Abstract
Although the molecules and cells involved in triggering immune responses against parasitic worms (helminths) remain enigmatic, research has continued to implicate expansions of T-helper type 2 (Th2) cells and regulatory T-helper (Treg) cells as a characteristic response to these organisms. An intimate association has also emerged between Th2 responses and wound-healing functions. As helminth infections in humans are associated with a strong Th2/Treg immunoregulatory footprint (often termed a ‘modified Th2’ response), plausible links have been made to increased susceptibility to microbial pathogens in helminth-infected populations in the tropics and to the breakdowns in immunological control (allergy and autoimmunity) that are increasing in frequency in helminth-free developed countries. Removal of helminths and their anti-inflammatory influence may also have hazards for populations exposed to infectious agents, such as malaria and influenza, whose worst effects are mediated by excessive inflammatory reactions. The patterns seen in the control of helminth immunity are discussed from an evolutionary perspective. Whilst an inability to correctly regulate the immune system in the absence of helminth infection might seem highly counter-adaptive, the very ancient and pervasive relationship between vertebrates and helminths supports a view that immunological control networks have been selected to function within the context of a modified Th2 environment. The absence of immunoregulatory stimuli from helminths may therefore uncover maladaptations that were not previously exposed to selection.
Keywords: coevolution, coinfection, hygiene hypothesis, regulation, Th2
Overview
A general consensus has emerged that effector T-helper cell type 2 (Th2) mechanisms evolved to counter infections with helminths (and perhaps other large metazoan parasites) and that these defences are generally deployed against such pathogens within the context of a significant regulatory T (Treg) cell response.1 There is also a growing appreciation that immune responses against helminths share some of the same signals and mediators as the body's damage repair systems.2 As the interaction between the vertebrate immune system and at least some of the major helminth groups extends back hundreds of millions of years,3–5 near to a time when the adaptive immunity first evolved6,7 (see Fig. 1), reciprocal co-adaptation may have fundamentally shaped the characteristics of both. Below we consider the mechanism of anti-helminth immune responses and how ancient adaptations to deal with helminth infection may have left their mark on the way the immune system is structured and controlled. We discuss how this relates to adverse immunological phenomena seen in modern helminth-free human populations, such as allergy, autoimmunity and responses to immunopathological infectious agents.
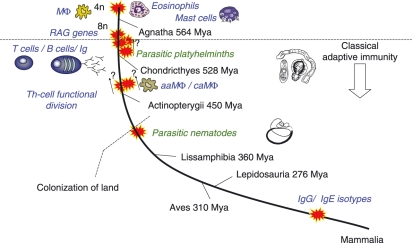
Figure 1.
The immune system evolved in the presence of helminths. The earliest dates of colonization by helminth lineages are mapped onto a phylogenetic history of vertebrates. Also mapped are major putative events in immune system evolution and the appearance of mediators of importance in anti-helminth responses. Divergence times of the major vertebrate groups from the mammalian lineage are indicated (Mya, million years ago).96 Macrophage-,97 eosinophil-98 and mast-99 like cells occur in Agnathans (lampreys) predating the appearance of classical adaptive immunity. The evolution of classical adaptive immunity was possibly preceded100 by two rounds of whole genome, or large-scale partial genome, duplication (4n and then 8n). Insertion of recombination activating (RAG) genes101 into the genome then allowed the evolution of the recombinatorial receptor system underpinning T-cell and B-cell functions. This occurred at some point between the divergences of the Agnatha (lampreys and hagfishes) and the Chondricthyes (sharks and rays). Colonization by platyhelminths occurred within this period, with all extant parasitic groups believed to be derived from a single common parasitic ancestor.4 The earliest evidence of Th2 cytokines102–104 and alternatively (aaMΦ) versus classically (caMΦ) activated macrophages105,106 is found in the Actinopterygii (bony fishes). Colonization of land probably allowed the invasion of primitive tetrapods by parasitic nematodes3,5 (molecular phylogenetic analyses suggest at least four independent invasions).107 The immunoglobulin E (IgE) isotype, important in anti-helminth responses, is a recent innovation in the mammalian lineage. Ig, immunoglobulin; Mφ, macrophage.108
Helminths
‘Helminth’ is a term used to group ecologically similar worm-like parasites actually derived from three unrelated phyla: the platyhelminths (tapeworms and flukes), the nematodes (roundworms) and the acanthocephalans (thorny-headed worms). A breathtaking diversity of species occur in vertebrates, the global fauna in an individual vertebrate host species typically numbering tens or even hundreds of different taxa. Life cycles may be direct, with transmission from one definitive host to another, or complex, involving distinct life-history stages cycling through different host species. Invading stages often undertake complex migrations through different organ systems and tissues once inside the definitive host. Adult helminths are large in comparison to microbial pathogens, individuals varying in size from hundreds of microns to tens of metres long. Generally, they produce propagules that are dispersed away from the existing host, sometimes by an arthropod vector. The relatively slow maturation time of individuals (a consequence of their size) and the requirement for host–host dispersal to complete the life cycle mean that the potential for the exponential population growth seen in microbes that reproduce in situ is very limited. Herein lies a fundamental difference. Whilst microbes represent an imminent threat and are usually countered by rapid, relatively violent inflammatory responses, helminths seem to be handled with gentler immune responses, with a strong regulatory component, that may take weeks, months or even years to reach their peak effectiveness.
