Abstract
The development of type 1 diabetes is influenced by both genetic and environmental factors. The current rise in the incidence of diabetes is occurring more rapidly than can be accounted for by genetic change, highlighting the influence of environmental modifiers. Considerable effort has been expended to identify infectious agents that might be responsible for this rise in incidence, but no single infectious agent has been linked to this dramatic increase in type 1 diabetes. There has been increasing interest in the possibility that infections of historical importance that might have shaped our immune systems over evolutionary time may also have played a role in down-modulating some autoimmune and allergic disorders. In this review, some of the ways in which certain organisms might have influenced the onset of autoimmunity are discussed.
Keywords: autoimmunity, type 1 diabetes, T cells
Introduction
Type 1 diabetes (T1D) is an autoimmune disease in which the pancreatic beta cells are selectively destroyed by the cells of the immune system. The development of this autoimmune condition is influenced by both genetic and environmental factors, with the concordance rate for diabetes development in identical twins being around 40%.1 T1D is a disease of juvenile onset that was lethal before the 1920s, when Banting and Best discovered insulin and provided a means of replacing the hormone lost by autoimmune destruction of the pancreatic beta cell.2 Given the importance of genetic background in the predisposition to diabetes, this suggests that potentially lethal allelic variants of certain genes have been retained, either because they have historically conferred a strong selective advantage or because they are in linkage disequilibrium with advantageous alleles. Such alleles might have historically provided increased resistance to infection.
In humans, overt onset of T1D is manifest when 70% of the β cell mass has been destroyed and there is insufficient insulin to maintain glucose homeostasis. Thereafter, daily insulin injections are required for survival. There are a range of complications, some of which are life threatening, which can arise following onset of diabetes, including retinopathy, nephropathy and neuropathy. Considerable effort has been, and is being, expended to prevent the onset of this autoimmune disease as well as to reverse it once it has been initiated. In terms of pathogenesis, analysis of pancreatic biopsies from diabetic individuals has shown the presence of mononuclear infiltration comprising CD4+ T cells, CD8+ T cells, B cells and macrophages in and around the islet area.3 The incidence of this autoimmune disease4,5 and some others such as systemic lupus erythematosus (SLE) has been increasing dramatically in the developed world, and there has been great interest in identifying causative agents of these autoimmune disorders. In terms of T1D, the agents that have been examined include both dietary factors and infectious agents. Dietary modifiers that have been studied include cow's milk and food containing nitrites, nitrates or nitrosamine, but no conclusive evidence has been obtained that such agents play a role in causing T1D. Antibodies to cow's milk proteins have been detected in the serum of individuals with T1D, leading to the proposition that early introduction of cow's milk protein to the infant diet may play a role in development of this autoimmune disease. It has also been postulated that beta-lactoglobulin, which is present in cow's milk but not in human milk, may initiate an immune response in babies that is cross-reactive with glycodelin and that this response against glycodelin interferes with the ability of this molecule to modulate immune responses.6 Several studies have been carried out to test the hypothesis of a link between cow's milk products and diabetes, but the conclusions drawn have varied.7–11
It has also been postulated that certain infections might initiate an autoimmune response against the pancreatic beta cells. A viral aetiology for the development of T1D appeared to be compelling following the isolation of a virus from the pancreas of a diabetic patient and the ability of certain viral infections to initiate diabetes in mice or non-human primates.12–15 The apparent seasonal onset of T1D further supported the proposition that infection might precipitate diabetes onset. However, further understanding of the aetiology of diabetes revealed that autoimmunity is present many years before the clinical onset of disease, with autoantibodies providing surrogate markers of beta cell autoreactivity.16 Extensive searches for a common viral insult in mothers of and children with T1D have not been conclusive,17 and alternative explanations for the dramatic rise in diabetes has therefore been sought. One possible explanation that arose from experimental observations in animal models of T1D is that infection might have inhibited the development of diabetes.18,19 There have been dramatic changes in the developed world over the last century, with marked improvements in social and economic conditions. This has led to reduced exposure to a range of infectious agents and has dramatically altered the balance between our immune systems and infections of historical importance. It has been proposed that such a change in exposure to certain infectious agents might be responsible for the increase in diabetes and other autoimmune conditions such as SLE and multiple sclerosis (MS).4,18,20,21 The situation in autoimmunity parallels that seen in the field of allergy, where it has been called the ‘hygiene hypothesis’.22 This does not preclude the possibility that certain infections might precipitate some autoimmune conditions and indeed there are some conditions where this is clearly the case, for example in the reactive arthritides. However, there are now many examples from animal models of human autoimmune diseases where certain infections have been shown to modulate either the spontaneous onset or the experimental induction of autoimmunity. These are summarized in Table 1. On the basis of some of these findings, infections with live worms have been carried out in the treatment of inflammatory bowel diseases, with some evidence of improvement in the clinical condition.23 In terms of human studies, the finding that MS patients with parasitic infections had a less severe disease course than non-infected individuals within a patient cohort is of considerable interest and provides possible support for the hypothesis that infection might inhibit the development of, or ameliorate, autoimmune pathology.24
Table 1.
