Abstract
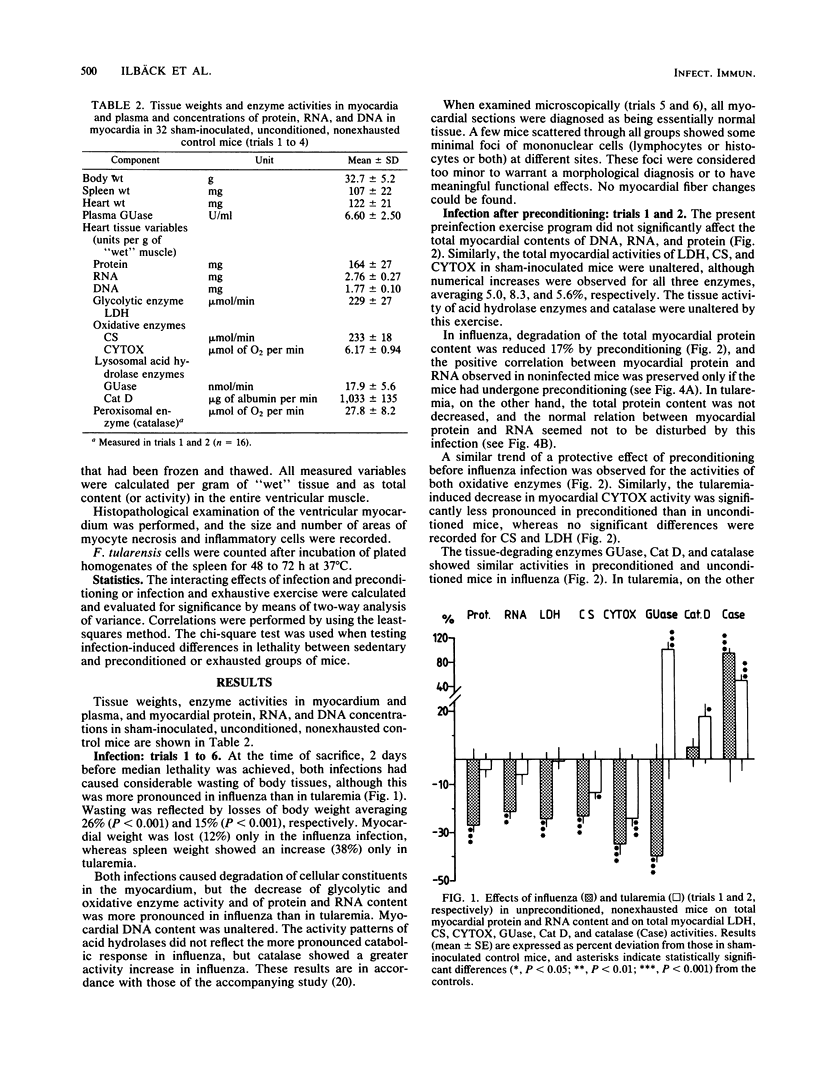
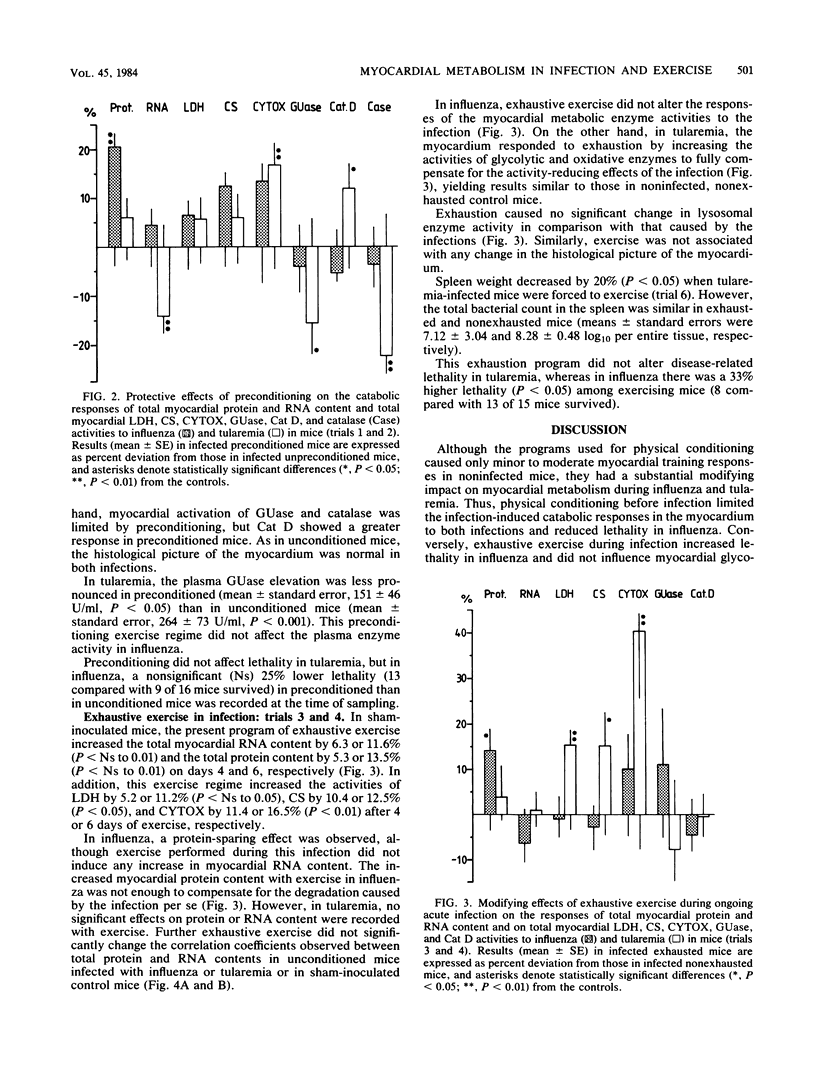
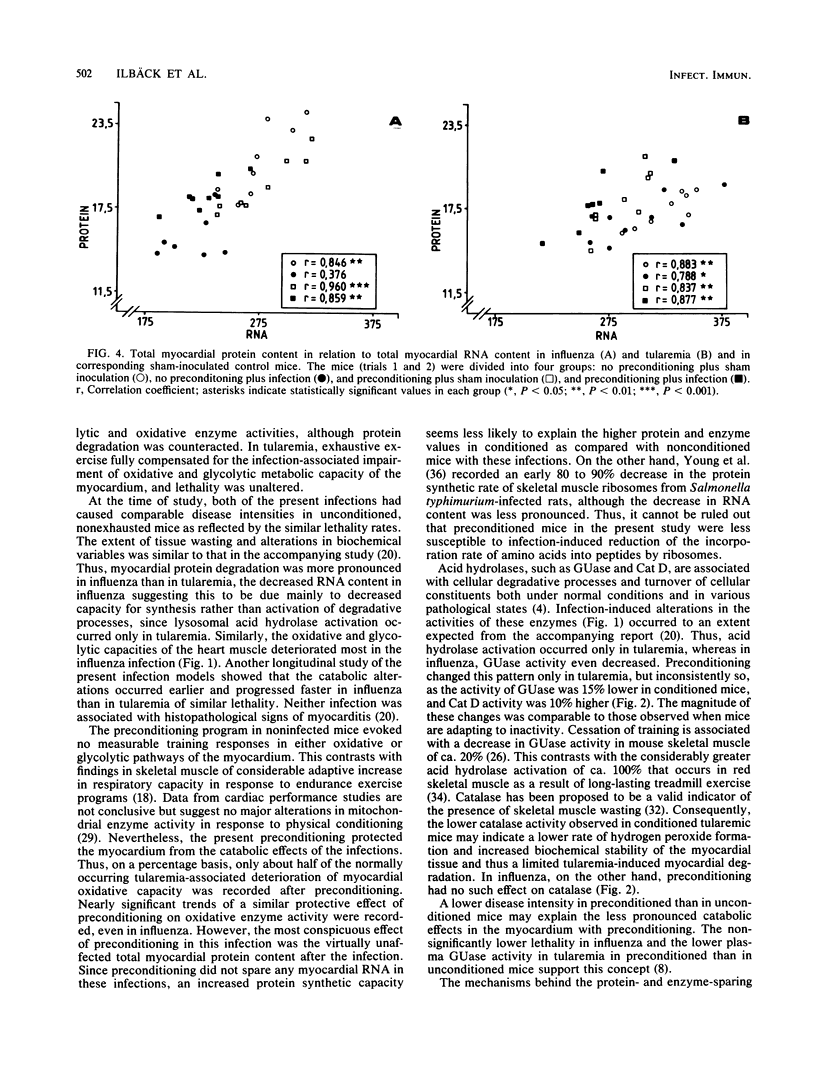
For a study of the interactions of strenuous physical exercise (daily swimming to exhaustion) and a viral as compared with a bacterial infection with regard to the clinical course and the biochemical response of the myocardium, influenza and tularemia of similar lethality were used in mice. In both infections, expected infection-induced catabolic alterations in the ventricular myocardium were evident 2 days before median lethality was achieved, with a more pronounced wasting in influenza than in tularemia. Exercise before inoculation (preconditioning) was beneficial in that the catabolic effects of both infections were limited and lethality in influenza was reduced. Thus, the myocardial protein-degrading effect of influenza did not occur with preconditioning, and oxidative tissue enzyme activities decreased less. In tularemia, cytochrome c oxidase activity was fully preserved with preconditioning, and activation of catalase was less pronounced. Exercise during ongoing infection counteracted the infection-induced decrease in the activities of glycolytic and oxidative enzymes in tularemia, but lethality and bacterial counts in the spleen were uninfluenced. Conversely, exhaustive exercise in influenza increased lethality and had no significant effect on cardiac enzymes. These exercise models caused no major alterations in activation of lysosomal enzymes (beta-glucuronidase and cathepsin D).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bass A., Brdiczka D., Eyer P., Hofer S., Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem. 1969 Sep;10(2):198–206. doi: 10.1111/j.1432-1033.1969.tb00674.x. [DOI] [PubMed] [Google Scholar]
- Bird J. W. Skeletal muscle lysosomes. Front Biol. 1975;43(4):75–109. [PubMed] [Google Scholar]
- Brown K. S., Milvy P. A critique of several epidemiological studies of physical activity and its relationship to aging, health, and mortality. Ann N Y Acad Sci. 1977;301:703–719. doi: 10.1111/j.1749-6632.1977.tb38239.x. [DOI] [PubMed] [Google Scholar]
