Abstract
Man has moved rapidly from the hunter–gatherer environment to the living conditions of the rich industrialized countries. The hygiene hypothesis suggests that the resulting changed and reduced pattern of exposure to microorganisms has led to disordered regulation of the immune system, and hence to increases in certain inflammatory disorders. The concept began with the allergic disorders, but there are now good reasons for extending it to autoimmunity, inflammatory bowel disease, neuroinflammatory disorders, atherosclerosis, depression associated with raised inflammatory cytokines, and some cancers. This review discusses these possibilities in the context of Darwinian medicine, which uses knowledge of evolution to cast light on human diseases. The Darwinian approach enables one to correctly identify some of the organisms that are important for the ‘Hygiene’ or ‘Old Friends’ hypothesis, and to point to the potential exploitation of these organisms or their components in novel types of prophylaxis with applications in several branches of medicine.
Keywords: evolution, hygiene hypothesis, immunoregulation, inflammation, interleukin-10
Introduction
Several categories of chronic inflammatory disorder have become much more prevalent in developed countries.1 The ‘hygiene hypothesis’ suggests that some of this increased prevalence is the result of defective regulation of the immune system resulting from diminished exposure to some classes of microorganism. During the 1980s and 1990s both Strachan and Matricardi and their colleagues observed that having many siblings, especially older ones, was correlated with a diminished risk of hay fever. These findings were considered consistent with a protective influence of postnatal infection that might be lost in the presence of modern hygiene.2–4 So the ‘hygiene hypothesis’ was born. Subsequent studies correlated protection with exposure to cowsheds,5 endotoxin,6 helminths7 and lactobacilli,8 thereby consolidating the view that microorganisms or their components were a crucial factor. Nevertheless, the concept was initially vague and lacked mechanistic explanations, so in the 28 years since Strachan's original study a multitude of different, sometimes overlapping, often mutually exclusive versions of this hypothesis have been considered. From time to time this has led to the ‘disproving’ of hypotheses or mechanisms that few had intended to propose in the first place.
This review approaches the issue from a different angle; Darwinian Medicine. It was Theodosius Dobzhansky who first made the now famous statement that ‘Nothing in biology makes sense except in the light of evolution’.9 This type of thinking is rapidly enhancing our understanding of all branches of medicine,10 and is particularly relevant to the hygiene hypothesis.
Environment of evolutionary adaptedness
The title of this section is taken from a book first published in 1969 by John Bowlby, who was concerned that those aspects of human behaviour that are genetically determined (such as instincts) might be adapted to the hunter–gatherer existence rather than to modern city life.11 The basis for this was the view that since the start of agriculture and pastoralism about 10 000 years ago, human adaptation to new environments has been cultural and technological rather than genetic. Interestingly, human genetic diversity appears to be increasing more rapidly than ever before, which might seem discordant with this view.12 However, this increase is mostly the result of the population explosion, rather than of adaptation to specific environments. For example, we have not adapted genetically to living in cold places: we have learnt to make fur coats. Humans easily detect problems with the physical environment and invent appropriate technological adaptations. We deal with excessive heat, cold, light, dark, water, drought etc. by technology. However, there are two physiological systems where we lack conscious awareness that there is something wrong, so we fail to seek technological solutions. For example, only since Bowlby have we been wondering if the brain is fully adapted to the modern social environment. The immune system, like the brain, is a learning system that requires the inputs that it has evolved to receive. Only since the hygiene hypothesis appeared have we been wondering if the immune systems of people living in clean modern cities are receiving the appropriate inputs.
Evolution turns the inevitable into a necessity
The human ‘Environment of evolutionary adaptedness’ is the hunter–gatherer environment. Does this allow us to define the microbial inputs that our immune systems have evolved to ‘expect’? This is a complex issue and views are changing rapidly.13,14 Here we discuss only a few organisms known to be relevant to the hygiene hypothesis.
First, there are harmless environmental organisms that will have been present throughout hominid (and indeed mammalian) evolution. These include commensal organisms, and also ‘pseudo-commensals’ (i.e. always present, but not actually replicating in the host) associated with rotting vegetation, soil and water supplies, such as lactobacilli and many actinomycetes including saprophytic mycobacteria.
Second, there are helminths. It used to be thought that man picked up helminths from his domesticated animals. If this were true they would not have impacted on the human genome until domestication began about 10 000 years ago. This represents about 500 generations, which is long enough to lead to changes in gene frequencies if selection pressure is very high. For example, farmers needed to be able to digest the lactose in milk from domesticated animals, and the frequency of relevant mutations in the gene encoding lactase has reached more than 90% in some populations.15 Nevertheless one might doubt whether 500 generations are enough for the presence of helminths to become a physiological requirement; for the inevitable to become a necessity (to paraphrase Jacques Monod). However, this is no longer an issue. Early hominids already carried some helminths,13 presumably inherited from ape-like ancestors, and detailed analysis of helminth genomes now suggests that more than one million years ago hominids had already picked up further species by sharing carrion with other scavenging mammals such as hyenas. Hominids shared the carnivore position in the helminth life cycle, and much later man passed the infection to his domesticated animals, so establishing the helminth strains and life cycles we see today.14
Interestingly, the groups of organisms (environmental saprophytes and helminths) that man and his ancestors encountered continuously and in large quantities for millions of years, are among those that are depleted from the modern environment,16 and have been shown to be relevant to immunoregulation, as discussed in detail later.
