Abstract
Natural killer (NK) cells belong to the innate immune system but can also affect adaptive immune reactions. This immune regulatory function is often ascribed to the CD56bright subpopulation of NK cells that is prevalent in secondary lymphoid tissues and has potent cytokine-producing ability. The NK cells have been described as affecting autoimmune disease and stimulating B-cell production of antibodies, but their role in systemic lupus erythematosus (SLE) pathology has not been extensively studied. We have studied NK cells in SLE, a B-cell-driven systemic autoimmune disease, and phenotypically characterized peripheral blood NK cells in comparison to NK cells from patients with immunoglobulin A nephritis, rheumatoid arthritis and healthy individuals. We have found an increased proportion of CD56bright NK cells in SLE, regardless of disease activity. We detected a somewhat increased expression of the activating receptor NKp46/CD335 on NK cells from SLE patients, although neither the percentage of NK cells of all lymphocytes nor the expression of other NK receptors analysed (LIR-1/CD85j, CD94, NKG2C/CD159c, NKG2D/CD314, NKp30/CD337, NKp44/CD336, CD69) differed between patient groups. We show that type I interferon, a proinflammatory cytokine known to be abundant in SLE, can cause increases of CD56bright NK cells in vitro. We confirmed that serum levels of interferon-α were increased in active, but not in inactive, disease in the SLE patient group. In conclusion, we found an increased proportion of CD56bright NK cells in the blood of SLE patients, although it remains to be examined whether and how this relates to the disease process.
Keywords: CD56bright natural killer cell, natural killer cells, systemic lupus erythematosus, type I interferon
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease affecting many organs, including kidneys and skin. SLE is characterized by many immunological abnormalities such as hyperactive B cells and overproduction of type I interferons (IFN, reviewed by Rönnblom et al.).1 Autoantibodies detectable in SLE are primarily specific for nuclear antigens that are possibly exposed to the immune system as the result of disease-associated defects in the clearance of apoptotic cells. The resulting antigen–antibody immune complexes accumulate in the skin and kidneys and cause inflammatory reactions including IFN responses,2 possibly through activation via Toll-like receptors.3 Interferon is considered central to innate immune activation and causes, among many other things, the activation of dendritic cells increasing their ability to promote subsequent adaptive immune responses.
Natural killer (NK) cells are lymphocytes belonging to the innate immune system. They mediate early protection against infections and lyse transformed cells and cells coated with antibody. They also secrete cytokines, predominantly IFN-γ.4 In humans, NK cells are defined by expression of CD56 and lack of CD3 and make up approximately 10% of peripheral blood lymphocytes. There are two major subpopulations of human NK cells, the CD56dim NK cells, which express killer immunoglobulin-like receptors (KIR) and CD16 (FcγRIIIa), and the CD56bright NK cells which lack expression of KIR and CD16 and which express the high-affinity interleukin-2 (IL-2) receptor α subunit CD25. These two sets of human NK cells differ in their function and distribution. The CD56dim NK cells make up approximately 90% of blood NK cells and have a high cytolytic capacity, while the CD56bright NK cell is the main NK cell type found in secondary lymphoid tissue and at sites of inflammation. It is conceivable that CD56bright NK cells may affect autoimmune diseases by promoting T-cell activation in lymphoid tissues5 and subsequently B-cell responses. It is also possible that NK cells may play a more direct role in promoting B-cell responses during SLE, in part through CD40/CD154 interactions between these cell types.6,7
We set out to analyse phenotypic abnormalities in peripheral blood NK cells from patients with SLE. As control groups, healthy donors and two other groups of patients affected by inflammatory disorders, IgA nephritis (IgAN) and rheumatoid arthritis (RA), were included. We found an increase in the proportion of CD56bright NK cells in patients with SLE regardless of disease activity. Furthermore, type I IFN could induce increased proportions of CD56bright NK cells in vitro. Levels of IFN-α were elevated in the patients with SLE in our study, although only in those with active disease.
Materials and methods
Patients
Twenty-three female patients (mean age 44 years, range 19–80) with SLE from the Department of Rheumatology at the Karolinska University Hospital were included in the study; their informed consent was obtained. All patients fulfilled at least four of the American College of Rheumatology (ACR) criteria for SLE.8 Disease manifestations, ongoing immunosuppressive therapy and routine laboratory analyses, including analysis of anti-double-stranded DNA antibodies (fluorescent enzyme immunoassay, normal < 10 U/ml), were recorded.
