Abstract
Aims
To conduct a preliminary clinical trial assessing whether adjunctive topical corticosteroids improve outcomes in bacterial keratitis and, if no difference is found, to determine the feasibility and sample size necessary for conducting a larger trial to answer this question.
Methods
In this single center, double-masked clinical trial, 42 patients with culture-confirmed bacterial keratitis at Aravind Eye Hospital in India were randomized to receive either topical prednisolone phosphate or placebo. All patients received topical moxifloxacin. The primary outcome was best spectacle-corrected visual acuity (BSCVA) at 3 months, adjusting for enrollment BSCVA and arm. Other pre-specified outcomes included re-epithelialisation time, infiltrate/scar size, and adverse events.
Results
Compared to placebo, the steroid group re-epithelialised more slowly (hazard ratio 0.47, 95% CI 0.23 to 0.94). There was no significant difference in BSCVA or infiltrate/scar size at 3 weeks or 3 months. To have 80% power to detect a 2-line difference in acuity, 360 cases would be required.
Conclusions
Although corticosteroid treatment resulted in a statistically significant delay in re-epithelialisation, this did not translate to a significant difference in visual acuity, infiltrate/scar size, or adverse events. To assess the effect of steroids on acuity, a larger trial is warranted and feasible.
Keywords: bacterial keratitis, corticosteroids, clinical trial
INTRODUCTION
Antimicrobial treatment of a bacterial corneal ulcer is commonly effective in eradicating infection. However, “successful” treatment is not always associated with a good visual outcome.[1, 2] The scarring that accompanies the resolution of infection leaves many eyes visually impaired or blind.[3] Some specialists advocate topical corticosteroids along with antibiotics to reduce immune-mediated tissue damage and scarring.[4] Others fear steroids will reduce the cornea’s immune response and prolong or exacerbate infection. Both approaches are acceptable according to the American Academy of Ophthalmology’s Preferred Practice Patterns.[5] Meta-analysis of retrospective studies on corticosteroid use in bacterial keratitis (BK) from 1950 to 2000 found that the efficacy of topical corticosteroids is unproven.[6] One small randomised controlled trial from South Africa addressed the effect of topical corticosteroids used in conjunction with topical antibiotics, but was inconclusive.[7]
The goal of this study was to conduct a randomized clinical trial to address 3 aims: 1) to assess for a large difference in efficacy between steroids and placebo which would make a larger trial inappropriate to conduct 2) to assess for a significant difference in adverse events, in particular corneal perforations, which would preclude conducting a larger trial and 3) if a difference is not found, to determine the feasibility and sample size of a larger trial by using the standard deviation of the outcome variable (3-month BSCVA) and the correlation coefficient between enrollment and 3-month BSCVA results from this study. In this paper, we present the results of this clinical trial designed to address these three aims.
MATERIALS AND METHODS
Study Design
This study was a single-center, randomised, double-masked clinical trial enrolling patients with culture-proven bacterial corneal ulcers, with the study intervention being adjunctive topical corticosteroid treatment. Institutional Review Board approval was obtained at UCSF and Aravind Eye Hospital. All bacterial corneal ulcer culture positive cases were considered for enrollment, and all patients provided written, informed consent for their study participation. Inclusion and exclusion criteria are listed in Table 1. A target enrollment of 42 patients was chosen because this sample size was deemed sufficient to achieve the goals of the pilot study listed above. Specifically, we estimated that 20.2 patients per arm would provide 80% power to detect a 0.4 logMAR effect size (4 Snellen lines) between the two study arms, assuming a standard deviation of 0.4 in the 3 month BSCVA, a correlation coefficient of 0.65 between enrollment and 3-month BSCVA, a dropout rate of 15%, and a two-tailed alpha of 0.05. If we were able to find a statistically significant difference in 3-month BSCVA or the proportion of corneal perforations between the steroid and placebo groups, a larger trial would not be warranted. If not, we would be able to use the standard deviation of the outcome variable (3-month BSCVA) and the correlation coefficient between enrollment and 3-month BSCVA results to calculate the sample size needed to conduct a larger trial.