Childhood virus infections
In sharp contrast, despite the observations on family size and birth order that gave impetus to the hygiene hypothesis, childhood virus infections seem less relevant. Domesticated and peri-domesticated animals are said to harbour approximately 184 different zoonotic diseases, so from about 10 000 years ago man will have increasingly encountered these, particularly viruses. Diseases that emerged during this period include influenza, measles, mumps and smallpox.13 However, until recently, human populations were too small to sustain these as endemic infections, so it is theoretically improbable that they became physiological necessities. As might be expected, therefore, the common virus infections of childhood (measles, mumps, rubella, chickenpox, cytomegalovirus and herpes simplex virus type 1) do not protect from allergic disorders.17,18 Most strikingly, children in daycare centres do not have an increased risk of atopy if they wash more often and reduce their infection rate.19
Mechanisms of protection from disorders of the immune system
Why might microorganisms such as helminths and some environmental saprophytes be essential for the correct functioning of the immune system? Several mechanisms are probably involved.
Idiosyncratic biological effects of hepatitis A virus
Although most childhood virus infections do not protect from allergic disorders,17–19 there is good evidence that exposure to infections transmitted by the orofaecal route has a protective role.18,20 This might be largely attributable to hepatitis A virus (HAV).21 The receptor for HAV on human lymphocytes is TIM-1, which is involved in the regulation of T-cell subsets, including regulatory T cells (Tregs) and T helper type 2 (Th2) cells.22 Exposure to HAV might selectively remove Th2 cells, or alter the balance of T-cell subsets.21,22 Before 1975 the incidence of infection with HAV approached 100% in the general population, but it has declined rapidly over the past two decades so HAV might explain the original observations of Strachan.2
Th1/Th2 balance; a weak hypothesis
The HAV might affect effector/regulator balance, or Th1/Th2 balance. Initially an imbalance between Th1 and Th2 was thought to explain all the epidemiology underlying the hygiene hypothesis. The idea was that lack of infections driving Th1 was leading to overproduction of Th2 cells. This was never a strong theory. First, Th1 cytokines such as interferon-γ are present in large quantities both in asthma23 and in established atopic dermatitis.24 Second, profound defects in the interleukin-12 (IL-12) or interferon-γ (Th1) pathways do not lead to an increased incidence or severity of allergic disorders, implying that in man Th1 is not a physiological regulator of Th2 responses.25 Third, superimposing polarized Th1 cells onto a Th2-mediated inflammatory site can lead to synergistic inflammation rather than to downregulation of immunopathology.26 In any case, the Th1/Th2 balance hypothesis has been untenable since as early as 1998,27 by which time it had been well-documented that there was a simultaneous increase in Th1-mediated (or perhaps Th17-mediated) chronic inflammatory diseases (type 1 diabetes, multiple sclerosis, inflammatory bowel disease),1 occurring in the same countries as the increases in allergic disorders.28 Moreover, individuals infected by helminths, which enhance Th2 responses, are paradoxically less likely to have allergic sensitization or allergic disorders, and treating the infection leads to increased allergic sensitization.7 The Th1/Th2 balance hypothesis has therefore been more or less abandoned (except in relation to HAV), and the mechanisms discussed below have taken its place.
Induction of immunoregulatory circuits
The points outlined in the previous section suggest that the critical problem is not Th1/Th2 balance, but rather an increasing failure in the rich, developed countries of immunoregulatory mechanisms that should terminate inappropriate inflammatory responses, whether Th1 (or Th17) or Th2, while allowing essential responses to proceed.