Infectious agents demonstrated to inhibit autoimmune pathology
| Autoimmune/ inflammatory disease | Infection/agents inhibiting pathology |
|---|---|
| Type 1 diabetes | Schistosoma mansoni (live infection, eggs, SEA, SWA)18,19Trichinella spiralis28Heligmosomoides polygyrus28Mycobacterium avium26Mycobacterium bovis56Salmonella typhimurium54 |
| Experimental autoimmune disease | Schistosoma mansoni (live infection, eggs) 57,58 |
| Encephalomyelitis | Mycobacterium bovis55Bordetella pertussis |
| Induced Graves' disease | Schistosoma mansoni59Mycobacterium bovis60 |
| Collagen-induced arthritis | Acanthocheilonema viteae (secreted product, ES-62)49Streptococcus sanguinis61Trypanosoma brucei62 |
| Inflammatory bowel disease | Trichinella spiralis63Schistosoma mansoni (eggs)64,65Heligmosomoides polygyrus66Hymenolepis diminuta67 |
| Crohn's disease | Trichurus suis68 |
SEA, soluble egg antigen; SWA, soluble worm antigen; ES-62, excretory-secretory product 62.
How might infection inhibit the development of autoimmunity?
The hypothesis as it applies to T1D is that certain infections of historical importance have shaped our immune systems to select for individuals who are able to survive the infection and do not develop a disproportionate response to the organism resulting in tissue pathology. It is also proposed that these infections would inhibit the development of T1D. With improved sanitation and living conditions, together with vaccination strategies, our exposure to infectious agents and development of disease has been markedly diminished. Therefore it is possible that an alteration of the environment over the last 60 years that might play a role in the increased incidence of T1D might be reduced exposure to infectious agents. In terms of infections that might have played such a role, analyses of coprolites and prehistoric mummies, such as the ‘ice man’ of the Otzal Alps and pre-Columbian mummies in Peru, have revealed the presence of a range of organisms to which these ancient ancestors were exposed, including mycobacteria and parasites. The hypothesis that infections with agents such as mycobacteria or helminth infections are able to inhibit the onset of T1D can be tested using robust animal models of human T1D. The NOD mouse spontaneously develops T1D and is widely used to investigate factors contributing to pancreatic β cell destruction.25 It has been shown that a mononuclear infiltrate appears in the pancreas when mice are around 5 weeks of age. As in humans with T1D, this infiltrate contains CD4+ T cells, CD8+ T cells, macrophages, dendritic cells and β cells. At around 10–12 weeks of age the infiltrate disrupts islet architecture, β cell destruction is observed and diabetes development occurs.
When NOD mice are infected with mycobacteria or helminths or exposed to products of these organisms, the spontaneous development of T1D is inhibited.18,26–28 It is now recognized that certain infectious agents, particularly those that chronically infect the host, induce immunoregulatory circuits. This regulation could not only dampen the host response against the pathogen but also reduce damage to host tissue. There is a range of ways in which this can be accomplished, and studies of the interactions between infectious agents and the immune system have highlighted several key pathways.