Table 1.
Inclusion and Exclusion Criteria for the SCUT trial
| Inclusion Criteria (All must be met) |
|
| Exclusion Criteria |
|
Study Intervention
All corneal ulcer patients presenting to Aravind Eye Hospital’s cornea clinic in Madurai, India, underwent corneal scrapings using a Kimura spatula for Gram stain and KOH wet mount as well as cultures plated on blood, chocolate, and Saboraud’s agar. Aravind Eye Hospital is both a primary and tertiary care eye hospital in South India with a well established cornea subspecialty clinic. Patients suspected of having a bacterial ulcer received topical antibiotic treatment with moxifloxacin (Vigamox®, Alcon Inc., Fort Worth TX) every hour while awake for the first 48 hours while culture results were pending. If the culture revealed bacterial growth and other inclusion and exclusion criteria were fulfilled (Table 1), then the patient was enrolled into the study. A positive corneal culture was defined by growth of bacteria on the plated C-streak on the culture plates. In the case of coagulase-negative Staphylococcus and diphtheroids, growth was necessary on 2 culture plates or on the smear as well as 1 culture in order to minimize the chance that the bacteria was a contaminant. After a minimum of 48 hours of moxifloxacin treatment, patients were randomised (block randomisation in groups of 10 generated by RAND command in Excel by TL; implementation including enrollment and assignment of participants by RM) to receive topical prednisolone phosphate 1% (Bausch & Lomb Pharmaceuticals, Inc., Tampa FL) or placebo drops (0.9% sodium chloride, prepared by Leiter’s pharmacy, San Jose CA), administered topically to the cornea 4 times a day for 1 week, followed by 2 times a day for 1 week, then once a day for 1 week, and then stopped. All patients continued to receive topical moxifloxacin every 2 hours while awake until re-epithelialisation, and then 4 times a day until 3 weeks after enrollment. Antibiotics were then discontinued unless the treating physician thought that longer treatment was warranted. For ethical reasons, physicians were allowed to change or add antibiotics at their discretion if they felt the ulcer was not responding. Note that enrollment, randomisation, and initiation of the study drug (steroid or placebo) occurred only after bacterial growth on culture and after at least 48 hours of moxifloxacin treatment. According to the standard of care at Aravind Eye Hospital, all patients were hospitalised from presentation until re-epithelialisation, with medications administered by the ward nurse. Patients were scheduled for follow-up at 3 weeks and 3 months after enrollment.
Double-masking of treatment assignment was achieved since the prednisolone phosphate solution could not be differentiated from the placebo. All study-site personnel and patients were masked to treatment assignment. Only the biostatisticians responsible for the randomisation coding and the study pharmacist were unmasked.
Study Assessments
Assessments of best spectacle-corrected visual acuity (BSCVA) and clinical characteristics (infiltrate/scar size, epithelial defect size) were performed at enrollment, 3 weeks, and 3 months. Visual acuity measurements were performed according to a protocol adapted from the Age Related Eye Disease Study (AREDS 1999), using a tumbling “E” chart at 4 meters and logMAR visual acuity. Acuities worse than logMAR 1.6 (~20/800) were recorded as: counting fingers 1.7, hand motion 1.8, light perception 1.9, and no light perception 2.0, as in the Herpetic Eye Disease Study (HEDS).[8] A Haag-Streit 900 slit lamp biomicroscope was used to assess the size of the infiltrate/scar and epithelial defect at study visits, and ocular adverse events such as corneal perforation. Infiltrate/scar size and epithelial defect size were measured according to a protocol adapted from HEDS. In brief, the longest dimension was measured, followed by the longest perpendicular to the first measurement. As in HEDS, no differentiation was made between infiltrate and scar when measuring infiltrate/scar size. Re-epithelialisation was defined as the absence of an epithelial defect with administration of fluorescein.