In support of this concept immunoregulation has been shown to be faulty in individuals suffering from allergic disorders,29 and some autoimmune diseases,30,31 and probably also in inflammatory bowel disease.32,33
It is clear that a failure of immunoregulatory mechanisms can indeed lead to simultaneous increases in diverse types of pathology, because genetic defects of Foxp3, a transcription factor that plays a crucial role in the development and function of Treg cells, leads to a syndrome known as X-linked autoimmunity–allergic dysregulation syndrome (XLAAD), which includes aspects of allergy, autoimmunity and enteropathy.34
Old friends hypothesis
The component of the hygiene hypothesis that implicates faulty induction of immunoregulation has been designated ‘The Old Friends’ hypothesis. The suggestion is that the environmental saprophytes (including mycobacteria and lactobacilli) needed to be tolerated by the immune system because they were harmless but always present in large numbers in food and water (i.e. ‘pseudo-commensals’). Similarly, the helminthic parasites needed to be tolerated because, although not always harmless, once they were established in the host any effort by the immune system to eliminate them was likely to cause tissue damage. For instance, a futile effort to destroy Brugia malayi microfilariae results in lymphatic blockage and elephantiasis.35
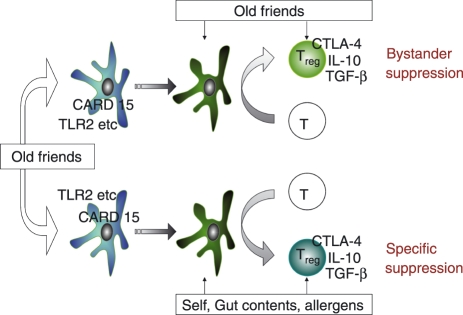
A cartoon of the pathway by which these organisms are currently thought to prime immunoregulation and mediate protection from allergies, autoimmunity and inflammatory bowel disease is shown in Fig. 1. The host–parasite relationship evolved so that rather than provoking needless damaging aggressive immune responses, these organisms cause a pattern of maturation of dendritic cells such that these drive Treg cells rather than Th1 or Th2 effector cells.36,37 This in turn leads to two mechanisms that help to control inappropriate inflammation. First, the constitutive presence of the ‘Old Friends’ causes continuous background activation of the regulatory dendritic cells and of Treg cells specific for the Old Friends themselves, resulting in constant background bystander suppression of inflammatory responses. Second, these regulatory dendritic cells inevitably sample self, gut contents and allergens, and so induce Treg cells specific for the illicit target antigens of the three groups of chronic inflammatory disorders. A striking example of this effect is provided by a recent experiment of nature. Patients in Argentina suffering from multiple sclerosis were followed up for 4·6 years. It was found that those who developed parasite infections (which were not treated) had significantly fewer exacerbations than those who did not.38 Moreover, they also developed specific Treg cells that responded to myelin basic protein by releasing IL-10 and transforming growth factor-β (TGF-β). In other words, the presence of the parasite appeared to drive the development of Treg cells that recognized the autoantigen, and inhibited the disease process.
Figure 1.
The Old Friends Hypothesis. Organisms such as helminths and environmental saprophytes, that are part of mammalian evolutionary history (‘Old Friends’) and must be tolerated, are detected by pattern recognition receptors such as Toll-like receptor 2 (TLR2) and CARD15 on dendritic cells (DC). The DC mature into regulatory DC that drive regulatory T cell (Treg) responses to the antigens of these organisms. The continuing presence of these antigens in the gut flora, in food, or resident as parasites such as microfilariae, leads to continuous background release of regulatory cytokines from these Treg, exerting bystander suppression of other responses, as shown in the upper arm of the figure. Meanwhile the increased numbers of regulatory DC lead to increased processing by such DC of self antigens, gut content antigens and allergens, as shown in the lower arm of the figure. Therefore the numbers of Treg specifically triggered by these antigens is also increased, downregulating autoimmunity, inflammatory bowel disease and allergies respectively. In addition to this ability to prime regulatory pathways, microorganisms also provide adjuvants (such as inhaled endotoxin) that might enhance development and polarization of responses to some allergens and tumour antigens (see main text). CTLA-4, cytotoxic T-lymphocyte antigen 4; IL-10, interleukin-10; TGF-β, transforming growth factor-β.
The validity of this hypothetical model is further supported by clinical trials and experimental models in which exposure to microorganisms that were ubiquitous during mammalian evolutionary history, but are currently ‘missing’ from the environment in rich countries (or from animal units with Specific Pathogen-Free facilities), will treat allergy,39–41 autoimmunity42 or intestinal inflammation.43
Adjuvant effects
Another aspect of immunoregulation is adjuvanticity leading to enhanced responses rather than downregulation. Clearly these two facets of immunoregulation are closely related. The interplay between the two is particularly clear in relation to cancer. Microbial components such as endotoxin (lipopolysaccharide) are powerful adjuvants that can enhance immune responses. Workers in the cotton industry in Shanghai are heavily exposed to lipopolysaccharide, and they are reported to have reduced incidences of cancers of the oesophagus, stomach,44 breast45 and lung.46 Similarly, dairy workers, who are exposed to a multitude of microbial components including lipopolysaccharide, are also protected from lung cancer.47 In the 1970s a number of authors claimed that bacillus Clamette–Guérin (BCG) vaccination protected against leukaemia and other haematological tumours, although the validity of their findings remains uncertain.48 More recently it has been suggested that BCG or smallpox vaccine can protect against melanoma.49 This issue is not discussed further in this review. Below we discuss situations where the problem may be failure to terminate inflammation, rather than failure to initiate it.
Disorders of immunoregulation
We suggest therefore that the largest effect of diminished exposure to helminths and saprophytic bacteria (the Old Friends) on patterns of disease in developed countries is the increase in disorders of immunoregulation. In this section we point out that in addition to the allergic disorders, autoimmunity and inflammatory bowel disease, all of which have been considered in detail in relation to the ‘Old Friends’ hypothesis in the past,1,16 there are several other disorders that are also increasing, and that are likely to be exacerbated or made more common by a switch in the balance of anti-inflammatory to inflammatory mechanisms (Fig. 2). We do not understand all of the ways in which regulatory dendritic cells and Treg cells evoked by the ‘Old Friends’ block or terminate inflammatory responses. However, we know that the release of the anti-inflammatory cytokines, IL-10 and TGF-β is often involved,39,40 and much of what follows is based on data showing defective Treg-cell function, or defective release of IL-10 and TGF-β.
