Summary
α6-containing (α6*) nicotinic ACh receptors (nAChRs) are selectively expressed in dopamine (DA) neurons and participate in cholinergic transmission. We generated and studied mice with gain-of-function α6* nAChRs, which isolate and amplify cholinergic control of DA transmission. In contrast to gene knockouts or pharmacological blockers, which show necessity, we show that activating α6* nAChRs and DA neurons is sufficient to cause locomotor hyperactivity. α6L9’S mice are hyperactive in their home cage and fail to habituate to a novel environment. Selective activation of α6* nAChRs with low doses of nicotine, by stimulating DA but not GABA neurons, exaggerates these phenotypes and produces a hyperdopaminergic state in vivo. Experiments with additional nicotinic drugs show that altering agonist efficacy at α6* provides fine-tuning of DA release and locomotor responses. α6*-specific agonists or antagonists may, by targeting endogenous cholinergic mechanisms, provide a new method for manipulating DA transmission in Parkinson’s disease, nicotine dependence, or attention deficit hyperactivity disorder.
Introduction
Identification of the relevant nAChRs involved in 1) normal DA transmission, 2) disorders of the DA system such as schizophrenia, Parkinson’s disease, and ADHD, and 3) nicotine dependence, is a major priority. α6* (* indicates that other subunits may be present in the pentameric receptor) nAChRs are highly and selectively expressed in DA neurons, with additional functional expression in locus coeruleus and retinal ganglion cells (Azam and McIntosh, 2006; Azam et al., 2002; Champtiaux et al., 2002; Gotti et al., 2005b; Le Novere et al., 1996). α6* nAChRs are selectively inhibited by the marine cone snail peptide α-conotoxin MII (αCtxMII) (Cartier et al., 1996; Whiteaker et al., 2000). Immunoprecipitation and αCtxMII binding studies demonstrated that α6β2β3* and α6 α4β2β3* pentamers are the predominant α6* nAChRs in striatum (Champtiaux et al., 2003; Zoli et al., 2002). α6β2* receptors account for ~ 30 % of nicotine-stimulated DA release in striatum (Grady et al., 2002; Kaiser et al., 1998; Kulak et al., 1997). β3 subunits are encoded by a gene adjacent to α6, are usually co-expressed with α6, and are essential for α6* nAChR biogenesis and function (Cui et al., 2003; Gotti et al., 2005a). α6* receptors exhibit the highest known sensitivity to nicotine and ACh in functional measurements on native receptors (Salminen et al., 2004), yet function poorly in heterologous expression systems (Drenan et al., 2008). As a result, there has been little progress in defining selective agonists for α6* nAChRs, or on other functional measurements. Furthermore, in midbrain DA neurons, studies of somato-dendritic α6* receptors are complicated by the presence of α4β2* (non-α6), and selective antagonists of α4β2* (non-α6) have not been identified.
Many experiments with selective destruction of DA neurons show that activity of such neurons is necessary for reinforcement of natural and artificial rewards. These cells exhibit tonic and phasic firing patterns (Grenhoff et al., 1986), where phasic or “burst” firing carries salient information thought to predict imminent reward status (Schultz, 2002). Pedunculopontine tegmentum (PPTg) and laterodorsal tegmentum (LDTg) fibers provide a cholinergic drive that strongly regulates DA neuron excitability and the transition to burst firing (Lanca et al., 2000). Midbrain nAChRs in three locations respond to mesopontine-derived ACh: 1) α7* nAChRs on glutamatergic terminals from cerebral cortex, 2) α4β2* nAChRs on GABAergic terminals and cell bodies, and 3) α4* and α6* somato-dendritic nAChRs on DA neurons (Calabresi et al., 1989). Nicotine interferes with normal cholinergic transmission to DA neurons, in part, by modifying the weights of these various nAChR synapses. For example, nicotine at concentrations found in the CSF of smokers preferentially desensitizes α4β2* nAChRs regulating midbrain GABA release (Mansvelder et al., 2002), yet still permits α7* nAChR-regulated glutamate release (Mansvelder and McGehee, 2000). This produces both disinhibition and direct excitation of DA neurons, increasing the probability of a switch to burst firing.
Nicotine also exaggerates the action of endogenous ACh in regulating DA release in striatum. Striatal cholinergic interneurons continually release ACh that activates nAChRs, which maintains background DA levels during tonic firing of midbrain DA neurons (Zhou et al., 2001). However, DA release in response to burst firing of DA neurons is facilitated by a reduction in nAChR activity (Rice and Cragg, 2004; Zhang and Sulzer, 2004). α6* receptors, due to their high sensitivity and their selective expression in DA cell bodies and presynaptic terminals, are probably key players in cholinergic control of DA release. We reasoned that genetic “sensitization” of these receptors would 1) amplify the role of α6* nAChRs in cholinergic control of DA transmission, and 2) allow for selective pharmacological stimulation of these neurons. This approach would complement previous experiments using α6 knock-out mice and α6* pharmacological blockade, demonstrating behavioral and physiological responses for which α6* nAChRs are sufficient.
We introduced a bacterial artificial chromosome (BAC) transgene into the mouse germline with a mutant copy of the mouse α6 nAChR subunit gene that rendered mutant α6* channels “hypersensitive” to endogenous ACh or exogenous nicotine. DA neuron excitability and DA release is greatly augmented in these mice, and they exhibit behavioral phenotypes consistent with increased DA neuron firing and/or DA release. These studies improve our knowledge of cholinergic regulation of the midbrain DA system and of α6* nAChR biology, and have implications for the treatment of disorders involving changes in DA.
Results
Production and Characterization of BAC α6L9’S Transgenic Mice
We selected a 156 kb mouse BAC clone containing the genomic Chrna6 (α6 nAChR) locus with substantial 5’ and 3’ flanking genomic regions for generating a targeting construct to faithfully recapitulate expression of the α6 gene. α6 Leu280 (the Leu 9’ residue in the M2 domain) was mutated to Ser via homologous recombination using a two-step selection / counter selection procedure in E. coli. (Figure S1A). The final construct was injected into fertilized mouse eggs and six transgenic offspring were identified by genomic DNA sequencing and diagnostic PCR (Figure S1B and C). To eliminate possible artifacts of transgene position/insertion, we analyzed two independently derived lines (lines ‘2’ and ‘5’), which have different transgene copy numbers (Figure S1D) and different genomic positions. Both mouse lines expressed mutant α6L9’S mRNA in addition to wild type (WT) α6 mRNA (Figure S1E).
We found no difference in location or intensity of [125I]-α-conotoxin MII (αCtxMII) labeling in α6L9’S versus WT brains, confirming correct regional expression of α6* nAChRs in mutant mice (Figure S1F). To corroborate this, we quantified α6* binding sites. Membranes were prepared from striatum (ST), olfactory tubercle (OT), superior colliculus (SC) (regions which account for most α6* binding sites), and thalamus (TH) (where most binding sites are α4β2*). [125I]-epibatidine binding, coupled with inhibition by unlabeled αCtxMII, revealed α6* and non-α6* (α4β2*) receptors (Figure S1G). Collectively, these results indicate that α6L9’S mice exhibit normal levels and localization of neuronal nAChRs.