Statistical Methods
Baseline characteristics were compared between the treatment and placebo groups using Student’s T-test for continuous variables and Fisher’s exact test for categorical variables. The primary efficacy endpoint was BSCVA at 3 months in the study eye, using a linear regression model with 3-month logMAR BSCVA as the outcome variable and treatment arm (placebo vs. steroid) and enrollment logMAR BSCVA as covariates. Other pre-specified endpoints included BSCVA at 3 weeks, adjusting for enrollment BSCVA, and infiltrate/scar size at 3 weeks and 3 months, adjusting for enrollment infiltrate/scar size. For analysis, infiltrate/scar size was characterized by the geometric mean of the longest dimension and the longest perpendicular. The association between enrollment and 3-month BSCVA was assessed using Pearson’s correlation coefficient. The validity of the model was checked by assessing normality of the residuals using a Q-Q plot. Bootstrap mixed model regression was also conducted to test the robustness. The time to re-epithelialisation was compared between the two treatment groups using Cox’s proportional hazards model, adjusting for baseline epithelial defect size. STATA 9.2 was used to conduct all of the statistical analyses. Efficacy endpoints were analysed on an intent-to-treat basis for all randomised patients enrolled in the study. The primary analysis included patients with both enrollment and 3-month data. A sensitivity analysis was also performed in which 3-week values were carried forward to 3 months if the 3-month visit was missed. Safety assessments included comparing the incidence of ocular and non-ocular adverse events, including corneal perforations, by Fisher’s exact test.
RESULTS
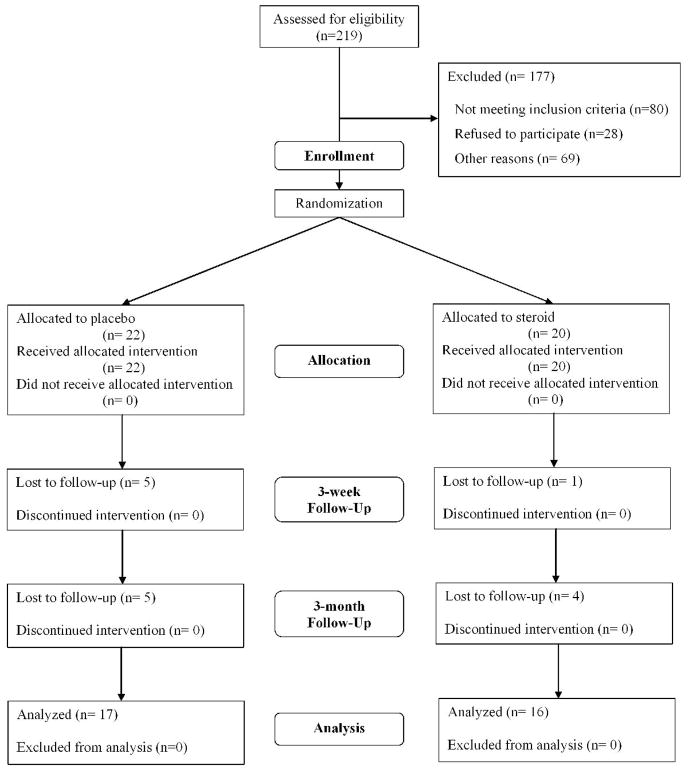
Two hundred and nineteen patients with a positive bacterial culture were assessed for eligibility during the enrollment period from 1/4/05 to 8/20/05 and 177 patients who did not satisfy all inclusion and exclusion criteria were excluded (Figure 1). Forty-two patients with culture-proven BK at Aravind Eye Hospital were enrolled: 22 in the placebo arm and 20 in the steroid arm. Thirty-three patients (79%) followed up at 3 months, and an additional 3 patients followed up for their 3-week visit but missed their 3-month visit (Figure 1). Enrollment characteristics for the 42 patients, including enrollment BSCVA, infiltrate/scar size, and distribution of organisms were not significantly different between the 2 study arms (Table 2). The enrollment BSCVA and infiltrate/scar size in the patients who did not complete the study were not significantly different between the 2 study arms and did not differ significantly from patients who had complete follow-up.
