hall FC, Gaston JS. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896–908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 25.Dhodapkar MV, Steinman RM. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–7. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 26.Cosmi L, Liotta F, Lazzeri E, et al. Human CD8+ CD25+ thymocytes share phenotypic and functional features with CD4+ CD25+ regulatory thymocytes. Blood. 2003;102:4107–14. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 27.Mahic M, Henjum K, Yaqub S, Bjørnbeth BA, Torgersen KM, Taskén K, Aandahl EM. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38:640–6. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 28.Singh RP, La CavaA, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in Lupus-Prone (NZB x NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol. 2008;180:2069–80. doi: 10.4049/jimmunol.180.4.2069. [DOI] [PubMed] [Google Scholar]
- 29.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13:6947–58. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, Vaslin B. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol. 2007;81:13444–55. doi: 10.1128/JVI.01466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugita S, Futagami Y, Horie S, Mochizuki M. Transforming growth factor beta-producing Foxp3(+)CD8(+)CD25(+) T cells induced by iris pigment epithelial cells display regulatory phenotype and acquire regulatory functions. Exp Eye Res. 2007;85:626–36. doi: 10.1016/j.exer.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Billerbeck E, Blum HE, Thimme R. Parallel expansion of human virus-specific FoxP3- effector memory and de novo-generated FoxP3+ regulatory CD8+ T cells upon antigen recognition in vitro. J Immunol. 2007;179:1039–48. doi: 10.4049/jimmunol.179.2.1039. [DOI] [PubMed] [Google Scholar]
- 33.Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol. 2007;178:7649–57. doi: 10.4049/jimmunol.178.12.7649. [DOI] [PubMed] [Google Scholar]
- 34.Peng J, Dicker B, Du W, Tang F, Nguyen P, Geiger T, Wong FS, Wen L. Converting antigen-specific diabetogenic CD4 and CD8 T cells to TGF-beta producing non-pathogenic regulatory cells following FoxP3 transduction. J Autoimmun. 2007;28:188–200. doi: 10.1016/j.jaut.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Popescu I, Macedo C, Abu-Elmagd K, et al. EBV-specific CD8+ T cell reactivation in transplant patients results in expansion of CD8+ type-1 regulatory T cells. Am J Transplant. 2007;7:1215–23. doi: 10.1111/j.1600-6143.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- 36.Kapp JA, Honjo K, Kapp LM, Xu X, Cozier A, Bucy RP. TCR transgenic CD8+ T cells activated in the presence of TGFbeta express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection. Int Immunol. 2006;18:1549–62. doi: 10.1093/intimm/dxl088. [DOI] [PubMed] [Google Scholar]
- 37.Hill N, Sarvetnick N. Cytokines: promoters and dampeners to autoimmunity. Curr Opin Immunol. 2002;14:791–7. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]
- 38.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 39.Weiner HL. Induction and mechanisms of action of transforming growth factor- β secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]