At the inclusion visit, disease activity was estimated using the SLE Activity Measurement (SLAM) and a score of 7 or more was defined as active disease. Of the 23 patients with SLE, three were sampled twice, once during active and again during inactive phases of disease. For patient characteristics see Table 1.
Table 1.
Patient characteristics
| Age (years) | Present disease manifestations | DMARD | Pred. (mg/day) | Anti-dsDNA (U/ml) | SLAM score |
|---|---|---|---|---|---|
| Active SLE patients | |||||
| 391 | Nephritis, arthritis | None | 5 | 68 | 8 |
| 26 | Skin, haematological | None | 6·25 | 251 | 14 |
| 56 | Arthritis | AZA | 10 | ND | 13 |
| 34 | Nephritis, arthritis | Chlorambucil | 15 | 139 | 9 |
| 43 | Nephritis, skin | None | 5 | > 400 | 26 |
| 52 | Nephritis | None | 10 | 314 | 8 |
| 64 | Haematological | None | 5 | ND | 12 |
| 19 | Nephritis | AZA | 20 | ND | 16 |
| 29 | Nephritis, lymphadenopathy | None | 15 | > 400 | 25 |
| 231 | Nephritis, APS | None | 0 | 65 | 9 |
| 47 | Skin, haematological, fever, nephritis | 302 | > 400 | 16 | |
| 36 | Arthritis, mental depression | AZA | ND | 56 | 12 |
| 371 | Nephritis | MMF | 8·75 | 52 | 9 |
| 45 | Nephritis | None | 7·5 | 15 | 10 |
| 44 | Haematological, hair loss, musculoskeletal | AZA, AM | 5 | Neg | 15 |
| 39 | 8·1 | 104 | 12 | ||
| Inactive SLE patients | |||||
| 38 | Inactive | AZA, AM | 2·5 | Neg | 1 |
| 59 | Inactive | None | 6 | Neg | 2 |
| 69 | Inactive | AZA | 4 | Neg | 4 |
| 39 | Inactive | AM2 | 0 | Neg | 4 |
| 35 | Inactive | None | 0 | 110 | 6 |
| 80 | Inactive | None | 0 | Neg | 4 |
| 58 | Inactive | None | 0 | ND | 4 |
| 44 | Inactive | None | 0 | Neg | 1 |
| 391 | Inactive | MMF | 25 | 24 | 6 |
| 231 | Inactive | None | 20 | 28 | 6 |
| 371 | Inactive | MMF | 10 | ND | 5 |
| 47 | 6 | Neg | 4 | ||
Medians shown below columns in bold types.
AM, antimalarials; APS, antiphospholipid syndrome; AZA, azathioprine; DMARD, disease modifying anti-rheumatic drug; MMF, mycophenolate mofetil; ND, not determined; Pred., prednisolone; SLAM, systemic lupus erythematosus activity measurement; SLE, systemic lupus erythematosus.
Patients analysed during active and inactive SLE.
Recent onset.
As control populations, 10 patients with biopsy-proven IgAN (seven men/three women, mean age 42 years, range 19–66), 13 patients with RA (four male/nine female, mean age 54, range 29–74, all fulfilling ACR criteria for RA;9 none treated with methotrexate, one with myocrisine, two patients had no disease-modifying drugs; seven patients had prednisolone doses below 10 mg/day; no patient was being treated with or had earlier been given biologicals) and 20 healthy controls (10 men/10 women, mean age 44 years, range 25–65) were included.
All patients gave their informed consent to participate and the study was approved by the local ethical committee.