- CHALMERS T. C., ECKHARDT R. D., REYNOLDS W. E., CIGARROA J. G., Jr, DEANE N., REIFENSTEIN R. W., SMITH C. W., DAVIDSON C. S. The treatment of acute infectious hepatitis. Controlled studies of the effects of diet, rest, and physical reconditioning on the acute course of the disease and on the incidence of relapses and residual abnormalities. J Clin Invest. 1955 Jul;34(7 Pt 2):1163–1235. doi: 10.1172/JCI103164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Kluger M. J. Endogenous pyrogen activity in human plasma after exercise. Science. 1983 May 6;220(4597):617–619. doi: 10.1126/science.6836306. [DOI] [PubMed] [Google Scholar]
- Canonico P. G., Bird J. W. Lysosomes in skeletal muscle tissue. Zonal centrifugation evidence for multiple cellular sources. J Cell Biol. 1970 May;45(2):321–333. doi: 10.1083/jcb.45.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico P. G., Powanda M. C., Cockerell G. L., Moe J. B. Relationship of serum beta-glucuronidase and lysozyme to pathogenesis of tularemia in immune and nonimmune rats. Infect Immun. 1975 Jul;12(1):42–47. doi: 10.1128/iai.12.1.42-47.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutilletta A. F., Edmiston K., Dowell R. T. Effect of a mild exercise program on myocardial function and the development of hypertrophy. J Appl Physiol Respir Environ Exerc Physiol. 1979 Feb;46(2):354–360. doi: 10.1152/jappl.1979.46.2.354. [DOI] [PubMed] [Google Scholar]
- Dawson C. A., Horvath S. M. Swimming in small laboratory animals. Med Sci Sports. 1970 Summer;2(2):51–78. [PubMed] [Google Scholar]
- ELSON S. H., ABELMANN W. H. EFFECTS OF MUSCULAR ACTIVITY UPON THE ACUTE MYOCARDITIS OF C3H MICE INFECTED WITH TRYPANOSOMA CRUZI. Am Heart J. 1965 May;69:629–636. doi: 10.1016/0002-8703(65)90245-0. [DOI] [PubMed] [Google Scholar]
- Edlung A. The effect of defined physical exercise in the early convalescence of viral hepatitis. Scand J Infect Dis. 1971;3(3):189–196. doi: 10.3109/inf.1971.3.issue-3.02. [DOI] [PubMed] [Google Scholar]
