Abstract
Increasing evidence suggests that primate visual cortex has a specialized architecture for processing discrete object categories such as faces. Human fMRI studies have described a localized region in the fusiform gyrus [the fusiform face area (FFA)] that responds selectively to faces. In contrast, in nonhuman primates, electrophysiological and fMRI studies have instead revealed 2 apparently analogous regions of face representation: the posterior temporal face patch (PTFP) and the anterior temporal face patch (ATFP). An earlier study suggested that human FFA is homologous to the PTFP in macaque. However, in humans, no obvious homologue of the macaque ATFP has been demonstrated. Here, we used fMRI to map face-selective sites in both humans and macaques, based on equivalent stimuli in a quantitative topographic comparison. This fMRI evidence suggests that such a face-selective area exists in human anterior inferotemporal cortex, comprising the apparent homologue of the fMRI-defined ATFP in macaques.
Keywords: face processing, fMRI, inferotemporal cortex, macaque-human homology
The fusiform face area (FFA) is one of the most distinctive regions in the human ventral temporal cortex. A wide range of noninvasive techniques, including PET, fMRI, ERP, and MEG have shown that FFA responds selectively to images of faces, relative to a wide variety of control objects (1–7). Such approaches have revealed an enormous amount about face selectivity in this distinctive region of human cerebral cortex.
Such noninvasive techniques cannot furnish the kind of incisive information that is available from classical neurobiological techniques (e.g., single-unit recording) in nonhuman primates. These 2 realms have begun to be bridged by recent fMRI studies in awake monkeys, which demonstrated that apparently homologous face-selective regions also exist in macaque inferotemporal (IT) cortex (8, 9). Subsequent experiments reported that ≈97% of the single units in the largest face-selective region (the so-called posterior temporal face patch, located in caudal TE) respond strongly and selectively to images of faces, compared with images of control objects (10). This fMRI and physiological evidence supports the idea that this region of monkey cortex is indeed selective for faces.
A computational transformation concluded that this posterior temporal face patch (PTFP) in macaques is located in a cortical region that corresponds topographically to area FFA in human subjects (8). This analysis assumes that neighborhood relations between specific cortical areas are evolutionarily conserved across the sheet of cortical gray matter, as validated in many previous human–monkey comparisons (11–14).
However, this conclusion raises a significant issue. In macaques, an additional face-selective region is consistently found further anterior in the temporal lobe, in rostral TE (8–10, 15, 16); here, this is termed the anterior temporal face patch (ATFP). However, in humans, no such area (i.e., anterior to FFA) has been reported by conventional fMRI mapping of face representations. If the human FFA corresponds to the PTFP in macaques [as suggested by Tsao et al. (8)], where is the human homologue of the macaque ATFP?
In fact, a few previous studies have suggested face-related activity in human anterior temporal cortex, using techniques other than fMRI (e.g., using neuropsychological lesions, invasive electrophysiology, and PET imaging) (see Discussion). However, because of limited spatial resolution in those techniques, that previously reported activity could not be well localized. Here, we used a sensory-based fMRI approach to precisely localize this face-selective activity in human cortex and directly compare its location relative to a homologous region in macaques.
We collected and analyzed fMRI maps from humans and awake-behaving monkeys, in response to identical visual stimuli (faces versus places and faces versus objects). The resultant evidence suggests that a face-selective area does exist in human anterior IT cortex, exactly where it is predicted by a macaque–human cortical deformation analysis. In addition, discrete face-responsive patches were observed in human and macaque parietal and frontal cortex, with a common topographic organization across species. Collectively this evidence suggests that the IT face patches are part of a larger network of activation throughout the brain, which is presumably homologous in humans and macaques.