Fluorescence-activated cell sorting (FACS) staining of patient samples
Peripheral blood mononuclear cells (PBMC) were separated by density centrifugation and stained for cell surface markers within 4 hr of sampling. Cells were multi-colour stained using monoclonal antibodies specific for: CD3 (cone SK7), CD56 (clone B159 or NCAM16.2), CD16 (clone NKP15) and CD94 (clone HP-3D9, all directly conjugated and from BD Biosciences, San José, CA), NKp30/CD337 (clone Z25, Beckman Coulter, Marseille, France), NKp44/CD336 (clone Z231, Beckman Coulter), CD69 (clone FN50, Dako, Glostrup, Denmark), NKG2D/CD314 (clone 149810, R&D Systems, Minneapolis, MN), NKG2C/CD159c (clone 134591, R&D Systems), NKp46/CD335 (kindly provided by Marco Colonna, Washington University, St Louis, MO) and LIR1/CD85j (clone M405, kindly provided by Amgen Inc., Thousand Oaks, CA). The unconjugated antibodies were detected by phycoerythrin-conjugated polyclonal goat anti-mouse IgG (Dako). As negative control, mouse IgG1 was used (Dako). The NK cells were defined as CD3− CD56+ lymphocytes and CD56bright NK cells were defined as CD3− CD56+ CD16− lymphocytes.
In vitro stimulation with cytokines
PBMC from healthy blood donors were grown in Iscove's modified Dulbeccos' medium (IMDM; Sigma-Aldrich, St Louis, MO) supplemented with antibiotics, l-glutamine, non-essential amino acids, β-mercaptoethanol, sodium pyruvate (all from Sigma-Aldrich), 5% inactivated human serum, 5% inactivated fetal calf serum (Sigma-Aldrich, IMDM complete), in the presence or absence of the following recombinant human cytokines: IL-2 (200 U/ml, PeproTech, London, UK), IL-15 (20 ng/ml, PeproTech) and universal type I IFN (1000 U/ml, PBL, Piscataway, NJ). After 3 days of culture the cells were stained for CD3, CD56 and CD16. As a negative control, phycoerythrin-conjugated IgG1 (BD Biosciences) was used. To determine the percentage of dead cells, cultured cells were stained using 7-amino-actinomycin D (7AAD; BD Biosciences) in combination with antibodies specific for CD3, CD56 and CD16, followed by analysis by flow cytometry. To delineate the degree of proliferation of CD56dim and CD56bright NK cells in response to cytokines, bromodeoxyuridine (BrDU) was added on day 2 of culture and PBMC were stained with monoclonal antibodies specific for BrDU in combination with CD3, CD56 and CD16, and analysed by flow cytometry. The NK cell function was analysed by adding 0·5 million K562 cells to PBMC cultures grown with medium or in the presence of IL-2, IFN or a combination of IFN and IL-2 at day 2 of culture. After 20 hr, GolgiStop (BD Biosciences) was added, and cells were stained intracellularly for IFN-γ after 4 hr, in combination with antibodies for CD3, CD56 and CD16.
Measurements of IFN-α in serum
To detect IFN-α levels in serum, a dissociation-enhanced lanthanide fluoroimmunoassay (DELFIA) with anti-IFN antibodies LT27:273 and LT27:293 was used as described elsewhere.10
Statistical analysis
Differences were calculated using analysis of variance (anova) combined with a Tukey post hoc test. For comparisons between two groups of patients, the Student's t-test was used. Data in the text are given as median (range).
Results
Patients with SLE have an increased proportion of CD56bright NK cells in blood
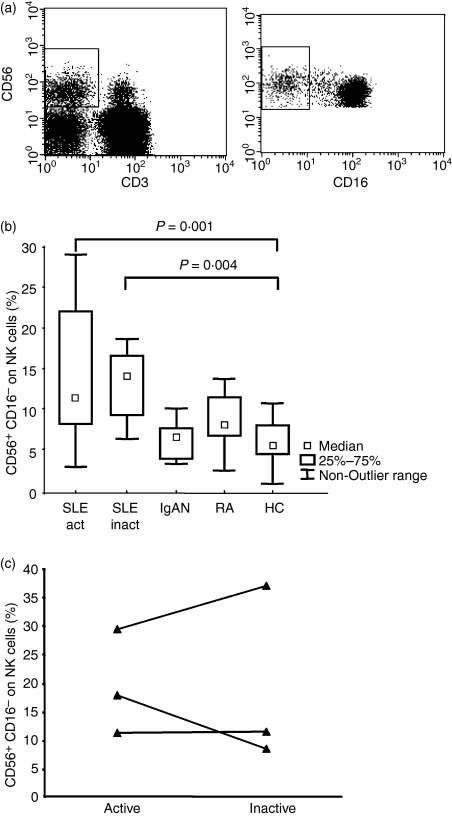