Spontaneous and Nicotine-Induced Hyperactivity in α6L9’S Mice
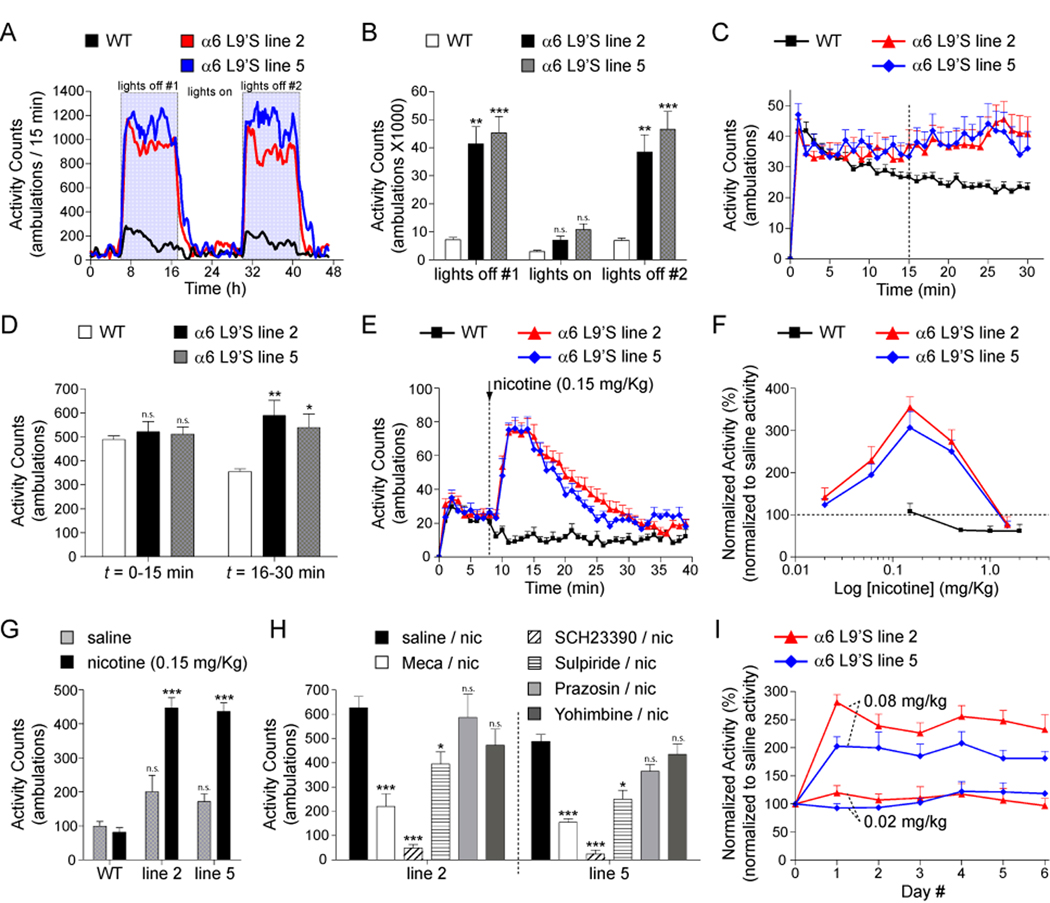
We measured home cage horizontal locomotor activity for WT and α6L9’S mice over a period of 48 h. α6L9’S mice were markedly hyperactive relative to WT control littermates (Figure 1A). This effect was restricted to lights off (Figure 1B), though there was a non-significant trend toward hyperactivity during lights on. Although WT mice show locomotor habituation when placed into a novel environment, α6L9’S mice exhibit sustained activity. We measured WT and α6L9’S locomotor activity for 30 min after placement in a new home cage environment (Figure 1C). Activity during the first 15 min of the session was unchanged, but from t = 16 to 30 min, α6L9’S mice exhibited significantly greater activity than WT littermates (Figure 1D).
Figure 1. α6L9’S mice are markedly hyperactive and hypersensitive to the psychomotor stimulant action of nicotine.
(A and B) α6L9’S mice are hyperactive in their home cage. Horizontal locomotor activity of mice (α6L9’S or WT cagemates) in their home cage environment was recorded during a 48 h period. Raw locomotor activity data (# ambulations / 15 min period) are reported (A). Total locomotor activity from “lights on” and “lights off” periods indicated in (A) are shown (B) for WT and α6L9’S mice.
(C and D) α6L9’S mice do not habituate to a novel environment. Mice (α6L9’S or WT cagemates) were removed from their home cage and immediately placed in a fresh cage. Locomotor activity was measured for 30 min. Raw locomotor activity data (# ambulations / min) are reported (C). Total locomotor activity from (C) for t = 0–15 min or t = 16–30 min is shown (D).
(E) Nicotine specifically stimulates locomotor activity in α6L9’S transgenic mice. After 8 min of baseline locomotor activity, mice (WT, α6L9’S line 2 and 5) were injected with 0.15 mg/kg, i.p. nicotine. Locomotor activity was recorded for an additional 30 min after injection. Raw locomotor activity data (# ambulations / min) are reported.
(F) Dose-response relationship for nicotine-stimulated locomotor activity in WT and α6L9’S mice. Mice were administered nicotine at the indicated dose, and total locomotor activity was measured as in (E). Locomotor activity for each mouse was normalized to a saline control injection for the same mouse. Data are expressed as the percentage of the response to saline (set to 100 %).
(G) Locomotor activity following saline or nicotine (0.15 mg/kg, i.p.) injection in WT and α6L9’S mice. Total raw locomotor activity data (# ambulations / min, 30 min total) are reported.
(H) Behavioral pharmacology of nicotine-stimulated locomotor activation in α6L9’S mice. Mice (WT or α6L9’S) were pre-injected with saline, mecamylamine (1 mg/kg, i.p.), SCH23390 (2 mg/kg, i.p.), sulpiride (20 mg/kg, i.p.), prazosin (1 mg/kg, i.p.), or yohimbine (1 mg/kg, i.p.) immediately prior to the start of data acquisition. Nicotine (0.15 mg/kg, i.p.) was injected a t = 8 min and locomotor activity was measured for an additional 30 min. Total raw locomotor activity data (# ambulations / min, 30 min total) are reported.
(I) Lack of sensitization or tolerance following repeated nicotine administration in α6L9’S mice. Groups of α6L9’S mice were injected with saline once daily for three consecutive days followed by nicotine (0.02 mg/kg, i.p. or 0.08 mg/kg, i.p.) once daily for six consecutive days. Locomotor activity was measured as in (E), and nicotine-stimulated locomotor activity for each mouse was normalized to saline control injections (average of three saline injections) for the same mouse. Data are expressed as the percentage of the response to saline (set to 100 %).
All data are expressed as mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
α6* nAChRs are highly expressed in DAergic neurons, and nicotine has psychomotor stimulant properties in rodents. We injected WT and α6L9’S mice with nicotine and measured locomotor activity. Low doses of nicotine (0.15 mg/kg, i.p.) strongly activated locomotion in α6L9’S mice but had no effect in WT mice (Figure 1E). To characterize the locomotor activation phenotype in α6L9’S mice, we constructed a nicotine dose-response relation. WT mice exhibited locomotor suppression at doses of nicotine between 0.5 and 2.0 mg/kg, i.p. (Figure 1F), consistent with previous work (Tapper et al., 2007). In contrast, α6L9’S mice exhibited steadily increasing locomotor responses between 0.02 and 0.15 mg/kg, i.p. nicotine, followed by a decline at 0.4 mg/kg, i.p and locomotor suppression similar to WT mice at 1.5 mg/kg, i.p. Thus, selective activation of α6* receptors results in locomotor activation rather than locomotor suppression. This phenotype was not a stress response, as saline injections did not produce locomotor activation (Figure 1G).
Locomotor activation in α6L9’S mice was dependent on activation of nicotinic receptors; we noted a strong block of the locomotor response in α6L9’S (but not WT) mice pre-injected with mecamylamine (1 mg/kg, i.p.) (Figure 1H). Further, α6L9’S locomotor activation acted through dopamine receptors; the response to 0.15 mg/kg, i.p. nicotine was completely inhibited by SCH23390 (D1DR antagonist; 2 mg/kg, i.p.) and partially inhibited by sulpiride (D2DR antagonist; 20 mg/kg, i.p.) (Figure 1H).
To determine whether α6L9’S mice develop tolerance or sensitization to nicotine psychomotor stimulation, we injected groups of α6L9’S mice once daily for six days with nicotine. Injection of 0.02 mg/kg, i.p. or 0.08 mg/kg, i.p. nicotine did not produce any change in locomotor behavior after repeated injections (Figure 1I). Nicotine also produces hypothermia in mice, and α4* hypersensitive mice exhibit this effect at nicotine doses ~ 50-fold lower than WT mice (Tapper et al., 2004). We found no difference between the hypothermia responses of WT and α6L9’S mice (data not shown).