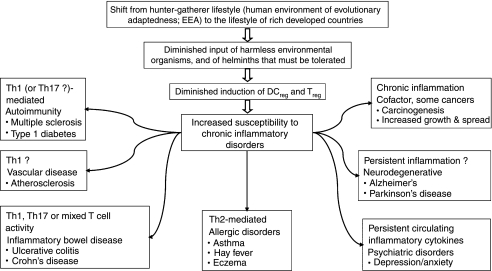
Figure 2.
Pathologies that are increasing, and that might be partly attributable to defective immunoregulation. Human evolution and physiology was shaped by the hunter–gatherer way of life, which is regarded as the human ‘Environment of Evolutionary Adaptedness’ (EEA). Despite increasing human genetic diversity, most human adaptation to novel environments has been cultural and technological rather than genetic, so a gene–environment misfit may be occurring, particularly in the immune system which is not linked to a conscious sensory modality that can warn us of problems. Harmless organisms that were abundant in food and water, and helminths that had to be tolerated, developed a role in the induction of immunoregulatory circuits. Without these there may be a failure to terminate inappropriate inflammatory responses, leading to an increased susceptibility to chronic inflammatory disorders, the precise nature of which depends on the genetics and history of the individual. These disorders may be mediated by T helper type 1 (Th1), Th17 or Th2 lymphocytes, or be mixed, as in ulcerative colitis and Alzheimer's disease, or in individuals with no obvious inflammatory pathology, who have persistently raised circulating cytokines that are associated with depression.
Cancer
In an earlier section we discussed the evidence that microbial components might provide adjuvants that enhance the immunogenicity of tumour antigens, and so abort development of some cancers. However, chronic inflammatory lesions, such as those induced by chronic infections (viruses, chlamydia or bacteria), asbestos, or chronic exposure to smoke or alcohol, are all associated with increased cancer risk.50 Oesophageal cancer provides a vivid example of the role of inflammation; reflux of gastric acid, alcohol and tobacco all predispose to it.51 This is partly because inflammatory mediators are involved in the control of cell replication, angiogenesis and cell migration, and they also drive increased levels of reactive oxygen intermediates that can cause DNA damage.50 Many of these functions of inflammation are regulated by the transcription factor nuclear factor-κB, and manipulating the activity of nuclear factor-κB has profound effects on tumorigenesis.52 Interestingly, tumour necrosis factor-α double-negative (TNF-α−/−) or TNF receptor 1 double-negative (TNFR1−/−) mice are more resistant to chemically induced carcinogenesis.50,53 Similarly, there are several single nucleotide polymorphisms (SNPs) of chemokines and cytokines that are associated with malignancy.50 Interestingly, a regular input of non-steroidal anti-inflammatory drugs such as aspirin that inhibit cyclooxygenase-2 (the inducible form of prostaglandin H synthase) is associated with reduced risk of colorectal cancer.54 It is therefore reasonable to suggest that diminished background immunoregulation in rich countries, as explained by the ‘Old Friends Hypothesis’, might explain some of the increase in certain cancers. This view is in no way incompatible with the view expressed earlier that transient adjuvant effects driving effector responses to tumour antigens can also be important.
Once the tumour is established the issue becomes more complex. A proinflammatory haplotype of SNPs in IL-6, IL-10 and TNF-α is associated with a poor prognosis in gastro-oesophageal malignancy,55 and it is clear that the inflammatory response can provide mediators that assist the growth and spread of the cancer.50 Nevertheless, it is obviously possible that in some situations excessively effective immunoregulation can impede the immune system's attempts to destroy the tumour cells.56
Atherosclerosis
The metabolic syndrome, which involves abdominal obesity, hypertriglyceridaemia, low high-density lipoprotein cholesterol and insulin resistance, has risen to a prevalence of 41% in New York.57 It has been observed recently that whereas women with uncomplicated obesity have increased serum levels of IL-10, those with the metabolic syndrome do not.58 Could this imply less regulatory cell activity? The hypothesis is strengthened by considering atherosclerosis, which is a T-cell-mediated inflammatory lesion in blood vessel walls, considerably more common in patients with the metabolic syndrome. Atherosclerotic plaques are inflammatory lesions driven mostly by Th1 cells.59 Several independent groups have found that IL-10 and TGF-β have a downregulatory effect on the development of atherosclerotic plaques.59 Atherosclerosis is exaggerated in IL-10-deficient mice.60 By contrast, mice with transgenic T cells overexpressing IL-10 are protected from atherosclerosis,61 and in several experimental models the transfer of Treg cells will also inhibit atherosclerosis.62 Infection with Schistosoma mansoni inhibits atherogenesis in mice, and although the authors attributed this to effects on lipid metabolism, the induction of Treg cells is likely.63 Further evidence is reviewed by Kuiper et al.64
There is similar evidence that IL-10 has a beneficial role in human atherosclerotic plaques,59,65–67 and serum levels of IL-10 are reduced in patients with unstable angina.68 Interestingly atherosclerotic lesions contain a very low percentage of Treg cells as determined by direct immunohistochemistry.69
Alzheimer's disease