To determine whether NK cells play a role in the pathogenesis of SLE, we decided to analyse the phenotype of peripheral blood NK cells in this patient group, and compare it to that of healthy controls. As additional control groups with chronic inflammatory conditions, patients with IgAN and RA were included. These patients have an organ-specific, B-cell-driven autoimmune disease and a systemic T-cell-driven inflammatory disease, respectively. There were no statistically significant differences in the proportion of NK cells of all lymphocytes among the patient groups [SLE 11% (0·3–34), IgAN 14% (3–27), RA 13% (1·6–28), healthy controls 15% (5–42), P = 0·7 by anova]. We also compared the proportions of all NK cells that were CD16− CD3− CD56+ (CD56bright) NK cells (Fig. 1a) among the patient groups. A statistically significant difference was found between SLE patients (n = 23), which had increased proportions of CD56bright NK cells, and the healthy donors (n = 20) (SLE 13% (3–58), healthy controls 5% (1–11), P < 0·001, t-test). Patients with IgAN and RA had normal levels of CD56bright NK cells (Fig. 1b). There was no difference in CD56bright NK cell proportions between healthy males and females (data not shown). To better understand the reason for this increased proportion of CD56bright NK cells, we divided the patients with SLE according to disease activity, defining patients with SLAM score of ≥ 7 as having ‘active disease’. In addition, samples were taken from three patients with SLE during active disease and again during inactive disease (Fig. 1c). There was no difference in the proportion of CD56bright NK cells in SLE in relation to severity of disease. No association between the proportion of CD56bright NK cells and presence of nephritis, medical treatment or anti-double-stranded DNA antibodies could be distinguished within the SLE patient group (data not shown).
Figure 1.
The percentage of CD56bright natural killer (NK) is increased in patients with systemic lupus erythematosus (SLE). (a) On the left, an example of gating of CD3− CD56+ NK cells on gated blood lymphocytes from a healthy control is shown. On the right, CD56bright NK cells defined as CD16− NK cells are shown in the square. (b) The percentage of CD56bright NK cells of total blood CD3− CD56+ NK cells is plotted for patients with SLE during active disease (n = 15), patients during inactive disease (n = 8), patients with immunoglobulin A nephritis (IgAN) (n = 10), patients with rheumatoid arthritis (RA) (n = 13) and healthy blood donors (n = 20). Analysis of variance test P = 0·0009, followed by Tukey's post hoc test: active SLE compared to healthy control P = 0·01, and inactive SLE compared to healthy control P = 0·004. (c) The percentage of CD56bright NK cells of total blood CD3− CD56+ NK cells is plotted for three patients with SLE sampled during active and again during inactive diseases. Results from the active sample are included in (b).
We also analysed the expression of different NK cell inhibitory and activating receptors (LIR-1/CD85j, CD94, NKG2C/CD159c, NKG2D/CD314, NKp30/CD337, NKp44/CD336, CD69) but found no differences in the expression of these markers on NK cells among the patient groups (data not shown). The expression of NKp46/CD335 was slightly increased on NK cells from patients with SLE, but only when analysed on CD56dim NK cells separately [median fluorescence intensity (MFI) on CD56dim cells was 159 (100–242) for active SLE, 118 (47–273) for inactive SLE, 91 (44–136) for IgAN, 89 (45–171) for healthy controls; P = 0·002 active SLE compared to healthy controls, anova followed by Tukey]. Expression of NKp46/CD335 on CD56bright NK cells was somewhat higher than that for CD56dim NK cells [MFI 199 (62–379) and 101 (47–242) respectively, for all individuals].