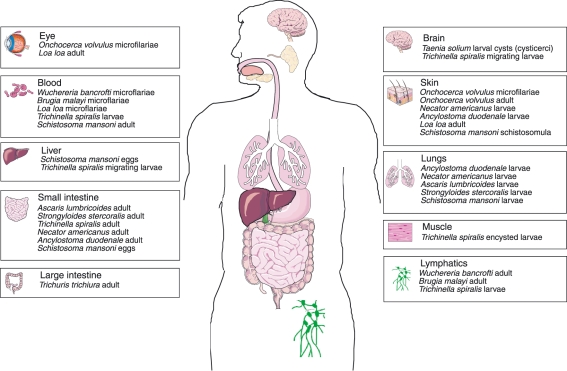
Any survey of a vertebrate wildlife population will generally yield an array of different helminths (see e.g. ref. 8), with many hosts infected by tens, hundreds or even thousands of individual worms. It also seems reasonable to assume the same for pre-industrial human populations, given the rates of helminth parasitism seen in the modern-day tropics. Almost every organ system and tissue in the human body (Fig. 2) is a potential target for infection, and well over 100 species have been recovered from humans.9 However, in terms of current global significance, a smaller number of species might be highlighted, all of which are primarily distributed in the tropics. In blood flukes of the genus Schistosoma, water-borne larvae penetrate the host's skin before establishing in the blood vascular system, producing eggs that migrate through tissues before release to the exterior. Larvae of the filarial nematode Onchocerca volvulus are transmitted by blood-feeding blackflies and establish in subcutaneous nodules, from which adult females release millions of microfilaria larvae into the dermis of the host. The ‘geohelminth’ soil-transmitted nematodes Ancylostoma duodenale, Necator americanus, Ascaris lumbricoides and Trichuris trichiura infect the host's gut, and from there eggs are passed to the exterior in faeces. Ancylostoma duodenaleand N. americanus (hookworms) invade the host by larval penetration of the skin, migrating through the lungs before arriving in the intestine. Ascaris lumbricoides (roundworm) and T. trichiura (whipworm) are transmitted by an oral route, the former undergoing a complex migration through the lungs, and the latter developing entirely in a mucosal site. Worm infection tends to be chronic, with maximum individual life-spans of months or a few years (the gastrointestinal nematodes) to decades (schistosomes). Pathology mediated by these helminths is relatively limited: overt symptoms are usually rare in human populations although adverse outcomes may be measurable, for example, in reduced growth or cognitive development. That this limited level of pathogenicity is a result of adaptation seems likely. For example, outbreaks in humans in the Philippines of an intestinal capillarid usually infecting fish-eating birds resulted in severe disease and a 6% mortality rate from > 1000 confirmed cases between 1967 and 1990.10
Figure 2.
The wide range of organ systems targeted by a selection of the helminth parasites occurring in humans.
A stereotypical immune response
The development of resistance to gastrointestinal nematodes is generally associated with a Th2 response in laboratory models11–13 (Fig. 3a). Findings consistent with this have been reported in epidemiological studies of hookworm,14 roundworm15–17 and whipworm15–17 in humans. The triggering of the Th2 response programme by intestinal nematodes, orchestrated by cytokines including interleukin (IL)-4,12 IL-5,18 IL-9,19 IL-13,20 IL-2121 and IL-2522 and IL-33,23 leads to a stereotyped cascade of effector mechanisms, potentially including immunoglobulin E (IgE) isotype-switched B-cell responses, mast cells, eosinophils, basophils and increased permeability, smooth muscle contractility and mucus production in the gut. Increases in IL-13-driven intestinal epithelial cell (IEC) turnover24 and production of the protein resistin-like molecule beta (RELMβ),25 which interferes with nematode chemotaxis, may also be involved in protection. However, only a subset of the recruited mediators26 may be important effectors in resistance against any individual gastrointestinal nematode species. For example, in mouse model systems, mucosal mast cell responses are critical for worm expulsion in Trichuris muris and Trichinella spiralis infection19,27,28 but not in Heligmosomoides bakeri29,30 or Nippostrongylus brasiliensis infection.31 Th2 responses are also triggered by tissue-dwelling (histozoic) species such as the schistosomes32 and filarial nematodes,33 although it is not clear-cut how important Th2-driven effector mechanisms are in protecting the host.34–38 In these cases Th1 mechanisms can be implicated in resistance and are perhaps especially important in killing incoming larval stages38,39 migrating through tissues, but once the definitive site is reached the adult parasite may survive for many years. Fibrotic responses can be a prominent feature of the host reaction against histozoic species: Th2-driven granulomas are focussed upon eggs in Schistosoma spp.38 and fibrosis in Onchocerca infection can be associated with microfilarial migrations and occur around the onchocercoma nodules enclosing adults.40
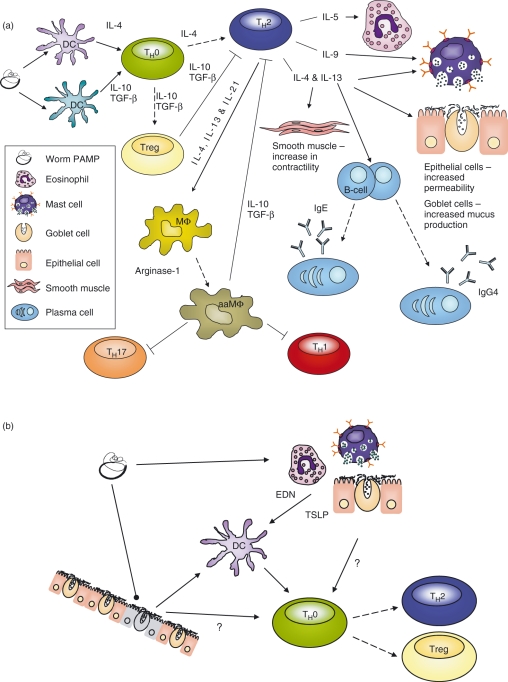
Figure 3.