- Friman G., Ilbäck N. G., Beisel W. R., Crawford D. J. The effects of strenuous exercise on infection with Francisella tularensis in rats. J Infect Dis. 1982 May;145(5):706–714. doi: 10.1093/infdis/145.2.706. [DOI] [PubMed] [Google Scholar]
- Gatmaitan B. G., Chason J. L., Lerner A. M. Augmentation of the virulence of murine coxsackie-virus B-3 myocardiopathy by exercise. J Exp Med. 1970 Jun 1;131(6):1121–1136. doi: 10.1084/jem.131.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckian J. C., Morrey B. F., Kirby H. B. Role of lysosomes and cathepsin inhibitor in plasma during pneumococcal infection. J Infect Dis. 1970 Oct;122(4):290–302. doi: 10.1093/infdis/122.4.290. [DOI] [PubMed] [Google Scholar]
- HORSTMANN D. M. Acute poliomyelitis relation of physical activity at the time of onset to the course of the disease. J Am Med Assoc. 1950 Jan 28;142(4):236–241. doi: 10.1001/jama.1950.02910220016004. [DOI] [PubMed] [Google Scholar]
- Hedin G., Friman G. Orthostatic reactions and blood volumes after moderate physical activation during acute febrile infections. Int Rehabil Med. 1982;4(2):107–109. doi: 10.3109/09638288209166891. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson E. W., Dominik J. W., Rowberg A. H., Higbee G. A. Influenza virus population dynamics in the respiratory tract of experimentally infected mice. Infect Immun. 1976 Feb;13(2):438–447. doi: 10.1128/iai.13.2.438-447.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A. M., Wilson F. M. Virus myocardiopathy. Prog Med Virol. 1973;15:63–91. [PubMed] [Google Scholar]
- Moe J. B., Canonico P. G., Stookey J. L., Powanda M. C., Cockerell G. L. Pathogenesis of tularemia in immune and nonimmune rats. Am J Vet Res. 1975 Oct;36(10):1505–1510. [PubMed] [Google Scholar]
- Neufeld H. A., Pace J. A., White F. E. The effect of bacterial infections on ketone concentrations in rat liver and blood and on free fatty acid concentrations in rat blood. Metabolism. 1976 Aug;25(8):877–884. doi: 10.1016/0026-0495(76)90120-7. [DOI] [PubMed] [Google Scholar]