Type I IFN increased the proportion of CD56bright cells in vitro
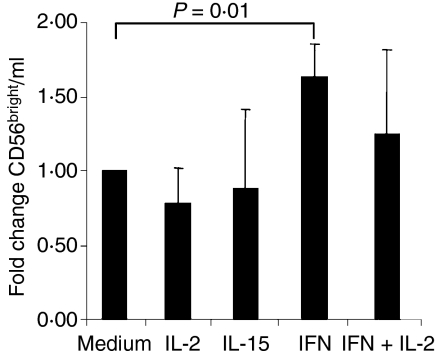
We hypothesized that cytokines with known stimulatory effects on NK cells may cause the increase in the proportion of CD56bright cells shown. We cultured PBMC from healthy donors for 3 days in the presence of IL-15, IL-2 or type I IFN, or in the presence of the combination of IL-2 and type I IFN. IL-15 and IL-2 decreased the proportion of CD56bright NK cells in culture after 3 days, possibly as a result of NK cell maturation. In contrast, type I IFN increased the proportion or CD56bright NK cells in culture significantly (Fig. 2), while a combination of IL-2 and type I IFN did not affect the proportion of CD56bright cells in the cultures compared with the medium control. As values differed considerably between blood donors, the figure shows normalized values, where the number of CD56bright NK cells per ml culture medium is expressed as the fold change of that found in the medium control. An increased proportion of CD56bright NK cells in presence of IFN could be the result of at least three different mechanisms: (1) increased proliferation of CD56bright NK cells, (2) increased death of CD56dim NK cells or (3) alterations in phenotype induced by IFN treatment. To study this, we analysed the proliferation of the respective NK cell subtype using BrDU incorporation and subsequent FACS staining. There was no proliferation in IFN-treated cells from healthy blood donors, while IL-2 and IL-15 induced proliferation predominantly of the CD56bright NK cells [56% (39–73) of CD56bright NK cells; 7·5% (2–17) of CD56dim NK cells, n = 4]. Stainings using 7AAD to analyse the deaths of the two NK cell subtypes indicated a slightly higher proportion of dead cells among the CD56bright compared to CD56dim NK cells (data not shown). Investigation of IFN-γ production in gated subsets of NK cells stimulated with the classical NK target cell line K562 determined no increased function on a per cell basis of NK cells from patients with SLE (data not shown). In general, CD56bright NK cells responded to a higher degree (data not shown), confirming earlier reports.11
Figure 2.
Culture of peripheral blood mononuclear cells (PBMC) in the presence of type I interferon (IFN) increases the number of CD56bright NK cells. The PBMC from healthy donors (n = 4) were cultured for 3 days in the presence of the indicated cytokines. The number of CD56bright NK cells per ml of cell culture was analysed and compared to the number found in the medium control. The fold increase in CD56bright NK cell is plotted. Paired t-test: P = 0·01 unstimulated compared to IFN-stimulated.
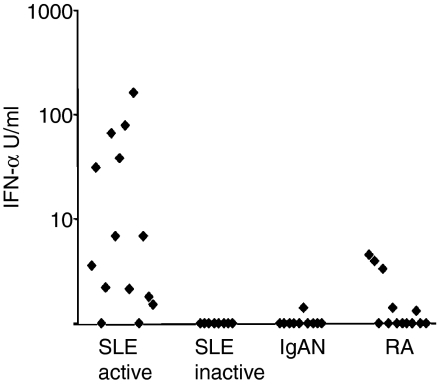
We hypothesized that the in vitro effect of type I IFN on proportions of NK subpopulations could also be operative in vivo and we therefore set out to search for a possible correlation between IFN-α serum levels and the proportion of CD56bright NK cells. Levels of IFN-α in serum were analysed for all patient groups and found to be highly elevated only in the SLE group with active disease (Fig. 3). There was no correlation between the IFN-α levels and the proportion of CD56bright NK cells in this group, nor in the SLE patient population as a whole (data not shown).
Figure 3.
Serum levels of interferon-α (IFN-α) are elevated in patients with systemic lupus erythematosus (SLE) with active disease. Serum from patients with active SLE (n = 14), inactive SLE (n = 8), immunoglobulin A nephritis (IgAN, n = 10) and rheumatoid arthritis (RA, n = 13) was analysed for levels of IFN-α. Levels are expressed as U/ml. P = 0·02 on analysis of variance.
Discussion
We have described an increase in the proportions of peripheral blood CD56bright NK cells in SLE, evident during inactive as well as during active disease. This argues against a therapy-associated effect because most patients with inactive disease did not receive any treatment. In addition, in vitro studies showed that IFN, a cytokine previously implicated in SLE, can increase the proportion of this NK cell subtype.
