Abstract
Dendritic cells (DCs) mediate interactions between innate and specific immunity and may induce regulatory mechanisms. We investigated the effects of modulated DCs in mice with collagen-induced arthritis (CIA) and tested the responses of cells to induced naturally occurring regulatory T cells. DCs were stimulated or not with DNA or lipopolysaccharide (LPS) for 24 hr. DC maturation was assayed, and then modulated DCs were intraperitoneally injected on day 14 into DBA/1 mice to treat CIA. In addition to arthritis scores and type 2 collagen (CII) response, the induction of CD4+ CD25+ T cells was analysed by flow cytometry in peripheral blood and the expression of Foxp3, transforming growth factor (TGF)-β, interleukin (IL)-10 and cytotoxic T-lymphocyte antigen (CTLA)-4 was quantified. Finally, the expression of indoleamine-2,3-dioxygenase (IDO) was assayed in DCs. In comparison with LPS-stimulated DCs, plasmid-stimulated DCs expressed lower levels of major histocompatibility complex (MHC) class II, CD40, CD80 and CD86 molecules and secreted less IL-12p70, interferon (IFN)-γ, IL-10 and TNF-α, displaying a semi-mature phenotype. Compared with non-stimulated DCs, stimulated DCs improved arthritis scores when injected after immunization, without modifying the T helper type 1 (Th1)/Th2 balance of the immune response against collagen. Stimulated DCs induced markers for regulatory T cells (Foxp3, TGF-β1 and CTLA-4) in vivo. Only LPS-stimulated DCs expressed IDO, which may explain their better therapeutic efficacy. Regulatory mechanisms were induced using DCs modulated by innate immunity stimulators. Innate immunity mechanisms do not require the presence of the disease-causing antigen, even in T- and B-cell specific diseases. Our results have implications for the treatment of rheumatoid arthritis, an autoimmune disease whose triggering antigen has not been identified, and substantially clarify the role of regulatory T cells in CIA.
Keywords: antigen-independent maturation, collagen-induced arthritis, dendritic cells, innate immunity, regulatory T cells
Introduction
Rheumatoid arthritis (RA), the most common inflammatory joint disease, exacts a huge toll in terms of disability, deformity, reductions in quality of life, premature death, and economic costs.1 RA is an autoimmune disease characterized by chronic inflammation of the synovial membrane, which is infiltrated by activated immune cells including CD4+ T cells, B cells, and antigen-presenting cells such as dendritic cells (DCs) and macrophages. The factors responsible for RA induction and progression are poorly understood but may involve interactions between innate and specific immunity.2 Although sound evidence indicates direct involvement of T cells and B cells in the immune process and supports a crucial role for class II major histocompatibility complex (MHC-II) antigens, no specific antigen has been proved to trigger the disease or to produce synovitis. Immune regulation mechanisms probably play a role in limiting RA.3 Regulatory T cells (Tregs) can suppress the activation and proliferation of CD4+ T cells, both in vivo4–6 and in vitro.7,8 Although Foxp3+ T cells account for only 2% of all lymph node cells,9 there is growing evidence that they are directly involved in regulating immune processes.10,11 Depletion of CD4+ CD25+ Tregs in animal models can lead to the spontaneous development of autoimmune diseases and also increases the severity of symptoms in mice with collagen-induced arthritis (CIA), a model for RA.12 Interestingly, functional and quantitative deficits in Tregs have been described in patients with active RA. Treg function was restored after successful tumour necrosis factor (TNF)-α antagonist therapy in patients with RA.13 The balance between effectors and regulatory cells may play a pivotal role in determining the speed and severity of disease progression, at least in CIA.14,15 Many cells are involved in the induction of Tregs. Of these, dendritic cells (DCs), which are highly plastic cells, may be among the most efficient given their ability to interact strongly with T cells. Defective stimulation of T cells by DCs was shown in a rat model of spondyloarthropathy and suggested as a possible contributor to the disease.16 The optimum DC maturation stage (immature, semi-mature or fully mature) and the best antigen for achieving tolerance in autoimmune disease remain matters of debate. DCs at all maturation stages have been reported to induce tolerance.17 In RA models, fully mature collagen-loaded DCs prevented CIA after the induction of a T helper type 2 (Th2) shift.18 Recently, injection of immature DCs before collagen immunization was shown to suppress the development of CIA by inducing a newly described subset of Tregs.19
Because the best antigen for inducing RA-like disease in animal models remains a matter of debate, we evaluated the effect of unloaded DCs in mice previously immunized against bovine type II collagen (CIIb). We used both semi-mature and fully mature DCs, comparing their effects on clinical arthritis severity, the anti-collagen immunological response and Treg induction to those of immature DCs or buffer. Because we observed that plasmid stimulation of DCs induced a stable state of semi-maturation, we compared plasmid-modulated DCs to lipopolysaccharide (LPS)-stimulated DCs.
Materials and methods
Mice
DBA/1 and C57BL/6 mice were purchased from Harlan (Gannat, France). The mice were used at 6–12 weeks of age. Animals were housed and acclimated according to National Animal Care guidelines (Agreement A-93-008-01). All experiments were approved by the Animal Care and Use Committee at Villetaneuse University, France.
Induction and evaluation of CIA
CIA was induced in male DBA/1J (H-2q) mice. CIIb (Chondrex™; Morwell Diagnostics, Zurich, Switzerland) was dissolved in 0·1 m acetic acid solution overnight at 4° to a concentration of 2 mg/ml. The dissolved CIIb was emulsified in an equal volume of complete Freund's adjuvant (Difco, Marne la Vallée, France) and 50 μl was injected subcutaneously into the base of the tail. A booster injection, also of 50 μl, was given 3 weeks later. Thereafter, each mouse was examined two to three times a week. Limb joint arthritis was scored using an established scoring system.20 Briefly, the clinical severity of the arthritis in each joint or group of joints (toes, tarsus, ankle, wrist and knee) was graded on a five-point scale (0, normal findings; 1, erythema; 2, swelling; 3, deformity; and 4, necrosis). The scores were summed to yield the arthritis score. In each group, arthritis severity was expressed as the mean score on a given day, or as the mean of the maximal arthritic index in each mouse during the course of the disease.