The neurodegenerative disorders, Alzheimer's disease (AD) and Parkinson's disease (PD) both appear to be mediated by inflammation.70 This is apparent both from the pathology70 and from the epidemiology. Japanese American men in Honolulu were examined 25 years after a blood sample was taken to measure C-reactive protein, using very sensitive assays (hsCRP). Raised C-reactive protein was associated with a higher incidence of AD and vascular dementia 25 years later.71 As mentioned earlier, the metabolic syndrome is itself associated with inflammatory cytokines, and a study in the USA yielded support for the hypothesis that the metabolic syndrome correlates with eventual cognitive impairment in the elderly, particularly in those with a high serum C-reactive protein and IL-6.72 Other studies have yielded preliminary evidence that AD is associated with SNP that lead to increased production of IL-673 or TNF.74 Moreover, a large meta-analysis concluded that prolonged intake of non-steroidal anti-inflammatory drugs can give some protection against AD.75,76
There is also some evidence that the neurodegenerative conditions are more common in individuals with SNP of their IL-10 genes that modulate the production of this cytokine. For instance, in an Italian population the presence of the -1082A allele was proposed as a genetic risk factor for AD.77 Other studies found similar associations, though not always with the same allele or haplotype.73,78,79 and a study based in Germany found no associations at all,80 so the matter remains unresolved.76 The role of IL-10 is equally unclear in relation to PD. Interleukin-10 SNPs were not related to sporadic PD in a Polish population,81 but a Swedish study that documented the -1082A/G SNP found that the age of onset of PD was delayed by 5 years in individuals who had two G alleles compared with individuals with two A alleles.82 Meanwhile, there is accumulating evidence that TGF-β might have anti-AD effects both because of its immunoregulatory and anti-inflammatory properties and because it enhances the clearance of amyloid-β.76
Depression and anxiety
Some stress-related psychiatric conditions, particularly depression and anxiety, are associated with markers of ongoing inflammation, even in the absence of any accompanying inflammatory disorder.83 Depressed subjects may have raised proinflammatory cytokines and evidence of an ongoing acute-phase response. Moreover, proinflammatory cytokines can induce depression, which is commonly seen in patients with cancer or hepatitis when they are treated with IL-2 or interferon-α. In these patients brain imaging shows a pattern similar to that which accompanies spontaneously occurring depression, and the depression can be treated with paroxetine, a serotonin reuptake inhibitor antidepressant.84 Similarly, there is evidence that depression can be associated with polymorphisms that lead to the overproduction of proinflammatory cytokines,85 while in sharp contrast, treatments that neutralize these cytokines can alleviate depression.86 A recent study with germ-free mice shows that the ratio of proinflammatory to anti-inflammatory cytokines also regulates nociception, and depression is often accompanied by exaggerated awareness of pain.87 Therefore some psychiatric disorders in developed countries might be attributable to failure of immunoregulatory circuits to terminate ongoing inflammatory responses, leading to prolonged ‘sickness behaviour’ and mood changes. This view is further supported by the fact that the depression is associated with low expression of TGF-β and IL-10 relative to the expression of proinflammatory cytokines.88,89 Moreover, antidepressants increase the secretion of IL-10.90 These issues have been extensively reviewed elsewhere.91
Recently unexpected improvements in mood were observed during clinical trials with an immunomodulatory vaccine that induces Treg cells. This led to an investigation of this material in a mouse model, and to the discovery that it activates a specific group of brain serotonergic neurons involved in the pathophysiology of mood disorders, and exerts a prozac-like effect in an industry standard test for antidepressant activity.91,92
Conclusions
This review attempts to show how focusing on major changes in lifestyle that accompany the shift from hunter–gatherer to industrialized society, passing via herding and farming, can lead to a hypothesis that falls within Darwinian Medicine and has considerable explanatory power. It is clear that multiple environmental changes must have contributed to changing patterns of disease, and this brief overview does not intend to imply that diminished input of helminths and harmless environmental saprophytes is the only factor. Nor does it claim that all the links suggested above are definitively proven. However, in relation to allergic disorders the hypothesis is supported by epidemiology, experimental models and therapeutic trials, and backed up by the identification of relevant pathways and gene–environment interactions. Therefore, in view of man's unique ability to adapt to novel environments by means of culture and technology, faster than he can adapt by genetic change, it is logical to anticipate manifestations of gene–environment misfit in other disease contexts. The list of disorders discussed here is illustrative, and others might need to be added. It is clear that this area is worth exploring in detail because unravelling the mechanism of action of the ‘Old Friends’ at the molecular level might lead to new drugs for prophylaxis and treatment in many areas of medicine.
