Abstract
The composition of lymphocyte subsets in the lung has been found to be compartment-specific. To characterize the effect of age, weanling, young adult and adult rats were studied in control conditions and after a single intratracheal dose of the Toll-like receptor 2/6 (TLR2/6) agonist macrophage activating lipopeptide-2 (MALP-2). In all age groups, T, B and natural killer (NK) cells increased dramatically in the epithelium and lamina propria of the bronchi. Male adult rats were found to have responded to MALP-2 to a much greater extent than females when lymphocyte subsets were counted in the epithelium and the lamina propria. In a second series of experiments the time kinetics of regulatory T-cell (Treg) subsets and dendritic cells (DCs) in the lung was studied after local stimulation with MALP-2. Different time-dependent patterns were found in the Treg subsets CD4+ CD25+, CD4+ CD25+ neuropilin 1+ and CD4+ CD25+ Foxp3+ cells. Neutrophils and DCs also showed different patterns. Thus, the local application of a TLR agonist increased the number of lymphocyte subsets in a compartment-specific pattern. However, data should not be generalized or extrapolated from one age group, sex or lymphocyte subpopulation to another.
Keywords: age, air-conducting parts, bronchus-associated lymphoid tissue, leucocyte subsets, lung, rat, TLR agonist
Introduction
After the intestinal tract, the lung has the second largest contact area of all organs to the outside and is continuously exposed to inhaled particles, microbial agents and allergens. In addition to non-specific defence mechanisms such as that provided by defensins, cellular non-specific cells (e.g. neutrophils and macrophages) and specific cells [e.g. lymphocyte subsets and dendritic cells (DCs)] play a critical role in protective and allergic immune reactions.
It has been documented that there are different compartments in the lung, each with a specific leucocyte subset composition and kinetics (for review see ref. 1), such as the air-conducting parts with intraepithelial and lamina propria lymphocytes as well as the bronchus-associated lymphoid tissue (BALT) and the gas exchange parts, for example the lung interstitium and the bronchoalveolar space also containing various leucocytes. The latter can now be routinely sampled in patients by bronchoalveolar lavage (BAL). However, the cellular composition of the BAL does not necessarily represent the whole lung. Furthermore, it is widely accepted that, to understand immune reactions in the lung, draining mediastinal lymph nodes have also to be considered.2
In recent years the family of regulatory T cells (Tregs) has been characterized and shown to be essential in preventing persistent immune reactions.3 These receptors also play a critical role in the control of allergic reactions of the lung.4 Meanwhile, it has been documented that Tregs can also sense pathogens directly via Toll-like receptors (TLRs).5 Very recently, the concept has been proposed that Treg responsiveness largely depends on the local microenvironment, with very different quantitative and qualitative functional results.6 TLR agonists might play a critical role in future preventive or therapeutic applications in inflammation and allergy. In a mouse model of respiratory influenza, infection resulted in a surprisingly long TLR bacterial ligand desensitization.7 Thus, TLR agonists might reduce the duration of this dangerous period and prevent bacterial superinfections. The lipopeptide macrophage activating lipopeptide-2 (MALP-2) is a TLR2 and 6 agonist, can be produced in vitro, is therefore lipopolysaccharide (LPS) free and can be applied locally, for example into the trachea, to modify immune reactions selectively in this organ.8,9
In previous studies in rats, we determined an effective dose of intratracheally applied MALP-2 and studied the kinetics of neutrophils, lymphocyte subsets and DCs.8 In this very dynamic situation, we tested in the present study the effects of intratracheally applied doses of MALP-2 on the number of lymphocyte subsets in additional compartments, namely the epithelial layer and the lamina propria of the bronchi, and also on the amount of BALT, and investigated potential differences among three age groups.
A further unknown aspect of immune modulation by the TLR2/6 agonist lipopeptide MALP-2 is when Treg subsets in the lung might appear after a single local intratracheal dose. Therefore, the time kinetics of the accumulation of Tregs and DCs was studied after a single dose of the TLR2/6 agonist using not only CD4, CD25 and Foxp3 but also neuropilin 1 (Nrp1) as a marker. This marker for a Treg subset had previously only been used in the mouse10 and has very recently also been shown to be a marker for a Treg subset in human lymph nodes, which was absent in the blood.11 The data of these studies demonstrate the high effectivity of a single dose of a TLR2/6 agonist and the necessity of studying not only different compartments of the lung containing several different leucocyte subsets, but also a series of time-points after immune stimulation.