Cells and molecules involved in the generation of immune responses in helminth infections. Solid arrows indicate signalling influences on cells (with important signalling molecules indicated alongside), and dashed arrows indicate the developmental trajectory of an individual cell type. (a) A widely accepted hypothesis for the function of mediators and effectors involved in the modified Th2 response against helminths. Pathogen-associated molecular patterns (PAMPs) from helminths may stimulate the differentiation of dendritic cells (DCs) that bias T-helper (Th) cell polarization towards Th2 or regulatory T (Treg) cell subsets. Th2 cells produce a range of cytokines driving effector responses, including: eosinophil responses, mast cell responses, isotype-switched B-cell responses, increases in intestinal permeability, smooth muscle contractility and mucus production, and the differentiation of alternatively activated macrophages (aaMΦ). Treg cells induced in the periphery and producing suppressive cytokines [interleukin (IL)-10 and transforming growth factor (TGF)-β] dampen levels of innate and adaptive immune activation. (b) A model incorporating alternative hypotheses for early events in the initiation of anti-helminth responses. Helminth PAMPs might stimulate signalling activity in other ‘front-line’ cell types (eosinophils, basophils, mast cells or intestinal epithelial cells) which, in turn, influences DCs or even naïve Th (Th0) cells. Damage-associated molecular patterns (DAMPs) released by dying cells, or signals resulting from apoptosis, might also influence either DCs or Th0 cells. EDN, eosinophil-derived neurotoxin; TSLP, thymic stromal lymphopoetin.
The other recurring immunological characteristic of helminth infections is that despite significant responses, for example vigorous antibody responses and eosinophilia, which may or may not be effective in resistance, there is often a marked systemic suppression of innate and adaptive immunity41 in the host. This may be linked to expansions of regulatory CD4+ CD25+ T cells producing the suppressive cytokines transforming growth factor (TGF)-β and/or IL-1042–44 and perhaps other suppressor T-cell subsets,45 including CD8+ T cells with cytokine-independent suppressive activity.46 Immunoepidemiological studies in humans have strongly associated regulatory cytokine activity and/or immunological hyporesponsiveness with increasing infection intensity in filarial nematodes,37,47Schistosoma flukes48,49 and intestinal nematodes.50 A further important dimension in helminth infections is the differentiation of alternatively activated macrophages (aaMΦs)2,51 under the influence of IL-4, IL-13 and IL-21. Whilst aaMΦs recruit to the site of infection52 and have been implicated as functional effectors,53 they also have strong anti-inflammatory properties, secreting IL-10 and TGF-β, and strongly expressing genes such as Arginase-1, Ym1 (chitinase-3-like protein 3) and FIZZ1 (resistin-like molecule alpha)54 whose functions relate to the repair of extracellular matrices, wound healing and fibrosis.
The overall outcome of a helminth infection may therefore be an environment with down-regulated proinflammatory responsiveness, activated damage repair mechanisms and a tightly controlled development of Th2 anti-parasite effector responses. Although exposure to helminths in exposed human populations typically begins in early childhood, delayed age-intensity peaks55 and substantial reinfection rates56 are a testament to the slow development of immunity.
Evolution of the Th2 arm: home win or score draw?
From a ‘worm's view’ the nature of the host response is such that primary worm infections are often successful, but secondary waves of invasion are likely to encounter progressively increasing levels of resistance. The apparently evolutionarily fixed nature of the stereotypical anti-helminth Th2/Treg response57 may stem from the fact that, within the constraints acting on both protagonists, it maximizes fitness in both the host and some individual parasites.58 For the host, the highly regulated Th2 anti-worm response and initial toleration of infection may lead to greater fitness than the generation of violent, energetically costly and potentially immunopathological responses to a low or intermediate threat. For the parasites in primary infections, relative fitness may be very high because they can survive and reproduce whilst later invaders succumb to the developing immune responses. There might therefore be strong host-mediated selection for parasite genotypes that co-operate with, rather than subvert, the Th2/Treg phenotype, perhaps even to the extent that there could have been synergistic coevolution of complementary pathogen-associated molecular pattern (PAMP)–host innate receptor recognition systems. Stabilizing features of such an interaction might be that the parasite cannot easily evolve mechanisms for incoming stages to evade or subvert a fully developed memory response in a well-adapted host and that primary infections that fail to stimulate a Th2 response might benefit late-arriving competitors, penalizing the fitness of early colonizers.