Results
fMRI data were collected from 10 human and 2 monkey (Macaca mulatta) subjects. The visual stimuli, scanner, general procedures, and analysis were identical in the human and monkey experiments, except as noted. All subjects were scanned in a 3T horizontal bore magnet. Results were analyzed in inflated and flattened cortical surface formats (using FreeSurfer), to optimally visualize and measure the cortical representations. To maximize the statistical sensitivity, data were extensively signal-averaged within each subject. In addition, the monkey scans included an exogenous contrast agent [monocrystalline iron oxide nanoparticle (MION)], and the functional activity in the monkey cortex was sampled at higher spatial resolution, because of the smaller size of the monkey brain (see Methods for more details).
In the first experiment, the face patches were localized by using face-based and (as a control) place-based images. These images were extracted from group photographs (for faces) and indoor scenes including multiple objects (for places) (Fig. S1). These face and place images were closely matched in visual complexity [e.g., spatial frequency distribution (Fig. S2), quantity of objects, and visual clutter] (26th Annual Meeting of the Cognitive Science Society, Chicago, 5–7 August, 2004). To minimize potential artifacts caused by differences in the spatial envelope of object stimuli (and as part of a parallel study on object retinotopy), the stimuli were confined to retinotopically specific ring apertures of equivalent size and spatial extent for the face and place images (e.g., ref. 17). Within a given functional scan, the stimuli were presented in a blocked design, with each block containing multiple examples of a particular stimulus condition (e.g., the foveal face images). All subjects fixated the center of the stimulus screen throughout the scans (passive viewing). The activation maps were generated by comparing all face stimuli vs. all place stimuli, with both conditions averaged across the full range of aperture locations.
Fig. 1 shows the location of face-selective patches in macaque visual cortex. As described (8–10), a large PTFP (the middle face patch in refs. 8 and 10), and a smaller ATFP were consistently localized in macaque IT cortex (see Fig. 1 A and B). Recently, these 2 main patches have been further subdivided into smaller face clusters (18). However, these subdivisions were not consistently detected in our macaque subjects; therefore, they are not discussed here.
Fig. 1.
Face-selective patches in the macaque. (A and B) Patches in the macaque temporal cortex that were significantly more activated by face stimuli (red-yellow pseudocolor) than by (nonface) place stimuli (blue-cyan). Activation maps are displayed on a flattened view of the right visual cortex in monkey J (A) and monkey R (B). In both monkeys, the most significant face patch was the PTFP, located in the fundus and lower bank of STS, in caudal TE. In addition, there was a smaller ATFP, located in the upper bank of AMTS, in rostral TE. In monkey J (A), PTFP spread posteriorly into area TEO/V4, and ATFP spread superiorly into rostral/anterior STS. A distinct face-responsive patch also appeared in the parietal cortex (PFP) of monkey J. (C) A lateral view of the inflated right (Upper) and left (Lower) hemispheres of monkey J, showing a distinct face-responsive patch in the frontal cortex (FFP). The map threshold is P < 10−4. Sulcal abbreviations: OTS, occipito-temporal; IPS, intraparietal; SF, Sylvian fissure; AMTS, anterior middle temporal; AS, arcuate; PS, principal.
In human subjects, the same stimulus comparison (faces vs. places) revealed the expected face-selective region in the fusiform gyrus (FFA) (4). More unexpectedly, we also found an additional face area in anterior IT cortex, in 50% of the subjects. Fig. 2 shows the location of this new face patch (the ATFP) in 4 individual human subjects. The human ATFP appeared in both hemispheres, without obvious lateral bias. Fig. 3A shows this face patch in the averaged map of all 10 human subjects. This new face patch was consistently located within the rostral collateral sulcus, and it was statistically robust in the average across subjects, strongly supporting the conclusion that it is a real site of face-related activity.
Fig. 2.
Human ATFP in individual subjects. The comparison between face versus place stimuli shows significant face-selective patches (FFA and ATFP) in human ventral temporal cortex. Activation maps are displayed on flattened views of the visual cortex in 4 individual hemispheres (the 2 maps on the right are on left hemispheres). The human ATFP was consistently located within the rostral CoS, with no obvious hemispheric laterality. Face- and place-related activations in the posterior occipito-temporal cortex were topographically organized/arranged in a large-scale dorso-ventral mirror symmetry of image category (see also ref. 50). The map threshold is P < 10−2. Sulcal abbreviations: IPS, intraparietal; CoS, collateral.
