Abstract
A transient increase in intracellular Ca2+ is the universal signal for egg activation at fertilization. Eggs acquire the ability to mount the specialized fertilization-specific Ca2+ signal during oocyte maturation. The first Ca2+ transient following sperm entry in vertebrate eggs has a slow rising phase followed by a sustained plateau. The molecular determinants of the sustained plateau are poorly understood. We have recently shown that a critical determinant of Ca2+ signaling differentiation during oocyte maturation is internalization of the plasma membrane calcium ATPase (PMCA). PMCA internalization is representative of endocytosis of several integral membrane proteins during oocyte maturation, a requisite process for early embryogenesis. Here we investigate the mechanisms regulating PMCA internalization. To track PMCA trafficking in live cells we cloned a full-length cDNA of Xenopus PMCA1, and show that GFP-tagged PMCA traffics in a similar fashion to endogenous PMCA. Functional data show that MPF activation during oocyte maturation is required for full PMCA internalization. Pharmacological and co-localization studies argue that PMCA is internalized through a lipid raft endocytic pathway. Deletion analysis reveal a requirement for the N-terminal cytoplasmic domain for efficient internalization. Together these studies define the mechanistic requirements for PMCA internalization during oocyte maturation.
Keywords: Plasma-membrane Ca2+ ATPase (PMCA), Calcium, oocyte maturation, endocytosis, trafficking, lipid-rafts, Xenopus laevis
Introduction
Oocyte maturation defines a cellular differentiation period that prepares the immature oocyte for egg activation at fertilization. Egg activation refers to the cellular events that take place at and shortly after fertilization, and which ensure a proper egg-to-embryo transition (Stricker, 1999). These include the block to polyspermy and the completion of meiosis and transition into embryonic mitosis in vertebrates. Egg activation is mediated by a rise in cytoplasmic Ca2+ with specialized spatial and temporal dynamics (Stricker, 1999). In Xenopus the specialized fertilization-specific Ca2+ signal takes the form of a slow sweeping Ca2+ wave followed by a high Ca2+ plateau that lasts for several minutes (Busa et al., 1985; Machaca, 2007). Ca2+ channels and transporters underlie and define the spatial and temporal dynamics of the Ca2+ signal in eggs. The activity and localization of these channels and transporters is modulated during Xenopus oocyte maturation to endow the egg with the capacity to produce the specialized Ca2+ signal at fertilization (Machaca et al., 2000; Machaca et al., 2002; Machaca, 2004; El Jouni et al., 2005; Machaca, 2007). A critical component of Ca2+ signaling differentiation during maturation is internalization of the plasma-membrane Ca2+ ATPase (PMCA) (El Jouni et al., 2005; Ullah et al., 2007). PMCA is a high-affinity, low capacity Ca2+ pump and represent a primary pathway for Ca2+ extrusion out of the cell following Ca2+ mobilization (Guerini et al., 2005). PMCA localizes to the cell membrane in immature oocytes, whereas in fully mature eggs, it translocates to an intracellular vesicular pool thus inhibiting Ca2+ extrusion (El Jouni et al., 2005).
Mathematical modeling argues that the absence of PMCA activity is critical to maintain a high cytoplasmic Ca2+ plateau in eggs, which is important for egg activation (Ullah et al., 2007). PMCA internalization during oocyte maturation is representative of a pervasive down-regulation of several oocyte integral membrane proteins and transport pathways, including β-integrin (Muller et al., 1993), the Na-pump (Schmalzing et al., 1990; Muller et al., 1993), and amino-acid and ionic transporters (Richter et al., 1984). This maturation-dependent endocytosis is presently best described for the Na-pump during maturation (Schmalzing et al., 1990; Pralong-Zamofing et al., 1992), however the molecular regulation of membrane proteins internalization remains obscure.
Maturation-dependent membrane protein internalization is coupled to a dramatic decrease in plasma membrane surface area (Kado et al., 1981; Machaca et al., 2000), which is believed to be due to an early block of exocytosis during maturation while endocytosis continues unchecked (Colman et al., 1985; Leaf et al., 1990). The exocytotic block appears to be between the trans-Golgi network (TGN) and the plasma membrane (Colman et al., 1985; Leaf et al., 1990). The decrease in the cell membrane surface area is revealed structurally by the loss of microvilli, which are enriched in oocytes but practically absent in eggs (Gardiner et al., 1983; Campanella et al., 1984). Despite the dramatic decrease in membrane area, the internalization of integral membrane proteins during maturation is not due to a non-specific bulk-flow phenomenon coupled to the decrease in membrane area. This is because some membrane proteins such as the Ca2+-activated Cl- channels, which are critical for the block to polyspermy (Machaca et al., 2001), and are in fact functionally enriched during maturation (Machaca et al., 2000). In addition, if bulk flow is to be invoked for membrane protein internalization, then the level of downregulation is expected to be proportional to the decrease in membrane surface area (~50%). This is not the case as both PMCA and the Na+-pump are fully internalized during maturation (Schmalzing et al., 1990; El Jouni et al., 2005). This argues that a subset of membrane proteins in the oocyte are specifically targeted for internalization during oocyte maturation.
The internalization of ionic transporters into an intracellular vesicular pool generates a pool of membranes and membrane proteins that will incorporate into the newly formed blastomeres during the rapid early embryonic mitotic divisions (Angres et al., 1991; Gawantka et al., 1992). This mechanism is thought to be the initiating step in the biogenesis of the first polarized epithelium during the blastocoele stage of Xenopus embryogenesis (Muller, 2001). As early blastomeres divide they incorporate intracellular vesicles enriched with ionic transporters into their basolateral membranes, while the plasma membrane -which is devoid of ionic transporters-forms the apical membrane. This endows this newly formed epithelium with the capacity to transport salts and water in a vectorial fashion, eventually contributing to the formation of the fluid-filled blastocoel cavity (Muller et al., 1995; Muller, 2001).
Given the critical role of PMCA internalization for the differentiation of Ca2+ signaling and as an example of integral membrane protein internalization during maturation, in this work we begin to define the regulatory mechanisms governing PMCA internalization during oocyte maturation.
Materials and Methods
Cloning of Xenopus oocyte PMCA, GFP-tagging and deletion constructs
Several Xenopus oocyte ESTs were obtained from EST projects in Japan (NIBB, NBRP) and sequenced. Based on these sequences specific PCR primers were designed to amplify the full length PMCA cDNA from a Xenopus oocyte cDNA library, a kind gift from Nigel Garret at the Wellcome Trust Cancer Research Institute. PMCA was amplified from the library either untagged or by engineering a Flag-tag at the C-terminal end using the following primers. Forward primer: GCTGGATCCGCTATGGCTAATA ATTCAGTTGCATAT. Reverse primer for untagged PMCA: GGCTCGAGTCAGAGT GATGTCTCCAGACTATGTAG. Reverse primer for Flag-tagged PMCA: GGCTCGAGTCATTTATCATCATCATCTTTATAATCGAGTGATGTCTCCAGACTATGTAGTGG. Multiple PCR clones were sequences in addition to the ESTs to confirm the Xenopus oocyte PMCA1b sequence (Accession number EU752492). The amplified full-length cDNA was sub-cloned into the BamH1-Xho1 sites of pSGEM (a generous from Michael Hollmann (Max-Planck-Institute) (Villmann et al., 1997).