Augmented Nicotine-Stimulated DA Release from Presynaptic Terminals in α6L9’S Mice
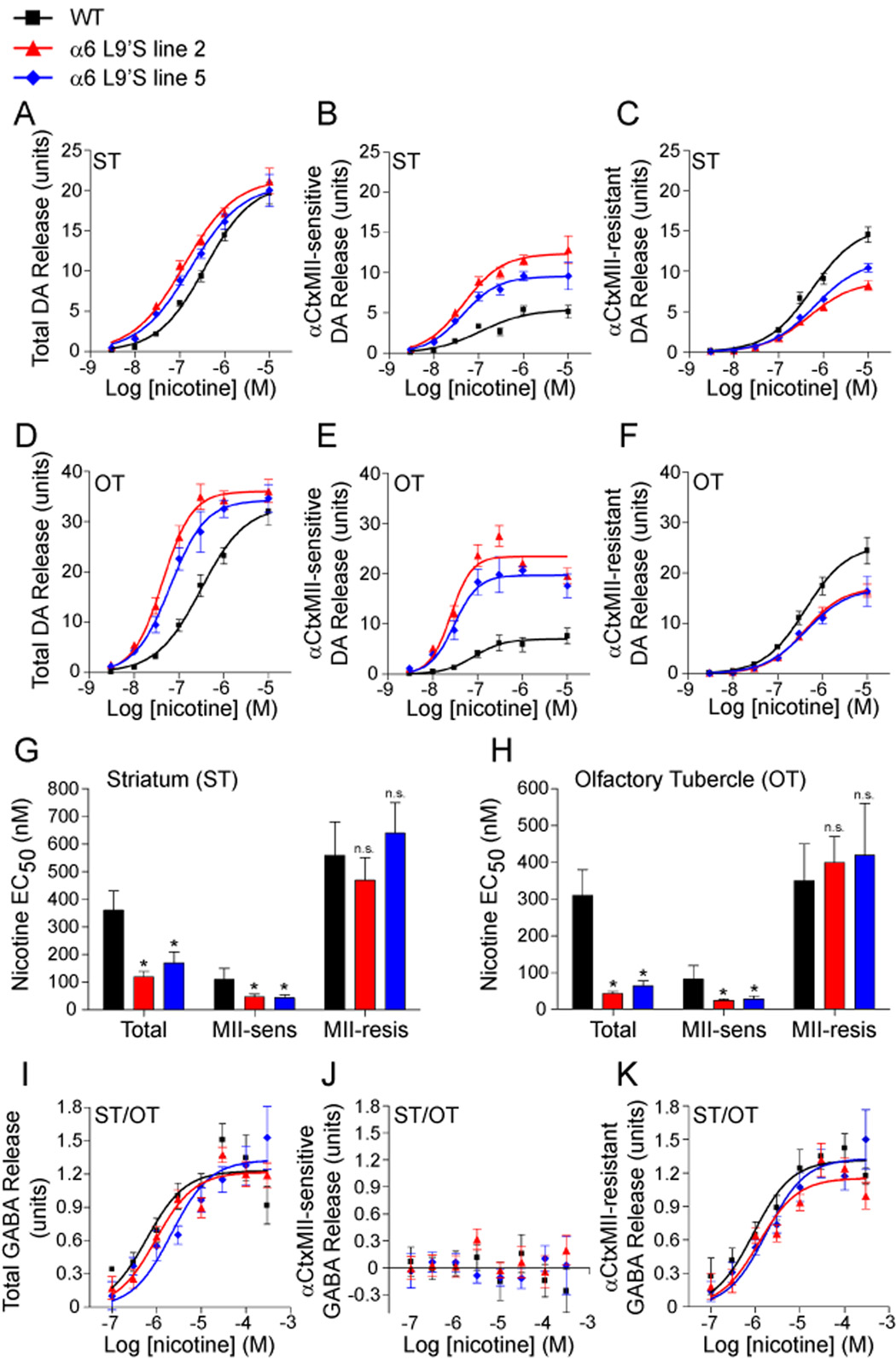
Mouse α6* nAChRs expressed in midbrain DA neurons are located both on the cell body and on presynaptic terminals in caudate/putamen (CPu), nucleus accumbens (NAc), and striatal aspects of the olfactory tubercle (OT). We measured nicotine-stimulated [3H]DA release from striatal synaptosomes of WT and α6L9’S mice. We made separate tissue samples containing striatum (ST; CPu and dorsal aspects of NAc) and olfactory tubercle (OT). Because CPu receives DAergic projections mainly from substantia nigra whereas OT receives DAergic projections exclusively from VTA, this preparation crudely separates the mesostriatal and mesolimbic pathways. For total DA release in ST, there was no difference in Rmax (Figure 2A, Figure S2), and a small but significant reduction in EC50 for both transgenic lines relative to WT (Figure 2G). In OT, there was a slight increase in Rmax (Figure 2D, Figure S2) and a greater reduction in the EC50 for α6L9’S lines compared to WT (Figure 2H). We used αCtxMII to inhibit α6* receptors, revealing the contribution of α6* and non-α6* nAChRs to this augmented DA release. In ST and OT, we observed a significant increase in total DA release mediated by α6 at most nicotine concentrations (Figure 2B and E). Interestingly, this was accompanied by a concomitant decrease in the non-α6* (α4β2* mediated) component (Figure 2C and F) in α6L9’S samples. In ST and OT, we measured a significant reduction in the EC50 for the α6-dependent component, and no change in EC50 for the non-α6 component (Figure 2G and H). Overall, DA release from α6L9’S striatal synaptosomes may be underestimated by this assay, as 20 mM K+ stimulated slightly less DA release in both α6L9’S lines relative to WT mice (Figure S2). These results directly reveal that selective α6* activation is capable of stimulating striatal DA release.
Figure 2. Presynaptic α6* nAChRs in striatum mediate hypersensitive, nicotine-stimulated DA release in α6L9’S transgenic mice.
(A–C) Hypersensitive DA release in striatum of α6L9’S transgenic mice is α6*-dependent. Striata (‘ST’; dorsal striatum and dorsal aspects of nucleus accumbens) from WT and α6L9’S mice were dissected and synaptosomes were prepared. DA release was stimulated with a range of nicotine concentrations (3 nM, 10 nM, 30 nM, 100nM, 300 nM, 1 µM, 10 µM), and a concentration-response relation for each mouse line (n = 6 mice / line) is shown for total release (A). To determine the relative contribution of α6* and non-α6* receptors, striatal synaptosome samples were incubated with αCtxMII (50 nM). α6* independent release is shown in (C), and αCtxMII-sensitive (α6* dependent) release is shown in (B).
(D–F) Hypersensitive DA release in olfactory tubercle of α6L9’S transgenic mice is α6*-dependent. Olfactory tubercle (‘OT’) was dissected from WT and α6L9’S mice, and samples were processed as in (A–C).
(G and H) Quantification of hypersensitive DA release in striatum (G) and olfactory tubercle (H). Average nicotine EC50 values for each concentration response curve from (A–F) are shown.
(I–K) α6* nAChRs do not participate in nicotine-stimulated GABA release in striatum. ST and OT were dissected and combined, followed by synaptosome preparation. GABA release was stimulated by nicotine at the following concentrations: 100 nM, 300 nM, 1 µM, 3 µM, 10 µM, 30 µM, 100 µM, and 300 µM. Total (I) release, and αCtxMII-sensitive (J) and resistant (K) components are shown.
Data are expressed as mean ± SEM. *, p < 0.05.
α4β2* nAChRs modulate striatal GABA release (Lu et al., 1998). We measured GABA release from striatal (ST and OT combined) synaptosomes of WT and α6L9’S mice to determine whether any α6*-dependent component was revealed by the gain-of-function L9’S mutation. There was no difference in total GABA release for any genotype comparison (Figure 2I, Figure S3), and there was no α6* component (Figure 2J and K, Figure S3). A student’s t-test on Rmax and EC50 values revealed no significant difference between total and αCtxMII-resistant GABA release for any genotype (WT Rmax p = 0.47, WT EC50 p = 0.73; line 2 Rmax p = 0.93, line 2 EC50 p = 0.31; line 5 Rmax p = 0.62, line 5 EC50 p = 0.63). Furthermore, there was no significant difference between any genotype on Rmax or EC50 (2-way ANOVA with Tukey post-hoc comparison - Rmax F(2,66) = 1.87, p = 0.163; EC50 F(2,66) = 0.744, p = 0.48) nor was there any effect of αCtxMII across all genotypes on Rmax or EC50 (Rmax F(1,66) = 0.35, p = 0.554; EC50 F(1,66) = 0.643, p = 0.426). Stimulation with 20 mM K+ showed no differences between WT and α6L9’S samples for GABA release (Figure S3). This indicates that GABAergic terminals in striatum, either derived locally or from VTA or SNr GABAergic neurons, contain no appreciable α6* nAChRs.
α6* receptors synthesized in retinal ganglion cells reside in the superficial layers of superior colliculus (SC) (Gotti et al., 2005b), and we measured nicotine-stimulated 86Rb+ efflux from SC synaptosomes. α6L9’S SC αCtxMII-sensitive receptors are hypersensitive to nicotine relative to WT, whereas αCtxMII-resistant 86Rb+ efflux is unchanged (Figure S4).