Fig. 3.
Human ATFP averaged across subjects. Patches in the averaged human visual cortex that were significantly more activated by faces (red-yellow) than by places (blue-cyan) are shown. Group-average activation maps are displayed on ventral (A) and lateral (B) views of the inflated right hemisphere. Spherical averaging was used for intersubject registration of anatomical surfaces and fMRI activity maps. The maps show a robust ATFP in the anterior IT cortex. Additional face areas were located along the fusiform gyrus: area FFA, the IOG–OFA, the LO, and the posterior STS. This extended network of face processing (24) also included regions in the AMG, the IFS, the OFC, and new face-responsive areas in the IPS and the PCG. The map threshold is P < 10−4.
Is this site the human homologue of the ATFP in macaques? To test for a topographic homology between macaque and human face patches, the macaque temporal face patches were computationally deformed onto a human flat map by using a landmark-based registration in Caret (see Methods). This analysis predicted the location of face-selective regions in human cortex, in relationship to those in macaques (Fig. 4). Consistent with a preliminary analysis (8), we found that PTFP in macaque corresponded to the classical FFA in human cortex. More importantly here, ATFP appeared in topographically equivalent locations in macaque and human. In these 2 species, the face patches were located differently relative to the major sulci (e.g., the superior temporal sulcus). However, current evolutionary comparisons of cortical maps typically give precedence to neighboring areas rather than to sulcal/gyral proximity, partly because the pattern and the number of sulci and gyri vary considerably across species, independent of the cortical area map (14).
Fig. 4.
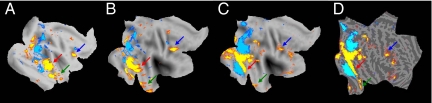
Computational deformation of macaque face patches onto a human flat map in Caret. (A) Face/place fMRI activation from the right hemisphere of monkey J, warped onto the macaque atlas flat map. (B) Deformed macaque face/place patches, projected onto the human atlas flat map. (C) Face/place fMRI activation from the right hemisphere of the averaged human brain, warped onto the human atlas flat map. (D) The same as C, except that the fMRI activity pattern was overlaid on the flat map of the averaged human brain (in the native space). The PTFP in macaque (B, red arrow) corresponded to the FFA in human cortex (C, red arrow). The ATFP appeared in topographically equivalent locations, in human and macaque (B and C, green arrows). In addition, the location of the macaque FFP matched with the location of the human FFP in the PCG (B and C, blue arrows).
To confirm that the human ATFP can be activated by additional, more conventional stimuli, we conducted a control experiment using large (full-screen) face/place stimuli (Fig. S3). This control experiment verified the original result: the ATFP was again activated, suggesting that the face-selective activity in this cortical region does not depend on a particular stimulus configuration.
In a further control experiment, the face patches were localized in a blocked-design comparison between single faces versus a wide range of object stimuli (Fig. S4). To stabilize attention, the human subjects performed male/female face discrimination (in the face blocks) and graspable/nongraspable object discrimination (in the object blocks), while fixating on the center of the stimulus screen. Again, the maps (Fig. 5), slice data (Fig. S5), and region of interest analysis (Fig. S6) clearly showed face selectivity in ATFP in the same human subjects. This finding confirmed that ATFP can be selectively and robustly activated in quite different stimulus conditions, even when conventional single faces are contrasted with different object categories and when visual attention is explicitly controlled.
Fig. 5.
Human ATFP is also activated in the face/object localizer experiment. The comparison between face versus object stimuli shows ATFP on magnified flattened views of the IT cortex in 5 human hemispheres (A–E) and 1 macaque hemisphere (F). The maps also show human FFA and macaque PTFP in the corresponding locations. The map threshold is P < 10−2.