Materials and methods
Animals
To study the effects of a single intratracheal dose of the TLR2/6 agonist MALP-2, the following groups of Lewis rats from the central animal facilities of the Medical School Hannover were used: weaned rats [3–4 weeks of age, male, 76 ± 4 g body weight (mean ± SEM), n = 6], young adults (8–10 weeks of age, male, 259 ± 60 g body weight, n = 5) and adults (12–14 months of age; male, 418 ± 24 g body weight, n = 6; female, 336 ± 20 g body weight, n = 6). The young adult rats were those that had been used primarily for other purposes.8 The weanling and adult rats were previously described in a study on the effects of MALP-2 on the bronchoalveolar space and lung interstitium.12 In the adult group, male and female rats were studied to determine gender effects at this age, at which significant early increases in monocyte chemotactic protein (MCP)-1 and tumour necrosis factor (TNF)-α after MALP-2 application had previously been documented.8 The time kinetics of the effect of MALP-2 on leucocyte subsets was studied in young adult Lewis rats (250 ± 4 g body weight, male, n = 6 per time-point). All rats were maintained in a separate minimal barrier facility and were regularly monitored microbiologically according to Federation of European Laboratory Animal Science Association recommendations. The experiments were approved by the Lower Saxony district government.
MALP-2
The lipopeptide MALP-2 (kindly provided by P. Mühlradt, BioTec Gründerzentrum, Braunschweig, Germany) was synthesized and purified as described previously.13 The advantage of this in vitro production is that it is free of LPS. The MALP-2 was kept in a stock solution in 33% propanol/water and diluted with pyrogen-free 0·9% NaCl immediately before use. The solvent controls consisted of the corresponding volume without MALP-2. The volumes were adjusted to the size of the lungs as described previously (500 μl final volume for adults and young adults; 250 μl final volume for weanling rats).12 For the time kinetic experiments 2·5 μg of MALP-2 in a 250-μl final volume was used, as this dose had been shown to be most appropriate for such studies.8 The intratracheal application has been described previously.12
Preparation of the tissues
Rats were killed under deep anaesthesia on day 3 after the intratracheal instillation of MALP-2 after blood samples had been taken from the abdominal aorta. The thorax was opened and the right main bronchus filled with 3 ml of optimal cutting temperature (OCT) (TissueTec; Sakura Finetek Europe, Zeeterwoude, the Netherlands) to achieve normal lung size. The inferior lobe was divided into a proximal, a middle and a distal part, frozen in liquid nitrogen and kept at −80°.
Cell suspensions of the lung and bronchial lymph nodes
The lung tissue and mediastinal lymph nodes were removed after exsanguination, passed through a metal sieve with two rounded tweezers, followed by rinsing of the sieve, centrifuged at 400 g and processed as described for blood cells.8
Differential cell counts and flow cytometry
Total cell numbers were determined in a Neubauer counting chamber (Hecht, Sondheim, Germany) and differentials were obtained on cytospots using Quick-Diff (DQ; Dade, Bering, Switzerland) by counting at least 300 cells. The flow cytometry techniques have been used and described before.12 The antibodies employed to identify subsets are listed in Table 1. To identify NK cells, an antibody against CD161 was used (Table 1). The Nrp1 staining was performed as described for the mouse.10 The antibody W3/25 also binds to CD4 T lymphocytes and to rat monocytes. The αβ T-cell receptor was also used and combined with the monocyte marker ED9. It was found to be advantageous to stain the surface first and then perform the permeabilization for Foxp3 staining. The following subsets of Tregs were identified: CD4+ CD25+ Nrp1+ and CD4+ CD25+ Foxp3+.
Table 1.