Infection of 4–5-week-old NOD mice with Schistosoma mansoni or injection of soluble egg antigen (SEA) or soluble worm antigen (SWA) from this helminth prevented the onset of diabetes. This protection was not seen if exposure to helminth antigens was delayed until the mice were 10 weeks of age. Helminth antigens are able to induce interleukin (IL)-10 production by dendritic cells and B cells as well as being able to induce alternatively activated macrophages, invariant natural killer T cells (iNKTs) and regulatory T cells.19,29–32 The exact way in which helminth antigens such as those from S. mansoni mediate these effects is under investigation by several groups. IL-10 and transforming growth factor (TGF)-β play immunomodulatory roles and are produced by cells of both the innate and the adaptive immune response. IL-10 is induced by several infectious agents and not only plays a role in delaying or inhibiting the host immune response but also in limiting tissue pathology.30,33–35 Its importance is underscored by the fact that surrogate IL-10 is encoded by some viruses. Exogenous administration of IL-10 has been shown to inhibit the development of diabetes in NOD mice and blockade of its receptor has been shown to accelerate the onset of diabetes.36
In the context of T1D, this cytokine and all of these cells have the potential to inhibit this Th1-mediated disease. NOD mice have been shown to have a deficiency in iNKT cells and increases in this cell type inhibit the development of diabetes.37–40 Glycolipids derived from S. mansoni activate this cell type and increase their numbers in NOD mice, providing a parasite-specific means of regulating the onset of diabetes.19,29 Apart from inducing IL-10 production by DCs, SEA has been shown to favour the generation of a Th2 response as well as being able to increase the proportional representation of regulatory T cells (Tregs).41–44 While such responses may limit host pathology and facilitate the complex life cycle of S. mansoni, they clearly also have the potential to modulate the onset of Th1-mediated autoimmune conditions. Little is known of the effect of S. mansoni infection on Th17-mediated autoimmune conditions.
The ability of the helminth products to skew the immune response to Th2 has been shown to extend to co-administered non-parasite antigens and even to influence the response to other infectious agents such as Leishmania major.45,46 Progress has been made in identifying components within the crude schistosome extracts that are involved in the skew towards Th2 and in mast cell recruitment.47 Identification of factors involved in the induction and expansion of Tregs could provide biomodulators of considerable therapeutic potential.
The effect of other infections on the onset of diabetes
Although many organisms or antigens derived from them have been shown be able to provide long-lasting prevention of T1D in NOD mice, it is important to recognize that this is not achieved with all infections nor with some products of parasites known to influence other autoimmune responses. Excretory-secretory glycoprotein (ES-62) is a phosphoryl choline containing a glycoprotein product of the filarial nematode Acanthocheilonema viteae, which has been shown to have profound immunomodulatory activities. This product is able to inhibit the onset of a range of inflammatory disorders, including collagen-induced arthritis,48,49 but to be unable to inhibit the spontaneous development of T1D in NOD mice (D. Thomas, A. Cooke and W. Harnett, unpublished data). Also, although some virus infections have been shown to inhibit the development of diabetes in NOD mice,50–52 infection with murine herpesvirus 68 (MHV68), a mouse gamma herpesvirus endowed with remarkable immunomodulatory strategies, is unable to provide long-term prevention of diabetes in NOD mice.53 The mechanisms by which some of these viruses prevent the onset of diabetes remain to be fully clarified, but in the case of coxsackievirus B4 were shown not to involve either IL-4 or interferon (IFN)-γ.51