As mentioned above, Th2 mediators are important in defence against metazoan parasites, but also for damage repair,57 and it is intuitive that the two roles might be evolutionarily linked. Worm infections are likely to produce high levels of local tissue damage associated with the feeding and migratory activities of individual parasites. There may also sometimes be a requirement for the host to physically contain (‘wall off’) dangerous motile stages by fibrotic reactions within tissues. It thus seems plausible that, during evolution, anti-worm mediators may have been ‘grafted’ onto the control networks that regulate wound repair and tissue remodelling. Mechanisms involved in wound healing and responses to helminths are not totally overlapping, of course, as the latter also involve genuine effector mechanisms (IgE, mast cells and esoinophils) not usually implicated in wound repair, and themselves capable of generating immunopathology. However, the intimacy of damage repair and anti-helminth responses may give clues as to the enigmatic signals that initiate dominant immune responses in worm infections.
The initiation and control of immune responses in worm infections
Whilst the exact signalling pathways by which Th2 responses are initiated remain obscure, helminth products seem able to stimulate the development of partially activated DCs,59 with suppressed expression of Toll-like receptors (TLRs) and activation markers,60,61 that promote Th2 and/or Treg phenotypes.59,62,63 From an analogy with the very well-known pathways by which Th1 cells are induced, the simplest model for the initiation of Th2 responses in helminth infections would be an interaction between a PAMP derived from a worm and a pattern recognition receptor (PRR) expressed by a DC. Worm PAMPs might act via one or more of the TLR family, or perhaps other classes of innate receptor64,65 such as C-type lectins, nucleotide-binding oligomerization domain-containing protein (NOD)-like receptors or protease-activated receptors. Prospecting of helminth excretory-secretory (ES) products for molecules with TLR-mediated immunomodulatory effects, mainly focussing on DCs, has yielded a number of examples that might be consistent with such a model. Synthetic lacto-N-fucopentanose III, equivalent to a molecule occurring naturally in soluble egg antigen (SEA), has been reported to interact with TLR4 to produce Th2 polarizing DCs66 and schistosome lyso-phosphatidylserine may interact with TLR2 to produce Treg polarizing DCs.67 ES-62 from the filarial nematode Acanthocheilonema viteae is reported to exert immunomodulatory effects on macrophages68 and DCs69 by a TLR4-dependent mechanism.70 However, the interaction of ES-62 with TLR4 on mast cells, which results in the inhibition of Th1 but not Th2 cytokine production, does not involve classical TLR signalling.71 Also, the Th2-inducing effects of SEA on DCs have recently been shown to be independent of MyD88, TLR2 and TLR4.72 A further consideration is that PRRs on cell types other than DCs may be important (Fig. 3b), as responses that favour a Th2 environment have been reported in basophils,73,74 mast cells,71 eosinophils75 and IECs.76 In the case of IECs, Trichuris muris infection stimulates inhibitor of kappa-B kinase beta (IKKβ)-dependent expression of the cytokine thymic stromal lymphopoetin (TSLP), which can stimulate the differentiation of Th2-inducing DCs. Innate responses by eosinophils include secretion of eosinophil-derived neurotoxin (EDN), which mediates TLR2/Myd88-dependent activation of DCs that drive in vivo antigen-specific adaptive responses towards a Th2 phenotype. A wide range of other alarmin-type signalling molecules can be released during infection (e.g. defensins, cathelicidins and high-mobility group 1 box proteins), including some with known pro-inflammatory influences,77 but perhaps others with undiscovered Th2-inducing activities. Even if DCs are pivotal in initiating expansions of Th2 cells, pattern recognition by these cells themselves might not therefore be necessary, as cytokines or alarmins released following pattern recognition by more ‘frontline’ cells, such as IEEs (Fig. 3b), could be sufficient for maturation of Th2-inducing DC phenotypes. It is even possible that some Th2-inducing signals might bypass DCs altogether and act directly on T lymphocytes prior to acquisition of a terminally differentiated phenotype.59
Apart from an exploration of cell types other than DCs, the overlap between effector and tissue repair functions in anti-helminth immune responses suggests that the search for innate ‘pattern recognition’ signals initiating anti-worm responses might need to consider endogenous host molecules that convey information about cellular damage (Fig. 3b) and that trigger anti-inflammatory programmes appropriate for wound healing. During helminth infection such damage-associated molecular patterns (DAMPs; here used to denote endogenous signals only) might be sensed by elements of the innate immune system, perhaps as combinatorial signals with PAMPs.57 A range of endogenous molecules have been shown to interact with receptors of the TLR2, TLR3, TLR4, TLR7, TLR8 and TLR9 families, including some that are released on cell damage and that can exert either stimulatory or anti-inflammatory effects.78 For example, suppressive IL-10-inducing ligands for TLR9 have been characterized,79 some of the most potent of which contain TTAGGG multimers characteristic of mammalian telomeres.80 Regulatory T cells may also be induced by other endogenous mechanisms: interactions with apoptotic cells, or phosphatidylserine from apoptotic cells, causes differentiation of immature DCs with tolerogenic properties favouring anti-inflammatory Th phenotypes.81–84 It even seems a possibility that much of the Treg activity in a worm infection could be initiated by signalling from endogenous molecules. In the case of anti-helminth Th2 responses, however, cross-talk between worm PAMPs and the innate immune system would be expected to be important, as many anti-helminth effectors (e.g. IgE and mast cells) are recruited that are apparently unconnected to wound healing.