Antibodies used
| Specificity | Clone | Isotype | Conjugate | Dilution | Supplier |
|---|---|---|---|---|---|
| CD6 | Ox 52 | IgG2a, κ | Unconjugated | 1 : 2000 | Serotec1 |
| T lymphocytes (α/β) | R73 | IgG1 | Unconjugated | 1 : 20 | Serotec |
| MHC class II | Ox 6 | IgG1 | Biotinylated | 1 : 50 | Serotec |
| CD4 | W3/25 | IgG1 | Biotinylated FITC APC | 1 : 100 1 : 1000 1 : 250 | Serotec |
| CD25 | Ox 39 | IgG1 | Biotinylated PE | 1 : 50 1 : 50 | Serotec |
| Neuropilin 1 (Nrp1) | 130604 | IgG1 | Unconjugated | 1 : 100 | RD Systems2 |
| Isotope control for Nrp1 | IgG1 | Unconjugated | 1 : 100 | RD Systems3 | |
| Foxp3 | FJK-16s | IgG2a, κ | FITC | 1 : 10 | BioLegend4 |
| Isotype control for Foxp3 (mAB) | IgG2a | FITC | 1 : 10 | BioLegend | |
| B cells [F (ab) fragment of rat IgG] | Ox 12 | IgG1 | Unconjugated | 1 : 1000 | Serotec |
| Monocytes, macrophages, DCs, granulocytes | ED9 | IgG1 | Unconjugated | 1 : 500 | Serotec |
| NK cells | CD161 | 100/78 | Unconjugated | 1 : 500 | Serotec |
Kidlington, Oxford, UK;
Abington, Oxford, UK;
Wiesbdaen, Germany;
San Diego, CA.
DC, dendritic cell; IgG, immunoglobulin G; mAb, monoclonal antibody; MHC, major histocompatibility complex; NK, natural killer.
Area of BALT
Slices (16–20 per antibody) at intervals of 42 μm were stained with the monoclonal antibodies and evaluated using an ocular grid with an area of 0·25 mm2 at ×20 magnification. For each antibody six slices were screened. Thus, the mean cut area of BALT was calculated and given in μm2.
Data analysis
Mean, standard error and level of significance were determined using graph pad prism 3·03 (Graph Pad Prism Software, San Diego, CA). For comparison of the experimental groups the Mann–Whitney U-test was applied. Values of P ≤ 0·05 were taken as statistically significant.
Results
Intraepithelial lymphocytes (IELs)
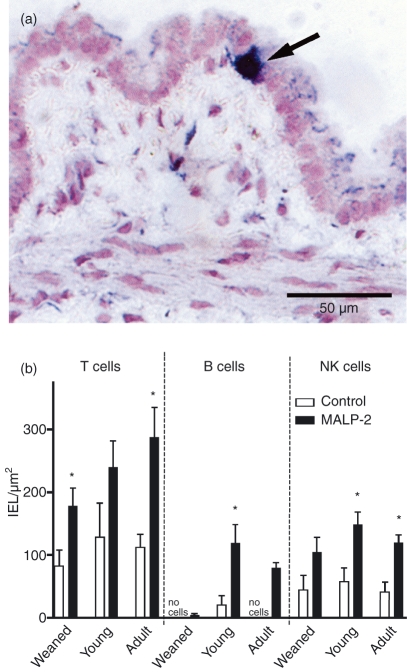
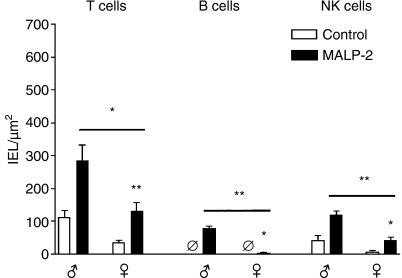
Under low-power magnification, no obvious differences were seen after MALP-2 treatment. Therefore, higher power was used to enumerate the individual subsets. Lymphocytes between the epithelial cells could be identified unambiguously as a result of the subset staining (Fig. 1a). The majority of these IELs were T lymphocytes, outnumbering the NK cells by a factor of 2 in all age groups. In contrast, B lymphocytes among the IELs were absent in weanling and adult rats. After the intratracheal MALP-2 instillation a dramatic increase in T, B and NK cell numbers was obvious in all age groups. Only the data for T cells in young adults, for NK cells in weaned rats and for B cells in weaned and adult rats did not reach the level of significance (Fig. 1b). A surprising finding was that the number of T, B and NK cells in the epithelium was significantly higher in male than in female rats (Fig. 2).
Figure 1.
Lymphocyte subsets in control and macrophage activating lipopeptide-2 (MALP-2)-treated rats 3 days after a single dose in the bronchial epithelium in different age groups. (a) Immunohistological staining of αβ T cells. A positive cell is indicated by the arrow. (b) Numbers of T, B and natural killer (NK) cells in the epithelium. *P < 0·05.