References
- 1.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 2.Strachan DP. Hay fever, hygiene, and household size. Br Med J. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan DP, Taylor EM, Carpenter RG. Family structure, neonatal infection, and hay fever in adolescence. Arch Dis Child. 1996;74:422–6. doi: 10.1136/adc.74.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matricardi PM, Franzinelli F, Franco A, et al. Sibship size, birth order, and atopy in 11,371 Italian young men. J Allergy Clin Immunol. 1998;101:439–44. doi: 10.1016/s0091-6749(98)70350-1. [DOI] [PubMed] [Google Scholar]
- 5.Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 6.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347:869–77. doi: 10.1056/NEJMoa020057. [DOI] [PubMed] [Google Scholar]
- 7.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 8.Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6. doi: 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 9.Dobzhansky T. Biology, molecular and organismic. Am Zool. 1964;4:443–52. doi: 10.1093/icb/4.4.443. [DOI] [PubMed] [Google Scholar]
- 10.Nesse R. Evolution and Medicine. Henry Stewart Talks 2007. (Audiovisual presentations by multiple authors); Available at: http://www.hstalks.com/evomed/
- 11.Bowlby J. Attachment and loss, Volume 1: Attachment. Harmondsworth, Middlesex: Penguin; 1971. [Google Scholar]
- 12.Hawks J, Wang ET, Cochran GM, Harpending HC, Moyzis RK. Recent acceleration of human adaptive evolution. Proc Natl Acad Sci USA. 2007;104:20753–8. doi: 10.1073/pnas.0707650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armelagos GJ, Harper KN. Genomics at the origins of agriculture, part two; evolutionary anthropology. Evol Anthropol. 2005;14:109–21. [Google Scholar]
- 14.Hoberg EP. Phylogeny of Taenia: species definitions and origins of human parasites. Parasitol Int. 2006;55(Suppl):S23–30. doi: 10.1016/j.parint.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Tishkoff SA, Reed FA, Ranciaro A, et al. Convergent adaptation of human lactase persistence in Africa and Europe. Nat Genet. 2007;39:31–40. doi: 10.1038/ng1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rook GA, Adams V, Hunt J, Palmer R, Martinelli R, Brunet LR. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin Immunopathol. 2004;25:237–55. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- 17.Benn CS, Melbye M, Wohlfahrt J, Bjorksten B, Aaby P. Cohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of life. BMJ. 2004;328:1223–7. doi: 10.1136/bmj.38069.512245.FE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matricardi PM, Rosmini F, Riondino S, Fortini M, Ferrigno L, Rapicetta M, Bonini S. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma; epidemiological study. Br Med J. 2000;320:412–7. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunder T, Tapiainen T, Pokka T, Uhari M. Infections in child day care centers and later development of asthma, allergic rhinitis, and atopic dermatitis: prospective follow-up survey 12 years after controlled randomized hygiene intervention. Arch Pediatr Adolesc Med. 2007;161:972–7. doi: 10.1001/archpedi.161.10.972. [DOI] [PubMed] [Google Scholar]
- 20.Matricardi PM, Rosmini F, Panetta V, Ferrigno L, Bonini S. Hay fever and asthma in relation to markers of infection in the United States. J Allergy Clin Immunol. 2002;110:381–7. doi: 10.1067/mai.2002.126658. [DOI] [PubMed] [Google Scholar]
- 21.McIntire JJ, Umetsu SE, Macaubas C, et al. Immunology: hepatitis A virus link to atopic disease. Nature. 2003;425:576. doi: 10.1038/425576a. [DOI] [PubMed] [Google Scholar]
- 22.Degauque N, Mariat C, Kenny J, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2007;118:735–41. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug N, Madden J, Redington AE, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Resp Cell Mol Biol. 1996;14:319–26. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 24.Klunker S, Trautmann A, Akdis M, Verhagen J, Schmid-Grendelmeier P, Blaser K, Akdis CA. A second step of chemotaxis after transendothelial migration: keratinocytes undergoing apoptosis release IFN-gamma-inducible protein 10, monokine induced by IFN-gamma, and IFN-gamma-inducible alpha-chemoattractant for T cell chemotaxis toward epidermis in atopic dermatitis. J Immunol. 2003;171:1078–84. doi: 10.4049/jimmunol.171.2.1078. [DOI] [PubMed] [Google Scholar]
- 25.Lammas DA, Casanova JL, Kumararatne DS. Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-gamma) pathway. Clin Exp Immunol. 2000;121:417–25. doi: 10.1046/j.1365-2249.2000.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–83. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rook GAW, Stanford JL. Give us this day our daily germs. Immunol Today. 1998;19:113–16. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- 28.Stene LC, Nafstad P. Relation between occurrence of type 1 diabetes and asthma. Lancet. 2001;357:607. doi: 10.1016/S0140-6736(00)04067-8. [DOI] [PubMed] [Google Scholar]