Generation of bone marrow-derived dendritic cells (BMDCs)
BMDCs were produced as described by Lutz and co-workers.21 Briefly, DBA/1 mice were killed and their femurs were rapidly harvested and washed in sterile phosphate-buffered saline (PBS) and then incubated for 2–5 min in 70% alcohol at room temperature. The bone marrow was flushed out and passed through a filter with a pore size of 0·70 mm. The leucocytes were washed and re-suspended in classical medium: RPMI 1640 (Invitrogen, Cergy Pontoise, France) supplemented with penicillin (100 U/ml; Eurobio, Les Ulis, France) and streptomycin (100 μg/ml; Eurobio), 2-mercaptoethanol (50 μm; Prolabo, Fontenay-sous-Bois, France), and 10% fetal calf serum (PAN; Dutscher, Illkirch, France). Leucocytes were seeded at 2 × 106 per 100-mm Petri dish, in 10 ml of RPMI 1640-supplemented medium containing 200 ng of recombinant murine granulocyte–macrophage colony-stimulating factor (rmGM-CSF; Difco). On day 3, 10 ml of supplemented medium containing 200 ng of rmGM-CSF was added to each dish. On day 6, the cells were collected, washed, and stimulated with 1 μg/ml of LPS (O26:B6 or O111:B4; Sigma, Lyon, France) or with LPS-free pcDNA3·1neo plasmid (Invitrogen) at a concentration of 10, 1 or 0·01 μg/ml, according to the Limulus Amebocyte Lysate assay (Biowhittaker, Walkersville, MD), for 24 or 48 hr.
Purity control, maturation and endocytosis evaluation by cytofluometry
BMDCs were incubated with specific antibodies to IA/IE (2G9), CD11b (M1/70), CD8a (53-6·7), CD45R/B220 (RA3-6B2), CD4 (GK1·5), CD3 (17A2), and NKR-P1B or 1C (PK136), all purchased from Becton-Dickinson (Le Pont de Claix, France). Briefly, the cells were incubated with the appropriate antibodies for 30 min at 4°, washed, and incubated with specific secondary antibodies for 30 min at 4°. The cells were then washed and incubated with fluorescein isothiocyanate (FITC)-CD11c (HL-3) antibody for 30 min at 4°. Finally, the cells were washed and analysed by flow cytometry (Coulter XL, Villepinte, France). The results (mean of 10 experiments) showed that 90% of BMDCs were CD11c+ and 20% were CD11b+; none of the BMDCs expressed CD4, CD3, CD8, B220, or NK1·1. To evaluate cell maturation, we washed BMDCs after 24 or 48 hr of stimulation then incubated them with specific antibodies to IA/IE, CD40 (3/23), CD80 (1G10), or CD86 (GL1), for 30 min at 4°. To evaluate endocytosis, 2 × 105 BMDCs stimulated for 24 or 48 hr were incubated with 200 μg of Texas Red Dextran [40 000 molecular weight (MW); Invitrogen, Cergy Pontoise, France] for 30, 60, 120, or 240 min or 24 hr. The cells were then stained as below with a FITC-CD11c antibody, washed, and analysed by flow cytometry.
Enzyme-linked immunosorbent assay (ELISA)
Murine interferon (IFN)-γ, IL-10, IL-12p70 and TNF-α were assessed using ELISA kits (R&D Systems, Lille, France) according to the manufacturer's instructions.
Type II collagen antibody titration
Serum CIIb-specific antibody was measured using a standard ELISA. Briefly, 96-well plates (Nunc ImmunoPlate Maxisorp Surface; VWR, Fontenay sous Bois, France) were coated with CIIb overnight at 4°. After washing, blocking was achieved by adding 1% bovine serum albumin (Sigma)/PBS solution. After 2 hr, the plates were washed, and diluted sera were incubated in the wells overnight at 4°. After washing, total immunoglobulin G (IgG), IgG2a or IgG1 CIIb-specific antibody was detected using alkaline phosphatase (AP)-conjugated anti-mouse IgG (Sigma), anti-mouse IgG2a or anti-mouse IgG1 (Clinisciences, Montrouge, France), respectively. The plates were washed, incubated with 50 μl of para-nitrophenyl phosphate (Sigma) at room temperature for 30 min, and finally read at 405 nm.
RNA isolation
The spleen, peripheral lymph nodes, and mesenteric lymph nodes were removed and crushed onto a filter with a pore size of 0·70 mm. If needed, red blood cells were lysed in red blood cell lysis (ACK) buffer. The cells were washed and re-suspended in TriReagent (Euromedex, Mondolsheim, France). RNA was then isolated as recommended by the manufacturer. Briefly, chloroform (Sigma) was added to the cell suspension, which was then centrifuged. Isopropanol was added to the aqueous phase, which was centrifuged. The RNA was washed with 75% ethanol, dried, and re-suspended in RNAse-free DNAse-free water. The RNA concentration was measured at 260 nm. For all samples, 260:280 ratios were at least equal to 1·6. In some cases, extractions were performed from BMDCs and CD4+ CD25+/− T cells as described below.
Reverse transcription
RNA (5 μg) was reverse-transcribed using the SuperScriptTMIII reverse transcriptase kit (Invitrogen). First, oligo dT (500 ng; Sigma) and dNTPmix (Invitrogen) were added to the samples, which were then incubated for 5 min at 65°. First-strand buffer and DTT were then added on ice and the mix was incubated for 2 min at 42°. Finally, SSIII reverse transcriptase was added and the mix was incubated for 50 min at 42°. Inactivation was achieved by incubation for 15 min at 70°.