DA Release and Locomotor Activity are Precisely Controlled by Varying α6* Agonist Efficacy
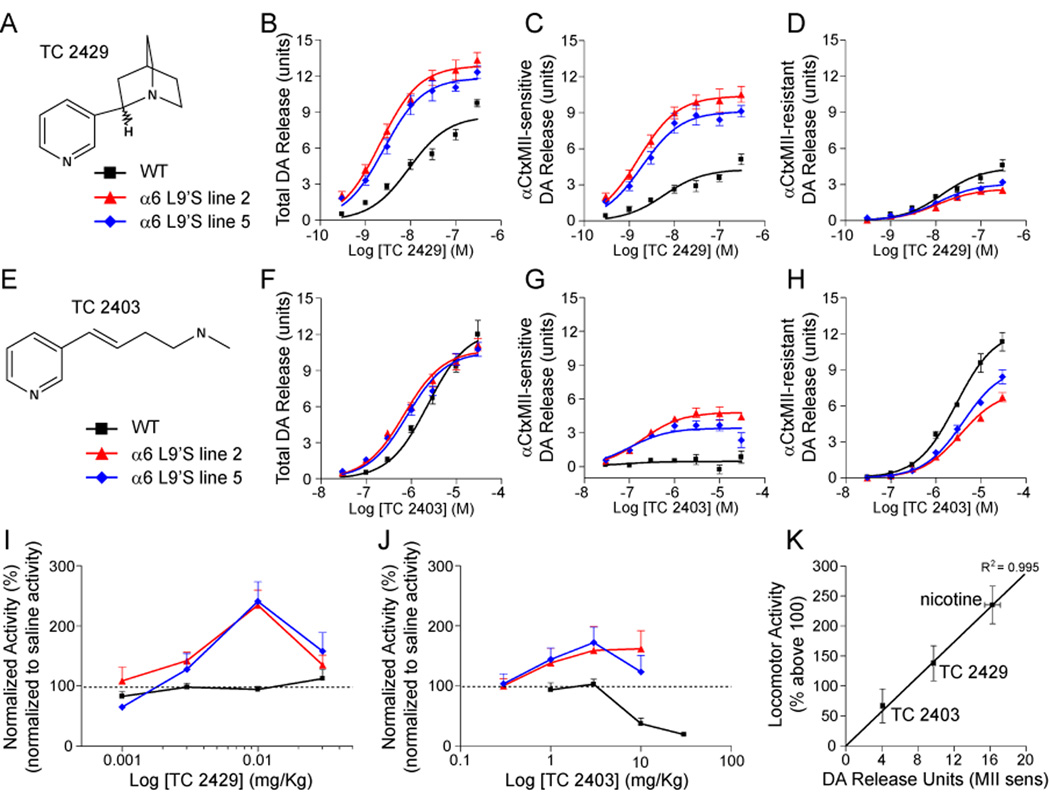
The DA release data suggest a mechanism involving dopamine for the psychomotor stimulant action of nicotine, as well as the spontaneous hyperactivity observed. For DA release in WT mice, TC 2429 (Figure 3A) (Bhatti et al., 2008) is a full agonist (vs. nicotine) with 3-fold selectivity at α6β2* and a weak partial agonist at α4β2, whereas TC 2403 (Figure 3E) (Bencherif et al., 1996; Lippiello et al., 1996) is a full agonist (vs. nicotine) at α4β2 and has no activity at α6β2* (Figure S5). TC 2429, like nicotine, was more efficacious and more potent for DA release from striatal synaptosomes of α6L9’S mice relative to WT (Figure 3B). In both α6L9’S lines, Rmax was greater, and the EC50 was reduced relative to WT. This was entirely attributable to α6* nAChRs (Figure 3C), which accounted for a greater proportion of the total response. There was a concomitant decline in the total response, but not the EC50, for α4β2* (αCtxMII-resistant) nAChRs (Figure 3D). We characterized the ability of TC 2429 to induce psychomotor activation in α6L9’S and WT control mice. Similar to nicotine, injections of TC 2429 stimulated locomotor activity in α6L9’S mice but not WT (Figure 3I). Unlike nicotine, TC 2429 did not produce locomotor suppression in WT mice.
Figure 3. α6-dependent neurotransmitter release and behavioral phenotypes are discriminated by subtype-selective nicotinic compounds.
(A) TC 2429 structure.
(B–D) An α6*-selective nicotinic agonist demonstrates enhanced striatal DA release in α6L9’S mice. Striatum (ST) and olfactory tubercle (OT) from WT and α6L9’S mice were dissected and combined, followed by synaptosome preparation. DA release was stimulated with a range of nicotine concentrations (see Figure 2A), and total (B) release, as well as αCtxMII-sensitive (C) and αCtxMII-resistant (D) components are shown for each genotype.
(E) TC 2403 structure.
(F–H) An α4*-selective nicotinic agonist modestly activates striatal α6* nAChRs in α6L9’S mice. Similar to (B–D), total DA release (F), as well as αCtxMII-sensitive (G) and αCtxMII-resistant (H) components are shown for TC 2403.
(I) An α6*-selective agonist stimulates locomotor activity in α63L9’S mice without affecting WT activity. Mice were administered TC 2429 at the indicated dose, and total locomotor activity was measured as in Figure 1. Locomotor activity for each mouse was normalized to saline control injections in the same mouse.
(J) α6* nAChR stimulation by TC 2403 induces modest locomotor activity in α6L9’S mice. Mice were administered TC 2403 at the indicated dose, and total locomotor activity was measured as in Figure 1. Locomotor activity for each mouse was normalized to saline control injections in the same mouse.
(K) Close correlation between α6*-mediated DA release and locomotor activity. For nicotine (Figure 2), TC 2429, and TC 2403, αCtxMII-sensitive DA release average Rmax values for line 2 and 5 (ST and OT combined) was plotted versus peak locomotor activity (% above 100). A regression line (R2 = 0.995) for the three compounds is shown.
Data are reported as mean ± SEM.
TC 2403 was slightly more potent and had equivalent efficacy for DA release in α6L9’S versus WT striatum (Figure 3F). The increased potency was due to the amplification of an αCtxMII-sensitive response not visible in WT tissue preparations (Figure 3G). TC 2403 was a partial agonist at this receptor (Figure S5). Consistent with competition between α6 and α4 subunits for common β2 subunits, there was a decline in Rmax in α6L9’S mice for α4β2* (αCtxMII-resistant) nAChRs (Figure 3H and S5). In locomotor assays, TC 2403 induced a slight locomotor activation in α6L9’S but not WT mice (Figure 3J), consistent with partial activity at α6* nAChRs. Nicotine (10 µM) was used as a positive control for DA release for TC 2429 and TC 2403 (Figure S5). For nicotine, TC 2429 and TC 2403, we noted a tight correlation between α6*-dependent DA release in vitro and peak locomotor activity in vivo (Figure 3K). These results suggest a mechanism whereby agonism at α6* nAChRs stimulates striatal DA release and produces locomotor stimulation, perhaps without GABAergic attenuation which is normally co-activated by α4β2* activation (Figure 2I–K).