To our knowledge, this is the first description of a treatment-independent increase in the proportion of blood CD56bright NK cells in non-infectious diseases. CD56bright NK cells have previously been described as increasing during treatment with IL-2 receptor blockade in uveitis12 and in multiple sclerosis,13 as well as during IFN-β treatment in multiple sclerosis,14 and there is an increased proportion of CD56bright NK cells during chronic human immunodeficiency virus infection.15 There are several possible mechanisms that may explain our finding, including differential homing of NK cell populations in disease,16 specific proliferation of CD56bright NK cells and an increased output of immature CD56bright NK cells17,18 from bone marrow or from lymphoid tissue.19 CD56bright NK cells develop in lymphoid tissue and can be found in close proximity to T cells where they can affect adaptive immune responses,5 with possible implications for autoimmune adaptive responses. Another possibility is a described difference in resistance to apoptosis, where CD56bright NK cells survive better in oxidant-rich environments.20,21 It is noteworthy that the increased proportion of CD56bright NK cells is not just a consequence of any autoimmune disease affecting the kidneys or the joints, because patients with IgAN and RA showed a different pattern.
Whether NK cells are disease promoting or provide protection from autoimmune disease is not clear, and this seems to depend on the particular disease studied. It is also conceivable that NK cells exert different functions depending on disease stage, i.e. in the initiation of disease processes compared with the perpetuation of inflammation (reviewed by Johansson et al.).22 Patients with SLE23 as well as the lpr mouse model for SLE,24 exhibit low NK cell activity which may be partly explained by a low expression of the adaptor protein DAP12 in SLE patients.25 Furthermore, an association in time between disease development and low NK cell activity has been described in the lpr mouse.26 In a mouse model of another B-cell-dependent autoimmune disease, experimental autoimmune myasthenia gravis (EAMG), Shi et al. showed that depletion of NK cells during the priming phase protected from disease development by reducing IFN-γ production by CD4+ T cells and increasing production of the anti-inflammatory cytokine transforming growth factor-β, while levels of pathogenic antibodies decreased.27
The connection between the accumulation of CD56bright NK cells and IFN stimulation in vitro is of particular interest because IFN has been implicated in SLE (reviewed by Rönnblom et al.).1 Serum levels of IFN are increased in patients with SLE28 and overproduction of IFN induces lupus-like manifestations.29 Furthermore, 129Sv mice deficient for interferon alpha receptor (IFNAR) are protected from experimental lupus30 and IFN-α therapy for unrelated disorders is associated with autoimmune manifestations, including lupus-like symptoms.31 We hypothesized that the SLE-associated increase in CD56bright NK cell proportion described here could be induced by high IFN-α levels. Interestingly, we found that IFN could increase the proportion of CD56bright NK cells in vitro, but that this was the result of neither an increased proliferation of CD56bright NK cells nor the death of CD56dim NK cells in cell cultures. We conclude that the IFN-induced increase of the number of CD56bright NK cells is the result of phenotypic changes. This could either be a result of a decreased rate of maturation from CD56bright to CD56dim NK cells, or of an increased rate of change from the CD56dim to the CD56bright NK cell phenotype. Considering the described ability of CD56bright NK cells to develop into CD56dim cells,17 we favour the first mentioned possibility.
We analysed IFN-α serum levels in our patient groups and found the levels to be increased in the SLE patients with active disease, while the group of patients with inactive SLE had undetectable levels. Hence, there is no straightforward correlation between increased serum levels of IFN-α and the accumulation or CD56bright NK cells even if some of the patients with heightened CD56bright counts were patients with active disease. PBMC from SLE patients with undetectable IFN serum levels display increased levels of the IFN-α-inducible protein MxA, which is indicative of in vivo IFN-α production.32 It is therefore possible that low levels of IFN-α, undectectable in serum by our method, induce the increase in proportion of CD56bright NK cells in SLE described here. The abundance of CD56bright NK cells in SLE, a cell type with high cytokine-secreting ability, may play a role in the pathology of SLE. It is intriguing to speculate on a genetic rather than a disease-associated cause of this accumulation of potentially proinflammatory NK cells, which may contribute to the risk of developing SLE.
Acknowledgments
Mathula Thangarajh is acknowledged for her enthusiasm. The project was supported by funds from the Swedish Medical Research Council, the King Gustav the V's 80th birthday fund, Karolinska Institutet, the Swedish Association against Rheumatism and the Swedish Foundation for Strategic Research.