Figure 1.
CONSORT Flowchart: Enrollment 1/4/05 – 8/20/05
Table 2.
Baseline Characteristics of Patients (n=42)
| Placebo N (%) | Steroid N (%) | P-value | |
|---|---|---|---|
| Age (mean years ± SD) | 44.1 ± 17.0 | 49.9 ± 13.0 | 0.22* |
| Sex | |||
| Male | 11 (50.0) | 11 (55.0) | 0.75** |
| Female | 11 (50.0) | 9 (45.0) | |
| Organisms | |||
| Coagulase-neg Staphylococcus | 3 (13.64) | 2 (9.52) | |
| Diphtheroids | - | 1 (4.76) | |
| Staphylococcus aureus | 2 (9.09) | - | |
| Streptococcus pneumoniae | 10 (45.45) | 9 (42.86) | |
| Viridans-group Streptococcus spp | 1 (4.55) | 3 (14.29) | 0.51** |
| Haemophilus influenzae | - | 1 (4.76) | |
| Moraxella spp | 1 (4.55) | - | |
| Pseudomonas spp (non-aeruginosa) | - | 1 (4.76) | |
| Pseudomonas aeruginosa | 4 (18.18) | 4 (19.05) | |
| Aeromonas hydophila | 1 (4.55) | - | |
| Mean enrollment visual acuity (logMAR ± SD) | 1.15 ± 0.63 | 1.28 ± 0.54 | 0.50* |
| Mean enrollment infiltrate/scar diameter (mm ± SD) | 1.8 ± 1.6 | 2.0 ± 1.2 | 0.63* |
Independent sample t-test
Fisher’s exact test
For the placebo group, the mean BSCVA at enrollment was 1.15 logMAR (Snellen equivalent 20/250), with a standard deviation (SD) of 0.63. At 3 weeks, the BSCVA was 0.75 logMAR (Snellen equivalent 20/125), with a SD of 0.75. At 3 months, the BSCVA had improved to 0.59 logMAR (Snellen equivalent 20/100), SD=0.75. For the steroid-treated group, the mean enrollment VA was 1.28 logMAR (Snellen equivalent 20/400), SD of 0.54, which improved to 0.66 logMAR (Snellen equivalent 20/100), SD of 0.68 at 3 weeks. At 3 months, the BSCVA was 0.71 logMAR (Snellen equivalent 20/100), SD=0.72.
The multiple linear regression model showed that compared to placebo treatment, steroid treatment was associated with 0.19 lower (better) logMAR acuity (1.9 lines) at 3 weeks (95% CI −0.52 to 0.15, P=0.26), and 0.09 lower logMAR acuity (0.9 line) at 3 months (95% CI −0.41 to 0.24, P=0.60) (Tables 3a and 3b). Enrollment and 3-month BSCVA were highly associated, with a Pearson correlation coefficient of 0.79. The standard deviation of our primary outcome, 3-month BSCVA, was 0.72.
Table 3.
| Table 3a. Linear Regression Predicting 3-week logMAR BSCVA (n=36 patients) | ||
|---|---|---|
| Covariate | Coefficient (95% CI) | P-value |
| Enrollment BSCVA (logMAR) | 0.88 (0.60, 1.16) | < 0.0001 |
| Steroid treatment | −0.19 (−0.52, 0.15) | 0.26 |
| Constant | −0.25 (−0.65, 0.15) | 0.21 |
| Table 3b. Linear Regression Predicting 3-month logMAR BSCVA (n=33 patients) | ||
| Covariate | Coefficient (95% CI) | P-value |
| Enrollment BSCVA (logMAR) | 0.99 (0.70, 1.27) | < 0.0001 |
| Steroid treatment | −0.09 (−0.41, 0.24) | 0.60 |
| Constant | −0.45 (−0.83, 0.07) | 0.02 |
Similar linear regression models were used to predict 3-week and 3-month infiltrate/scar size, using enrollment infiltrate/scar size and treatment arm as covariates. At 3 weeks, steroid treatment was associated with 0.57 mm smaller infiltrate/scar size diameter in mm (95% CI 1.5 smaller to 0.37mm larger, P=0.23) compared to the placebo group. At 3 months, steroid treatment was associated with 0.33 mm smaller infiltrate/scar size diameter (95% CI 1.4 smaller to 0.75 larger, P=0.53) compared to the placebo group.