α6L9’S Receptors Sensitize DA Neurons to Activation by Nicotine
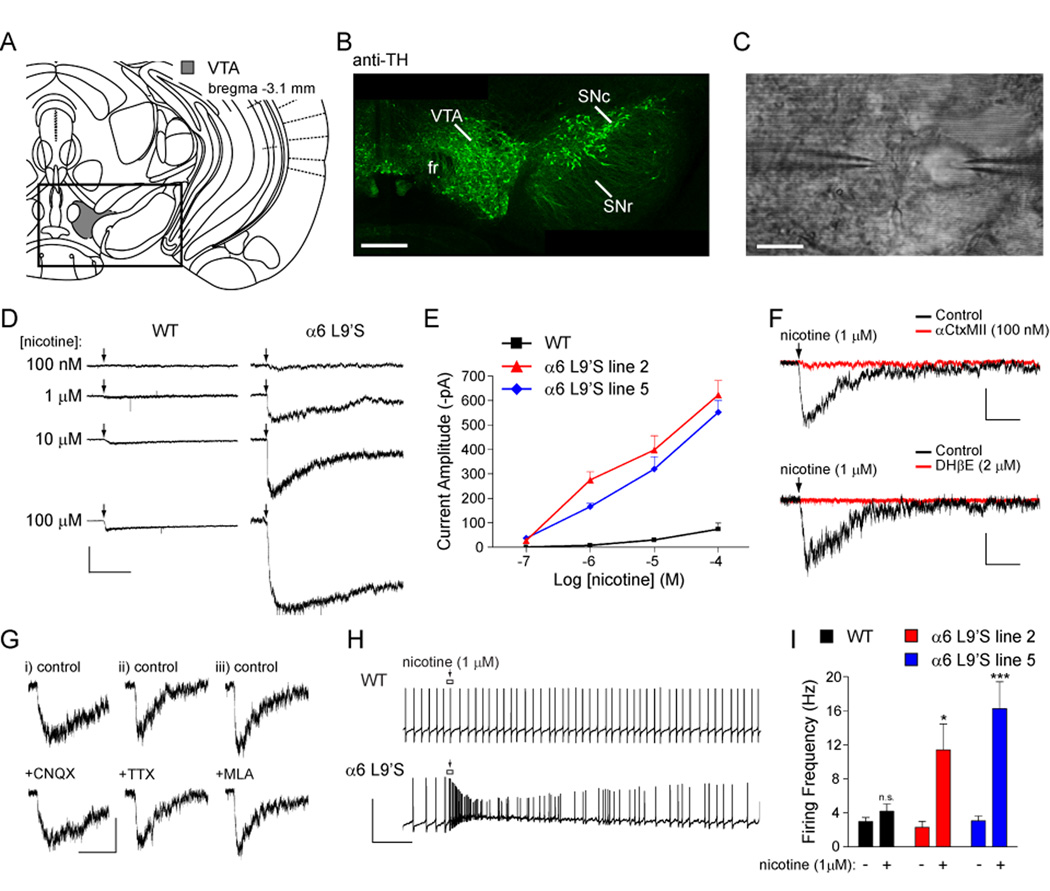
To directly determine whether DA neurons in α6L9’S mice express hypersensitive α6* nAChRs, we prepared coronal midbrain slices (Figure 4A) and made patch-clamp recordings from VTA DA neurons in whole-cell configuration. DA neurons can be identified based on expression of tyrosine hydroxylase (TH) (Figure 4B), and with electrophysiology (Wooltorton et al., 2003); DA neurons exhibit pacemaker firing (1–5 Hz), membrane potential adaptations in response to hyperpolarizing current injections, and often express Ih. We recorded currents induced by local nicotine application (Figure 4C) at a range of concentrations. Nicotinic currents in α6L9’S neurons were markedly hypersensitive to nicotine; puffs of nicotine (1 µM) elicited inward currents that were larger than those seen in WT neurons at any concentration (Figure 4D). At all nicotine concentrations, α6L9’S neurons of both transgenic lines showed larger average peak current responses than WT neurons (Figure 4E). Normalizing the peak current amplitude to cell capacitance yielded identical results (Figure S6).
Figure 4. Ventral tegmental area (VTA) DA neurons express hypersensitive nAChRs.
(A) Diagram of coronal sections from mouse brain containing ventral tegmental area (Franklin and Paxinos, 2008).
(B) Tyrosine hydroxylase (TH) staining of DA neurons in coronal slices. Coronal slices (250 µm; bregma −3.1 mm) from an α6L9’S mouse were fixed and stained for TH as described in Supplementary Experimental Procedures. The boxed area from (A) is shown. Scale bar: 350 µm.
(C) Micrograph of VTA neuron studied with local nicotine application. A VTA neuron is recorded via the patch pipette (right) and the cell body is stimulated with a nicotine-filled micropipette (left). Scale bar: 20 µm.
(D) Hypersensitive nAChRs in VTA neurons. Patch-clamp recordings were taken from VTA DA neurons in coronal slices from α6L9’S and WT mice. Neurons were voltage clamped at −60 mV and nicotine at the indicated concentration was locally applied to the cell body using a picospritzer (250 ms). Except during drug application, the micropipette tip was kept several hundred µm from the cell to prevent receptor desensitization. Scale bars: 200 pA, 5 s.
(E) Concentration-response relationship for hypersensitive nicotinic responses in WT and α6L9’S mouse lines. The average peak amplitude of the cellular response for each mouse line and each nicotine concentration is plotted.
(F) Hypersensitive nAChRs are inhibited by αCtxMII and dihydro-β-erythroidine (DHβE). Nicotine (1 µM) was locally applied to VTA DA neurons in α6L9’S slices before (black trace) and after (red trace) 10 min bath application of βCtxMII (100 nM) or DHβE (2 µM) (n = 5 for each antagonist). Scale bars: 100 pA, 3 s.
(G) Hypersensitive nAChR activation is independent of AMPA receptor function, action potentials, and α7* nAChRs. The indicated drug was bath applied to VTA DA neurons in α6L9’S slices, followed by activation of nAChRs with local application of 1 µM nicotine. Peak current amplitudes in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), tetrodotoxin (TTX), or methyllycaconitine (MLA) were unchanged. Results from both mouse lines were identical in all cell tested (n = 3 for each drug). Scale bars: i) 100 pA, 3 s; ii) 60 pA, 3 s; iii) 70 pA, 3 s.
(H) Dopamine neuron firing is augmented by a low nicotine concentration only in α6L9’S mice. VTA dopamine neurons were held in I = 0 mode and nicotine (1 µM) was puff applied (arrow) as in (D) following a stable recording of baseline firing. A representative firing response is shown for a neuron from each genotype. Scale bars: 100 mV, 2 s.
(I) Quantification of firing responses in WT and α6L9’S VTA DA neurons. Peak instantaneous firing frequency values for each nicotine (1 µM) application (one per cell) were derived and averaged for each mouse line. Average peak values are compared with the average baseline firing rate for the same group of cells.
Data are reported as mean ± SEM. *, p < 0.05; ***, p < 0.001.
Whole-cell responses to 1 µM nicotine were ≥ 90 % blocked when αCtxMII (100 nM) was added to the perfusate (Figure 4F, upper panel). αCtxMII block persisted 30 min after washout, consistent with previous work (McIntosh et al., 2004). Likewise, responses to 1 µM nicotine were 100 % blocked in the presence of dihydro-β-erythroidine (DHβE, 2 µM), a potent inhibitor of most β2* receptors (Figure 4F, lower panel). Although the general kinetics of these hypersensitive responses suggests that α6L9’S receptors are post-synaptic, a presynaptic mechanism is not excluded by the data. To address this we inhibited AMPA/Kainate receptors with 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 15 µM) and voltage gated Na+ channels with tetrodotoxin (TTX, 0.5 µM). Inward current responses to 1 µM nicotine were unaffected by either CNQX or TTX (Figure 4G). Furthermore, we found no effect (n = 6, 0 % block) on peak current amplitude (1 µM nicotine) in the presence of methyllycaconitine (MLA, 10 nM) (Figure 4G). Thus, hypersensitive α6* responses were consistent with a post-synaptic mechanism.
VTA DA neuron pacemaker firing was unaltered in WT versus α6L9’S slices (Figure 4I). To determine whether hypersensitive nicotinic receptors in α6L9’S neurons were capable of acutely altering the excitability of VTA DA neurons, we recorded action potential firing in response to local nicotine application. Nicotine (1 µM) induced a transient increase in the instantaneous firing rate and a depolarization of the membrane potential (Figure 4H). There was a significant increase in the firing rate for α6L9’S, but not WT, cells (Figure 4I).