References
- 1.Rönnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–20. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 2.Vallin H, Perers A, Alm GV, Rönnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol. 1999;163:6306–13. [PubMed] [Google Scholar]
- 3.Barrat FJ, Meeker T, Gregorio J, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–9. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–29. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 6.Blanca IR, Bere EW, Young HA, Ortaldo JR. Human B cell activation by autologous NK cells is regulated by CD40–CD40 ligand interaction: role of memory B cells and CD5+ B cells. J Immunol. 2001;167:6132–9. doi: 10.4049/jimmunol.167.11.6132. [DOI] [PubMed] [Google Scholar]
- 7.Gao N, Dang T, Yuan D. IFN-gamma-dependent and -independent initiation of switch recombination by NK cells. J Immunol. 2001;167:2011–8. doi: 10.4049/jimmunol.167.4.2011. [DOI] [PubMed] [Google Scholar]
- 8.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 9.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 10.Vallin H, Blomberg S, Alm GV, Cederblad B, Rönnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin Exp Immunol. 1999;115:196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–91. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]
- 13.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saraste M, Irjala H, Airas L. Expansion of CD56bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci. 2007;28:121–6. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- 15.Titanji K, Sammicheli S, De Milito A, et al. Altered distribution of natural killer cell subsets identified by CD56, CD27 and CD70 in primary and chronic human immunodeficiency virus-1 infection. Immunology. 2008;123:164–70. doi: 10.1111/j.1365-2567.2007.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 17.Chan A, Hong DL, Atzberger A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 18.Romagnani C, Juelke K, Falco M, et al. CD56bright D16− killer Ig-like receptor NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol. 2007;178:4947–55. doi: 10.4049/jimmunol.178.8.4947. [DOI] [PubMed] [Google Scholar]
- 19.Freud AG, Becknell B, Roychowdhury S, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Thoren FB, Romero AI, Hermodsson S, Hellstrand K. The CD16−/CD56bright subset of NK cells is resistant to oxidant-induced cell death. J Immunol. 2007;179:781–5. doi: 10.4049/jimmunol.179.2.781. [DOI] [PubMed] [Google Scholar]
- 21.Harlin H, Hanson M, Johansson CC, Sakurai D, Poschke I, Norell H, Malmberg KJ, Kiessling R. The CD16− CD56(bright) NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16+ CD56(dim) subset. J Immunol. 2007;179:4513–9. doi: 10.4049/jimmunol.179.7.4513. [DOI] [PubMed] [Google Scholar]
- 22.Johansson S, Berg L, Hall H, Hoglund P. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26:613–8. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Erkeller-Yusel F, Hulstaart F, Hannet I, Isenberg D, Lydyard P. Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus. 1993;2:227–31. doi: 10.1177/096120339300200404. [DOI] [PubMed] [Google Scholar]
- 24.Pan LZ, Dauphinee MJ, Ansar Ahmed S, Talal N. Altered natural killer and natural cytotoxic cellular activities in lpr mice. Scand J Immunol. 1986;23:415–23. doi: 10.1111/j.1365-3083.1986.tb03073.x. [DOI] [PubMed] [Google Scholar]
- 25.Toyabe S, Kaneko U, Uchiyama M. Decreased DAP12 expression in natural killer lymphocytes from patients with systemic lupus erythematosus is associated with increased transcript mutations. J Autoimmun. 2004;23:371–8. doi: 10.1016/j.jaut.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–64. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi FD, Wang HB, Li H, Hong S, Taniguchi M, Link H, Van Kaer L, Ljunggren HG. Natural killer cells determine the outcome of B cell-mediated autoimmunity. Nat Immunol. 2000;1:245–51. doi: 10.1038/79792. [DOI] [PubMed] [Google Scholar]
- 28.Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science (New York, NY) 1982;216:429–31. doi: 10.1126/science.6176024. [DOI] [PubMed] [Google Scholar]
- 29.Zhuang H, Kosboth M, Lee P, et al. Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 2006;54:1573–9. doi: 10.1002/art.21800. [DOI] [PubMed] [Google Scholar]
- 30.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–88. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rönnblom LE, Alm GV, Oberg K. Autoimmune phenomena in patients with malignant carcinoid tumors during interferon-alpha treatment. Acta Oncol (Stockholm, Sweden) 1991;30:537–40. doi: 10.3109/02841869109092414. [DOI] [PubMed] [Google Scholar]
- 32.von Wussow P, Jakschies D, Hochkeppel H, Horisberger M, Hartung K, Deicher H. MX homologous protein in mononuclear cells from patients with systemic lupus erythematosus. Arthritis Rheum. 1989;32:914–8. [PubMed] [Google Scholar]