Quantitative polymerase chain reaction (PCR)
PCR was performed using the Light Cycler FastStart DNA MasterPlus SYBR Green I kit (Roche Diagnostics, Mannheim, Germany). Denaturation consisted of 8 min of incubation at 95° and fusion in a sequence of 10 seconds at 95°, 30 seconds at 65°, and a final signal-acquiring phase during which the temperature was increased from 65° to 95° in steps of 1° per second in order to check the amplicon fusion temperature. Cycling parameters for each oligonucleotide are listed below.
Actin. Ten seconds at 95°, 5 seconds at 63°, and 8 seconds at 72°, repeated 40 times; forward primer, 5′-AGAGGGAAATCGTGCGTGAC-3′; reverse primer, 5′-CAATAGTGATGACCTGGCCGT-3′; amplicon size 148 bp; amplicon fusion temperature, about 86°; and final primer concentration, 0·4 μm.
IL-10. Same cycle as actin, repeated 40 times; forward primer, 5′-GGTTGCCAAGCCTTATCGGA-3′; reverse primer, 5′-ACCTGCTCCACTGCCTTGCT-3′; amplicon size, 190 bp; amplicon fusion temperature, about 86°; and final primer concentration, 0·2 μm.
Foxp3. Ten seconds at 95°, 10 seconds at 63°, and 10 seconds at 72°, repeated 45 times; forward primer, 5′-CAGCCTGCCTCTGACAAGAA-3′; reverse primer, 5′-GGGGGTTCAAGGAAGAAGAG-3′; amplicon size, 272 bp; amplicon fusion temperature, about 90°, and final primer concentration, 0·33 μm.
Indoleamine-2,3-dioxygenase (IDO). Ten seconds at 95°, 10 seconds at 64°, and 10 seconds at 72°, repeated 40 times; forward primer, 5′-AGTCGGAAGAGCCCTCAAAT-3′; reverse primer, 5′-GGTGTTTTCTGTGCCCTGAT-3′; amplicon size, 152 bp; amplicon fusion temperature, about 84°, and final primer concentration, 0·5 μm.
Glucocorticoid-induced TNF protein-related (GITR). Ten seconds at 95°; 10 seconds at 60°, and 12 seconds at 72°, repeated 45 times; forward primer, 5′-AGAACGGAAGTGGCAACAAC-3′; reverse primer, 5′-TTGCAGATCTTGCACTGAGG-3′; amplicon size, 130 bp; amplicon fusion temperature, about 83°; and final primer concentration, 0·5 μm.
Transforming growth factor β1 (TGF-β1). Ten seconds at 95°, 10 seconds at 66°, and 9 seconds at 72°, repeated 40 times; forward primer, 5′-TTGCTTCAGCTCCACAGAGA-3′; reverse primer, 5′-TGGTTGTAGAGGGCAAGGAC-3′; amplicon size, 183 bp; amplicon fusion temperature, about 87·5°; and final primer concentration, 0. 33 μm.
Quantification was performed using realquant 1·01 software, by creating automatically normalized ratios (NRs) as follows: NR = [C(sample target gene)/C(sample reference gene)]/[C(calibrator target gene)/C(calibrator reference gene)], where C is the crossing point.
T-cell isolation and CD4+ CD25+ cell isolation from blood
For splenic and lymph node T-cell isolation, organs were crushed onto a nylon filter of pore size 0·70 mm. Peripheral blood lymphocytes were obtained through conservative (e.g. on D25) or lethal (e.g. D38) intakes and purified after a conventional gradient separation and red blood cell lysis in ACK. The cells were washed and isolated by negative selection using the mouse CD4+ T Cell Isolation Kit (Miltenyi Biotec, Paris, France) according to the manufacturer's instructions. In some cases, CD4+ CD25+/− T lymphocytes were isolated using the CD4+ CD25+ Regulatory T Cell Isolation Kit (Miltenyi Biotec) according to the manufacturer's instructions.
Statistical analysis
Means were compared using unpaired Student's t-tests or analysis of variance (ANOVA). P values smaller than 0·05 were considered significant.
Results
Plasmid-stimulated dendritic cells are semi-mature dendritic cells
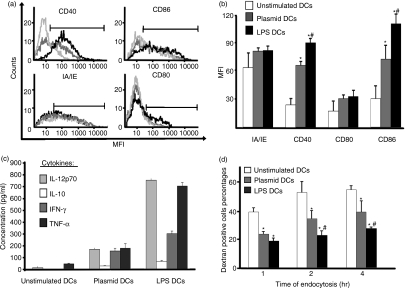
DCs from bone marrow of DBA1/J mice were stimulated with DNA (naked plasmid) and compared with non-stimulated DCs (immature DCs) and LPS-stimulated DCs (fully mature DCs). After 24 or 48 hr, cells were harvested and analysed by flow cytometry for expression of MHC class II and costimulatory molecules; results are shown for the CD11c+ DC population. CD40 and CD86 expression levels of plasmid-stimulated DCs were higher than those of non-stimulated DCs but lower than those of LPS-stimulated DCs (Fig. 1a). Interestingly, plasmid-stimulated and LPS-stimulated DCs expressed CD80 and MHC class II molecules at the same level. These experiments were replicated five times, and the results were used to determine the mean fluorescence intensity (MFI) for each molecule (Fig. 1b). LPS-stimulated DCs expressed more CD40 and CD86 than did non-stimulated or plasmid-stimulated DCs. Plasmid-stimulated DCs expressed more CD40 and CD86 than did non-stimulated DCs. The cytokine assay results showed that non-stimulated DCs produced small amounts of IL-12p70 and TNF-α and no IL-10 or IFN-γ. Plasmid-stimulated DCs produced larger amounts of IL-12p70 and TNF-α and also produced IL-10 and IFN-γ. LPS-stimulated DCs produced considerably larger amounts of cytokines than did non-stimulated and plasmid-stimulated DCs; in increasing order, IFN-γ, TNF-α and IL-12. Interestingly, we found no shift in maturation neither when we increased the stimulation time to 48 hr (data not shown), nor using more than 10 μg/ml of plasmid. We then evaluated the functional capabilities of the DCs. The percentages of dextran-positive cells suggested that endocytosis ability was highest for non-stimulated DCs, intermediate for plasmid-stimulated DCs, and lowest for LPS-stimulated DCs (Fig. 1d). Finally, in mixed leucocyte reactions, allogeneic stimulation was weakest for non-stimulated DCs, intermediate for plasmid-stimulated DCs, and strongest for LPS-stimulated DCs (data not shown). Cells expressing CD80 and class II MHCs were not modified. Overall, these results showed that plasmid-stimulated DCs were more mature than non-stimulated DCs but less mature than LPS-stimulated DCs. We considered that non-stimulated DCs were immature, LPS-stimulated DCs fully mature, and plasmid-stimulated DCs semi-mature, and chose an optimum concentration of plasmid of 10 μg/ml.