Figure 2.
Numbers of intraepithelial lymphocytes (IELs) in the bronchial wall of male (♂) and female (♀) adult rats 3 days after a single intratracheal dose of macrophage activating lipopeptide-2 (MALP-2). Ø, no positive cells detectable. *P < 0·05; **P < 0·01.
Lamina propria lymphocytes
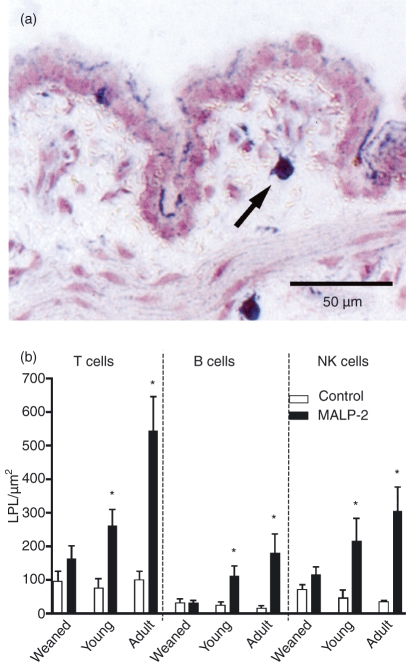
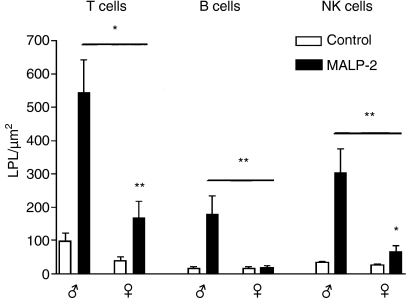
The area between the basal lamina of the epithelium and the muscularis mucosae is the lamina propria (Fig. 3a). In the control rats no difference was found among the three age groups (Fig. 3b). After MALP-2 stimulation the number of T, B and NK cells was increased in all age groups; however, the effects in weanling rats did not reach significance and the adult animals had much stronger responses than the other age groups. As for the IELs, the male animals in the adult group showed a much more dramatic response to MALP-2 than the female animals (Fig. 4).
Figure 3.
Lymphocytes in the bronchial lamina propria 3 days after a single intratracheal dose of macrophage activating lipopeptide-2 (MALP-2) or solvent. (a) A T lymphocyte is indicated by the arrow. (b) The effect of MALP-2 stimulation is demonstrated by the increase in T, B and natural killer (NK) cells, the extent of which differs with age. *P < 0·05.
Figure 4.
The increase in T, B and natural killer (NK) cells 3 days after macrophage activating lipopeptide-2 (MALP-2) treatment is significantly smaller in female (♀) than in male (♂) adult rats. *P < 0·05; **P < 0·01.
BALT
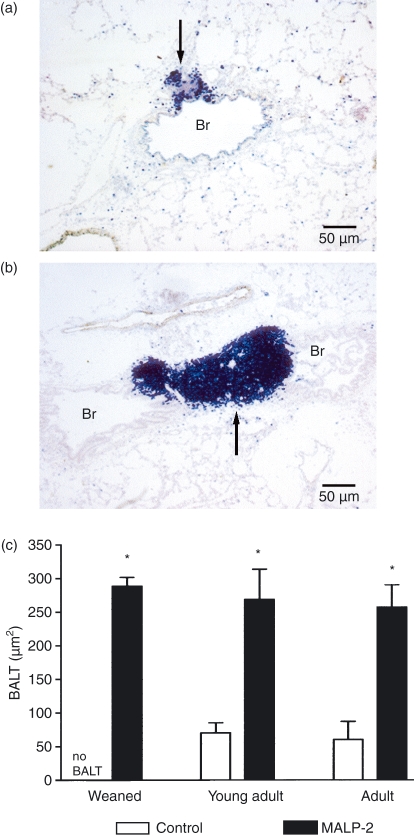
In weanling rats no BALT could be found, while it was present in 77 and 50% of young adult and adult male rats, respectively. The size of the BALT could readily be measured as the borders were demarcated as a result of the immunohistological staining (Fig. 5a,b). In all age groups the size of the BALT increased dramatically 3 days after MALP-2 application (Fig. 5c).