- 29.Akdis M, Verhagen J, Taylor A, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kriegel MA, Lohmann T, Gabler C, Blank N, Kalden JR, Lorenz HM. Defective suppressor function of human CD4+ CD25+ regulatory T cells in autoimmune polyglandular syndrome type II. J Exp Med. 2004;199:1285–91. doi: 10.1084/jem.20032158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771–8. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 33.Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–8. [PubMed] [Google Scholar]
- 34.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–56. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 36.van der Kleij D, Latz E, Brouwers JF, et al. A novel host–parasite lipid cross-talk schistosomal lyso-phosphatidylserine activates Toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 37.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260–7. doi: 10.1016/j.jaci.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 38.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 39.Zuany-Amorim C, Sawicka E, Manlius C, et al. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002;8:625–9. doi: 10.1038/nm0602-625. [DOI] [PubMed] [Google Scholar]
- 40.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricklin-Gutzwiller ME, Reist M, Peel JE, Seewald W, Brunet LR, Roosje PJ. Intradermal injection of heat-killed Mycobacterium vaccae in dogs with atopic dermatitis: a multicentre pilot study. Vet Dermatol. 2007;18:87–93. doi: 10.1111/j.1365-3164.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 42.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 43.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wernli KJ, Fitzgibbons ED, Ray RM, et al. Occupational risk factors for esophageal and stomach cancers among female textile workers in Shanghai, China. Am J Epidemiol. 2006;163:717–25. doi: 10.1093/aje/kwj091. [DOI] [PubMed] [Google Scholar]
- 45.Ray RM, Gao DL, Li W, et al. Occupational exposures and breast cancer among women textile workers in Shanghai. Epidemiology. 2007;18:383–92. doi: 10.1097/01.ede.0000259984.40934.ae. [DOI] [PubMed] [Google Scholar]
- 46.Astrakianakis G, Seixas NS, Ray R, et al. Lung cancer risk among female textile workers exposed to endotoxin. J Natl Cancer Inst. 2007;99:357–64. doi: 10.1093/jnci/djk063. [DOI] [PubMed] [Google Scholar]
- 47.Mastrangelo G, Grange JM, Fadda E, Fedeli U, Buja A, Lange JH. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol. 2005;161:1037–46. doi: 10.1093/aje/kwi138. [DOI] [PubMed] [Google Scholar]
- 48.Hoover RN. Bacillus Calmette–Guérin vaccination and cancer prevention: a critical review of the human experience. Cancer Res. 1976;36:652–4. [PubMed] [Google Scholar]
- 49.Krone B, Kolmel KF, Henz BM, Grange JM. Protection against melanoma by vaccination with Bacille Calmette–Guérin (BCG) and/or vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur J Cancer. 2005;41:104–17. doi: 10.1016/j.ejca.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 51.Murphy SJ, Anderson LA, Johnston BT, Fitzpatrick DA, Watson PR, Monaghan P, Murray LJ. Have patients with esophagitis got an increased risk of adenocarcinoma? Results from a population-based study. World J Gastroenterol. World J Gastroenterol. 2005;11:7290–5. doi: 10.3748/wjg.v11.i46.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 53.Arnott CH, Scott KA, Moore RJ, Robinson SC, Thompson RG, Balkwill FR. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene. 2004;23:1902–10. doi: 10.1038/sj.onc.1207317. [DOI] [PubMed] [Google Scholar]
- 54.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–13. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 55.Deans C, Rose-Zerilli M, Wigmore S, Ross J, Howell M, Jackson A, Grimble R, Fearon K. Host cytokine genotype is related to adverse prognosis and systemic inflammation in gastro-oesophageal cancer. Ann Surg Oncol. 2007;14:329–39. doi: 10.1245/s10434-006-9122-9. [DOI] [PubMed] [Google Scholar]
- 56.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Rundek T, White H, Boden-Albala B, Jin Z, Elkind MS, Sacco RL. The metabolic syndrome and subclinical carotid atherosclerosis: the Northern Manhattan study. J Cardiometab Syndr. 2007;2:24–9. doi: 10.1111/j.1559-4564.2007.06358.x. [DOI] [PubMed] [Google Scholar]
- 58.Esposito K, Pontillo A, Giugliano F, Giugliano G, Marfella R, Nicoletti G, Giugliano D. Association of low interleukin-10 levels with the metabolic syndrome in obese women. J Clin Endocrinol Metab. 2003;88:1055–8. doi: 10.1210/jc.2002-021437. [DOI] [PubMed] [Google Scholar]
- 59.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc Med. 2007;17:113–18. doi: 10.1016/j.tcm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- 61.Pinderski LJ, Fischbein MP, Subbanagounder G, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–71. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 62.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 63.Doenhoff MJ, Stanley RG, Griffiths K, Jackson CL. An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology. 2002;125:415–21. doi: 10.1017/s0031182002002275. [DOI] [PubMed] [Google Scholar]
- 64.Kuiper J, van Puijvelde GH, van Wanrooij EJ, van Es T, Habets K, Hauer AD, van den Berkel TJ. Immunomodulation of the inflammatory response in atherosclerosis. Curr Opin Lipidol. 2007;18:521–6. doi: 10.1097/MOL.0b013e3282efd0d4. [DOI] [PubMed] [Google Scholar]