Figure 1.
Phenotype of bone marrow-derived dendritic cells (BMDCs) after stimulation. (a, b, c) BMDCs were stimulated for 24 hr with 10 μg/ml plasmid (grey line) or 1 μg/ml lipopolysaccharide (LPS) (black line) or left unstimulated (light grey or white line). (a) Expression of costimulatory molecules was determined by flow cytometry. (b) Mean value of mean fluorescence intensity (MFI) in five separate experiments. Expression of costimulatory molecules by DCs was determined by flow cytometry, which showed statistically significant differences (*P<0·05 for LPS- and plasmid-stimulated DCs versus unstimulated DCs; #P<0·05 for LPS-stimulated DCs versus plasmid-stimulated DCs). (c) Cytokine production by BMDCs. LPS- and plasmid-stimulated DCs produced larger amounts of cytokines than did unstimulated DCs (plasmid-stimulated DCs versus unstimulated DCs, for all cytokines: P<0·001; LPS-stimulated DCs versus unstimulated DCs: P<0·0001). LPS-stimulated DCs produced greater amounts of cytokines than did plasmid-stimulated DCs (P<0·01). The means of five independent experiments are shown. (d) Dextran endocytosis: percentage of dextran-positive DCs after 1, 2 and 4 hr (means of three separate experiments). Endocytosis was less marked with plasmid- and LPS-stimulated DCs than with unstimulated DCs (*P<0·05). Plasmid-stimulated DCs showed stronger endocytosis than did LPS-stimulated DCs (#P<0·05 for LPS-stimulated DCs versus plasmid-stimulated DCs). Plasmid DCs, plasmid-stimulated DCs; LPS DCs, LPS-stimulated DCs.
In vivo prevention of CIA using semi-mature and fully mature antigen-independent DCs
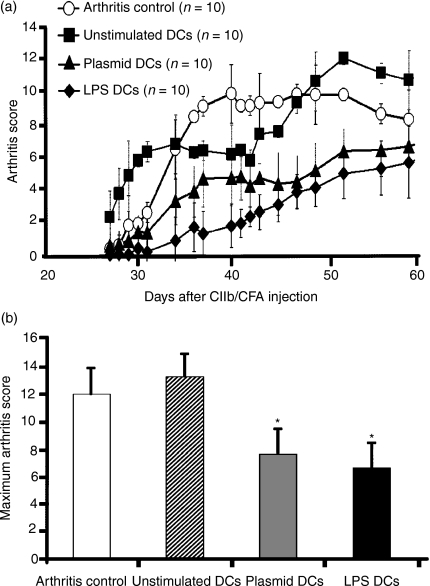
We tested the ability of semi-mature unpulsed DCs to modulate CIA induction. We chose non-stimulated DCs as the immature control, and LPS-stimulated DCs as the fully mature DCs. DCs were given by intraperitoneal injection, which has been used successfully to treat experimental autoimmune encephalomyelitis22 and to induce a Th2 shift, after numeration (less than 10% of dead cells in each injected sample). Plasmid-stimulated DCs markedly decreased CIA severity (Fig. 2a). After a single injection, protection was statistically significant from day 34 to day 60 (P<0·05 to P<0·005 for plasmid-stimulated DCs and P<0·01 to P<0·001 for LPS-stimulated DCs, compared with the group given buffer and the non-stimulated DC group). This statistically significant protection was confirmed by ANOVAs comparing the buffer group with the plasmid-stimulated DC group (P<0·05) and the LPS-stimulated DC group (P<0·001) and comparing the non-stimulated DC group with the two other DC groups (P<0·05 for both). No difference was found between the buffer group and the non-stimulated DC group. Arthritis scores were lower in the LPS-stimulated group than in the plasmid-stimulated DC group, but the differences were not statistically significant. The maximal arthritis score was significantly lower in the plasmid-stimulated DC and LPS-stimulated DC groups (P<0·01) than in the buffer and non-stimulated DC groups (Fig. 2b). The onset and incidence of CIA were not significantly different in the four groups (data not shown).
Figure 2.