Figure 5.
(a) In an adult control rat only a small area of bronchus-associated lymphoid tissue (BALT) (arrow) can be seen. (b) At 3 days after a single intratracheal dose of macrophage activating lipopeptide-2 (MALP-2) the BALT (arrow) is much larger. Immunohistology was performed with staining of T lymphocytes. Br, bronchus. (c) The area of BALT in different age groups 3 days after intratracheal MALP-2. The mean area of BALT increased significantly in all age groups. *P < 0·05.
Kinetics of Tregs and DCs after MALP-2 stimulation in the lung
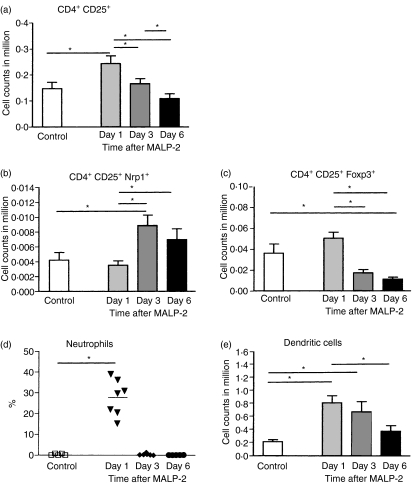
One to six days after the intratracheal instillation of the TLR2/6 agonist the number of leucocytes in the blood did not change (data not shown), indicating a lung-restricted effect. In the lung tissue the relative and absolute numbers of T lymphocytes as well as CD4+ T cells did not show significant differences between control and treated animals (data not shown). Granulocytes, however, in the lung interstitium increased from hardly any cells in the control animals to a mean of 27% in the treated animals on day 1, but on days 3 and 6 the neutrophil numbers were back to normal values. The different Treg subpopulations in the lung tissue showed a subset-specific pattern, which was comparable when the relative and absolute numbers were compared. The CD4+ CD25+ cells had already reached a maximum on day 1, which was also true for the CD4+ CD25+ Foxp3+ Tregs. However, the Tregs expressing Nrp1 (CD4+ CD25+ Nrp1+) reached a maximum later, on day 3 (Fig. 6). The number of neutrophils showed a rapid increase on day 1 but already on day 3 the numbers were back to control levels (Fig. 6d). The DCs reached a maximum on day 1 and showed a gradual decrease until day 6 after MALP-2 application (Fig. 6e).
Figure 6.
Time kinetics of different regulatory T cell (Treg) subsets (a–c) in lung tissue on days 1, 3 and 6 after a single intratracheal dose of macrophage activating lipopeptide-2 (MALP-2). The neutrophils (d) and dendritic cells (DCs) (e) show different time kinetics. *P < 0·05.
Tregs and DCs in the lung draining lymph node
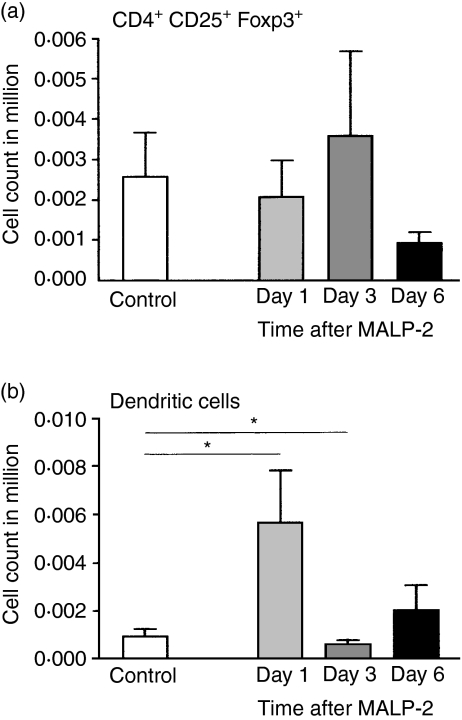
The relative and absolute numbers of CD4+ CD25+ (data not shown) and CD4+ CD25+ Foxp3+ Treg subsets did not show significant differences in the bronchial lymph node (Fig. 7a), while the relative number of DCs increased significantly from 2·4 to 7·6% on day 1, but on day 3 the numbers were back to control levels (Fig. 7b).
Figure 7.
