Abstract
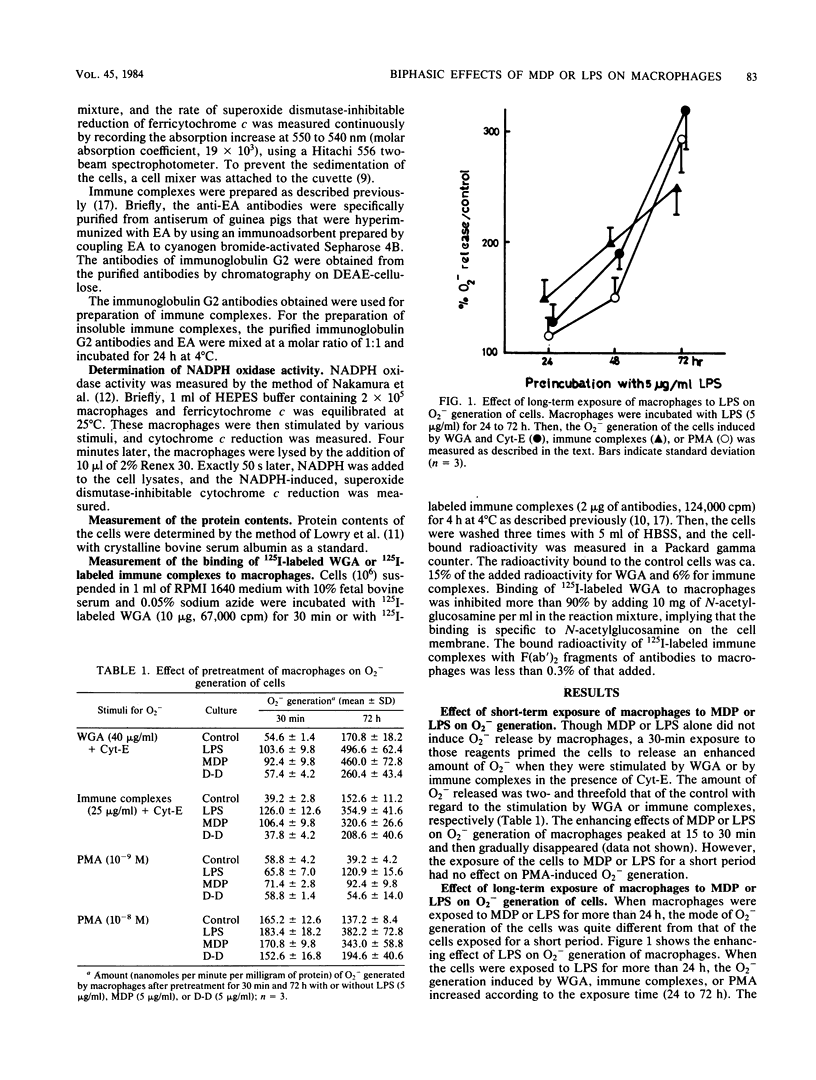
The superoxide anion (O2-)-generating activity of guinea pig macrophages stimulated by wheat germ agglutinin (WGA), immune complexes, or phorbol myristate acetate (PMA) was studied after short- and long-term exposures of the cells to muramyl dipeptide (MDP) or lipopolysaccharide (LPS). Neither MDP nor LPS alone induced O2- release in macrophages. Short-term (30 min) exposure to these agents caused the enhanced release of O2- in response to WGA or immune complexes, though the PMA-induced O2- generation was not affected. On the other hand, long-term exposure (more than 24 h) to MDP or LPS progressively enhanced O2- generation of the cells induced by WGA, immune complexes, or even PMA. These results suggest that the mechanism for O2- generation of macrophages stimulated by WGA or immune complexes differs from that stimulated by PMA and that the differences also exist between short- and long-term exposure to MDP or LPS.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Babior B. M. Endogenous protein phosphorylation by resting and activated human neutrophils. Blood. 1983 Feb;61(2):333–340. [PubMed] [Google Scholar]
- Baxter M. A., Leslie R. G., Reeves W. G. The stimulation of superoxide anion production in guinea-pig peritoneal macrophages and neutrophils by phorbol myristate acetate, opsonized zymosan and IgG2-containing soluble immune complexes. Immunology. 1983 Apr;48(4):657–665. [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Dewald B., Baggiolini M., Curnutte J. T., Babior B. M. Subcellular localization of the superoxide-forming enzyme in human neutrophils. J Clin Invest. 1979 Jan;63(1):21–29. doi: 10.1172/JCI109273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Kinnes D. A. Prostaglandin E1 and prostaglandin I2 modulation of superoxide production by human neutrophils. Biochem Biophys Res Commun. 1983 Jun 15;113(2):506–512. doi: 10.1016/0006-291x(83)91754-0. [DOI] [PubMed] [Google Scholar]
- Goodwin B. J., Weinberg J. B. Receptor-mediated modulation of human monocyte, neutrophil, lymphocyte, and platelet function by phorbol diesters. J Clin Invest. 1982 Oct;70(4):699–706. doi: 10.1172/JCI110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma K., Yamaguchi T., Kaneda M., Shimada K., Tomita Y., Chance B. A determination of H2O2 release by the treatment of human blood polymorphonuclear leukocytes with myristate. J Biochem. 1979 Jul;86(1):87–95. [PubMed] [Google Scholar]
- Kaku M., Yagawa K., Nagao S., Tanaka A. Enhanced superoxide anion release from phagocytes by muramyl dipeptide or lipopolysaccharide. Infect Immun. 1983 Feb;39(2):559–564. doi: 10.1128/iai.39.2.559-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakamura M., Baxter C. R., Masters B. S. Simultaneous demonstration of phagocytosis-connected oxygen consumption and corresponding NAD(P)H oxidase activity: direct evidence for NADPH as the predominant electron donor to oxygen in phagocytizing human neutrophils. Biochem Biophys Res Commun. 1981 Feb 12;98(3):743–751. doi: 10.1016/0006-291x(81)91175-x. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Mizel D. Role of transmethylation in the elicitation of an oxidative burst in macrophages. Cell Immunol. 1982 Sep 15;72(2):277–285. doi: 10.1016/0008-8749(82)90475-0. [DOI] [PubMed] [Google Scholar]
- Yagawa K., Itoh T., Tomoda A. Effect of transmethylation-reaction and increased levels of cAMP on superoxide generation of guinea-pig macrophages induced with wheat germ agglutinin and phorbor myristate. FEBS Lett. 1983 Apr 18;154(2):383–386. doi: 10.1016/0014-5793(83)80187-2. [DOI] [PubMed] [Google Scholar]
- Yagawa K., Okamura J. Role of adenosine deaminase in activation of macrophages. Infect Immun. 1981 Apr;32(1):394–397. doi: 10.1128/iai.32.1.394-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagawa K., Onoue K., Aida Y. Structural studies of Fc receptors. I. Binding properties, solubilization, and partial characterization of fc receptors of macrophages. J Immunol. 1979 Jan;122(1):366–373. [PubMed] [Google Scholar]
- Young J. D., Unkeless J. C., Kaback H. R., Cohn Z. A. Mouse macrophage Fc receptor for IgG gamma 2b/gamma 1 in artificial and plasma membrane vesicles functions as a ligand-dependent ionophore. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1636–1640. doi: 10.1073/pnas.80.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]