We also conducted tests to ensure that our linear regression model was valid given the distribution of our data. Q-Q plots did not reveal gross departures from normality, which would necessitate alternative analyses. In addition, results were comparable using bootstrap regression, showing similar coefficients, P-values and standard errors. A sensitivity analysis which carried forward 3-week values for patients with missing 3-month values demonstrated results consistent with the primary analysis.
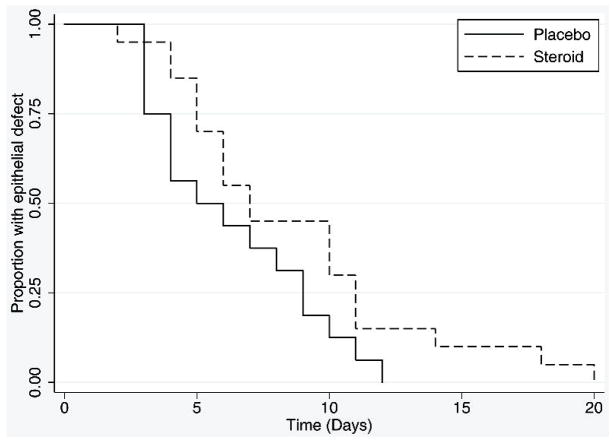
The average time to re-epithelialisation was 6.3 days (SD 3.1) in the placebo group and 8.6 days (SD 4.7) in the steroid treated patients. Re-epithelialisation in the steroid group was slower than the placebo group after adjusting for baseline epithelial defect size (hazard ratio 0.47; 95% CI 0.23 to 0.94, P=0.03). Figure 2 shows the Kaplan-Meier curves for re-epithelialisation for the steroid and placebo groups.
Figure 2.
Time to Re-epithelialisation in Steroid and Placebo Groups
No systemic adverse events occurred in this study. Four patients had fortified antibiotics added to the moxifloxacin regimen because the treating physician deemed this to be in the best interest of the patient, 3 in the steroid group and 1 in the placebo group. There were 4 ocular adverse events (2 corneal perforations requiring corneal glue, 1 uncontrolled elevated intraocular pressure despite medical therapy, and 1 worsening infiltrate at 7 days). One perforation occurred with Staphylococcus aureus and one with Streptococcus pneumoniae.. All these events occurred in the placebo group. The Fisher’s exact test comparing the proportion of corneal perforations between the steroid and placebo group was not statistically significant (P=0.49).
DISCUSSION
In this study of bacterial corneal ulcers, steroid treatment was associated with a statistically significant delay in re-epithelialisation compared to placebo. However, this did not translate to worse clinical outcomes. Steroid use was associated with a nearly 2-line improvement in BSCVA at 3 weeks, and a nearly 1-line improvement by 3 months, although neither of these were statistically significant. Of note, our results suggest that any benefit from steroid treatment may be most apparent before 3 months, indicating that steroids may be associated with faster recovery and resolution. Compared to placebo, steroid treatment was also associated with a greater decrease in infiltrate/scar size at 3 weeks and 3 months, although these improvements were also not statistically significant.