Worm versus man: coevolutionary legacies?
An ideal organism would be able to perfectly control its immune system under any combination of infection challenges and antigenic stimuli. However, organisms are not perfect and have been selected to perform optimally within the set of conditions encountered by their ancestors. In humans, one of these past conditions is arguably exposure to a diverse community of helminth parasites. The relationship between helminths and vertebrates is ancient and can be traced back to when nematodes invaded primitive tetrapods3 and the ancestral platyhelminths4 first appeared in fishes prior to the colonization of land (Fig. 1). In the intervening time, individual helminth lineages may have diverged, switched between host lineages, or become extinct many times. However, if the pattern of infection seen in present-day wildlife populations can be extrapolated into the past, then almost every natural vertebrate population would have been exposed to a varied assemblage of worm-like pathogens. Because helminth infection is and (probably) has been so ubiquitous, mammalian immune systems may have been selected to anticipate that they would suffer chronic exposure to large-bodied Th2/Treg-inducing pathogens. In other words, there would have been selection for immunoregulatory systems that work well in the context of worm infection. However, there would rarely have been selection for immunoregulatory systems that work optimally when worms are absent (the situation seen in modern human populations). The absence of parasitic worms and their strong Th2/Treg-inducing influences may therefore uncover flaws in immunoregulatory networks that have previously been hidden from selection.85,86 Such a scenario might explain the dramatic increases in allergy and autoimmune conditions in Western countries and is supported by the protective effect of helminth infection, or helminth-derived products, in laboratory models of allergy43,87 and automimmunity.85,88
The future
The strong Th2/Treg-inducing influence of worms, and the possibility that the immune system may malfunction in the absence of such stimuli, has global public health implications: both for the parts of the world population that are helminth-free and for the parts that remain exposed. In the first case, a search for molecules that mimic the immunoregulatory stimuli seen in worm infections may one day yield pharmaceuticals that can be used routinely to induce healthier, better regulated immune systems. Given the intimate link between immune responses to worms and tissue repair, it might be necessary to go beyond a search for PAMPs that interact with DCs and to extend this to include other cell types65 and other molecular patterns57 including DAMPs. For helminth-exposed populations it has been demonstrated that the immunosuppressive activities of worm infections may make individuals less responsive to microbial pathogens and to vaccination. This is a major concern because wherever helminth infection is prevalent so too are dangerous microbial infectious agents, such as human immunodeficiency virus (HIV), Mycobacterium tuberculi and malarial parasites. Anthelmintic treatment programmes might result in significant health benefits from the increased immunological responsiveness against such pathogens. However, a ‘flip-side’ to this coin might be foreseen in the context of highly immunopathogenic coinfections such as malaria or influenza, whose worst effects are caused by excessive immune responses. The potential realism of this scenario is supported by laboratory model studies suggesting that gastrointestinal nematode infections suppress immunopathology during influenza.89 It is also at least hinted at by the contradictory body of epidemiological90,91 and laboratory model92–95 data for interactions in malaria–helminth coinfections. The consequences of de-worming for susceptibility to the worst symptoms of important immunopathogenic infections are therefore of urgent interest for further laboratory model studies and should be carefully monitored in the field.58
Acknowledgments
JEB is grateful for research funding from The Wellcome Trust, The Royal Society, The Natural Environment Research Council, the European Commission and The University of Nottingham. IMF and SL are respectively supported by research funding from the European Commission (Marie Curie Early Stage Research Training) and The University of Nottingham.