In macaques, additional face-responsive foci also appeared in parietal cortex [the parietal face patch (PFP)] and frontal cortex [the frontal face patch (FFP)] (see Fig. 1C). PFP was located in the lateral intraparietal area (LIP) and anterior intraparietal area (AIP) (19). FFP was topographically located between the principal sulcus and the inferior limb of the arcuate sulcus, near the boundary between areas 45 and 8A (20). As one might hope, the human maps also revealed face-responsive patches in apparently homologous regions, within intraparietal sulcus (IPS), precentral gyrus (PCG), and inferior frontal sulcus (IFS) (see Fig. 3B). In the macaque–human cortical deformation analysis, the macaque FFP corresponded to a human FFP located in the PCG (see Fig. 4). In both species, this frontal face area was located near (anterior and inferior to) area FEF (the frontal eye field) (21).
In addition to these evolutionary common regions of face responsiveness, faces activated a more extended cortical network in humans. The additional face-responsive regions were located within human occipito-temporal cortex [inferior occipital gyrus (IOG)–occipital face area (OFA) (22), lateral occipital (LO) (23), and posterior superior temporal (STS) (5)], in localized sites beyond the classic ventral visual pathway, including the amygdala (AMG), and the orbitofrontal cortex (OFC) (24, 25) (Fig. 3). This extended face network may reflect additional cognitive processing of faces in humans.
Discussion
Here, we addressed an obvious discrepancy in the current maps of face-selective regions in humans versus macaques. fMRI maps from awake macaques predict that an area of high face selectivity should exist in a specific location anterior to human FFA. However, such an area has not been systematically demonstrated in conventional fMRI maps of face selectivity in humans. Here, we predicted the exact location of that area in humans, using a quantitative topographic modeling based on macaque maps. By using extensive signal averaging and common fMRI procedures in macaque and human subjects, we then demonstrated that this area (ATFP) does exist in the human brain.
How has this distinctive human region escaped notice in of the many face-mapping studies done previously? A partial explanation is that some previous studies actually have suggested face-related activity in the anterior temporal region, although that activity has not been well localized, nor explicitly predicted by evolutionary comparisons. For instance, using fMRI, Kriegeskorte et al. (26) found face-exemplar information in human anterior IT cortex, but not in conventionally defined FFA. ERP studies have attributed a source of face selectivity (face-specific AP350s) to the anterior ventral temporal pole (6), and this ERP component has been sensitive for priming and habituation paradigms involving faces (27). Previous PET studies have also reported face-related activity in the anterior temporal lobe during identification of famous/familiar faces (e.g., refs. 1 and 28). The critical role of the anterior temporal lobe in face recognition is further supported by a recent structural imaging study, which showed a significant reduction in the volume of white/gray matter in the anterior IT cortex, in individuals with congenital prosopagnosia, relative to the normal subjects (29). This volumetric reduction of cortex was correlated with the extent of behavioral deficits in face recognition.
Analogously, neuropsychological studies by Damasio et al. (30) have reported that lesions in the anterior temporal lobes can cause a particular type of prosopagnosia termed amnesic associative prosopagnosia. Such patients have preserved perception of faces, but impaired recognition of faces. Similar deficits in face recognition can be also produced after anterior temporal lobectomy (e.g., ref. 31). In these patients, face recognition difficulties were more commonly associated with resection of the right anterior temporal lobe, whereas resection of the left anterior temporal lobe caused anomia, difficulties in naming of famous or personally familiar faces (31). This evidence suggests that the anterior temporal lobe may be critical for social aspects of face processing.