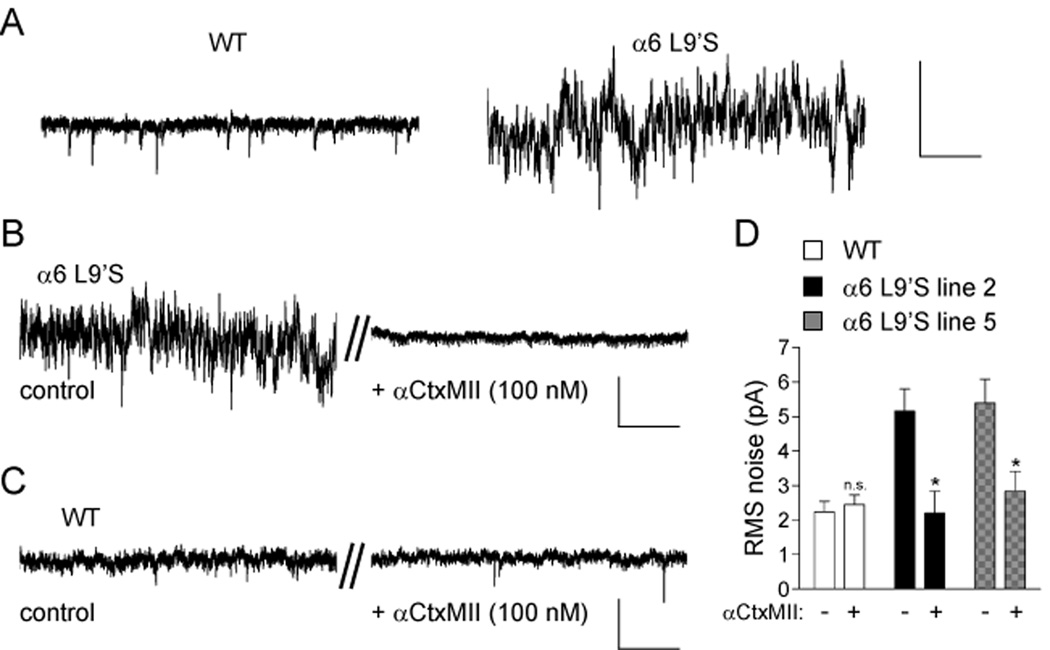
We noticed greater fluctuations in the holding current in α6L9’S VTA DA neurons than in WT (Figure 5A). This suggests that some α6L9’S channels are tonically active, reminiscent of previous observations in α4 nAChR L9’S and L9’A knock-in mice (Labarca et al., 2001; Shao et al., 2008). To determine whether this change in resting membrane conductance was due to active α6L9’S channels, α6* receptors were blocked by application of αCtxMII. α6 blockade completely eliminated this increased channel noise in all α6L9’S cells tested (Figure 5B). WT neurons bathed in αCtxMII served as a control (Figure 5C). To quantify this, we measured the root-mean-square (RMS) value of the fluctuations in WT and α6L9’S voltage clamp recordings. There was a significant decrease in the noise for α6L9’S cells in the presence of αCtxMII (Figure 5D). We found no effect of CNQX, picrotoxin, or TTX on the increased membrane noise in α6L9’S cells (Figure S7). DHβE, however, completely eliminated the increased noise similar to αCtxMII (Figure S7). These results further demonstrate that VTA DA neurons express functional, hypersensitive α6* nAChRs, and that these receptors may be activated by local ACh.
Figure 5. Spontaneous α6 channel activity in α6L9’S VTA DA neurons.
(A) Increased current fluctuations in voltage-clamp recordings from α6L9’S neurons. VTA DA neurons from WT or α6L9’S mice were voltage clamped at −60 mV. A segment from a representative voltage clamp recording is shown. Scale bars: 40 pA, 0.8 s.
(B) Noise in α6L9’S neurons is dependent on α6* nAChRs. VTA DA neurons were voltage clamped at −60 mV and a baseline recording was taken (control). αCtxMII was bath applied for 10 min, and a representative (n = 4, each line) segment of the voltage clamp record after this 10 min period is shown (+αCtxMII, 100 nM) for the same cell.
(C) αCtxMII inhibition has no effect in WT control recordings. As a negative control for perfusion artifacts, WT neurons were assayed as in (B).
(D) Quantification of channel noise increase in α6L9’S mice. RMS noise values for voltage clamp recordings from VTA DA neurons in the presence and absence of αCtxMII is shown. Data are reported as mean ± SEM. *, p < 0.05. Scale bars (B and C): 20 pA, 0.5 s.
We examined the properties of hypersensitive α6* receptors in DA neurons of the substantia nigra pars compacta (SNc) and found similar results to the VTA. SNc DA neurons from α6L9’S mice expressed hypersensitive nicotinic currents relative to WT, and firing rates were excited by 1 µM nicotine in α6L9’S but not WT cells (Figure S8A and B). Finally, α6L9’S SNc DA neurons expressed tonically active, αCtxMII-sensitive α6* receptors (Figure S8C).
Selective Expression of Functional α6* Receptors in Midbrain DA Neurons
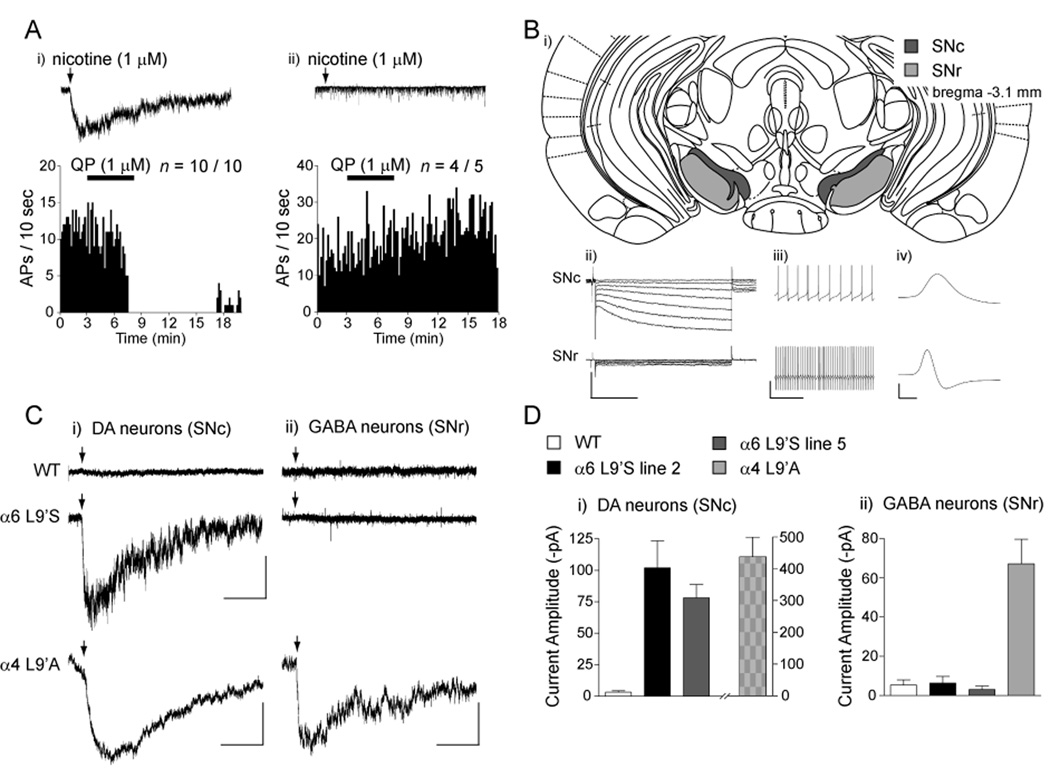
Although expression data suggest selective expression of α6* nAChRs in DA neurons, no electrophysiological experiments supporting this idea have been published. Midbrain DA neurons typically express D2-class autoreceptors, in contrast to midbrain GABAergic neurons (Nashmi et al., 2007). We recorded from 15 VTA neurons, ten of which expressed α6* nAChRs (based on large inward current responses to 1 µM nicotine), and five which did not. All cells expressing α6* nAChRs were sensitive to the inhibitory properties of the D2 DA receptor agonist quinpirole (Figure 6A, panel i), indicating that these cells were likely DAergic. In contrast, most α6*-negative cells did not express D2 receptors (Figure 6A, panel ii). To more accurately determine whether α6* receptors are functionally expressed in DA and/or GABA cells in midbrain, we recorded from substantia nigra (SN) neurons in slices from WT and α6L9’S mice. The spatial partitioning of DA and GABA neurons in the SN pars compacta (SNc) and pars reticulata (SNr) (Figure 6B, panel i) was combined with electrophysiological signatures (Figure 6B, panel ii–iv; see Experimental Procedures) to unambiguously identify these cell types. In whole-cell recordings from α6L9’S and WT SN neurons, we observed hypersensitive nicotinic responses in α6L9’S DA neurons, but not in GABA neurons (Figure 6C). Average peak current amplitudes were comparable between α6L9’S line 2 and 5 in SNc DA neurons (Figure 6D, panel i). Average responses in WT and α6L9’S GABA neurons were ≤ 10 pA (Figure 6D, panel ii). Responses to nicotine at 1 µM were undetectable in WT DA and GABA neurons (Figure 6C, panel i), however, these cells responded predictably to 100 µM or 1 mM nicotine (data not shown). As a control for the specificity of our results, we recorded from SNc and SNr neurons in slices from α4L9’A mice. We found hypersensitive nicotinic responses in SNc and SNr neurons from these mice (Figure 6C and D), consistent with the idea that α4 is expressed in both DA and GABA cells (Nashmi et al., 2007). These results, coupled with the absence of α6* receptors in GABAergic presynaptic terminals in striatum (Figure 2I–K), indicate that functional α6* receptors, in contrast to α4* receptors, are restricted to DA neurons in the midbrain.
Figure 6. Functional α6* nAChRs are selectively expressed in midbrain DA, but not GABA, neurons.