To add a GFP tag in frame at the N-terminus of PMCA, eGFP was cut out of peGFPc1 with NheI-BamH1 and ligated into pSGEM-PMCA-FLAG cut with XbaI-BamH1. PMCA deletions were generated using Quick-Change II XL site-directed mutagenesis kit (Stratagene). Primers used for generating the deletions are listed in table 1. RNAs from the different constructs were transcribed in vitro using the mMessage mMachine transcription kit (Ambion). The GFP-PMCA constructs were linearized with Sph1 and RNA transcribed using T7 polymerase. RNAs for Cyclin B1, Mos and Wee1 were prepared as previously described (Sun et al., 2004).
Table 1.
For each deletion the forward primer is listed in the top row and the reverse primer in the second row.
| Deletion | Primers | Residues |
|---|---|---|
| DEL 1 | ggtaccgcgggcccgggatccgctatggctaataattcagttgcaggaatttgttctaggttgaaaacctctccacatgaa | 8-54 |
| ttcatgtggagaggttttcaacctagaacaaattcctgcaactgaattattagccatagcggatcccgggcccgcggtacc | ||
| DEL 2 | aagttattcaaatccccgtggctgacatagttgttggagatattgcacaaattaaactttcaggaacacatgttatggaggggtct | 217-257 |
| agacccctccataacatgtgttcctgaaagtttaatttgtgcaatatctccaacaactatgtcagccacggggatttgaataactt | ||
| DEL 3 | tgcaatttgttcagataaaactggaacactaaccatgaacaggatgacagtagtccaatctttcataaatgaaaagcatagcaagggtgcttctgagattatccttaaaaagtgctaca | 491-593 |
| tgtagcactttttaaggataatctcagaagcacccttgctatgcttttcatttatgaaagattggactactgtcatcctgttcatggttagtgttccagttttatctgaacaaattgca | ||
| DEL 4 | cattgtgaaagcagtgatgtggggaaggtttcttcagtttcagcttactgtcaatg | 841-848 |
| cattgacagtaagctgaaactgaagaaaccttccccacatcactgctttcacaatg | ||
| DEL 5 | gatgacgctccaacaaaacgacactcaactgctcttgacagtggaatacaccttat | 1173-1182 |
| ataaggtgtattccactgtcaagagcagttgagtgtcgttttgttggagcgtcatc |
Imaging and Immunocytochemistry
Oocytes were activated with progesterone 5 days after injection of 16.5ng of GFP-PMCA RNA. Cells were imaged to confirm GFP-PMCA expression and then fixed in 2% paraformaldehyde for 2 h at 4°C, embedded in tissue freezing media (Electron Microscopy Science) using Tissue Tek cryomold, and frozen at −20°C overnight. Sections of 8μm were collected on super frost plus Gold slides (Electron Microscopy Science). Slides were washed in TBST (20 mM Tris pH 7.4, 150 mM NaCl, and 0.1% Tween) for 1 hr and then blocked in blocking buffer (10 mM Tris–HCl pH 7.5, 1% BSA, 0.3%Triton-X100, 1% Gelatin, 0.02 M Glycine, 150 mM NaCl, and 5% goat serum) for 1 hr at room temperature. Slides were then incubated with primary antibodies for 90 min in 20 mM Tris pH 7.4, 150 mM NaCl, and 0.05% goat serum, followed by 1 hr incubation with the appropriate Alexa546 labeled secondary (Molecular Probes). Antibodies used were: a pan-specific anti-PMCA antibody that recognizes all 4 PMCA isoforms (MA3-914, Affinity BioReagents) and anti-FLAG (#2004712) antibody. For every experiment, slices were stained with the secondary alone as a control and showed no detectable staining at the confocal settings used to collect the experimental images. For Wheat Germ Agglutinin (WGA) staining cells were incubated in Normal Ringer Solution at 4°C for 20min then switched to fixing solution for 10min at 4°C and then WGA-633 was added at a concentration of 4.8 μg/ml and incubated for 2 hrs at 4°C. Transmission and fluorescence images were collected on a Zeiss LSM510 confocal at RT using a Plan-Apochromat 63× oil objective (NA 1.4). Figures were compiled using Adobe Photoshop.
For live cell imaging GFP-PMCA was visualized directly on an LSM510 confocal using a 40x water objective (NA 1.2). The cell membrane was stained with Wheat germ agglutinin-Alexa633 (4.8μg/ml) in PBS for 15min at RT. Cells were scanned at a focal plane 250μm above the coverslip unless otherwise indicated. For cholera toxin B (CTB) labeling GFP-PMCA expressing cells were incubated with Alexa555-labeled CTB (4.8μg/ml) in OR2 for 3.5hrs on ice, rinsed in OR2 to wash out excess CTB and chased for 12-33min before imaging. For transferrin labeling, GFP-PMCA expressing cells at GVBD were incubated with Alexa633-labeled transferrin (125μg/ml) in OR2 solution for 0.5-1.5 hr, washed extensively to remove excess transferrin and chased for different time points before imaging.
Westerns
For PMCA Westerns cells were dounced in lysis buffer (20 mM HEPES-KOH pH 7.5, 1 mM EGTA, 2 mM β-Mercaptoethanol, 10% sucrose, 1 mM PMSF, and 0.1 mM AEBSF, 80 nM Aprotinin, 5 μM Bestatin, 1.5 μM E–64, 2 μM Leupeptin, and 1 μM Pepstatin A) and centrifuged at 1000×g two times to remove the yolk. Samples were separated on 5% polyacrylamide gels, transferred to PVDF membrane and blotted with the primary antibodies (PMCA Pan antibody MA3-914 from ABR or PMCA1 isoform specific antibody PA1-914). This was followed by the appropriate HRP conjugated secondary antibody (Jackson ImmunoResearch) and detected by ECL-Plus (Amersham Pharmacia Biotech). Westerns were visualized using a STORM™ system (Molecular Dynamics).
For phospho-MAPK and phospho-Cdc2 lysates were prepared in extraction buffer (80 mM ß-glycerophosphate, 20 mM Hepes, pH 7.5, 20 mM EGTA, 15 mM MgCl2, 1 mM sodium vanadate, 50 mM NaF, 1 mM DTT, 10 μg/ml aprotinin, 50 μg/ml leupeptin, 1 mM PMSF) and typically the equivalent of one oocyte per lane was loaded. Anti-phopho-MAPK, anti-phospho-Tyr15 of Cdc2 antibodies were from Cell Signaling.