Three days after a single intratracheal dose of macrophage activating lipopeptide-2 (MALP-2), the number of CD4+ CD25+ Foxp3+ regulatory T cells (Tregs) does not show a significant increase in absolute numbers in the bronchial lung draining lymph node (a), while the dendritic cells (DCs) display a rapid early increase (b).
Discussion
The synthetic lipopeptide MALP-2 has the advantage of being free of LPS. It stimulates neutrophils via TLR2 and TLR614 to secrete interleukin (IL)-8 and macrophage inflammatory protein (MIP)-1β and enhances phagocytic capacity. It can be locally applied to the upper respiratory tract, being effective, for example, as an adjuvant in measles vaccination,15 and when given tracheally stimulates leucocyte subsets in a cell- and time-specific pattern.8 When MALP-2 was locally applied with interferon (IFN)-γ in the mouse ovalbumin (OVA) asthma model, eosinophilic and T helper type 2 (Th2) cytokines were reduced to control levels.16 This activation potential was also reported to reduce the number and size of lung metastases when MALP-2 was intratracheally applied on the same day that tumour cells were injected intravenously.9 Furthermore, it has been documented that MALP-2 has a direct stimulatory effect on neutrophils via TLR2/6 binding.17
In a recent paper, dramatic differences among age groups were reported in the effects of intratracheally instilled MALP-2 on the number of leucocyte subsets in the lung interstitium and the bronchoalveolar space and the kinetics of MCP-1 and TNF-γ in the BAL.12 It was concluded that data on immune reactions should not be extrapolated from one compartment of the lung to others, or from young adults to weaned or older animals.12 Additional compartments of the lung containing leucocytes are the epithelial layer, the lamina propria and the BALT.1 The number of IELs in the bronchial wall has not been studied in different age groups in parallel to date, although this is an interesting subpopulation mainly consisting of T lymphocytes.18 The clear increase on day 3 after a single dose of MALP-2 in T and NK cells in this compartment and also of B cells, a population normally not present at this site, was surprising. The functional relevance of this finding remains to be determined in future work. This stimulation by the TLR2/6 agonist also resulted in many more lymphocytes in the lamina propria, with the highest numbers in the adult animals. Thus, in future vaccination or infection studies of the lung, the different compartments should be examined in parallel and the results not extrapolated to all age groups. Furthermore, gender differences such as those documented here for adult male and female rats should be kept in mind, as there is increasing evidence of human gender differences, for example in chronic lung diseases.19,20
BALT is a unique structure in the mucosal immune system as it differs from Peyer's patches – a typical secondary lymphoid organ – in that it is not present constantly.21 However, this might be an advantage for the initiation of protective immune reactions in the lung: in a first step BALT might be induced, activated and enlarged and in a second step particulate antigen might be taken up by BALT and protective immune reactions initiated. The data presented here show that a single dose of MALP-2 resulted in dramatic increases in the size and frequency of BALT in all age groups. Further studies are needed to evaluate the functional role of this BALT in protective immune reactions and how long this increased size and activity will persist.
An immune modifier such as MALP-2 might also influence the number and subset composition of Tregs. As this was expected to show a kinetic pattern, different time-points after a single dose of MALP-2 were studied. Tregs were initially described as effective regulators in preventing autoimmune diseases but were later described as having a control function in inflammation initiated by infectious pathogens or allergens (for a review, see ref. 22). The phenotype of Tregs in rats has also been characterized.23 Meanwhile, an ever-increasing number of subsets of Tregs have been described (for a review, see ref. 24) and their roles in mucosal immune reactions have been discussed.25 In addition to CD25+ on CD4+ lymphocytes, Nrp1 has been described as a marker on Tregs in the mouse.10 Recently Nrp1 was described on a subset of Tregs in humans. However, surprisingly, it was identified on some Tregs in the lymph nodes but not on lymphocytes in the blood.11 Thus, it is of great interest that we have also characterized a subset of Tregs as Nrp1+ in the rat.