Some bacterial infections have been shown to inhibit the development of diabetes in NOD mice. For example, infection of NOD mice with Salmonella typhimurium at around 10 weeks of age, relatively late in the progression towards the onset of diabetes, provides protection from the development of diabetes. As Salmonella infection elicits potent Th1 responses in NOD mice, and T cells from previously infected NOD mice were able to transfer diabetes to NOD recipients with severe combined immunodeficiency (SCID), this suggested protective mechanisms other than Th2 skewing or induction of Tregs, which were dominant in situ. Exploration of the mechanism of diabetes prevention identified a role for dendritic cells in modulating the trafficking of diabetogenic T cells to the pancreas in mice infected with S. typhimurium.54 Such effects of infection on trafficking have been noted in other autoimmune situations. It has been shown that infection with Mycobacterium bovis strain bacillus Calmette–Guerin (BCG) redirected the trafficking of T cells specific for myelin antigen away from the central nervous system and into granulomas, resulting in prevention of experimental autoimmune encephalomyelitis (EAE).55
Concluding comments
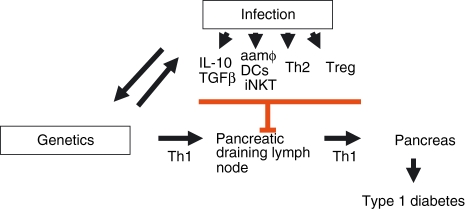
It has become clear from a range of studies that the spontaneous development of diabetes in NOD mice can be inhibited by several different infectious agents but not by all infections. The timing of infection is important, with some infections being able to inhibit the onset of diabetes only if they occur before there is substantial pancreatic infiltration. In the case of infection with S. typhimurium, in contrast, optimal protection from diabetes is achieved if infection occurs when there is an established infiltrate. In the context of infection with the helminth S. mansoni, it has become apparent that the mechanism of diabetes prevention may differ with different stages of the parasite life cycle and be mediated by a range of parasite-induced immunoregulatory processes. Some of the ways in which infectious agents may influence the onset of diabetes are represented in Figure 1.
Figure 1.
Infection can inhibit the development of type 1 diabetes through a range of mechanisms. The development of type 1 diabetes is influenced by several genes with MHC Class II playing a major role. Over time there has been an interplay between the genetics of the host and infectious agents leading to a mutual adaptation. Infections which induce immunoregulatory cytokines such as IL-10 and TGFβ are able to downmodulate inflammation and also reinforce regulatory T cell activity and induce iNKT cell activity. Alternatively activated macrophages and ‘tolerogenic’ dendritic cells have been shown to inhibit Th1 responses through a range of mechanisms including skewing of the response to Th2, inducing regulatory T cells and producing amino acid catabolising enzymes such as arginase or indoleamine 2,3-dioxygenase. The induction of such responses limits host tissue pathology and benefits both infectious agent and host. aamφ, alternatively activated macrophages; DC, dendritic cell; IL, interleukin; iNKT, invariant natural killer T cell; TGF, transforming growth factor; Treg, regulatory T cell; Th, T helper.
By identifying the ways in which infectious agents manipulate the host response and inhibit autoreactivity, it will become possible to develop novel therapeutic approaches that may not require infection with a live organism.
References
- 1.Redondo MJ, Fain PR, Eisenbarth GS. Genetics of type 1A diabetes. Recent Prog Horm Res. 2001;56:69–89. doi: 10.1210/rp.56.1.69. [DOI] [PubMed] [Google Scholar]
- 2.Banting FG, Best CH. The internal secretion of the pancreas. Lab Clin Med. 1922;7:465–80. [Google Scholar]
- 3.Imagawa A, Hanafusa T, Itoh N, et al. Immunological abnormalities in islets at diagnosis paralleled further deterioration of glycaemic control in patients with recent-onset Type I (insulin-dependent) diabetes mellitus. Diabetologia. 1999;42:574–8. doi: 10.1007/s001250051197. [DOI] [PubMed] [Google Scholar]
- 4.Dunne DW, Cooke A. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nat Rev Immunol. 2005;5:420–6. doi: 10.1038/nri1601. [DOI] [PubMed] [Google Scholar]