Results
Cloning of the Xenopus oocyte PMCA
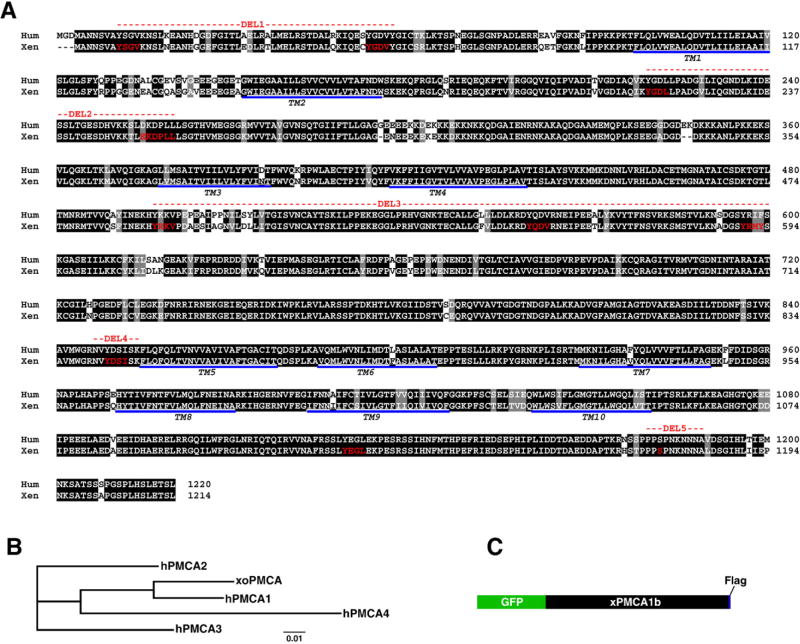
As a first step toward characterizing PMCA trafficking during oocyte maturation, we needed to obtain a full-length cDNA of the Xenopus oocyte PMCA gene to allow molecular manipulation. However, no full-length cDNA are available for any of the four Xenopus laevis PMCA isoforms. To clone a full-length cDNA, we searched Xenopus EST databases focusing on ESTs from oocytes, ovaries and embryos. We obtained and sequenced several EST clones with high sequence homology to the N- and C-termini of human PMCA from the Xenopus EST projects in Japan (NIBB, NBRP). Based on the EST sequences, we designed primers targeted at amplifying the full length PMCA cDNA from a Xenopus oocyte cDNA library. We were successful at amplifying a 3699 bp PMCA clone that covered the entire coding sequence (Fig. 1A, Accession number EU752492). To rule out PCR errors multiple PCR products in addition to the ESTs were sequenced to obtain a minimum 4 fold coverage of the entire coding sequence. Blast searches and Clustal analyses reveal that the gene amplified from the oocyte library (xoPMCA) codes the for PMCA1b isoform (Fig. 1B), which is the ubiquitous form of the pump (Strehler et al., 2007). Xenopus oocyte PMCA1b is highly conserved with 90% sequence identity to human PMCA1b (Fig. 1A).
Figure 1. Cloning of Xenopus oocyte PMCA 1b.
A. Sequence alignment of Xenopus (Xen) and human (Hum) PMCA 1b reveals 90% sequence identity. Several features are highlighted: 10 trans-membrane domains (underlined), the nine linear endocytic motifs (red) and the consensus MAPK/MPF phosphorylation site (S1176 in red). The red dashed lines indicate the segments deleted in the various deletion constructs (see Fig. 5). B. Phylogenetic analysis of Xenopus oocyte PMCA (xoPMCA) with the 4 human PMCA isoforms show that the cloned PMCA is the PMCA1 isoform. The sequences used for this analysis are: hPMCA1 (Accession number P20020), hPMCA2 (NP_001674), hPMCA3 (Q16720), hPMCA4 (AAA36455). C. Cartoon of the GFP- and Flag-tagged Xenopus PMCA.
Trafficking of PMCA during maturation
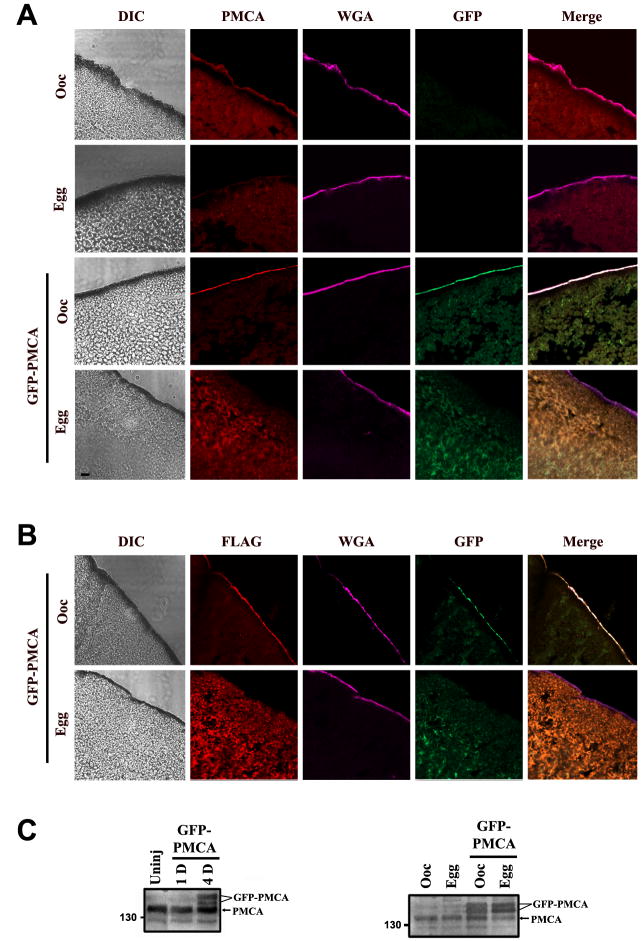
To be able to follow PMCA trafficking in real time during oocyte maturation, we generated a GFP-tagged form of the pump with a flag-tag at the C-terminus (Fig. 1C). We then tested the trafficking of GFP-PMCA as compared to endogenous PMCA (Fig. 2). Oocytes were injected with GFP-PMCA RNA and allowed to express for several days. GFP-PMCA expression could be detected as early as 4 days after injection (Fig. 2C, left), but varied up to 7 days depending on the batch of oocytes used. Once GFP fluorescence could be readily detected, oocytes were matured with progesterone and both immature oocytes and fully mature eggs were fixed, sectioned and stained with either anti-Flag or anti-PMCA antibodies as indicated in Figure 2. As previously shown (El Jouni et al., 2005), in control cells PMCA is enriched at the cell membrane and colocalizes with wheat germ agglutinin in oocytes (Fig. 2A, top row, Ooc). Whereas in eggs PMCA is not detectable at the cell membrane as it is internalized during maturation (Fig. 2A, Egg).
Figure 2. GFP-PMCA traffics in a similar fashion to endogenous PMCA.
A. Subcellular localization of endogenous PMCA and GFP-PMCA. Oocytes and eggs were fixed, sectioned, and stained with a pan PMCA antibody (red signal). Differential interference contrast (DIC), GFP (green), and wheat germ agglutinin (WGA, pink) images of the same slice are also shown. WGA-Alexa633 (pink) was used to stain the plasma membrane. The scale bar is 10μm. B. Similar staining as in panel A, except that an anti-Flag antibody was used to detect injected GFP-PMCA (Flag). For panels A & B the oocyte examples are representative of at least 30 similar cells and the egg examples are representative of 5-15 similar cells. All cells examined showed robust PMCA enrichment at the cell membrane in oocytes and undetectable levels in eggs. C. Left panel: Time course of PMCA expression using the pan PMCA antibody at 1 (1D) and 4 days (4D) after injection, as compared to uninjected cells (Uninj) (left panel). An equivalent of one cell was loaded in each lane. Right panel: Expression of GFP-PMCA as compared to endogenous PMCA using a PMCA1 isoform specific antibody (right panel). Oocytes refer to cells before progesterone treatment (Ooc) and eggs are mature cells (>3hrs after GVBD). An equivalent of ½ cell was loaded per lane.