Clinical efficacy of modulated dendritic cells (DCs). (a) Mice immunized against bovine type II collagen (CIIb) were injected with 5 × 105 DCs on day 14 after the first injection of CIIb and complete Freund's adjuvant (CFA). The DCs were either not stimulated (▪) or stimulated with plasmid (▴) or lipopolysaccharide (LPS) (♦). The arthritis scores are shown relative to those in CIIb-immunized mice injected with buffer instead of DCs (○). Plasmid-stimulated DCs were associated with significantly lower arthritis scores from day 34 to day 42 (P≤0·05) and from day 43 to day 60 (P≤0·02). Arthritis scores with LPS-stimulated DCs were significantly lower than those with unstimulated DCs or buffer from day 34 to day 60 (P≤0·01); this was confirmed by analysis of variance (ANOVA). Arthritis scores were not significantly different between unstimulated DCs and buffer. Arthritis scores were lower with LPS-stimulated DCs than with plasmid-stimulated DCs, but the difference was not statistically significant. (b) Maximum arthritis scores in each group. The values were significantly lower with plasmid- or LPS-stimulated DCs than with unstimulated DCs or buffer (*P<0·01). The experiments were repeated twice, with similar results. Plasmid DCs, plasmid-stimulated DCs; LPS DCs, LPS-stimulated DCs.
Toll-like receptor (TLR)-modulated DC-induced decrease in CIIb immunoglobulin production with no Th2 shift
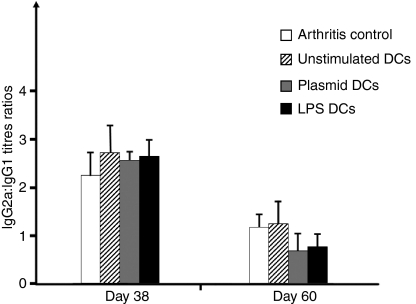
We investigated whether the clinical arthritis protection seen with TLR-modulated DCs (plasmid- or LPS-stimulated DCs) was associated with a Th2 shift in the collagen response. In the mice given plasmid- or LPS-stimulated DCs, anti-CIIb IgG1 and IgG2a titres were decreased compared with the mice given buffer or non-stimulated DCs on day 38 after collagen injection. Nevertheless, the IgG2a:IgG1 ratios were similar (Fig. 3). Thus, none of the three types of DC modified consistently the Th1/Th2 balance. The plasmid- and LPS-stimulated DCs significantly inhibited the Th1 and Th2 responses to type II collagen. On day 60, IgG2a titres but not IgG1 titres were slightly decreased in the mice given plasmid- or LPS-stimulated DCs. At the end of the experiment, no statistically significant change in the anti-collagen IgG2a:IgG1 ratio was observed, suggesting a slight Th1 response inhibition with no Th2 shift.
Figure 3.
Ratio of bovine type II collagen (CIIb)-specific immunoglobulins. CIIb-specific IgG2a:IgG1 ratios were taken as indicators of the T helper type 1 (Th1)/Th2 balance after injection of 5 × 105 dendritic cells (DCs) (10 animals per group). On day 38, mice given plasmid- or lipopolysaccharide (LPS)-stimulated DCs had lower levels of CIIb-specific IgG1 and IgG2a compared with mice given unstimulated DCs or buffer (P<0·05). The IgG2a:IgG1 ratios were not significantly different. On day 60, no significant differences were found for IgG1 titres, whereas IgG2a titres remained lower in the plasmid- and LPS-stimulated DC groups than in the unstimulated DC and buffer groups (P<0·05); nevertheless, the IgG2a:IgG1 ratios were not significantly different. The experiment was repeated twice with similar results. Plasmid DCs, plasmid-stimulated DCs; LPS DCs, LPS-stimulated DCs.
In vivo induction of the Treg markers Foxp3, TGF-β1, and IL-10
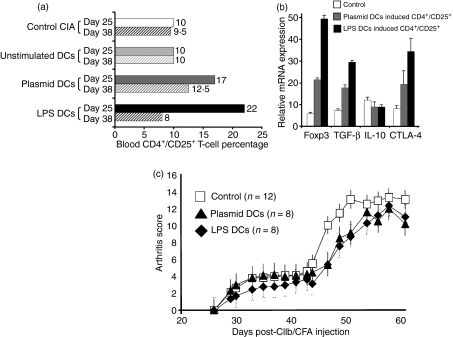
We then investigated the induction of Tregs in lymphoid organs and blood following in vivo injection of DCs on day 14. Using flow cytometry, we documented induction of T cells exhibiting the conventional CD4+ CD25+ phenotype in blood at day 25 post-immunization, after the injection of plasmid- or LPS-stimulated DCs only (Fig. 4a). These CD4+ CD25+ T cells decreased at day 38 but seemed to be maintained in mice injected with plasmid-stimulated DCs. It was noteworthy that we did not observe any difference in CD4+ CD25+ T-cell percentages in lymphoid organs or spleen using flow cytometry analyses of these markers (data not shown). To determine whether these cells were Tregs, we extracted them from blood and looked for expression of Treg markers. Over-expression of Foxp3 was noted in CD4+ CD25+ T cells induced by plasmid- or LPS-stimulated DCs (Fig. 4b). These CD4+ CD25+ Foxp3+ T cells expressed the ctla-4 gene but not the GITR gene (not shown). Strong TGF-β1 expression by these cells was noted, suggesting a possible mechanism of activation. However, similar to non-activated CD4+ CD25− T cells, the CD4+ CD25+ Foxp3+ T cells expressed little or none of the Tr1-specific cytokine IL-10.
Figure 4.