In humans, Foxp3 has been reported to play a crucial role in the function of Tregs,26 in protection against airway hyperreactivity27,28 and in the induction of tolerance to inhaled allergens.29 The reversal of airway hyperresponsiveness by induction of Tregs in a rat asthma model has been described30 and the resolution of a Th2 cell-driven response to allergen by Tregs documented.31 In the present study the number of Tregs increased very rapidly with surprising differences between Foxp3+ and Nrp1+ Tregs. In a very recent clinical viewpoint article,32 the contrasting data for mice and humans and the relevance of Foxp3 as a functional marker were discussed. However, the kinetics of increases in the number of Tregs in immune reactions were not mentioned. The functional consequences have to be defined in more detail in future experiments. In a recent study, a rapid turnover of Tregs was documented in humans using deuterium labelling.33 It has still to be determined to what extent influx and/or local proliferation results in the increase of Tregs in immune reactions.34
In the light of a report that MALP-2 acts via the TLR2/614 and descriptions of the important role of TLR on Tregs,5,35 the present data can be interpreted as a further example of an increase in the number of Tregs produced by a locally applied TLR2/6 agonist, which might be of great relevance in manipulating immune reactions in the lung, for example in preventing the long-lasting TLR desensitization that occurs after an influenza infection.7
It was a surprise that the draining bronchial lymph node did not show an increase of Tregs and DCs in a time-dependent manner after the MALP-2 instillation into the lung, as these lymph nodes play an important role in the immune reactions of the lung.1 The effects of the TLR2/6 agonist might be too small to be detected in the lymph node.
In conclusion, there are major differences in the number of lymphocyte subsets in lung compartments depending on the age and sex of the experimental animals. The TLR2/6 agonist MALP-2 applied locally into the trachea induced dramatic increases of lymphocyte subsets. The functional relevance of the different patterns of Treg subsets remains to be elucidated in future studies.
Acknowledgments
These studies were supported by the German Research Foundation (DFG), SFB 587/B1. We would like to thank M. Peter, S. Weber and K. Westermann for technical support and S. Fryk for secretarial help and for correcting the English.
Abbreviations
- BAL
bronchoalveolar lavage
- BALT
bronchus-associated lymphoid tissue
- DC
dendritic cell
- IEL
intraepithelial lymphocyte
- MALP-2
macrophage activating lipopeptide-2
- Nrp1
neuropilin 1
- OVA
ovalbumin
- TLR
Toll-like receptor
- Treg
regulatory T cell
References
- 1.Pabst R, Tschernig T. Control of lymphocyte trafficking through the lung. In: Lambrecht BN, Hoogsteden HC, Diamant Z, editors. The Immunological Basis of Asthma. New York: Dekker; 2003. pp. 293–310. [Google Scholar]
- 2.Kraal G, Samsom JN, Mebius RE. The importance of regional lymph nodes for mucosal tolerance. Immunol Rev. 2006;213:119–30. doi: 10.1111/j.1600-065X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 3.Hawrylowicz CM. Regulatory T cells and IL-10 in allergic inflammation. J Exp Med. 2005;202:1459–63. doi: 10.1084/jem.20052211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umetsu DT, Akbari O, DeKruyff RH. Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112:480–7. [PubMed] [Google Scholar]
- 5.Sutmuller RPM, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–93. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Matarese G, De Rosa V, La Cava A. Regulatory CD4 T cells: sensing the environment. Trends Immunol. 2008;29:12–7. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Didierlaurent A, Goulding J, Patel S, et al. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J Exp Med. 2008;205:323–9. doi: 10.1084/jem.20070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lührmann A, Deiters U, Skokowa J, et al. In vivo effects of a synthetic 2-Kilodalton macrophage-activating lipopeptide of Mycoplasma fermentans after pulmonary application. Infect Immun. 2002;70:3785–92. doi: 10.1128/IAI.70.7.3785-3792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shingu K, Kruschinski C, Lührmann A, Grote K, Tschernig T, von Hörsten S. Intratracheal macrophage-activating lipopeptide-2 reduces metastasis in the rat lung. Am J Respir Cell Mol Biol. 2003;28:316–21. doi: 10.1165/rcmb.2002-0106OC. [DOI] [PubMed] [Google Scholar]