In GFP-PMCA injected cell, the GFP-tagged PMCA traffics to the plasma membrane where it co-localizes with endogenous PMCA (Fig. 2A, GFP-PMCA, Ooc). Fortunately, fixation did not abrogate the GFP signal allowing us to co-localize endogenous PMCA (antibody staining) and GFP-PMCA. Following maturation GFP-PMCA is internalized in a similar fashion to endogenous PMCA. Staining GFP-PMCA with an anti-flag antibody confirms the GFP results (Fig. 2B). GFP-PMCA is not grossly over-expressed as compared to endogenous PMCA, and both GFP-tagged and endogenous PMCA run as two species with different electrophoretic mobility most likely representing differentially glycosylated forms (Fig. 2C, right). Together these data show that GFP-tagged PMCA traffics normally to the plasma membrane in oocytes, and that it is internalized during oocyte maturation in a similar fashion to endogenous PMCA. Therefore, GFP-PMCA is a good marker for endogenous PMCA internalization during maturation, and allows us to monitor PMCA trafficking in live cells.
Role of the meiotic kinase cascade in PMCA internalization
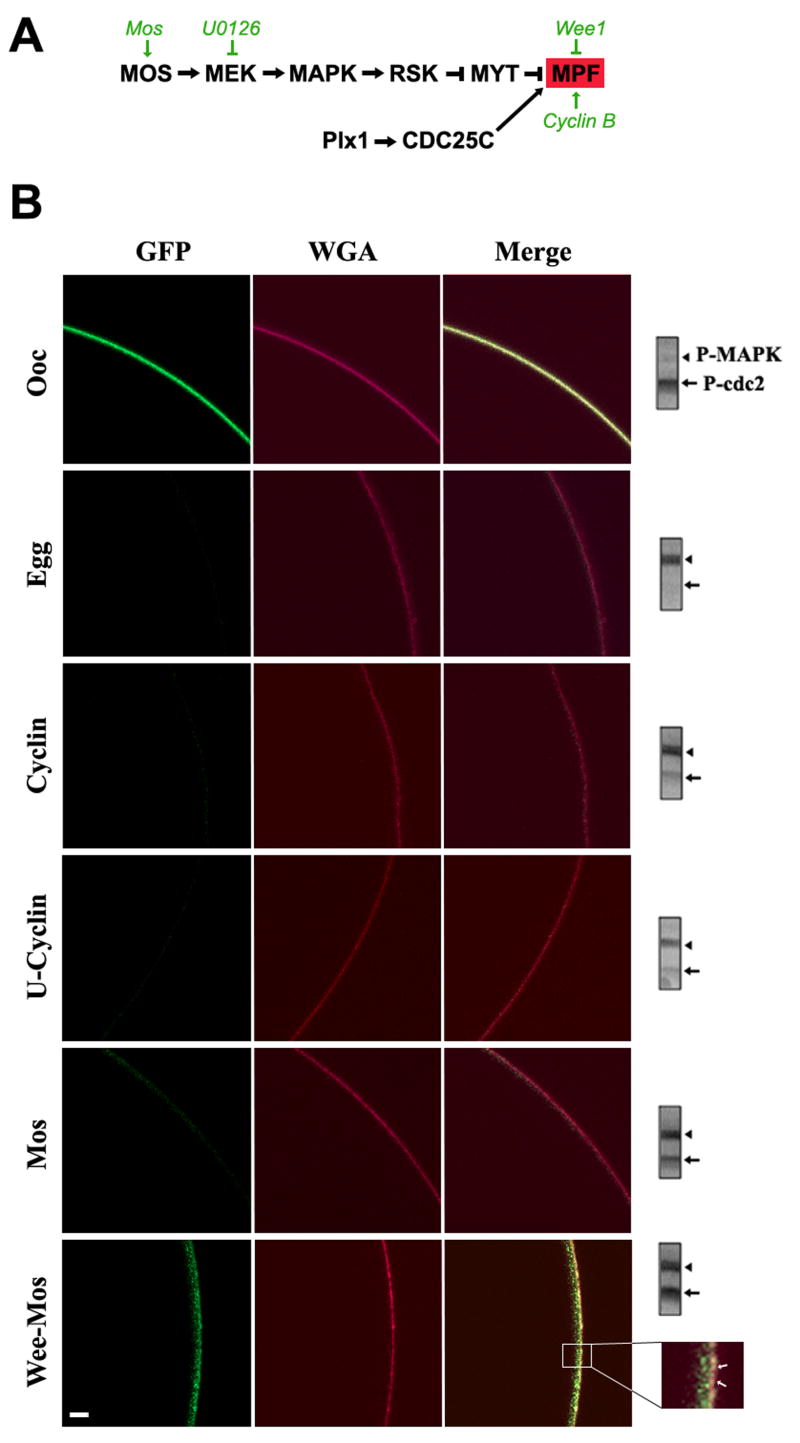
Oocyte maturation is driven by kinase cascades that regulate both cytoplasmic and nuclear (meiosis) maturation. The highlights of these cascades are summarized in Figure 3A. Progesterone through multiple steps induces mRNA polyadenylation leading to translation of several mRNAs including Mos (Nebreda et al., 2000). Mos accumulation activates the MAPK cascade culminating in MPF activation. An additional critical component for MPF activation during oocyte maturation is Cdc25C, which is a dual specificity phosphatase that dephosphosphorylates cdc2 leading to pre-MPF activation (Perdiguero et al., 2004). MPF activation commits the cells to maturation and meiosis progression.
Figure 3. Kinase-dependent modulation on PMCA internalization.
A. Signaling cascade regulating Xenopus oocyte meiosis entry. The steps at which the molecular and pharmacological manipulations used act are shown in green. B. Live cell imaging of PMCA internalization. Oocytes expressing GFP-PMCA were subjected to one of the following treatments: injected with Cyclin B1 RNA (Cyclin); pre-treated with U0126 (50μM) for 1hr before cyclin RNA injection (U-Cyclin); injected with Mos RNA (Mos); or injected with Wee1 RNA and incubated overnight before Mos RNA injection (Wee-Mos). The cell membrane was stained with WGA-Alexa633. The inset shows that in the Wee-Mos treatment, GFP-PMCA internalization is delayed but not completely inhibited. The examples shown are representative of 76 and 29 oocytes and eggs respectively, and 11-15 cells each for treatments used to manipulate the kinase cascades. All cells analyzed showed a similar pattern of PMCA localization to the examples shown, except for the Wee-Mos treatment where in 3/11 cells PMCA was internalized to similar levels as Mos. Western blot analysis for phospho-MAPK (P-MAPK) and phospho-MPF (P-MPF) of each of the scanned cells is shown on the right. The equivalent of ½ a cell was loaded. The scale bar is 20μm.
To test the role of the MAPK cascade and MPF in PMCA internalization we differentially activated these kinases as previously described (Sun et al., 2004). To activate the MAPK cascade in the absence of MPF activation, oocytes were injected with the Wee1 kinase to inhibit MPF, followed by Mos injection to activate the MAPK cascade. To activate MPF while inhibiting MAPK, cells were pre-treated with U0126 (a MEK inhibitor), and then injected with cyclin B1 RNA to induce MPF (Fig. 3A). We confirmed that the kinase cascades were modulated as expected using phospho-specific antibodies (Fig. 3B). MAPK phosphorylation leads to its activation, thus a positive band using phospho-specific anti-MAPK antibodies indicates its activation. In contrast, MPF is activated following cdc2 dephosphorylation. Therefore, loss of phospho-specific cdc2 reactivity is indicative of MPF activation (Fig. 3B, blots).