- 5.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes. 2002;51:3353–61. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 6.Goldfarb MF. Relation of time of introduction of cow milk protein to an infant and risk of type-1 diabetes mellitus. J Proteome Res. 2008;7:2165–7. doi: 10.1021/pr800041d. [DOI] [PubMed] [Google Scholar]
- 7.Fort P, Lanes R, Dahlem S, Recker B, Weyman-Daum M, Pugliese M, Lifshitz F. Breast feeding and insulin-dependent diabetes mellitus in children. J Am Coll Nutr. 1986;5:439–41. doi: 10.1080/07315724.1986.10720146. [DOI] [PubMed] [Google Scholar]
- 8.Martin JM, Trink B, Daneman D, Dosch HM, Robinson B. Milk proteins in the etiology of insulin-dependent diabetes mellitus (IDDM) Ann Med. 1991;23:447–52. doi: 10.3109/07853899109148088. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbauer J, Herzig P, Giani G. Early infant feeding and risk of type 1 diabetes mellitus-a nationwide population-based case-control study in pre-school children. Diabetes Metab Res Rev. 2008;24:211–22. doi: 10.1002/dmrr.791. [DOI] [PubMed] [Google Scholar]
- 10.Schrezenmeir J, Jagla A. Milk and diabetes. J Am Coll Nutr. 2000;19(Suppl. 2):176S–90S. doi: 10.1080/07315724.2000.10718087. [DOI] [PubMed] [Google Scholar]
- 11.Wasmuth HE, Kolb H. Cow's milk and immune-mediated diabetes. Proc Nutr Soc. 2000;59:573–9. doi: 10.1017/s0029665100000811. [DOI] [PubMed] [Google Scholar]
- 12.Onodera T, Jenson AB, Yoon JW, Notkins AL. Virus-induced diabetes mellitus: reovirus infection of pancreatic beta cells in mice. Science. 1978;201:529–31. doi: 10.1126/science.208156. [DOI] [PubMed] [Google Scholar]
- 13.Toniolo A, Onodera T, Jordan G, Yoon JW, Notkins AL. Virus-induced diabetes mellitus. Glucose abnormalities produced in mice by the six members of the Coxsackie B virus group. Diabetes. 1982;1:496–9. doi: 10.2337/diab.31.6.496. [DOI] [PubMed] [Google Scholar]
- 14.Yoon JW, Austin M, Onodera T, Notkins AL. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–9. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 15.Yoon JW, London WT, Curfman BL, Brown RL, Notkins AL. Coxsackie virus B4 produces transient diabetes in nonhuman primates. Diabetes. 1986;35:712–6. doi: 10.2337/diab.35.6.712. [DOI] [PubMed] [Google Scholar]
- 16.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7:550–7. doi: 10.1016/j.autrev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Green J, Casabonne D, Newton R. Coxsackie B virus serology and Type 1 diabetes mellitus: a systematic review of published case-control studies. Diabet Med. 2004;21:507–14. doi: 10.1111/j.1464-5491.2004.01182.x. [DOI] [PubMed] [Google Scholar]
- 18.Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–76. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaccone P, Fehervari Z, Jones FM, Sidobre S, Kronenberg M, Dunne DW, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur J Immunol. 2003;33:1439–49. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- 20.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 21.Cooke A, Zaccone P, Raine T, Phillips JM, Dunne DW. Infection and autoimmunity: are we winning the war, only to lose the peace? Trends Parasitol. 2004;20:316–21. doi: 10.1016/j.pt.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summers RW, Elliott DE, Qadir K, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol. 2003;98:2034–41. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- 24.Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61:97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- 25.Kikutani H, Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- 26.Bras A, Aguas AP. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology. 1996;89:20–5. doi: 10.1046/j.1365-2567.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins TC, Aguas AP. Mechanisms of Mycobacterium avium-induced resistance against insulin-dependent diabetes mellitus (IDDM) in non-obese diabetic (NOD) mice: role of Fas and Th1 cells. Clin Exp Immunol. 1999;115:248–54. doi: 10.1046/j.1365-2249.1999.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders KA, Raine T, Cooke A, Lawrence CE. Inhibition of autoimmune type 1 diabetes by gastrointestinal helminth infection. Infect Immun. 2007;75:397–407. doi: 10.1128/IAI.00664-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faveeuw C, Angeli V, Fontaine J, et al. Antigen presentation by CD1d contributes to the amplification of Th2 responses to Schistosoma mansoni glycoconjugates in mice. J Immunol. 2002;169:906–12. doi: 10.4049/jimmunol.169.2.906. [DOI] [PubMed] [Google Scholar]