A second possible explanation for the relative obscurity of ATFP is technical in nature. It is well known that the increased MR signal drop-out and distortion caused by susceptibility artifacts occur within and surrounding this anterior IT region (Fig. S7). Primarily they are caused by air-filled cavities in the auditory canal and surrounding sinus and temporal bone areas (32). Here, the consequently reduced signal-to-noise in anterior temporal structures was compensated by extensive signal averaging of functional data in each subject (see Methods). In addition, the face stimulus in the initial experiment consisted of a group of faces, which may well have increased the functional sensitivity in detecting the face patches (14th Annual Meeting of the Organization for Human Brain Mapping, Melbourne, Australia, 15–19 June, 2008). The ability to demonstrate a functionally distinct region in this part of cortex, despite the significant MR signal drop-out, strengthens the conclusion that this face-related activity is robust and real. Technical improvements in detection of fMRI activity from temporal lobe (e.g., ref. 33) can facilitate further studies of this anterior temporal face-selective area.
In the current study, the ATFP was reliably activated in 50% of the subjects tested (e.g., Figs. 2 and 5). Thus, it is possible that statistical methods relying on group activation data would fail to detect such areas. In our macaque maps, this ATFP also tends to be small and less consistent, relative to the PTFP (the homologue of human FFA). Thus, even the difficulty of mapping this anterior region is consistent across species.
Obviously further research is needed to clarify the response properties and the cortical connections of ATFP and to reveal its place in the network of face areas. Nevertheless, the location of this area in humans strongly suggests that it occupies a higher-tier (downstream) position in the visual hierarchy, compared with the classical FFA, analogous to the hierarchical relationship of areas TE and TEO in macaque visual cortex (34). It has been suggested that perceptual processing of complex or highly similar visual stimuli that share features (e.g., faces) relies disproportionately on more anterior temporal regions (35). Thus, posterior temporal regions (like FFA) may be involved mainly in face (versus nonface) categorization, whereas anterior temporal regions may be required in more detailed visual computations such as within-category face identification (26).
In the macaque, previous electrophysiological reports have described face- (or shape-) related activity, in specific sites in parietal cortex (e.g., in LIP and AIP) (36–38) and ventrolateral prefrontal cortex (the inferior prefrontal convexity) (39–41). These electrophysiological areas appear to coincide with the fMRI-based, face-responsive patches shown here in monkey. The direct comparison here demonstrates that apparently homologous areas also exist in the human brain. Circumstantial evidence (mostly collected before the localization of the face patches per se) suggests that these face-responsive regions are anatomically interconnected in a wider network of face processing throughout the brain (18, 40, 42–44).
Because the cortical topography of the face-selective network is evolutionarily so conserved in humans and macaques, the results of electrophysiological studies on macaque face patches (e.g., ref. 10) could be safely generalized to their homologous areas in humans.
Methods
Subjects.
Two juvenile (4–6 kg) male rhesus monkeys (M. mulatta) were used in the monkey fMRI experiments. Surgical details and the training procedure have been described (8, 45, 46) and are summarized here. Each monkey was implanted with an MRI-compatible plastic headset. All surgical procedures conformed to local (Massachusetts General Hospital animal protocol 2005N-000201) and National Institutes of Health guidelines. After recovery, monkeys were adapted to sit in a sphinx position inside a plastic restraining chair. They were trained to fixate a small fixation spot (0.35° × 0.35° in size) at the center of the visual display, and eye position was monitored by using an infrared pupil tracking system (ISCAN) at 120 Hz. Monkeys were rewarded for maintaining fixation within a square-shaped central fixation window (2° × 2° in size), surrounding the fixation spot. After 20–40 training sessions, when fixation performance had reached asymptote, we began functional scanning. Only scanning sessions with adequately high behavioral performance (> 90% fixation stability throughout the duration of each scan) were considered for statistical analysis. Before each scanning session, an exogenous contrast agent (MION) was injected i.v. (concentration: 8–10 mg/kg) to enhance the contrast-to-noise ratio and functional sensitivity (45, 47). For ease of comparison, the polarity of the MION MR response was inverted.
Ten human subjects (with normal or corrected-to-normal vision) were tested in multiple imaging sessions, using the same stimuli, scanner, general procedures, and analysis. Informed written consent was obtained from each human subject before the scanning session, and the experimental procedure was approved by Massachusetts General Hospital Institutional Review Board protocol 2000P-001155. During the functional scans, human subjects were instructed to fixate a small fixation spot at the center of the visual display.