In oocytes GFP-PMCA localizes to the cell membrane, which is indicated by wheat-germ agglutinin (WGA) staining (Fig. 3B). Internalization of PMCA during maturation is indicated by the almost complete absence of the GFP signal in eggs in this cross-sectional view of live cells (Fig. 3B). As expected both MAPK and MPF are in the inactivate state in oocytes and are activated in eggs (Fig. 3B, blots). When cells are injected with cyclin B RNA both MPF and MAPK are activated due to a positive feedback loop between both kinases (Fig. 3B, Cyclin), and pre-treatment with U0126 before cyclin RNA injection significantly reduced -but did not eliminate- MAPK activation (Fig. 3B, U-cyclin). Nonetheless, in both cases GFP-PMCA was internalized (Fig. 3B). This argues that the activation state of MAPK does not affect the ability of PMCA to be internalized during maturation, at least given the levels of MAPK inhibition attained in these experiments.
To test the role of MPF in PMCA internalization cells were injected with Wee1 RNA followed by Mos RNA injection (Wee-Mos). This resulted in robust activation of MAPK but not MPF (Fig. 3B, Wee-Mos). The control Mos only injected cells activated both MAPK and MPF and in this case PMCA was internalized (Fig. 3B, Mos). However, in cells where MPF activation was inhibited PMCA internalization was significantly delayed (Fig. 3B, Wee-Mos). Inhibition of MPF did not fully block PMCA endocytosis as some internalization was evident (Fig. 3B, inset). This partial internalization could be due to the dramatic endocytosis associated with membrane area decrease during oocyte maturation. Nonetheless, these data show that when MPF is inhibited PMCA internalization is hampered, strongly arguing that MPF activation is important for PMCA internalization.
Endocytic pathway
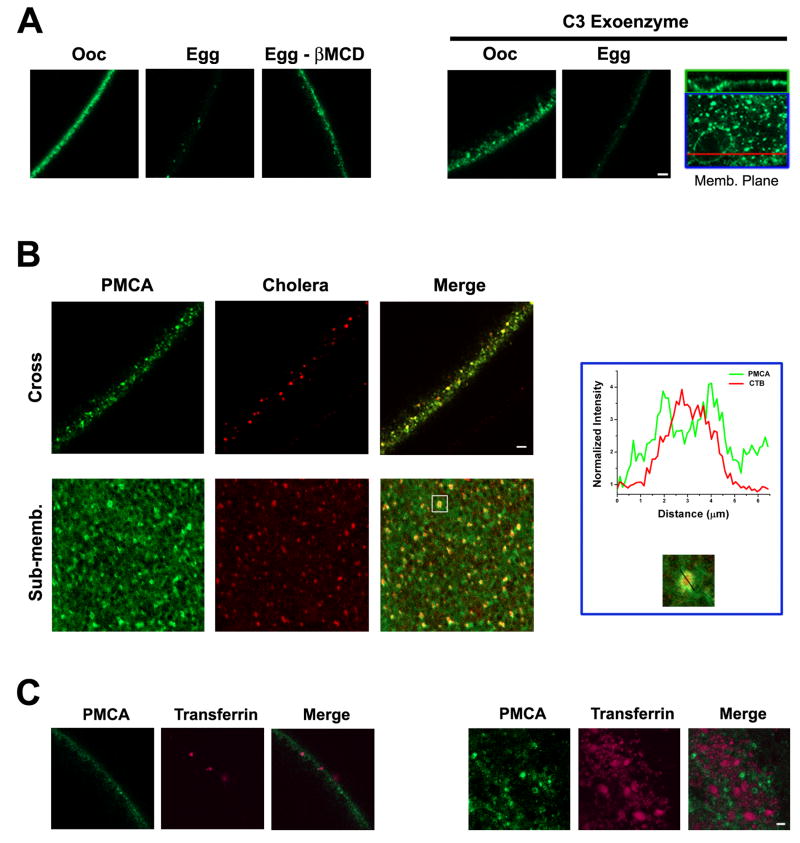
To begin to define the endocytic pathway(s) through which PMCA is internalized during maturation, we co-localized GFP-PMCA with endocytic markers and tested its sensitivity to endocytosis inhibitors (Fig. 4). The constitutive endocytosis pathway in Xenopus oocytes has been shown to be RhoA-dependent and to be inhibited by C3 exoenzyme, which ADP-rybosilates RhoA (Schmalzing et al., 1995). To determine whether PMCA is internalized through the constitutive endocytic pathway, we blocked it using C3 exoenzyme (Fig. 4A). C3 exoenzyme treatment increases membrane area (El Jouni et al., 2007), leading to large membrane invaginations that in cross section give the membrane a broader appearance (Fig. 4A). In confocal images at the membrane focal plane a large invagination appears as a circular structure (Fig. 4A, Memb. Plane). However, despite the larger membrane area that is enriched with PMCA as indicated by the GFP signal, C3 exoenzyme treated cells internalize PMCA normally during maturation (Fig. 4A). This shows that PMCA is not internalized through the constitutive endocytosis pathway during oocyte maturation.
Figure 4. PMCA internalization.
A. To interfere with raft-dependent endocytosis oocytes were treated 2% methyl-β-Cyclodextrin (βMCD) 9hrs before progesterone stimulation. GFP-PMCA internalization is inhibited in cells pretreated with βMCD during oocyte maturation (Egg – βMCD) as compared to control cells (Egg). For the C3 Exoenzyme treatment, oocytes were injected with 1.7ng C3 exoenzyme 1hr before progesterone stimulation (Schmalzing et al., 1995). The C3 exoenzyme treatment increases membrane invaginations leading to a diffuse membrane appearance in the oocyte (Ooc). These invaginations appear as large circular structures at the cell membrane focal plane (inset). A cross section through these circular regions (red line) shows the invagination at the membrane (inset top). Even though membrane area is increased in C3 exoenzyme treated cells PMCA internalization is unaffected (Egg). The scale bar is 10 μm. B. GFP PMCA expressing cells were labelled with Alexa555-labeled B subunit of the cholera toxin (CTB) and imaged around GVBD to visualize PMCA internalization. In a cross-section the GFP-PMCA and CTB signals co-localize (Cross). In a focal plane that is 10-20 μm below the cell membrane (Sub-memb) the CTB and GFP-PMCA signals also co-localize. A close up view and line scan of fluorescence intensity through one of the PMCA and CTB positive vesicles shows CTB in the luminal core of the vesicle, while PMCA localizes the vesicle membrane. The scale bar is 5 μm. C. GFP-PMCA expressing cells at GVBD were labeled Alexa633-labeled transferrin and chased for different time points before imaging. No co-localization is observed between GFP-PMCA and transferrin. The scale bar is 10 μm. The examples shown are representative of 5-8 cells for each treatment.