Induction of T cells with a regulatory phenotype (Treg). (a) Blood CD4+ CD25+ T-cell percentages on day 25 (solid bars) and day 38 (hatched bars) after injection of modulated or unstimulated dendritic cells (DCs) into bovine type II collagen (CIIb)-immunized mice. Statistically significant differences were found between mice injected with buffer or unstimulated DCs and mice injected with plasmid- or lipopolysaccharide (LPS)-stimulated DCs (P<0·05 for injection on day 25). Results are representative of three separate experiments. (b) Expression of Treg markers in purified CD4+ CD25+ T cells from mice injected with plasmid-stimulated DCs (grey) or LPS-stimulated DCs (black) or control cells (white). mRNA expression of foxp3, transforming growth factor (TGF)-β1, interleukin (IL)-10, and cytotoxic T-lymphocyte antigen (CTLA-4) was determined. All differences were statistically significant (P<0·05) except for IL-10. Each result is the mean of two separate experiments. Control cells were purified CD4+ CD25+ T cells from mice injected with unstimulated DCs or non-injected mice. (c) In vivo injection of plasmid or LPS-stimulated DCs induced Tregs. Tregs were obtained after injection on day 14 of 5 × 105 plasmid- or LPS-stimulated DCs, being extracted at 25 days after immunization against CIIb. These cells were then injected into independent immunized mice on day 25 (the purity of the population was > 95% of CD4+ CD25+ T cells; intraperitoneal injection of 2·5 × 106 cells). Arthritis scores were significantly lower from day 47 to day 60 (P≤0·05) in animals injected with plasmid-stimulated DCs (triangles) or LPS-stimulated DCs (rhombuses). Controls were purified CD4+ CD25+ T cells, obtained from unstimulated DC injected mice and/or CIIb-immunized mice, which did not display any prevention effect on arthritis. The experiment shown was repeated twice, with similar results. Plasmid DCs, plasmid-stimulated DCs; LPS DCs, LPS-stimulated DCs; CFA, complete Freund's adjuvant.
We then injected 2·5 × 106 of CD4+ CD25+ T cells obtained on day 25 (maximum expression of Treg markers; Fig. 4a) from mice injected with modulated DCs (plasmid- or LPS-stimulated DCs) on day 14. As shown in Fig. 4c, transfer of Tregs on day 25 after immunization to recipient mice decreased significantly the arthritis score if Tregs were obtained from animals injected with plasmid- or LPS-stimulated DCs (P<0·05 at days 47, 49, 51 and 56). The injection of CD4+ CD25+ cells obtained from animals with active arthritis process (CIA or non-stimulated DCs) did not alter the course of arthritis (controls in Fig. 4c).
Thus, Treg activation occurred in vivo within secondary lymphoid organs from day 16 to day 28 (Table 1) and in blood on day 25 (Fig. 4a).
Table 1.
In vivo induction of regulatory T cell (Treg) markers in lymphoid organs after injection of dendritic cells (DCs)
| Non-stimulated DCs | Plasmid DCs | LPS DCs | Non-stimulated DCs | Plasmid DCs | LPS DCs | ||||
|---|---|---|---|---|---|---|---|---|---|
| foxp3 | IL-10 | ||||||||
| PLNs | Day 16 | 1 | 43 | 36 | PLNs | Day 18 | 3 | 12 | 5 |
| Spleen | Day 18 | 4 | 35 | 48 | PLNs | Day 21 | 4 | 18 | 12 |
| MLNs | Day 24 | 3 | 6 | 31 | PLNs | Day 24 | 4 | 8.5 | 18 |
| TGF-β | TGF-β | ||||||||
| Spleen | Day 18 | 6 | 23 | 25 | PLNs | Day 18 | 8 | 26 | 16 |
| Spleen | Day 21 | 32 | 88 | 124 | PLNs | Day 21 | 17 | 26 | 22 |
| Spleen | Day 24 | 12 | 28 | 54 | PLNs | Day 24 | 14 | 57 | 33 |
Expression of the foxp3, interleukin-1- (IL-10) and transforming growth factor-β1 (TGF-β1) genes in the spleen, peripheral lymph nodes (PLNs), and mesenteric lymph nodes (MLNs) on days 16, 18, 21, 24 and 28 after immunization with bovine type II collagen and complete Freund's adjuvant. On day 14, the mice were injected with non-stimulated DCs, plasmid-stimulated DCs, or lipopolysaccharide (LPS)-stimulated DCs. Organs were harvested on day 16, 18, 21, 24 or 28, and total mRNA was extracted. After reverse transcription, total cDNA was determined using quantitative reverse transcriptase–polymerase chain reaction (Q-RT-PCR). Only detectable levels of gene expression are represented (e.g. there was no detectable expression of foxp3 on days 24 and 28 or IL-10 in PLNs and spleen). Plasmid- and LPS-stimulated DCs induced over expression of foxp3, IL-10, and TGF-β compared with non-stimulated DCs (P<0·05) or buffer (not shown) for all the results shown in the table. This experiment was repeated twice, with similar results.
LPS-stimulated DCs express a tolerogenic phenotype
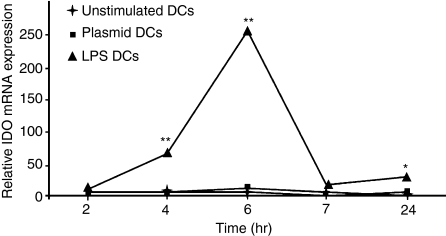
To investigate whether CIA prevention was mediated by a tolerogenic DC phenotype, we used quantitative reverse transcriptase–polymerase chain reaction (Q-RT-PCR) to test the expression of IDO, a key molecule in Treg education. IDO expression by DCs peaked 6 hr after the start of a 24-hr period of LPS stimulation (Fig. 5). Non-stimulated DCs or plasmid-stimulated DCs did not express IDO in detectable amounts. These data suggest that LPS stimulation of DCs may enhance Treg development via IDO expression and, in light of the clinical efficacy of plasmid-stimulated DCs, possibly other additional mechanisms.
Figure 5.