Most prior clinical trials on BK have focused on “healing time” or “cure rate” as their main outcome, defining success by re-epithelialisation.[9–15] Re-epithelialisation is not an optimal outcome measure when the intervention, steroids in this case, may cause a delay in healing while still translating to better visual acuity and infiltrate/scar size. In practice, a slight delay in re-epithelialisation would be acceptable if a more clinically relevant outcome such as visual acuity improved. The only other published trial on this topic also found a trend towards improved outcomes with steroids, but not a delay in re-epithelialisation.[7]
Since we did not find a significant difference in safety or efficacy which would make it unethical to conduct a larger trial, we used the standard deviation of the 3-month BSCVA and the correlation coefficient between enrollment and 3-month BSCVA to determine the optimal sample size for a larger trial. Since our regression model predicting 3-month BSCVA adjusts for the enrollment BSCVA, the high correlation coefficient between enrollment and 3-month BSCVA increases the power of both this study and future studies with this design. When considering prognostic factors for outcomes in bacterial corneal ulcers, presenting visual acuity is an important factor determining final visual acuity. We anticipate that a sample size of 360 patients will be necessary to have 80% power to detect an effect size of 0.2 logMAR (2 lines of visual acuity) between the steroid and placebo groups, assuming 15% dropout, and a two-tailed alpha of 0.05. Furthermore, given the large sample size needed, multiple centers will likely be needed to enroll sufficient patients in a timely fashion.
Steroid treatment in our patient population did not appear to be associated with any trend in adverse effects, such as perforation. There was a delay in epithelialisation with steroids, but this was not associated with other adverse events. These numbers are too small to ensure that steroids do not pose a safety risk but do not present evidence that would preclude proceeding with a larger trial.
There is considerable precedent in the medical literature for the use of corticosteroids in patients with fulminant bacterial infections.[16, 17] Corticosteroids are used to reduce tissue damage associated with the immune response to the infection.[18] In ophthalmology, controlled studies have demonstrated a benefit from the use of intravitreal corticosteroids in the treatment of bacterial endophthalmitis. Bacterial cytopathic effects and the host inflammatory response both contribute to corneal damage associated with BK. Thus, it is not unreasonable to consider their use in controlling immune-mediated corneal damage associated with BK.
In this trial, although the steroid-treated group had a significant delay in re-epithelialization, steroids were not associated with a statistically significant difference in BSCVA or infiltrate/scar size. In addition, there were no major safety concerns raised in this trial which would preclude conducting a larger study. Our results indicate the need for a larger study that would be sufficiently powered to address these research questions definitively. A larger study would also be able to address whether the effect of steroids differs by subgroup of bacteria. Using the infrastructure established from this trial, we have initiated a separate, larger randomised clinical trial (Steroids for Corneal Ulcers Trial, NEI U10-EY015114) that we anticipate will provide sound evidence to guide the optimal treatment of bacterial corneal ulcers.
Acknowledgments
Funding for this research was from That Man May See and the South Asia Research Fund. The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162. Dr. Acharya is supported by a National Eye Institute K23EY017897 grant and a Research to Prevent Blindness Career Development Award. Dr. Lietman is supported by a National Eye Institute grant U10-EY015114 and a Research to Prevent Blindness award. Dr. Zegans is supported by a K08 EY13977-01 NEI grant.
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in BJO and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://bjo.bmj.com/ifora/licence.pdf).
COMPETING INTERESTS
None declared.