Depletion of cholesterol from the membrane using β-methyl-cyclodextrin (βMCD) was able to significantly slow down PMCA internalization (Fig. 4A). This shows that PMCA is endocytosed through a cholesterol-depletion sensitive pathway, which typically argues for a lipid raft-dependent endocytic pathway (Kirkham et al., 2005b). Nonetheless, severe cholesterol depletion can inhibit clathrin-dependent endocytosis (Subtil et al., 1999). To determine whether PMCA is internalized through the clathrin-dependent or lipid raft dependent pathway, we co-localized GFP-PMCA with the B subunit of cholera toxin (CTB) as a marker for lipid-raft dependent endocytosis (Fig. 4B), and transferrin as a marker for clathrin-dependent endocytosis (Fig. 4C). For these experiments GFP-PMCA expressing cells were treated with CTB and imaged within ½ hour after GVBD with the goal of catching PMCA early after internalization. PMCA co-localizes with CTB under these conditions (Fig. 4B). In fact vesicles that contain both PMCA and CTB were detected, with PMCA enriched in the vesicle membrane and CTB enriched in the vesicle lumen (Fig. 4B, inset). This is illustrated by the line scan across the vesicle showing the enrichment of CTB in the core of the vesicle, with the CTB signal flanked by the GFP-PMCA signal at the vesicle membrane (Fig. 4B, inset). These data show that PMCA is endocytosed during maturation through the same endocytic pathway as CTB. CTB binds the ganglioside GM1, which is enriched on the outer leaflet of the cell membrane (Spangler, 1992; Wolf et al., 1998), and localizes preferentially to lipid rafts and caveolae (Montesano et al., 1982; Parton, 1994). In contrast, no co-localization was observed between GFP-PMCA and transferring (Fig. 5C). Therefore, our data argue that PMCA is internalized through a lipid raft-dependent and not the clathrin-dependent endocytic pathway.
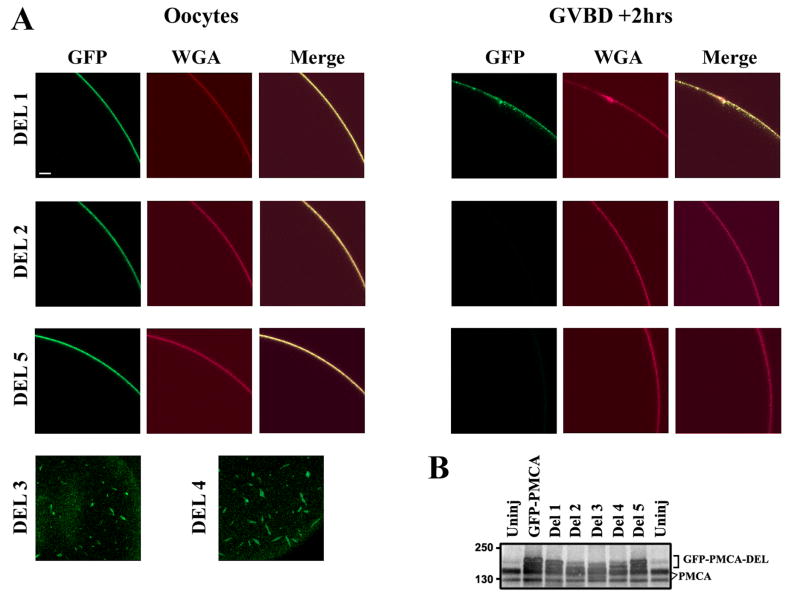
Figure 5. Deletion Analyses.
A. Oocytes were injected with RNA (5-7ng) of each of the GFP-PMCA deletions (DEL1, DEL2, and DEL5), then activated with progesterone 4-5days after injection. Internalization was assayed by imaging cells stained with WGA-Alexa633. For DEL3 and DEL4 no signal was observed at the cell membrane following injection of different RNA concentrations up to 12 days for DEL3 and 16 days for DEL4. Rather the products of these deletions localize intracellularly to elongated vesicle-like structures (Del3, Del4). The examples shown are representative of 5-10 cells for each deletion. Scale bar is 20μm. B. Expression of the full length and deletions. Anti-PMCA blot from uninjected oocytes (Uninj), oocytes injected with full-length GFP-PMCA and the 5 deletions. For the full-length, DEL1, DEL2, and DEL5 lysates were collected 5-7 days after injection. DEL3 and DEL4 lysates were collected 9 days and 16 days post-injection respectively. The bracket marks the electrophoretic mobility of full-length and different deletion mutants (GFP-PMCA-DEL). Endogenous PMCA is also indicated (PMCA).
Deletion analyses
Two major classes of linear motifs target proteins to endosomal compartments, have been identified in the cytosolic domains of transmembrane proteins: the ‘tyrosine based’ and ‘dileucine based’ motifs (Bonifacino et al., 2003). The tyrosine based motifs include NPXY and YXXØ where Ø is a hydrophobic amino acid with a bulky side chain (V, I, F, L, M). The dileucine motif is [DE]XXXLL or DXXLL. Xenopus PMCA1b has 8 sites that match the YXXØ motif and are conserved in human PMCA1b, and one site that matches the dileucine motif in its cytoplasmic domains (Fig. 1A, red). To begin to test whether specific structural determinants target PMCA for endocytosis during maturation, we performed a deletion analysis that removes all the tyrosine-based motifs except for the last one in the cytoplasmic domain, and the dileucine-based internalization motif as indicated in Figure 1. We also deleted a region around a consensus MAPK/MPF phosphorylation site (Ser1176, in xPMCA1b) given the importance of MPF activation in PMCA internalization (Fig. 3). Three of the five engineered deletions, Del1, Del2 and Del5, trafficked normally to the cell membrane where they were enriched (Fig. 5A). In contrast, the products of DEL3 and DEL4 were trapped in intracellular structures (Fig. 5A). Increasing the amount of injected RNA did not produce a membrane signal for these deletions. The inability of Del3 and Del4 to traffic to the cell membreane could be due to improper folding. DEL3 removes a large portion of the cytoplasmic region between TM4 and TM5 which represents the catalytic loop. DEL4 is very close to TM5, which may disrupt membrane insertion and thus prevent proper targeting of the protein to the cell membrane.
Of the three remaining deletions DEL2 and DEL5 were internalized normally during oocyte maturation (Fig. 5A). In contrast, a significant proportion of DEL1 product remained at the cell membrane (Fig. 5A), indicating the there exists important structural determinant(s) in the N-terminal cytoplasmic domain of PMCA1b that are required for targeting PMCA for internalization during oocyte maturation.
Discussion
The Ca2+ rise at fertilization is a prototypical example illustrating how Ca2+ signals elegantly regulate cellular physiology at multiple levels in a temporally and spatially controlled fashion. An important pathway for molding Ca2+ dynamics and maintaining Ca2+ homeostasis is Ca2+ extrusion out of the cell, which relies primarily on the Na-Ca-exchanger (NCE) and PMCA (Guerini et al., 2005). The presence of NCE in Xenopus oocytes is somewhat controversial, however new evidence supports the expression of a functional NCE in the oocyte membrane (Supplisson et al., 1991; Solis-Garrido et al., 2004). PMCA has a high affinity for Ca2+ and fine tunes Ca2+ levels back to the resting state after a Ca2+ transient (Guerini et al., 2005). PMCA is internalized during oocyte maturation, leading to inhibition of Ca2+ extrusion out of the egg (El Jouni et al., 2005). Mathematical modeling argues that PMCA inhibition is essential to generate the specific spatial and temporal Ca2+ dynamics in eggs (Ullah et al., 2007). These Ca2+ dynamics are critical to activate the egg at fertilization. Furthermore, PMCA internalization represents an example of integral membrane protein endocytosis during maturation, a process important for embryogenesis (Muller, 2001). It is therefore important to better define the molecular mechanisms controlling PMCA trafficking during maturation.