Imaging Procedures and Data Analysis.
Monkeys were scanned in a Siemens Allegra 3-T scanner (horizontal bore magnet), and a radial surface transmit-receive coil (11-cm diameter) was used for the acquisition of functional volumes. Two MRI pulse sequences were used for functional imaging in monkeys: (i) a gradient echo EPI sequence (TR 3,000 ms, TE 24 ms, flip angle 90°, 1.25 × 1.25 × 1.25-mm3 voxels, and 45 coronal slices covering almost the entire brain), and (ii) a multiecho sequence (TR 4,000 ms, TE 32 ms/73 ms, flip angle 90°, 1.25 × 1.25 × 1.25-mm3 voxels, and 28 coronal slices). The multiecho sequence was used to reduce spatial distortions (susceptibility artifacts) caused by gross body motion within the B0 field. Each monkey session consisted of 20–25 functional runs, with each run containing 14 blocks (block duration = 30 or 40 s). Each monkey was scanned for 4–5 sessions, and data from all sessions were averaged together. In an additional scan session using a Siemens Trio 3-T scanner, high-resolution anatomical images (3D MP-RAGE sequence, 0.35 × 0.35 × 0.35-mm3 voxels) were obtained while the monkeys were anesthetized.
Human subjects were scanned in the same Allegra 3-T scanner, using a single-channel CP head coil for the acquisition of functional volumes. The blood-oxygen-level-dependent (BOLD) functional data were based on a GE-EPI sequence (TR 2,000 ms, TE 30 ms, flip angle 90°, 3.1-mm isotropic voxels, and 35 axial slices covering almost the entire brain). A 3D MP-RAGE sequence (1.0-mm isotropic voxels) was also used for anatomical imaging. Each human session consisted of 10–15 functional runs, with each run containing 14 blocks (block duration = 16 or 24 s).
Data were analyzed by using FreeSurfer and FS-FAST (http://surfer.nmr.mgh.harvard.edu). The monkey-human comparison and the cortical deformation analysis were performed in Caret (http://brainmap.wustl.edu/caret). See SI Text for details of FreeSurfer and Caret analysis.
Visual Stimuli.
Stimuli were generated on a PC (running Windows XP), and presented through an LCD projector (Sharp; 1,024 × 768-pixels resolution, 60-Hz refresh rate) onto a rear-projection screen. Matlab 7.0 and Psychophysics Toolbox (48, 49) were used to program the experiments.
The stimuli were presented in a blocked design. Within a given functional scan, the first and last blocks were always null epochs (i.e., a fixation point on a black background), to allow the hemodynamic response to reach a steady state. The remaining stimulus blocks were ordered pseudorandomly, without a rest period between the stimulus blocks.
In the face/place localizer experiments, the initial stimuli were based on 10 face and 10 place images, which were all presented within retinotopically limited apertures, on a black background. The face images were extracted from group photographs (face mosaics: multiple equal-sized faces, adjacent to each other). The place images were extracted from familiar indoor scenes (pictures of the training and scanning rooms). The retinotopic ring apertures included: (i) a foveal (central) disk (3° diameter); (ii) a para-foveal (midperipheral) annulus (3° inner diameter and 10° outer diameter); and (iii) a peripheral annulus (10° inner diameter and 20° outer diameter). In the control experiment, the aperture was a large disk subtending 20° of visual angle. Within a stimulus block, multiple examples of a particular stimulus condition (e.g., foveal disks containing face images) were randomly presented, with each image presented for 1 s. The subjects passively viewed this set of images while fixating.