- Pilström L., Vihko V., Aström E., Arstila A. U. Activity of acid hydrolases in skeletal muscle of untrained, trained and detrained mice of different ages. Acta Physiol Scand. 1978 Oct;104(2):217–224. doi: 10.1111/j.1748-1716.1978.tb06269.x. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Interferon and neutralizing antibody in sera of exercised mice with coxsackievirus B-3 myocarditis. Proc Soc Exp Biol Med. 1976 Feb;151(2):333–338. doi: 10.3181/00379727-151-39204. [DOI] [PubMed] [Google Scholar]
- Saltin B. The interplay between peripheral and central factors in the adaptive response to exercise and training. Ann N Y Acad Sci. 1977;301:224–231. doi: 10.1111/j.1749-6632.1977.tb38201.x. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Tipton C. M. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–251. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- Squibb R. L., Lyons M. M., Beisel W. R. Virus involvement in the avian heart: effect on protein synthesis. J Nutr. 1968 Dec;96(4):509–512. doi: 10.1093/jn/96.4.509. [DOI] [PubMed] [Google Scholar]
- Stauber W. T., Bird J. W., Schottelius B. A. Catalase: an enzymatic indicator of the degree of muscle wasting. Exp Neurol. 1977 May;55(2):381–389. doi: 10.1016/0014-4886(77)90008-5. [DOI] [PubMed] [Google Scholar]
- Tottmar S. O., Pettersson H., Kiessling K. H. The subcellular distribution and properties of aldehyde dehydrogenases in rat liver. Biochem J. 1973 Dec;135(4):577–586. doi: 10.1042/bj1350577a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihko V., Salminen A., Rantamäki J. Exhaustive exercise, endurance training, and acid hydrolase activity in skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):43–50. doi: 10.1152/jappl.1979.47.1.43. [DOI] [PubMed] [Google Scholar]
- Wannemacher R. W., Jr, Banks W. L., Jr, Wunner W. H. Use of a single tissue extract to determine cellular protein and nucleic acid concentrations and rate of amino acid incorporation. Anal Biochem. 1965 May;11(2):320–326. doi: 10.1016/0003-2697(65)90020-5. [DOI] [PubMed] [Google Scholar]
- Young V. R., Chen S. C., Newberne P. M. Effect of infection on skeletal muscle ribosomes in rats fed adequate or low protein. J Nutr. 1968 Mar;94(3):361–368. doi: 10.1093/jn/94.3.361. [DOI] [PubMed] [Google Scholar]