(A) VTA DA neurons expressing hypersensitive α6* nAChRs express dopamine D2-class autoreceptors. VTA DA neurons expressing α6L9’S nAChRs were identified by the presence of large (> 100 pA) inward nicotinic currents similar to Figure 4. Following nicotine application, cells were held in I = 0 mode and quinpirole (QP; 1 µM) was bath applied. Action potential firing in response to QP is shown for a representative neuron expressing (panel i; n = 10/10) or not expressing (panel ii; n = 4/5) α6L9’S receptors.
(B) Identification of substantia nigra (SN) DA and GABA neurons. A diagram of coronal sections (bregma −3.1 mm) from mouse brain containing SN pars compacta (SNc) and pars reticulata (SNr) is shown (Franklin and Paxinos, 2008). SN DA versus GABA neurons were identified by i) location: SNc contains DA neurons whereas SNr is largely GABAergic; ii) DA neurons express hyperpolarization-activated cation current (Ih); iii) DA neurons exhibit pacemaker firing (1–5 Hz) whereas GABA neurons fire at > 10 Hz; iv) DA neurons have broad spikes (> 2 ms) whereas GABA neurons have narrow (≤ 1 ms) spikes. Scale bars: ii) 400 pA (current traces) / 30 mV (voltage step), 0.5 s; iii) 50 mV, 1 s; iv) 50 mV, 1 ms.
(C) Hypersensitive α6* nAChRs reveal selective expression in DA neurons. SN DA and GABA neurons were identified according to criteria in (B). Neurons in slices from WT, α6L9’S, and α4L9’A mice were patch clamped in whole cell configuration, and nicotine (1 µM) was locally applied (arrow) to activate hypersensitive nAChRs. Representative recordings for each are shown. Scale bars: WT and α6L9’S) 100 pA, 5 s; α4L9’A) 200 pA (DA) / 80 pA (GABA), 5 s.
(D) Quantification of current amplitudes from (C). Average peak current amplitude in response to 1 µM nicotine for each genotype and cell type is shown.
Data are expressed as mean ± SEM.
The results thus far suggest increased DA tone in midbrain and /or striatum. To examine this, we studied pacemaker and nicotine-induced firing of VTA DA neurons in the absence and presence of sulpiride, a D2 DA receptor antagonist. There was no change in the ability of sulpiride to modestly increase baseline firing between WT and α6L9’S VTA DA cells (Figure S9). Further, sulpiride did not affect 1 µM nicotine-induced increases in firing in α6L9’S cells, or its lack of effect in WT cells (Figure S9). To determine whether augmented striatal DA release in α6L9’S mice (Figure 2) could be detected in brain slices using patch-clamp recordings, we studied striatal cholinergic interneurons. These cells are readily identifiable (Figure S10A), their activity is modulated by DA, and they are the source of ACh that activates α6* receptors on presynaptic DA terminals. We detected no change in spontaneous firing between WT and α6L9’S cells (Figure S10B). The resting membrane potential for α6L9’S line 2 (but not line 5) was hyperpolarized compared to WT (Figure S10C). Although DA may modulate Ih currents in cholinergic interneurons (Deng et al., 2007), we found no difference between WT and α6L9’S Ih expression or function in these cells (Figure S10D and E).
α6L9’S nAChRs in Locus Coeruleus Neurons do not Account for Behavioral Phenotypes in Mutant Mice
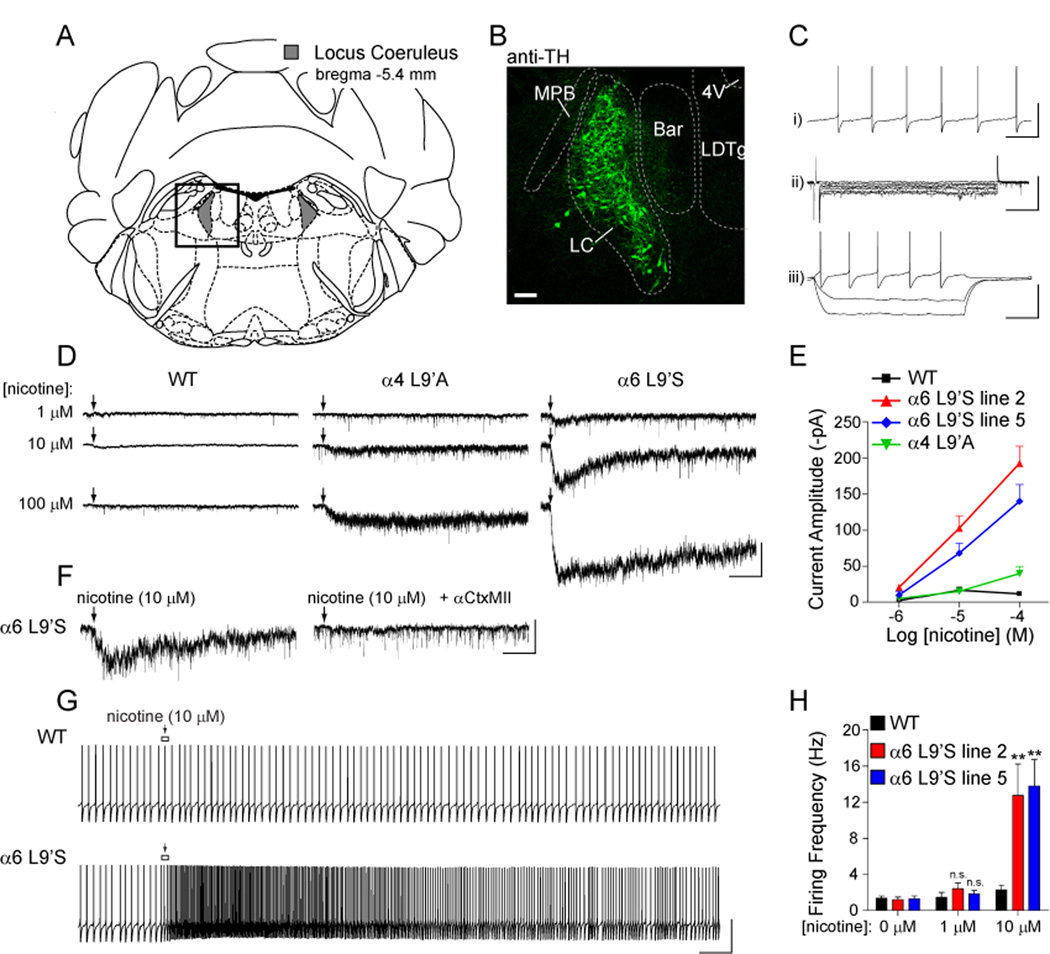
To determine whether α6* activation in α6L9’S mice might also stimulate locus coeruleus (LC) neuron firing, we recorded from LC neurons in coronal slices from WT and α6L9’S mice (Figure 7A). LC neurons express tyrosine hydroxylase (TH) (Figure 7B), exhibit spontaneous firing (1–2 Hz; Figure 7C, panel i) and lack Ih (Figure 7C, panel ii and iii). LC neurons from α6L9’S mice exhibited larger responses to locally applied nicotine compared to WT cells and cells from α4L9’A knock-in mice (Figure 7D and E). Although these responses were sensitive to αCtxMII (Figure 7F) and therefore α6-dependent, they were smaller and approximately 10-fold less sensitive to nicotine than were receptors on VTA DA neurons (compare Figure 4E and 7E). LC neurons were also able to fire action potentials in response to nicotine (Figure 7G), but at concentrations of nicotine 10-fold higher than for DA neurons (Figure 7H). The reduced sensitivity of LC α6* nAChRs relative to receptors on DA neurons suggests that they do not participate in the psychostimulant response to nicotine we observed. In support of this, we found no change in the locomotor response to nicotine when prazosin (α1AR antagonist) or yohimbine (α2AR antagonist) were administered prior to challenge with nicotine (Figure 1H).
Figure 7. Modest hypersensitivity to nicotine at α6L9’S nAChRs in noradrenergic locus coeruleus neurons.
(A) Diagram of coronal sections (bregma −5.4 mm) containing locus coeruleus (Franklin and Paxinos, 2008).
(B) Tyrosine hydroxylase (TH) staining of NE neurons in coronal slices. Coronal slices (200 µm; bregma −5.4 mm) from an α6L9’S mouse were fixed and stained for TH as described in Supplementary Experimental Procedures. The boxed area from (A) is shown. Scale bar: 100 µm. MPB, medial parabrachial nucleus; LC, locus coeruleus; Bar, Barrington’s nucleus; LDTg, laterodorsal tegmental nucleus; 4V, 4th ventricle.