- 30.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–56. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 31.Noel W, Raes G, Hassanzadeh Ghassabeh G, De Baetselier P, Beschin A. Alternatively activated macrophages during parasite infections. Trends Parasitol. 2004;20:126–33. doi: 10.1016/j.pt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Velupillai P, Secor WE, Horauf AM, Harn DA. B-1 cell (CD5+B220+) outgrowth in murine schistosomiasis is genetically restricted and is largely due to activation by polylactosamine sugars. J Immunol. 1997;158:338–44. [PubMed] [Google Scholar]
- 33.Gaubert S, Viana da Costa A, Maurage CA, et al. X-linked immunodeficiency affects the outcome of Schistosoma mansoni infection in the murine model. Parasite Immunol. 1999;21:89–101. doi: 10.1046/j.1365-3024.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 34.Higgins SC, Lavelle EC, McCann C, et al. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J Immunol. 2003;171:3119–27. doi: 10.4049/jimmunol.171.6.3119. [DOI] [PubMed] [Google Scholar]
- 35.Wohlleben G, Trujillo C, Muller J, Ritze Y, Grunewald S, Tatsch U, Erb KJ. Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol. 2004;16:585–96. doi: 10.1093/intimm/dxh062. [DOI] [PubMed] [Google Scholar]
- 36.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167:6087–91. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]
- 37.Baxter AG, Kinder SJ, Hammond KJ, Scollay R, Godfrey DI. Association between alphabetaTCR+CD4-CD8- T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–82. doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- 38.Beaudoin L, Laloux V, Novak J, Lucas B, Lehuen A. NKT cells inhibit the onset of diabetes by impairing the development of pathogenic T cells specific for pancreatic beta cells. Immunity. 2002;17:725–36. doi: 10.1016/s1074-7613(02)00473-9. [DOI] [PubMed] [Google Scholar]
- 39.Hong S, Wilson MT, Serizawa I, et al. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–6. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 40.Naumov YN, Bahjat KS, Gausling R, et al. Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets. Proc Natl Acad Sci USA. 2001;98:13838–43. doi: 10.1073/pnas.251531798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EJ, La Flamme A, Sabin E, Brunet LR. The initiation and function of Th2 responses during infection with Schistosoma mansoni. Adv Exp Med Biol. 1998;452:67–73. doi: 10.1007/978-1-4615-5355-7_9. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald AS, Pearce EJ. Cutting edge: polarized Th cell response induction by transferred antigen-pulsed dendritic cells is dependent on IL-4 or IL-12 production by recipient cells. J Immunol. 2002;168:3127–30. doi: 10.4049/jimmunol.168.7.3127. [DOI] [PubMed] [Google Scholar]
- 43.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–47. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 44.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–31. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 45.Kullberg MC, Pearce EJ, Hieny SE, Sher A, Berzofsky JA. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–70. [PubMed] [Google Scholar]
- 46.La Flamme AC, Scott P, Pearce EJ. Schistosomiasis delays lesion resolution during Leishmania major infection by impairing parasite killing by macrophages. Parasite Immunol. 2002;24:339–45. doi: 10.1046/j.1365-3024.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 47.Schramm G, Mohrs K, Wodrich M, Doenhoff MJ, Pearce EJ, Haas H, Mohrs M. Cutting edge: IPSE/alpha-1, a glycoprotein from Schistosoma mansoni eggs, induces IgE-dependent, antigen-independent IL-4 production by murine basophils in vivo. J Immunol. 2007;178:6023–7. doi: 10.4049/jimmunol.178.10.6023. [DOI] [PubMed] [Google Scholar]
- 48.Harnett W, Harnett MM. Filarial nematode secreted product ES-62 is an anti-inflammatory agent: therapeutic potential of small molecule derivatives and ES-62 peptide mimetics. Clin Exp Pharmacol Physiol. 2006;6:511–8. doi: 10.1111/j.1440-1681.2006.04400.x. [DOI] [PubMed] [Google Scholar]