Lipopolysaccharide (LPS) stimulates indoleamine-2,3-dioxygenase (IDO) expression by dendritic cells (DCs). Unstimulated DCs and DCs stimulated with plasmid or LPS were used. Stimulations were stopped after 2, 4, 6, 7 and 24 hr for mRNA collection. IDO expression was measured using quantitative reverse transcriptase–polymerase chain reaction (Q-RT-PCR). Only LPS induced IDO expression (**P<0·01 for 4 and 6 hr; *P<0·05 for 24 hr; compared with unstimulated DCs or plasmid-stimulated DCs). The experiment was repeated for times, with similar results. Plasmid DCs, plasmid-stimulated DCs; LPS DCs, LPS-stimulated DCs.
Discussion
In our study, unpulsed modulated DCs affected the occurrence and severity of CIA. We chose to use DNA-stimulated DCs exhibiting a semi-mature phenotype based on previous evidence suggesting the involvement of semi-mature DCs in tolerance induction.23 The phenotype induced by nude plasmid (pcDNA) was stable with plasmid concentrations of 10 μg/ml and was not the result of LPS contamination, as checked carefully at each evaluation. The high CpG concentration in the plasmid may explain this efficacy on DCs. Phosphodiester oligonucleotides were not effective, probably because they underwent rapid degradation. Thus, in future studies, phosphorothioate endotoxin-free oligonucleotides containing CpG sequences may be the best choice for the assay.
As expected, plasmid-stimulated DCs expressing suboptimal levels of costimulation molecules were effective in treating CIA. Interestingly, an even larger effect was achieved using fully mature LPS-stimulated DCs. In earlier studies, mature DCs stimulated CD4+ CD25+ T cells exhibiting a regulatory phenotype.24,25 CpG involvement in several regulation mechanisms was demonstrated recently.26 It is notable that injection of unloaded immature DCs on day 14 was not effective for the in vivo prevention of arthritis. Differences in the route and timing of injections are probably the main explanations for the multiplicity of effects observed in the literature with DC injections.
The specific B-cell response to collagen was diminished by semi-mature plasmid-stimulated DCs and by fully mature LPS-stimulated DCs, despite the absence of antigen loading. A major advantage of CIA is that it involves both B- and T-cell mediated effects, thus mimicking part of the pathophysiology of RA. Interestingly, we found no evidence of a shift in the B- and T-cell response (using in vitro re-stimulation of specific T cells against CIIb; data not shown) via a Th2 response. We could exclude a direct effect of the stimulators we used because we injected washed cells; as mentioned above, we carefully checked for the presence of contamination in the preparation. Despite a modest decrease in antibody production, the clinical prevention we observed was not caused by a Th2 shift, as measured using the IgG1:IgG2a ratio. An original feature of our study is that we chose to use unloaded DCs, as the antigen or antigens involved in RA have not been identified. Similar effects were observed in another study with unloaded DCs injected before collagen immunization of mice.19 Unloaded DCs induced tolerance when IL-2 was added;19 thus, the tolerogenic effect, which seemed ascribable to the mature DC phenotype, was dependent on IL-2. Our data are consistent with induction by semi-mature and fully mature DCs of cellular regulation mechanisms independently from the Th2/Th1 balance of the response to collagen. Moreover, Th17 cell stimulation, which are deleterious T cells in arthritis, could hardly be predicted as we clearly observed prevention of arthritis. However, IL-23 secretion by different types of modulated DCs will have to be assayed.
Semi-mature TNF-modulated DCs pulsed with collagen and injected intravenously were effective in preventing CIA.18 Unloaded and collagen-loaded immature DCs injected before collagen immunization exhibited a CIA-preventing effect possibly dependent on a new regulatory population.19 Our results are consistent with the effectiveness of cell-based therapy in CIA, but show particularity because of the route of injection of DCs. Local injection of collagen-loaded mature DCs has been shown to induce a local increase in the severity of arthritis.27 In contrast, Duivenwoorde et al. injected DCs intravenously, a route of injection known to induce tolerance through injections of high concentration of antigen, and were able to prevent CIA.18 Together with previous work on the use of DCs in CIA, our work, using intraperitoneal injections to promote regulation of collagen-induced polyarthritis, emphasizes the critical importance of the route of injection. A major advantage of our technique is that it is based on injections of unpulsed modulated DCs after collagen immunization. Thus, our method highlights the importance of using unpulsed DC injections to treat RA.
CpG motifs may either stimulate or inhibit immune responses depending on their route of administration.28 Subcutaneous injections exert adjuvant effects in lymph nodes, whereas intravenous injection may lead splenic cells to produce IDO, which is known to induce T-cell tolerance. In our study, clinical improvements in CIA were greatest with LPS-stimulated DCs, which secreted IFN-γ and also expressed IDO. IDO induction is both dependent and independent of IFN-γ in DCs.29–31 Moreover, IFN-γ has a previously known protective role in CIA.32,33 The involvement of IDO in arthritis needs to be investigated in future studies, as well as the role of IFN-γ in its production. Mechanisms independent of IDO production and activation are involved, as plasmid-modulated DCs showed neither IDO production nor IDO activation.
The best route of injection will have to be determined. Knowledge of the route followed by injected DCs in the body would help to determine whether the injected DCs educate pre-existing CD4+ CD25+ Foxp3+ cells in peripheral lymphoid organs or instead act as antigens. Our data showing expansion of CD4+ CD25+ Foxp3+ Tregs after injection of DCs (at various stages of maturity) are consistent with stimulation of pre-existing Tregs within lymphoid organs, including nodes draining joints or pseudo-germinal centres in the rheumatoid pannus.34 Nevertheless, another possibility is that semi-mature or fully mature DCs promote the emergence of Tr1 cells, as findings suggest that Tr1 cell formation may be antigen-related.35
Experiments with purified CD4+ CD25+ T cells could be optimized with flow cytometry cell sorting to obtain more Tregs. Purification from blood seems to be the best procedure, even though we observed a transient expression of foxp3 in other peripheral lymphoid organs, first in draining lymph nodes and then in the spleen. Overall, this work shows the kinetics of in vivo Treg induction after DC injections: they appeared in lymph nodes 48 hr after DC injections and then in the spleen, and thereafter were detected and could be isolated in blood until day 25.