Here we clone a full length Xenopus PMCA1b cDNA, and generate a GFP-tagged form to allow visualization of PMCA trafficking in live cells (Fig. 1). GFP-PMCA traffics in a similar fashion to endogenous PMCA, where it is enriched on the cell membrane and endocytosed during oocyte maturation (Fig. 2). Interestingly, MPF activation is required for complete PMCA internalization (Fig. 3). MPF is the master kinase that commits the oocytes to meiosis and maturation. The oocyte is arrested at the G2/M transition of the meiotic cell cycle and commitment to maturation depends on MPF activation. Interestingly PMCA is internalized rapidly (within 1hr) after MPF induction, which is indicated by germinal vesicle breakdown (GVBD). Inhibition of MPF activation under conditions where the MAPK cascade is fully activated inhibits PMCA internalization (Fig. 3).
We also investigated the pathways through which PMCA is endocytosed during maturation. Our results show that PMCA is not internalized through the constitutive RhoA-dependent pathway, because of its lack of sensitivity to C3 exoenzyme (Fig. 4A) (Schmalzing et al., 1995). In addition, PMCA does not co-localize with transferrin arguing that it is not internalized through the clathrin pathway. In contrast, internalized PMCA co-localizes with cholera toxin B and internalization is sensitivity to cholesterol depletion (Fig. 4). CTB binds to the gangioside GM1, which is enriched in lipid rafts, before internalization (Parton, 1994; Wolf et al., 1998).
Interestingly, multiple reports in different cell types showed that PMCA clusters into lipid rich plasma membrane domains, including caveolae (Fujimoto, 1993; Amino et al., 1997; Pang et al., 2005; Sepulveda et al., 2006; Jiang et al., 2007). Together these data argue that PMCA is internalized through a lipid raft-dependent endocytic pathway. It is not clear whether PMCA is endocytosed through caveolae-dependent endocytosis as there are several examples of caveolae-independent non-coated vesicle endocytosis that depend on lipid rafts but not caveolin per se (Kirkham et al., 2005b). In addition, it remains possible that additional endocytic pathways contribute to PMCA internalization, especially that recent reports show that in different cell types a significant proportion of CTB can be internalized through clathrin-dependent and caveolae- and clathrin-independent pathways (Torgersen et al., 2001; Kirkham et al., 2005a).
Interestingly, deletion analyses show that the N-terminus of PMCA1b is important for targeting PMCA for internalization during maturation (Fig. 5). These experiments were initially designed to test the role of Tyr-based motifs, which target proteins for endocytosis through clathrin-coated pits pathway (Bonifacino et al., 2003). However, it is unlikely that the Tyr-based motifs in the N-terminus are important given our functional data which argue against a role for clathrin-dependent endocytosis in PMCA internalization (Fig. 4). Another possibility is that DEL1 disrupts the structure of the N-terminal region of PMCA is such a way that precludes protein-protein interactions or post-translational modifications that target PMCA for internalization. One possibility in that regard in the direct interaction of PMCA with 14-3-3ε, which binds to the N-terminal domain of PMCA, and has been shown to inhibit PMCA function (Rimessi et al., 2005; Linde et al., 2008). In fact the 14-3-3 binding site in the N-terminal region localizes to residues 62-67 of human PMCA1 as part of an α-helix (Linde et al., 2008). The 14-3-3 consensus site corresponds to residues 59-64 in Xenopus PMCA1b, immediately following DEL1 (Fig. 1A). It is therefore possible that DEL1 disrupts the α-helical folding nature of the region close to the 14-3-3 binding site, thus disrupting 14-3-3 and PMCA interaction. In addition, recently the Na-pump has been shown to interact with 14-3-3ε, with evidence that this interaction is important for the Na+-pump endocytosis (Kimura et al., 2007).
PMCA trafficking during maturation represents an important example of internalization of membrane transporters in preparation for embryonic development (Muller, 2001). Such transporters are endocytosed during maturation and then inserted into the basolateral membrane of newly forming blastomeres leading to the formation of the first polarized epithelium during embryogenesis. Studies presented here afford a better delineation of the molecular mechanisms regulating PMCA internalization with important implications on our understanding of the developmental role of vesicular trafficking during oocyte maturation.
Acknowledgments
We are grateful to Nigel Garret (Xenopus oocyte cDNA library) and Michael Hollman (pSGEM) for reagents. This work was funded by a grant from NIH-NIGMS (GM61829) and a UAMS Graduate Student Research Fund grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amino K, Honda Y, Ide C, Fujimoto T. Distribution of plasmalemmal Ca(2+)-pump and caveolin in the corneal epithelium during the wound healing process. Curr Eye Res. 1997;16:1088–1095. doi: 10.1076/ceyr.16.11.1088.5098. [DOI] [PubMed] [Google Scholar]
- 2.Angres B, Muller AH, Kellermann J, Hausen P. Differential expression of two cadherins in Xenopus laevis. Development. 1991;111:829–844. doi: 10.1242/dev.111.3.829. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 4.Busa WB, Nuccitelli R. An Elevated Free Cytosolic Ca2+ Wave Follows Fertilization in Eggs of the Frog, Xenopus laevis. J Cell Biol. 1985;100:1325–1329. doi: 10.1083/jcb.100.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campanella C, Andreuccetti P, Taddei C, Talevi R. The modifications of cortical endoplasmic reticulum during in vitro maturation of Xenopus laevis oocytes and its involvement in cortical granule exocytosis. J Exp Zool. 1984;229:283–293. doi: 10.1002/jez.1402290214. [DOI] [PubMed] [Google Scholar]
- 6.Colman A, Jones EA, Heasman J. Meiotic maturation in Xenopus oocytes: a link between the cessation of protein secretion and the polarized disappearance of golgi apprati. J Cell Biol. 1985;101:313–318. doi: 10.1083/jcb.101.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Jouni W, Haun S, Hodeify R, Hosein WA, Machaca K. Vesicular traffic at the cell membrane regulates oocyte meiotic arrest. Development. 2007;134:3307–3315. doi: 10.1242/dev.005454. [DOI] [PubMed] [Google Scholar]
- 8.El Jouni W, Jang B, Haun S, Machaca K. Calcium signaling differentiation during Xenopus oocyte maturation. Dev Biol. 2005;288:514–525. doi: 10.1016/j.ydbio.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993;120:1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardiner DM, Grey RD. Membrane junctions in Xenopus eggs: their distribution suggests a role in calcium regulation. J Cell Biol. 1983;96:1159–1163. doi: 10.1083/jcb.96.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gawantka V, Ellinger-Ziegelbauer H, Hausen P. Beta 1-integrin is a maternal protein that is inserted into all newly formed plasma membranes during early Xenopus embryogenesis. Development. 1992;115:595–605. doi: 10.1242/dev.115.2.595. [DOI] [PubMed] [Google Scholar]