- 49.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 50.Oldstone MB. Prevention of type I diabetes in nonobese diabetic mice by virus infection. Science. 1988;239:500–2. doi: 10.1126/science.3277269. [DOI] [PubMed] [Google Scholar]
- 51.Serreze DV, Wasserfall C, Ottendorfer EW, et al. Diabetes acceleration or prevention by a coxsackievirus B4 infection: critical requirements for both interleukin-4 and gamma interferon. J Virol. 2005;79:1045–52. doi: 10.1128/JVI.79.2.1045-1052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon JW. Induction and prevention of type 1 diabetes mellitus by viruses. Diabete Metab. 1992;18:378–86. [PubMed] [Google Scholar]
- 53.Smith KA, Efstathiou S, Cooke A. Murine gammaherpesvirus-68 infection alters self-antigen presentation and type 1 diabetes onset in NOD mice. J Immunol. 2007;179:7325–33. doi: 10.4049/jimmunol.179.11.7325. [DOI] [PubMed] [Google Scholar]
- 54.Raine T, Zaccone P, Mastroeni P, Cooke A. Salmonella typhimurium infection in nonobese diabetic mice generates immunomodulatory dendritic cells able to prevent type 1 diabetes. J Immunol. 2006;177:2224–33. doi: 10.4049/jimmunol.177.4.2224. [DOI] [PubMed] [Google Scholar]
- 55.Sewell DL, Reinke EK, Co DO, Hogan LH, Fritz RB, Sandor M, Fabry Z. Infection with Mycobacterium bovis BCG diverts traffic of myelin oligodendroglial glycoprotein autoantigen-specific T cells away from the central nervous system and ameliorates experimental autoimmune encephalomyelitis. Clin Diagn Lab Immunol. 2003;10:564–72. doi: 10.1128/CDLI.10.4.564-572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baxter AG, Horsfall AC, Healey D, Ozegbe P, Day S, Williams DG, Cooke A. Mycobacteria precipitate an SLE-like syndrome in diabetes-prone NOD mice. Immunology. 1994;83:227–31. [PMC free article] [PubMed] [Google Scholar]
- 57.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, Fabry Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15:59–69. doi: 10.1093/intimm/dxg012. [DOI] [PubMed] [Google Scholar]
- 59.Nagayama Y, Watanabe K, Niwa M, McLachlan SM, Rapoport B. Schistosoma mansoni and alpha-galactosylceramide: prophylactic effect of Th1 Immune suppression in a mouse model of Graves' hyperthyroidism. J Immunol. 2004;173:2167–73. doi: 10.4049/jimmunol.173.3.2167. [DOI] [PubMed] [Google Scholar]
- 60.Nagayama Y, McLachlan SM, Rapoport B, Oishi K. Graves' hyperthyroidism and the hygiene hypothesis in a mouse model. Endocrinology. 2004;145:5075–9. doi: 10.1210/en.2004-0683. [DOI] [PubMed] [Google Scholar]
- 61.Costalonga M, Hodges JS, Herzberg MC. Streptococcus sanguis modulates type II collagen-induced arthritis in DBA/1J mice. J Immunol. 2002;169:2189–95. doi: 10.4049/jimmunol.169.4.2189. [DOI] [PubMed] [Google Scholar]
- 62.Mattsson L, Larsson P, Erlandsson-Harris H, Klareskog L, Harris RA. Parasite-mediated down-regulation of collagen-induced arthritis (CIA) in DA rats. Clin Exp Immunol. 2000;122:477–83. doi: 10.1046/j.1365-2249.2000.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan WI, Blennerhasset PA, Varghese AK, Chowdhury SK, Omsted P, Deng Y, Collins SM. Intestinal nematode infection ameliorates experimental colitis in mice. Infect Immun. 2002;70:5931–7. doi: 10.1128/IAI.70.11.5931-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF, Jr, Weinstock JV. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385–91. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 65.Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, Fallon PG. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557–66. doi: 10.4049/jimmunol.178.7.4557. [DOI] [PubMed] [Google Scholar]
- 66.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr, Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–8. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 67.Reardon C, Sanchez A, Hogaboam CM, McKay DM. Tapeworm infection reduces epithelial ion transport abnormalities in murine dextran sulfate sodium-induced colitis. Infect Immun. 2001;69:4417–23. doi: 10.1128/IAI.69.7.4417-4423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Summers RW, Elliott DE, Urban JF, Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]