References
- 1.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–44. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Kreider T, Anthony RM, Urban JF, Jr, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–53. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RC. Why do fish have so few roundworm (nematode) parasites? Environ Biol Fishes. 1996;46:1–5. [Google Scholar]
- 4.Littlewood DTJ, Rohde K, Clough KA. The interrelationships of all major groups of Platyhelminthes: phylogenetic evidence from morphology and molecules. Biol J Linn Soc Lond. 1999;66:75–114. [Google Scholar]
- 5.Clark WC. Origins of the parasitic habit in the Nematoda. Int J Parasitol. 1994;24:1117–29. doi: 10.1016/0020-7519(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 6.Flajnik MF, Kasahara M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity. 2001;15:351–62. doi: 10.1016/s1074-7613(01)00198-4. [DOI] [PubMed] [Google Scholar]
- 7.Laird DJ, De Tomaso AW, Cooper MD, Weissman IL. 50 million years of chordate evolution: seeking the origins of adaptive immunity. Proc Natl Acad Sci USA. 2000;97:6924–6. doi: 10.1073/pnas.97.13.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esch GW, Bush AO, Aho JM. Parasite Communities: Patterns and Processes. New York: Chapman and Hall; 1990. [Google Scholar]
- 9.Coombs I. Helminth species recovered from humans. In: Crompton DWT, Savioli L, editors. Handbook of Helminthiasis for Public Health. Boca Raton, FL: CRC Press, Taylor & Francis Group; 2006. pp. 12–24. [Google Scholar]
- 10.Cross JH. Intestinal capillariasis. Clin Microbiol Rev. 1992;5:120–9. doi: 10.1128/cmr.5.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4 Ralpha, and Stat 6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–64. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 13.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol. 1998;28:1145–58. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 14.Quinnell RJ, Pritchard DI, Raiko A, Brown AP, Shaw M-A. Immune responses in human necatoriasis: association between interleukin-5 responses and resistance to reinfection. J Infect Dis. 2004;190:430–8. doi: 10.1086/422256. [DOI] [PubMed] [Google Scholar]
- 15.Jackson JA, Turner JD, Rentoul L, et al. T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. J Infect Dis. 2004;190:1804–11. doi: 10.1086/425014. [DOI] [PubMed] [Google Scholar]
- 16.Jackson JA, Turner JD, Rentoul L, et al. Cytokine response profiles predict species-specific infection patterns in human GI nematodes. Int J Parasitol. 2004;34:1237–44. doi: 10.1016/j.ijpara.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Turner JD, Faulkner H, Kamgno J, et al. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J Infect Dis. 2003;188:1768–75. doi: 10.1086/379370. [DOI] [PubMed] [Google Scholar]
- 18.Ovington KS, McKie K, Matthaei KI, Young IG, Behm CA. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology. 1998;95:488–93. doi: 10.1046/j.1365-2567.1998.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun. 1998;66:3832–40. doi: 10.1128/iai.66.8.3832-3840.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–61. [PubMed] [Google Scholar]
- 21.Pesce J, Kaviratne M, Ramalingam TR, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–55. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 24.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–5. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 25.Artis D, Wang ML, Keilbaugh SA, et al. RELMB/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc Natl Acad Sci USA. 2004;101:13596–600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–77. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 27.Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–9. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 28.Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N. brasiliensis infection. Blood. 1995;86:1968–76. [PubMed] [Google Scholar]
- 29.Behnke JM, Lowe A, Clifford S, Wakelin D. Cellular and serological responses in resistant and susceptible mice exposed to repeated infection with Heligmosomoides polygyrus bakeri. Parasite Immunol. 2003;25:333–40. doi: 10.1046/j.1365-3024.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- 30.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–56. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann KF, Wynn TA, Dunne DW. Cytokine-mediated host responses during schistosome infections; walking the fine line between immunological control and immunopathology. Adv Parasitol. 2002;52:265–307. doi: 10.1016/s0065-308x(02)52014-5. [DOI] [PubMed] [Google Scholar]
- 33.Rachel A, Lawrence ED. Lymphatic filariasis: parallels between the immunology of infection in humans and mice. Parasite Immunol. 2001;23:353–61. doi: 10.1046/j.1365-3024.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 34.Joseph S, Jones FM, Kimani G, et al. Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect Immun. 2004;72:728–34. doi: 10.1128/IAI.72.2.728-734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth M, Shaw MA, Carpenter D, et al. Carriage of DRB1*13 is associated with increased posttreatment IgE levels against Schistosoma mansoni antigens and lower long-term reinfection levels. J Immunol. 2006;176:7112–18. doi: 10.4049/jimmunol.176.11.7112. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence RA, Allen JE, Gregory WF, Kopf M, Maizels RM. Infection of IL-4-deficient mice with the parasitic nematode Brugia malayi demonstrates that host resistance is not dependent on a T helper 2-dominated immune response. J Immunol. 1995;154:5995–6001. [PubMed] [Google Scholar]
- 37.Elson LH, Calvopina M, Paredes W, Araujo E, Bradley JE, Guderian RH, Nutman TB. Immunity to onchocerciasis: putative immune persons produce a Th1-like response to Onchocerca volvulus. J Infect Dis. 1995;171:652–8. doi: 10.1093/infdis/171.3.652. [DOI] [PubMed] [Google Scholar]
- 38.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 39.Babu S, Nutman TB. Proinflammatory cytokines dominate the early immune response to filarial parasites. J Immunol. 2003;171:6723–32. doi: 10.4049/jimmunol.171.12.6723. [DOI] [PubMed] [Google Scholar]
- 40.Okulicz JF, Stibich AS, Elston DM, Schwartz RA. Cutaneous onchocercoma. Int J Dermatol. 2004;43:170–2. doi: 10.1111/j.1365-4632.2004.02279.x. [DOI] [PubMed] [Google Scholar]