Alcon donated moxifloxacin for the study. The sponsors had no role in the design or conduct of the study, data analysis or manuscript preparation. None of the authors have any financial disclosures related to this manuscript.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bulletin of the World Health Organization. 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Titiyal JS, Negi S, Anand A, et al. Risk factors for perforation in microbial corneal ulcers in north India. Br J Ophthalmol. 2006;90:686–9. doi: 10.1136/bjo.2005.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erie J, Nevitt M, Hodge D, et al. Incidence of ulcerative keratitis in a defined population from 1950 through 1988. Archives of Ophthalmology. 1993;111:1665–71. doi: 10.1001/archopht.1993.01090120087027. [DOI] [PubMed] [Google Scholar]
- 4.O’Day DM. Corticosteroids - An Unresolved Debate. Ophthalmology. 1991;98:845–6. doi: 10.1016/s0161-6420(91)32212-7. [DOI] [PubMed] [Google Scholar]
- 5.Matoba AY, et al. Bacterial Keratitis: Preferred Practice Pattern: American Association of Ophthalmology. 2000 [Google Scholar]
- 6.Wilhelmus KR. Indecision about corticosteroids for bacterial keratitis - An evidence-based update. Ophthalmology. 2002;109:835–42. doi: 10.1016/s0161-6420(02)00963-6. [DOI] [PubMed] [Google Scholar]
- 7.Carmichael TR, Gelfand Y, Welsh NH. Topical Steroids in the Treatment of Central and Paracentral Corneal Ulcers. British Journal of Ophthalmology. 1990;74:528–31. doi: 10.1136/bjo.74.9.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barron BA, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–82. doi: 10.1016/s0161-6420(13)31155-5. [DOI] [PubMed] [Google Scholar]
- 9.Ofloxacin monotherapy for the primary treatment of microbial keratitis: a double-masked, randomized and controlled trial with conventional dual therapy. The Ofloxacin Study Group. Ophthalmology. 1997;104:1902–9. [PubMed] [Google Scholar]
- 10.Booranapong W, Kosrirukvongs P, Prabhasawat P, et al. Comparison of topical lomefloxacin 0.3 per cent versus topical ciprofloxacin 0.3 per cent for the treatment of presumed bacterial corneal ulcers. J Med Assoc Thai. 2004;87:246–54. [PubMed] [Google Scholar]
- 11.Constantinou M, Daniell M, Snibson GR, et al. Clinical efficacy of moxifloxacin in the treatment of bacterial keratitis: a randomized clinical trial. Ophthalmology. 2007;114:1622–9. doi: 10.1016/j.ophtha.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Hyndiuk RA, Eiferman RA, Caldwell DR, et al. Comparison of ciprofloxacin ophthalmic solution 0.3% to fortified tobramycin-cefazolin in treating bacterial corneal ulcers. Ciprofloxacin Bacterial Keratitis Study Group. Ophthalmology. 1996;103:1854–62. doi: 10.1016/s0161-6420(96)30416-8. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien TP, Maguire MG, Fink NE, et al. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group. Arch Ophthalmol. 1995;113:1257–65. doi: 10.1001/archopht.1995.01100100045026. [DOI] [PubMed] [Google Scholar]
- 14.Parmar P, Salman A, Kalavathy CM, et al. Comparison of topical gatifloxacin 0.3% and ciprofloxacin 0.3% for the treatment of bacterial keratitis. Am J Ophthalmol. 2006;141:282–6. doi: 10.1016/j.ajo.2005.08.081. [DOI] [PubMed] [Google Scholar]
- 15.Prajna NV, George C, Selvaraj S, et al. Bacteriologic and clinical efficacy of ofloxacin 0.3% versus ciprofloxacin 0.3% ophthalmic solutions in the treatment of patients with culture-positive bacterial keratitis. Cornea. 2001;20:175–8. doi: 10.1097/00003226-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Cisneros JR, Murray KM. Corticosteroids in Tuberculosis. Annals of Pharmacotherapy. 1996;30:1298–303. doi: 10.1177/106002809603001115. [DOI] [PubMed] [Google Scholar]
- 17.de Gans J, van de Beek D. Dexamethasone in adults with bacterial meningitis. New England Journal of Medicine. 2002;347:1549–56. doi: 10.1056/NEJMoa021334. [DOI] [PubMed] [Google Scholar]
- 18.Tunkel AR, Scheld WM. Corticosteroids for everyone with meningitis? New England Journal of Medicine. 2002;347:1613–5. doi: 10.1056/NEJMe020131. [DOI] [PubMed] [Google Scholar]