In the face/object localizer, the stimuli were based on 30 face and 30 object images, all presented on a black background. The face images were single faces (≈12° x 16° in size), selected from the Max-Planck face database (http://faces.kyb.tuebingen.mpg.de). The object images were collected from the pictures of manmade objects, which had approximately the same size and aspect ratio as the single faces. Within a stimulus block, multiple examples of face or object stimuli were randomly presented. Each stimulus was presented for 1,500 ms, with a 500-ms interstimulus interval. In the face blocks, the human subjects reported whether each face is male or female. In the object blocks, the subjects reported whether each object is graspable (e.g., for the cell phone image) or nongraspable (e.g., for the refrigerator image). The average performance of the subjects in these 2 subcategory discrimination tasks was ≈80%.
Supplementary Material
Acknowledgments.
We thank Wim Vanduffel for help in the monkey surgery and monkey fMRI setup, Mukund Balasubramanian for providing cortical flattening code, David Van Essen for help in the Caret analysis, and 2 anonymous reviewers for their scientific input on an earlier version of this manuscript. This work was supported by the National Institutes of Health Grants R01 MH67529 and R01 EY017081 (to R.B.H.T.), the Martinos Center for Biomedical Imaging, National Center for Research Resources Grant P41RR14075, and the Mental Illness and Neuroscience Discovery Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807304106/DCSupplemental.
References
- 1.Sergent J, Signoret JL. Functional and anatomical decomposition of face processing: Evidence from prosopagnosia and PET study of normal subjects. Philos Trans R Soc London Ser B. 1992;335:55–61. doi: 10.1098/rstb.1992.0007. [DOI] [PubMed] [Google Scholar]
- 2.Haxby JV, et al. Face encoding and recognition in the human brain. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. J Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halgren E, et al. Location of human face-selective cortex with respect to retinotopic areas. Hum Brain Mapp. 1999;7:29–37. doi: 10.1002/(SICI)1097-0193(1999)7:1<29::AID-HBM3>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and nonface stimuli. Cereb Cortex. 1999;9:415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- 7.Halgren E, Raij T, Marinkovic K, Jousmaki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cereb Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- 8.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinsk MA, DeSimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: A functional MRI study. Proc Natl Acad Sci USA. 2005;102:6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Essen DC, et al. Mapping visual cortex in monkeys and humans using surface-based atlases. Vision Res. 2001;41:1359–1378. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]
- 12.Tootell RB, Tsao D, Vanduffel W. Neuroimaging weighs in: Humans meet macaques in “primate” visual cortex. J Neurosci. 2003;23:3981–3989. doi: 10.1523/JNEUROSCI.23-10-03981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cognit Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Sereno MI, Tootell RB. From monkeys to humans: What do we now know about brain homologies? Curr Opin Neurobiol. 2005;15:135–144. doi: 10.1016/j.conb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Tanaka K, Tanifuji M. Optical imaging of functional organization in the monkey inferotemporal cortex. Science. 1996;272:1665–1668. doi: 10.1126/science.272.5268.1665. [DOI] [PubMed] [Google Scholar]
- 16.Afraz SR, Kiani R, Esteky H. Microstimulation of inferotemporal cortex influences face categorization. Nature. 2006;442:692–695. doi: 10.1038/nature04982. [DOI] [PubMed] [Google Scholar]
- 17.Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- 18.Moeller S, Freiwald WA, Tsao DY. Patches with links: A unified system for processing faces in the macaque temporal lobe. Science. 2008;320:1355–1359. doi: 10.1126/science.1157436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JW, Van Essen DC. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J Comp Neurol. 2000;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Petrides M, Cadoret G, Mackey S. Orofacial somatomotor responses in the macaque monkey homologue of Brocas area. Nature. 2005;435:1235–1238. doi: 10.1038/nature03628. [DOI] [PubMed] [Google Scholar]