- 41.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J Immunol. 2006;176:3248–56. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 42.Setiawan T, Metwali A, Blum AM, Ince MN, Urban JF, Jr, Elliott DE, Weinstock JV. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect Immun. 2007;75:4655–63. doi: 10.1128/IAI.00358-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med. 2005;202:1199–212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ince MN, Elliott DE, Setiawan T, Blum A, Metwali A, Wang Y, Urban JF, Jr, Weinstock JV. Cutting edge: Heligmosomoides polygyrus induces TLR4 on murine mucosal T cells that produce TGFbeta after lipopolysaccharide stimulation. J Immunol. 2006;176:726–9. doi: 10.4049/jimmunol.176.2.726. [DOI] [PubMed] [Google Scholar]
- 45.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metwali A, Setiawan T, Blum AM, Urban J, Elliott DE, Hang L, Weinstock JV. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am J Physiol Gastrointest Liver Physiol. 2006;291:G253–9. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 47.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis. 1998;178:1133–8. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 48.Mutapi F, Winborn G, Midzi N, Taylor M, Mduluza T, Maizels R. Cytokine responses to Schistosoma haematobium in a Zimbabwean population: contrasting profiles for IFN-gamma, IL-4, IL-5 and IL-10 with age. BMC Infect Dis. 2007;7:139. doi: 10.1186/1471-2334-7-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silveira AM, Gazzinelli G, Alves-Oliveira LF, et al. Human schistosomiasis mansoni: intensity of infection differentially affects the production of interleukin-10, interferon-gamma and interleukin-13 by soluble egg antigen or adult worm antigen stimulated cultures. Trans R Soc Trop Med Hyg. 2004;98:514–19. doi: 10.1016/j.trstmh.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 50.Turner JD, Jackson JA, Faulkner H, Behnke J, Else KJ, Kamgno J, Boussinesq M, Bradley JE. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J Infect Dis. 2008;197:1204–12. doi: 10.1086/586717. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Sosa M, Satoskar AR, Calderon R, Gomez-Garcia L, Saavedra R, Bojalil R, Terrazas LI. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2-biasing ability. Infect Immun. 2002;70:3656–64. doi: 10.1128/IAI.70.7.3656-3664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ alternatively activated macrophages control CD4+ T cell hyporesponsiveness at sites peripheral to filarial infection. J Immunol. 2006;176:6918–27. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 53.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van Rooijen N, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–60. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nair MG, Gallagher IJ, Taylor MD, Loke P, Coulson PS, Wilson RA, Maizels RM, Allen JE. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–94. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Woolhouse MEJ. Patterns in parasite epidemiology: the peak shift. Parasitol Today. 1998;14:428–34. doi: 10.1016/s0169-4758(98)01318-0. [DOI] [PubMed] [Google Scholar]
- 56.Evans AC, Stephenson LS. Not by drugs alone: the fight against parasitic helminths. World Health Forum. 1995;16:258–61. [PubMed] [Google Scholar]
- 57.Diaz A, Allen JE. Mapping immune response profiles: the emerging scenario from helminth immunology. Eur J Immunol. 2007;37:3319–26. doi: 10.1002/eji.200737765. [DOI] [PubMed] [Google Scholar]
- 58.Bradley JE, Jackson JA. Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunol. 2004;26:429–41. doi: 10.1111/j.0141-9838.2004.00730.x. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald AS, Maizels RM. Alarming dendritic cells for Th2 induction. J Exp Med. 2008;205:13–17. doi: 10.1084/jem.20072665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454–61. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 61.Tolouei Semnani R, Goel Venugopal P, Leifer CA, Mostbock S, Sabzevari H, Nutman TB. Inhibition of TLR3 and TLR4 function and expression in human dendritic cells by helminth parasites. Blood. 2008 doi: 10.1182/blood-2008-04-149856. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–27. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 63.Jenkins SJ, Mountford AP. Dendritic cells activated with products released by schistosome larvae drive Th2-type immune responses, which can be inhibited by manipulation of CD40 costimulation. Infect Immun. 2005;73:395–402. doi: 10.1128/IAI.73.1.395-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkins SJ, Hewitson JP, Jenkins GR, Mountford AP. Modulation of the host's immune response by schistosome larvae. Parasite Immunol. 2005;27:385–93. doi: 10.1111/j.1365-3024.2005.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–64. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thomas PG, Carter MR, Atochina O, Da'Dara AA, Piskorska D, McGuire E, Harn DA. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol. 2003;171:5837–41. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 67.van der Kleij D, Latz E, Brouwers JF, et al. A novel host-parasite lipid cross-talk. schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 68.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–5. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 69.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 Cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 70.Goodridge HS, Marshall FA, Else KJ, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–93. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 71.Melendez AJ, Harnett MM, Pushparaj PN, Wong WSF, Tay HK, McSharry CP, Harnett W. Inhibition of FcεRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375–81. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- 72.Kane CM, Jung E, Pearce EJ. Schistosoma mansoni egg antigen-mediated modulation of TLR-induced activation occurs independently of TLR2, TLR4, MyD88. Infect Immun. 2008 doi: 10.1128/IAI.00497-08. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Phillips C, Coward WR, Pritchard DI, Hewitt CRA. Basophils express a type 2 cytokine profile on exposure to proteases from helminths and house dust mites. J Leukoc Biol. 2003;73:165–71. doi: 10.1189/jlb.0702356. [DOI] [PubMed] [Google Scholar]