- 12.Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38:281–289. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, Fernandes D, Mehta N, Bean JL, Michaelis ML, Zaidi A. Partitioning of the plasma membrane Ca2+-ATPase into lipid rafts in primary neurons: effects of cholesterol depletion. J Neurochem. 2007;102:378–388. doi: 10.1111/j.1471-4159.2007.04480.x. [DOI] [PubMed] [Google Scholar]
- 14.Kado RT, Marcher K, Ozon R. Electrical membrane properties of the Xenopus laevis oocyte during progesterone-induced meiotic maturation. Dev Biol. 1981;84:471–476. doi: 10.1016/0012-1606(81)90417-6. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Allen PB, Nairn AC, Caplan MJ. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell. 2007;18:4508–4518. doi: 10.1091/mbc.E06-08-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005a;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkham M, Parton RG. Clathrin-independent endocytosis: New insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005b;1746:350–363. doi: 10.1016/j.bbamcr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Leaf DS, Roberts SJ, Gerhart JC, Moore HP. The secretory pathway is blocked between the trans-Golgi and the plasma membrane during meiotic maturation in Xenopus oocytes. Dev Biol. 1990;141:1–12. doi: 10.1016/0012-1606(90)90097-3. [DOI] [PubMed] [Google Scholar]
- 19.Linde CI, Di Leva F, Domi T, Tosatto SC, Brini M, Carafoli E. Inhibitory interaction of the 14-3-3 proteins with ubiquitous (PMCA1) and tissue-specific (PMCA3) isoforms of the plasma membrane Ca(2+) pump. Cell Calcium. 2008;43:550–561. doi: 10.1016/j.ceca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Machaca K. Increased sensitivity and clustering of elementary Ca2+ release events during oocyte maturation. Dev Biol. 2004;275:170–182. doi: 10.1016/j.ydbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Machaca K. Ca2+ signaling differentiation during oocyte maturation. Journal of Cellular Physiology. 2007;213:331–340. doi: 10.1002/jcp.21194. [DOI] [PubMed] [Google Scholar]
- 22.Machaca K, Haun S. Store-operated Calcium Entry Inactivates at the Germinal Vesicle Breakdown Stage of Xenopus Meiosis. J Biol Chem. 2000;275:38710–38715. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machaca K, Haun S. Induction of maturation-promoting factor during Xenopus oocyte maturation uncouples Ca2+ store depletion from store-operated Ca2+ entry. J Cell Biol. 2002;156:75–85. doi: 10.1083/jcb.200110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machaca K, Qu Z, Kuruma A, Hartzell HC, McCarty N. The Endogenous Calcium-Activated Cl Channel in Xenopus Oocytes: A Physiologically and Biophysically Rich Model System. In: Fuller CM, editor. Calcium activates chloride channels. Academic Press; San Diego: 2001. pp. 3–39. [Google Scholar]
- 25.Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 26.Muller AH, Gawantka V, Ding X, Hausen P. Maturation induced internalization of beta 1-integrin by Xenopus oocytes and formation of the maternal integrin pool. Mech Dev. 1993;42:77–88. doi: 10.1016/0925-4773(93)90100-c. [DOI] [PubMed] [Google Scholar]
- 27.Muller HA. Of mice, frogs and flies: generation of membrane asymmetries in early development. Dev Growth Differ. 2001;43:327–342. doi: 10.1046/j.1440-169x.2001.00587.x. [DOI] [PubMed] [Google Scholar]
- 28.Muller HA, Hausen P. Epithelial cell polarity in early Xenopus development. Dev Dyn. 1995;202:405–420. doi: 10.1002/aja.1002020410. [DOI] [PubMed] [Google Scholar]
- 29.Nebreda AR, Ferby I. Regulation of the meiotic cell cycle in oocytes. Curr Opin Cell Biol. 2000;12:666–675. doi: 10.1016/s0955-0674(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 30.Pang Y, Zhu H, Wu P, Chen J. The characterization of plasma membrane Ca2+-ATPase in rich sphingomyelin-cholesterol domains. FEBS Lett. 2005;579:2397–2403. doi: 10.1016/j.febslet.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- 32.Perdiguero E, Nebreda AR. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle. 2004;3:733–737. [PubMed] [Google Scholar]
- 33.Pralong-Zamofing D, Qi-Han Y, Schmalzing G, Good P, Geering K. Regulation of α1–β3-Na+-K+-ATPase isozyme during meiotic maturation of Xenopus laevis oocytes. Am J Physiol. 1992;262:C1520–C1530. doi: 10.1152/ajpcell.1992.262.6.C1520. [DOI] [PubMed] [Google Scholar]
- 34.Richter HP, Jung D, Passow H. Regulatory changes of membrane transport and ouabain binding during progesterone-induced maturation of Xenopus oocytes. Journal of Membrane Biology. 1984;79:203–210. doi: 10.1007/BF01871059. [DOI] [PubMed] [Google Scholar]
- 35.Rimessi A, Coletto L, Pinton P, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the 14-3-3{epsilon} protein with isoform 4 of the plasma membrane Ca(2+)-ATPase pump. J Biol Chem. 2005;280:37195–37203. doi: 10.1074/jbc.M504921200. [DOI] [PubMed] [Google Scholar]
- 36.Schmalzing G, Eckard P, Kroner S, Passow H. Downregulation of surface sodium pumps by endocytosis during meiotic maturation of Xenopus laevis oocytes. Am J Physiol. 1990;258:C179–C184. doi: 10.1152/ajpcell.1990.258.1.C179. [DOI] [PubMed] [Google Scholar]
- 37.Schmalzing G, Richter HP, Hansen A, Schwarz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J Cell Biol. 1995;130:1319–1332. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sepulveda MR, Berrocal-Carrillo M, Gasset M, Mata AM. The plasma membrane Ca2+-ATPase isoform 4 is localized in lipid rafts of cerebellum synaptic plasma membranes. J Biol Chem. 2006;281:447–453. doi: 10.1074/jbc.M506950200. [DOI] [PubMed] [Google Scholar]
- 39.Solis-Garrido LM, Pintado AJ, Andres-Mateos E, Figueroa M, Matute C, Montiel C. Cross-talk between native plasmalemmal Na+/Ca2+ exchanger and inositol 1,4,5-trisphosphate-sensitive ca2+ internal store in Xenopus oocytes. J Biol Chem. 2004;279:52414–52424. doi: 10.1074/jbc.M408872200. [DOI] [PubMed] [Google Scholar]
- 40.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strehler EE, Filoteo AG, Penniston JT, Caride AJ. Plasma-membrane Ca(2+) pumps: structural diversity as the basis for functional versatility. Biochem Soc Trans. 2007;35:919–922. doi: 10.1042/BST0350919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 43.Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci U S A. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Machaca K. Ca2+cyt negatively regulates the initiation of oocyte maturation. J Cell Biol. 2004;165:63–75. doi: 10.1083/jcb.200309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Supplisson S, Kado RT, Bergman C. A possible Na/Ca exchange in the follicle cells of Xenopus oocyte. Dev Biol. 1991;145:231–240. doi: 10.1016/0012-1606(91)90122-j. [DOI] [PubMed] [Google Scholar]
- 46.Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–3747. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 47.Ullah G, Jung P, Machaca K. Modeling Ca(2+) signaling differentiation during oocyte maturation. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.01.010. In Press. [DOI] [PubMed] [Google Scholar]
- 48.Villmann C, Bull L, Hollmann M. Kainate binding proteins possess functional ion channel domains. J Neurosci. 1997;17:7634–7643. doi: 10.1523/JNEUROSCI.17-20-07634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolf AA, Jobling MG, Wimer-Mackin S, Ferguson-Maltzman M, Madara JL, Holmes RK, Lencer WI. Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia. J Cell Biol. 1998;141:917–927. doi: 10.1083/jcb.141.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]