- 21.Koyama M, et al. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: Comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- 22.Gauthier I, et al. The fusiform “face area” is part of a network that processes faces at the individual level. J Cognit Neurosci. 2000;12:495–504. doi: 10.1162/089892900562165. [DOI] [PubMed] [Google Scholar]
- 23.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 24.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cognit Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 25.Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 26.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puce A, Allison T, McCarthy G. Electrophysiological studies of human face perception. III: Effects of top-down processing on face-specific potentials. Cereb Cortex. 1999;9:445–458. doi: 10.1093/cercor/9.5.445. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, et al. Functional delineation of the human occipito-temporal areas related to face and scene processing. A PET study. Brain. 2000;123:1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- 29.Behrmann M, Avidan G, Gao F, Black S. Structural imaging reveals anatomical alterations in inferotemporal cortex in congenital prosopagnosia. Cereb Cortex. 2007;17:2354–2363. doi: 10.1093/cercor/bhl144. [DOI] [PubMed] [Google Scholar]
- 30.Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Annu Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- 31.Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–86. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- 32.Ojemann JG, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- 33.Goense JB, Ku SP, Merkle H, Tolias AS, Logothetis NK. fMRI of the temporal lobe of the awake monkey at 7 T. NeuroImage. 2008;39:1081–1093. doi: 10.1016/j.neuroimage.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 34.Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- 35.Bright P, Moss HE, Stamatakis EA, Tyler LK. The anatomy of object processing: The role of anteromedial temporal cortex. Q J Exp Psychol B. 2005;58:361–377. doi: 10.1080/02724990544000013. [DOI] [PubMed] [Google Scholar]
- 36.Sakata H, et al. Neural coding of 3D features of objects for hand action in the parietal cortex of the monkey. Philos Trans R Soc London Ser B. 1998;353:1363–1373. doi: 10.1098/rstb.1998.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sereno AB, Maunsell JH. Shape selectivity in primate lateral intraparietal cortex. Nature. 1998;395:500–503. doi: 10.1038/26752. [DOI] [PubMed] [Google Scholar]
- 38.Lehky SR, Sereno AB. Comparison of shape encoding in primate dorsal and ventral visual pathways. J Neurophysiol. 2007;97:307–319. doi: 10.1152/jn.00168.2006. [DOI] [PubMed] [Google Scholar]
- 39.Pigarev IN, Rizzolatti G, Scandolara C. Neurons responding to visual stimuli in the frontal lobe of macaque monkeys. Neurosci Lett. 1979;12:207–212. doi: 10.1016/0304-3940(79)96063-4. [DOI] [PubMed] [Google Scholar]
- 40.Scalaidhe SP, Wilson FA, Goldman-Rakic PS. Areal segregation of face-processing neurons in prefrontal cortex. Science. 1997;278:1135–1138. doi: 10.1126/science.278.5340.1135. [DOI] [PubMed] [Google Scholar]
- 41.Scalaidhe SP, Wilson FA, Goldman-Rakic PS. Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: Evidence for intrinsic specialization of neuronal coding. Cereb Cortex. 1999;9:459–475. doi: 10.1093/cercor/9.5.459. [DOI] [PubMed] [Google Scholar]
- 42.Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson S, Trojanowski JQ. Prefrontal granular cortex of the rhesus monkey. I. Intrahemispheric cortical afferents. Brain Res. 1977;132:209–233. doi: 10.1016/0006-8993(77)90417-6. [DOI] [PubMed] [Google Scholar]
- 44.Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 45.Vanduffel W, et al. Visual motion processing investigated using contrast agent-enhanced fMRI in awake behaving monkeys. Neuron. 2001;32:565–577. doi: 10.1016/s0896-6273(01)00502-5. [DOI] [PubMed] [Google Scholar]
- 46.Tsao DY, et al. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron. 2003;39:555–568. doi: 10.1016/s0896-6273(03)00459-8. [DOI] [PubMed] [Google Scholar]
- 47.Leite FP, et al. Repeated fMRI using iron oxide contrast agent in awake, behaving macaques at 3 Tesla. NeuroImage. 2002;16:283–294. doi: 10.1006/nimg.2002.1110. [DOI] [PubMed] [Google Scholar]
- 48.Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- 49.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- 50.Hasson U, Harel M, Levy I, Malach R. Large-scale mirror-symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.