- 10.Bruder D, Probst-Kepper M, Westendorf AM, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur J Immunol. 2004;34:623–30. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia A, Buzzonetti A, Monego G, et al. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology. 2008;123:129–38. doi: 10.1111/j.1365-2567.2007.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lührmann A, Grote K, Stephan M, Tschernig T, Pabst R. Local pulmonary immune stimulation by the Toll-like receptor 2 and 6 ligand MALP-2 in rats is age dependent. Immunol Lett. 2007;108:167–73. doi: 10.1016/j.imlet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Mühlradt PF, Kiess M, Meyer H, Süssmuth R, Jung G. Isolation, structure, elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J Exp Med. 1997;185:1951–8. doi: 10.1084/jem.185.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi O, Kawai T, Mühlradt PF, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 15.Lührmann A, Tschernig T, Pabst R, Niewiesk S. Improved intranasal immunization with live-attenuated measles virus after co-inoculation of the lipopeptide MALP-2. Vaccine. 2005;23:4721–6. doi: 10.1016/j.vaccine.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Weigt H, Nassenstein C, Tschernig T, Mühlradt PF, Krug N, Braun A. Efficacy of macrophage-activating lipopeptide-2 combined with interferon-g in a murine asthma model. Am J Respir Crit Care Med. 2005;172:566–72. doi: 10.1164/rccm.200411-1490OC. [DOI] [PubMed] [Google Scholar]
- 17.Wilde I, Lotz S, Engelmann D, et al. Direct stimulatory effects of the TLR2/6 ligand bacterial lipopeptide MALP-2 on neutrophil granulocytes. Med Microbiol Immunol. 2007;196:61–71. doi: 10.1007/s00430-006-0027-9. [DOI] [PubMed] [Google Scholar]
- 18.Erle DJ, Pabst R. Intraepithelial lymphocytes in the lung. A neglected lymphocyte population. Am J Respir Cell Mol Biol. 2000;22:398–400. doi: 10.1165/ajrcmb.22.4.f182. [DOI] [PubMed] [Google Scholar]
- 19.Sin DD, Cohen SBZ, Day A, Coxson H, Paré PD. Understanding the biological differences in susceptibility to chronic obstructive pulmonary disease between men and women. Proc Am Thorac Soc. 2007;4:671–4. doi: 10.1513/pats.200706-082SD. [DOI] [PubMed] [Google Scholar]
- 20.Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease. Why it matters. Am J Respir Crit Care Med. 2007;176:1179–84. doi: 10.1164/rccm.200704-553CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pabst R. Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol Lett. 2007;112:1–8. doi: 10.1016/j.imlet.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Wei S, Kryczek I, Zou W. Regulatory T-cell compartmentalization and trafficking. Blood. 2006;108:426–31. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephens LA, Barclay AN, Mason D. Phenotypic characterization of regulatory CD4+CD25+ T cells in rats. Int Immunol. 2004;16:365–75. doi: 10.1093/intimm/dxh033. [DOI] [PubMed] [Google Scholar]
- 24.Taams LS, Palmer DB, Akbar AN, Robinson DS, Brown Z, Hawrylowicz CM. Regulatory T cells in human disease and their potential for therapeutic manipulation. Immunology. 2006;118:1–9. doi: 10.1111/j.1365-2567.2006.02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izcue APF. Special regulatory T-cell review: regulatory T cells and the intestinal tract – patrolling the frontier. Immunology. 2008;123:6–10. doi: 10.1111/j.1365-2567.2007.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 27.Stock P, Akbari O, Berry G, Freeman GJ, DeKruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nature Immunol. 2004;5:1149–56. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 28.Lewkowich IP, Herman NS, Schleifer KW, et al. CD4+CD25+ cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med. 2005;202:1549–61. doi: 10.1084/jem.20051506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostroukhova M, Seguin-Devaux C, Oriss TB, et al. Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane-bound TGF-b and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strickland DH, Stumbles PA, Zosky GR, et al. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med. 2006;203:2649–60. doi: 10.1084/jem.20060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–47. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaguchi S. Regulatory T cells in the past and for the future. Eur J Immunol. 2008;38:901–3. doi: 10.1002/eji.200890012. [DOI] [PubMed] [Google Scholar]
- 33.Vukmanovic-Stejic M, Zhang Y, Cook JE, et al. Human CD4+CD25hiFoxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–33. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker LSK. CD4+ CD25+ Treg: divide and rule? Immunology. 2004;111:129–37. doi: 10.1111/j.0019-2805.2003.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutmuller R, Garritsen A, Adema GJ. Regulatory T cells and toll-like receptors: regulating the regulators. Ann Rheumat Dis. 2007;66(Suppl. 3):iii91–5. doi: 10.1136/ard.2007.078535. [DOI] [PMC free article] [PubMed] [Google Scholar]