- 65.Uyemura K, Demer LL, Castle SC, et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J Clin Invest. 1996;97:2130–8. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mallat Z, Heymes C, Ohan J, Faggin E, Leseche G, Tedgui A. Expression of interleukin-10 in advanced human atherosclerotic plaques: relation to inducible nitric oxide synthase expression and cell death. Arterioscler Thromb Vasc Biol. 1999;19:611–6. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- 67.Pinderski Oslund LJ, Hedrick CC, Olvera T, Hagenbaugh A, Territo M, Berliner JA, Fyfe AI. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler Thromb Vasc Biol. 1999;19:2847–53. doi: 10.1161/01.atv.19.12.2847. [DOI] [PubMed] [Google Scholar]
- 68.Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC. Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation. 2001;104:746–9. doi: 10.1161/hc3201.094973. [DOI] [PubMed] [Google Scholar]
- 69.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS ONE. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A. Neuroinflammation in Alzheimer's disease and Parkinson's disease: are microglia pathogenic in either disorder? Int Rev Neurobiol. 2007;82:235–46. doi: 10.1016/S0074-7742(07)82012-5. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–74. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 72.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 73.Arosio B, Trabattoni D, Galimberti L, et al. Interleukin-10 and interleukin-6 gene polymorphisms as risk factors for Alzheimer's disease. Neurobiol Aging. 2004;25:1009–15. doi: 10.1016/j.neurobiolaging.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Ramos EM, Lin MT, Larson EB, et al. Tumor necrosis factor alpha and interleukin 10 promoter region polymorphisms and risk of late-onset Alzheimer disease. Arch Neurol. 2006;63:1165–9. doi: 10.1001/archneur.63.8.1165. [DOI] [PubMed] [Google Scholar]
- 75.Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer's disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–15. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 77.Lio D, Licastro F, Scola L, et al. Interleukin-10 promoter polymorphism in sporadic Alzheimer's disease. Genes Immun. 2003;4:234–8. doi: 10.1038/sj.gene.6363964. [DOI] [PubMed] [Google Scholar]
- 78.Bagnoli S, Cellini E, Tedde A, Nacmias B, Piacentini S, Bessi V, Bracco L, Sorbi S. Association of IL10 promoter polymorphism in Italian Alzheimer's disease. Neurosci Lett. 2007;418:262–5. doi: 10.1016/j.neulet.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 79.Ma SL, Tang NL, Lam LC, Chiu HF. The association between promoter polymorphism of the interleukin-10 gene and Alzheimer's disease. Neurobiol Aging. 2005;26:1005–10. doi: 10.1016/j.neurobiolaging.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 80.Depboylu C, Du Y, Muller U, et al. Lack of association of interleukin-10 promoter region polymorphisms with Alzheimer's disease. Neurosci Lett. 2003;342:132–4. doi: 10.1016/s0304-3940(03)00231-3. [DOI] [PubMed] [Google Scholar]
- 81.Bialecka M, Klodowska-Duda G, Kurzawski M, Slawek J, Opala G, Bialecki P, Safranow K, Drozdzik M. Interleukin-10 gene polymorphism in Parkinson's disease patients. Arch Med Res. 2007;38:858–63. doi: 10.1016/j.arcmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Hakansson A, Westberg L, Nilsson S, et al. Investigation of genes coding for inflammatory components in Parkinson's disease. Mov Disord. 2005;20:569–73. doi: 10.1002/mds.20378. [DOI] [PubMed] [Google Scholar]
- 83.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Musselman DL, Lawson DH, Gumnick JF, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 85.Wilson AG, Gordon C, di Giovine FS, de Vries N, van de Putte LB, Emery P, Duff GW. A genetic association between systemic lupus erythematosus and tumor necrosis factor alpha. Eur J Immunol. 1994;24:191–5. doi: 10.1002/eji.1830240130. [DOI] [PubMed] [Google Scholar]
- 86.Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm Bowel Dis. 2002;8:237–43. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 87.Amaral FA, Sachs D, Costa VV, et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc Natl Acad Sci USA. 2008;105:2193–7. doi: 10.1073/pnas.0711891105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Myint AM, Leonard BE, Steinbusch HW, Kim YK. Th1, Th2, and Th3 cytokine alterations in major depression. J Affect Disord. 2005;88:167–73. doi: 10.1016/j.jad.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Parissis JT, Adamopoulos S, Rigas A, Kostakis G, Karatzas D, Venetsanou K, Kremastinos DT. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. Am J Cardiol. 2004;94:1326–8. doi: 10.1016/j.amjcard.2004.07.127. [DOI] [PubMed] [Google Scholar]
- 90.Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 91.Rook GAW, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29:150–8. doi: 10.1016/j.it.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 92.Lowry CA, Hollis JH, de Vries A, et al. Identification of an immune-responsive mesolimbocortical serotonergic system: potential role in regulation of emotional behavior. Neuroscience. 2007;146:756–72. doi: 10.1016/j.neuroscience.2007.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]