Our data confirm that unloaded modulated DCs can prevent the development of CIA in mice. These cells were effective even when they were injected 2 weeks after collagen immunization, consistent with earlier studies. Our results establish that DCs can induce CD4+ CD25+ Foxp3+ T cells in vivo in a non-antigenic-specific manner. Moreover, our study opens up new possibilities for studying the promotion of peripheral tolerance in arthritis via IDO induction in DCs.
Acknowledgments
We are deeply grateful to Prof. F. Cymbalista (Avicenne Teaching Hospital, Bobigny, France) for helping with the cytofluometry tests and Stephane Chambris for valuable assistance with animal care.
Abbreviations
- BMDC
bone marrow-derived dendritic cells
- CIA
collagen-induced arthritis
- CIIb
bovine type II collagen
- DC
dendritic cell
- IDO
indoleamine-2,3-dioxygenase
- LPS-DC
LPS (lipopolysaccharide)-modulated DC
- MFI
mean fluorescence intensity
- MHC-II
class II major histocompatibility complex
- RA
rheumatoid arthritis
References
- 1.Rat AC, Boissier MC. Rheumatoid arthritis: direct and indirect costs. Joint Bone Spine. 2004;71:518–24. doi: 10.1016/j.jbspin.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Falgarone G, Jaen O, Boissier MC. Role for innate immunity in rheumatoid arthritis. Joint Bone Spine. 2005;72:17–25. doi: 10.1016/j.jbspin.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Fournier C. Where do T cells stand in rheumatoid arthritis? Joint Bone Spine. 2005;72:527–32. doi: 10.1016/j.jbspin.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 5.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 7.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 8.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 11.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 12.Morgan ME, Sutmuller RP, Witteveen HJ, et al. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003;48:1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005;7:R402–15. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Londei M. Role of regulatory T cells in experimental arthritis and implications for clinical use. Arthritis Res Ther. 2005;7:118–20. doi: 10.1186/ar1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacquard-Bouder C, Falgarone G, Bosquet A, Smaoui F, Monnet D, Ittah M, Breban M. Defective costimulatory function is a striking feature of antigen-presenting cells in an HLA-B27-transgenic rat model of spondylarthropathy. Arthritis Rheum. 2004;50:1624–35. doi: 10.1002/art.20211. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 18.van Duivenvoorde LM, Louis-Plence P, Apparailly F, van der Voort EI, Huizinga TW, Jorgensen C, Toes RE. Antigen-specific immunomodulation of collagen-induced arthritis with tumor necrosis factor-stimulated dendritic cells. Arthritis Rheum. 2004;50:3354–64. doi: 10.1002/art.20513. [DOI] [PubMed] [Google Scholar]
- 19.Charbonnier LM, van Duivenvoorde LM, Apparailly F, et al. Immature dendritic cells suppress collagen-induced arthritis by in vivo expansion of CD49b+ regulatory T cells. J Immunol. 2006;177:3806–13. doi: 10.4049/jimmunol.177.6.3806. [DOI] [PubMed] [Google Scholar]
- 20.Boissier MC, Feng XZ, Carlioz A, Roudier R, Fournier C. Experimental autoimmune arthritis in mice. I. Homologous type II collagen is responsible for self-perpetuating chronic polyarthritis. Ann Rheum Dis. 1987;46:691–700. doi: 10.1136/ard.46.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhang GX, Kishi M, Xu H, Rostami A. Mature bone marrow-derived dendritic cells polarize Th2 response and suppress experimental autoimmune encephalomyelitis. Mult Scler. 2002;8:463–8. doi: 10.1191/1352458502ms857oa. [DOI] [PubMed] [Google Scholar]
- 23.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 24.Brinster C, Shevach EM. Bone marrow-derived dendritic cells reverse the anergic state of CD4+CD25+ T cells without reversing their suppressive function. J Immunol. 2005;175:7332–40. doi: 10.4049/jimmunol.175.11.7332. [DOI] [PubMed] [Google Scholar]
- 25.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur J Immunol. 2004;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 26.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–42. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 27.Leung BP, Conacher M, Hunter D, McInnes IB, Liew FY, Brewer JM. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J Immunol. 2002;169:7071–7. doi: 10.4049/jimmunol.169.12.7071. [DOI] [PubMed] [Google Scholar]
- 28.Wingender G, Garbi N, Schumak B, et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur J Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 29.Jung ID, Lee CM, Jeong YI, Lee JS, Park WS, Han J, Park YM. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007;581:1449–56. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 30.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 31.Fujigaki S, Saito K, Sekikawa K, et al. Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is mediated dominantly by an IFN-gamma-independent mechanism. Eur J Immunol. 2001;31:2313–8. doi: 10.1002/1521-4141(200108)31:8<2313::aid-immu2313>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Boissier MC, Chiocchia G, Bessis N, Hajnal J, Garotta G, Nicoletti F, Fournier C. Biphasic effect of interferon-gamma in murine collagen-induced arthritis. Eur J Immunol. 1995;25:1184–90. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- 33.Kelchtermans H, Struyf S, De Klerck B, et al. Protective role of IFN-gamma in collagen-induced arthritis conferred by inhibition of mycobacteria-induced granulocyte chemotactic protein-2 production. J Leukoc Biol. 2007;81:1044–53. doi: 10.1189/jlb.0806486. [DOI] [PubMed] [Google Scholar]
- 34.Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med. 2002;195:1325–36. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J Invest Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]