- 74.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–18. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang D, Chen Q, Su SB, et al. Eosinophil-derived neurotoxin ac ts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-β expression regulates intestinal immune homeostasis. Nature. 2007;446:552–6. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 77.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 78.Ehlers M, Ravetch JV. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007;28:74–9. doi: 10.1016/j.it.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Jarnicki AG, Conroy H, Brereton C, et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J Immunol. 2008;180:3797–806. doi: 10.4049/jimmunol.180.6.3797. [DOI] [PubMed] [Google Scholar]
- 80.Gursel I, Gursel M, Yamada H, Ishii KJ, Takeshita F, Klinman DM. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J Immunol. 2003;171:1393–400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 81.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stuart LM, Lucas M, Simpson C, Lamb J, Savill J, Lacy-Hulbert A. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–35. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 83.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21:643–53. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005;3:166–75. doi: 10.3121/cmr.3.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–76. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 86.Maizels R. Regulation of the immune system in metazoan parasite infections. Novartis Found Symp. 2007;281:192–204. doi: 10.1002/9780470062128.ch16. [DOI] [PubMed] [Google Scholar]
- 87.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–56. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 88.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 89.Furze RC, Hussell T, Selkirk ME. Amelioration of influenza-induced pathology in mice by coinfection with Trichinella spiralis. Infect Immun. 2006;74:1924–32. doi: 10.1128/IAI.74.3.1924-1932.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nacher M, Singhasivanon P, Traore B, et al. Helminth infections are associated with protection from cerebral malaria and increased nitrogen derivatives concentrations in Thailand. Am J Trop Med Hyg. 2002;66:304–9. doi: 10.4269/ajtmh.2002.66.304. [DOI] [PubMed] [Google Scholar]
- 91.Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, Vouldoukis I, Looareesuwan S. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22:107–13. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 92.Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect Immun. 2005;73:3531–9. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Helmby H, Kullberg M, Troye-Blomberg M. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect Immun. 1998;66:5167–74. doi: 10.1128/iai.66.11.5167-5174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Souza B, Helmby H. Concurrent gastro-intestinal nematode infection does not alter the development of experimental cerebral malaria. Microbes Infect. 2008;10:916–21. doi: 10.1016/j.micinf.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Graham AL, Lamb TJ, Read AF, Allen JE. Malaria–filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J Infect Dis. 2005;191:410–21. doi: 10.1086/426871. [DOI] [PubMed] [Google Scholar]
- 96.Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–20. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 97.Ellis LC, Youson JH. Ultrastructure of the pronephric kidney in upstream migrant sea lamprey, Petromyzon marinus L. Am J Anat. 1989;185:429–43. doi: 10.1002/aja.1001850406. [DOI] [PubMed] [Google Scholar]
- 98.Rowley AF, Page M. Ultrastructural, cytochemical and functional studies on the eosinophilic granulocytes of larval lampreys. Cell Tissue Res. 1985;240:705–9. [Google Scholar]
- 99.Mulero I, Sepulcre MP, Meseguer J, Garcia-Ayala A, Mulero V. Histamine is stored in mast cells of most evolutionarily advanced fish and regulates the fish inflammatory response. Proc Natl Acad Sci USA. 2007;104:19434–9. doi: 10.1073/pnas.0704535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasahara M. The 2R hypothesis: an update. Curr Opin Immunol. 2007;19:547–52. doi: 10.1016/j.coi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 101.Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–51. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]
- 102.Kaiser P, Rothwell L, Avery S, Balu S. Evolution of the interleukins. Dev Comp Immunol. 2004;28:375–94. doi: 10.1016/j.dci.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Li J-H, Shao J-Z, Xiang L-X, Wen Y. Cloning, characterization and expression analysis of pufferfish interleukin-4 cDNA: the first evidence of Th2-type cytokine in fish. Mol Immunol. 2007;44:2078–86. doi: 10.1016/j.molimm.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 104.Ohtani M, Hayashi N, Hashimoto K, Nakanishi T, Dijkstra J. Comprehensive clarification of two paralogous interleukin 4/13 loci in teleost fish. Immunogenetics. 2008;60:383–97. doi: 10.1007/s00251-008-0299-x. [DOI] [PubMed] [Google Scholar]
- 105.Joerink M, Savelkoul HFJ, Wiegertjes GF. Evolutionary conservation of alternative activation of macrophages: structural and functional characterization of arginase 1 and 2 in carp (Cyprinus carpio L.) Mol Immunol. 2006;43:1116–28. doi: 10.1016/j.molimm.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 106.Joerink M, Forlenza M, Ribeiro CMS, de Vries BJ, Savelkoul HFJ, Wiegertjes GF. Differential macrophage polarisation during parasitic infections in common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2006;21:561–71. doi: 10.1016/j.fsi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Dorris M, De Ley P, Blaxter ML. Molecular analysis of nematode diversity and the evolution of parasitism. Parasitol Today. 1999;15:188–93. doi: 10.1016/s0169-4758(99)01439-8. [DOI] [PubMed] [Google Scholar]
- 108.Taylor AI, Gould HJ, Sutton BJ, Calvert RA. Avian IgY binds to a monocyte receptor with IgG-like kinetics despite an IgE-like structure. J Biol Chem. 2008;283:16384–90. doi: 10.1074/jbc.M801321200. [DOI] [PubMed] [Google Scholar]