(C) Electrophysiological identification of LC neurons. LC neurons are identified by i) pacemaker firing at 1–2 Hz, ii) lack of Ih, and iii) lack of membrane potential “sag” for hyperpolarizing current pulses (responses shown for injection of −80, −40, and +20 pA). Scale bars: i) 70 mV, 1.5 s; ii) 150 pA, 350 ms; iii) 70 mV, 500 ms.
(D) Hypersensitive nicotinic responses in LC neurons. Patch-clamp recordings were taken from LC neurons in coronal slices from WT, α4L9’A, and α6L9’S mice. Neurons were voltage clamped at −60 mV and nicotine at the indicated concentration was locally applied (arrow) to the cell body (250 ms).
(E) Concentration-response relationship for hypersensitive nicotinic responses in WT, α4L9’A and α6L9’S mice. The average peak amplitude of the cellular response for each mouse line and each nicotine concentration is plotted.
(F) Hypersensitive α6L9’S nAChRs are inhibited by αCtxMII. Nicotine (10 µM) was locally applied (arrow) to LC neurons in α6L9’S slices before and after 10 min bath application of αCtxMII (100 nM) (n = 3). Scale bars: 50 pA, 4 s.
(G) Noradrenergic neuron firing is augmented by a moderate nicotine concentration only in α6L9’S mice. Following a stable recording of baseline action potential firing, nicotine (10 µM) was puff applied (arrow) as in (D). Exemplar responses are shown for a WT and α6L9’S neuron. Scale bars: 70 mV, 3 s.
(H) Quantification of firing responses in WT and α6L9’S LC neurons. Peak instantaneous firing frequency values for each nicotine concentration were derived and averaged. Average peak values are compared with the average baseline firing rate (0 µM nicotine) for the same group of cells.
Data are reported as mean ± SEM. **, p < 0.01.
Discussion
α6L9’S Mice Reveal a Role for α6* nAChRs in Cholinergic Modulation of DA Transmission
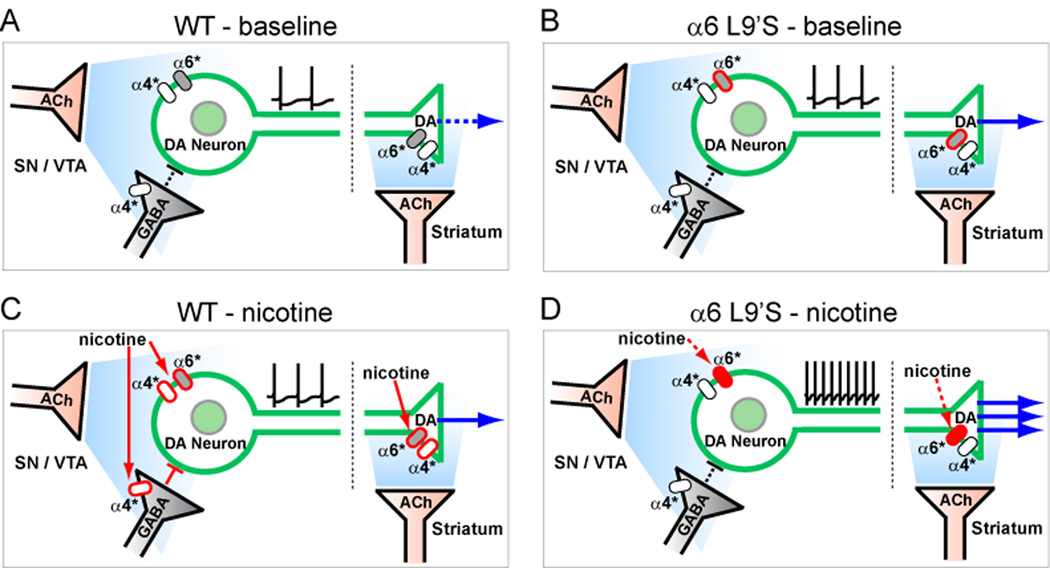
Here we report several new aspects of α6* nAChR biology and its role in cholinergic regulation of DA transmission. Also, we show the behavioral effects of selectively activating DA neurons (Figure 1). Our electrophysiology and neurochemistry experiments reveal a major role for α6* nAChRs in regulating both DA neuron firing (Figure 4 and S8) and synaptic release of DA in the striatum (Figure 2 and 3). Our finding that α6* nAChRs are largely excluded from midbrain GABA neurons (Figure 6) and from striatal GABAergic terminals (Figure 2I–K), in stark contrast to α4β2* nAChRs, is sustained by our behavioral data suggesting relatively unrestrained DA transmission in α6L9’S mutant mice. We propose a model in which specific functional expression of high-sensitivity α6L9’S nAChRs in DA neurons renders these cells selectively hypersensitive (Figure S11) to activation by endogenous ACh (Figure 8B) or by exogenous nicotine (Figure 8D). This likely reflects that in WT mice, or in humans, high-affinity α6* nAChRs are specifically poised to modulate the activity of monoamine neurotransmitters such as DA. These studies provide long-sought sufficiency data for α6* nAChRs, complementing studies utilizing α6* loss-of-function mutations and pharmacological blockade.
Figure 8. Hypersensitive DA neurons alter basal ganglia circuitry for cholinergic transmission and responses to nicotine.
(A–D) Schematic for baseline and nicotine-induced activation of DA neurons. In WT mice, background (A) ACh weakly stimulates somatic and presynaptic α4* and α6* nAChRs, leading to tonic DA release in ST. In α6L9’S mice, baseline ACh (B) activation of α6* nAChRs may cause heightened DA release via increased somatic and presynaptic nAChR activity. In WT (C), systemic nicotine (≥ 0.5 mg/kg, i.p.) activates α4* and α6* nAChRs on DA neurons, and α4* nAChRs on GABA neurons/terminals in midbrain. This, coupled with activation of presynaptic α4* and α6* nAChRs, increases DA release in ST. In α6L9’S mice (D), systemic nicotine (≤ 0.15 mg/kg, i.p.) specifically activates somatic and presynaptic α6* nAChRs on DA neurons without activating α4* nAChRs on inhibitory GABAergic inputs, which strongly stimulates DA release.
In vivo α6* nAChR Stoichiometry and Biophysical Properties Revealed by α6L9’S Mice
We observed similar levels of αCtxMII binding in α6L9’S brains compared to WT controls (Figure S1F and G). This is interesting in light of the fact that the two α6L9’S lines harbor multiple copies of the transgene (Figure S1D). Unlike an exon-replacement knock-in approach, α6L9’S BAC transgenic mice retain two copies of the WT α6 locus. In DA neurons, α6 and α4 subunits compete for common, limiting nAChR subunits such as β2, possibly β3 and α5, and probably for unknown assembly factors or chaperone proteins that may be specific to this cell type. As a result, the level of functional α6* expression in α6L9’S neurons is determined by a competition between WT and L9’S α6 subunits. Indeed, for every agonist tested, we observed diminished peak αCtxMII-resistant (α4β2*-dependent) DA release in α6L9’S ST/OT (Figure 2 and 3) despite equal levels of α4β2* binding sites (Figure S1G). Future crosses of α6L9’S mice to α4, β2, and β3 nAChR heterozygous or homozygous KO mice will yield further insights into nAChR subunit stoichiometry in vivo.
We noted a large increase in the potency and efficacy of nicotine in whole-cell recordings from α6L9’S DA neurons (Figure 4D and E). With respect to nicotinic channel engineering, the present studies are analogous to electrophysiological and Ca2+ flux-based measurements of hypersensitive nicotinic responses in α4L9’S, α4L9’A, and α7L9’T knock-in mice (Fonck et al., 2005; Labarca et al., 2001; Tapper et al., 2004; Wooltorton et al., 2003). The augmented responses (increased efficacy) to agonist likely reflect an increased maximal probability of channel opening, Popen, conferred by the L9’S mutation (Labarca et al., 1995), while the increased potency results from this mutation shifting the agonist concentration-response relation to lower concentrations (Revah et al., 1991). The increased noise in voltage clamp recordings from α6L9’S neurons probably arises from one of two effects: 1) the presence of ACh in the slice preparation that is secreted from terminals of severed mesopontine cholinergic axons, or 2) unliganded openings. Future in vivo studies, such as recordings from awake, behaving animals, will help confirm whether α6L9’S mouse spontaneous behavioral phenotypes are caused by the former or the latter.
Contrasting Cell-Specific Expression of α4* vs. α6* nAChRs Leads to Behavioral Differences in α4 and α6 Gain-of-Function Mutant Mice
α6L9’S mice are viable and fertile, whereas full expression of α4L9’S* receptors causes neonatal lethality due to excitotoxic death of DA neurons (Labarca et al., 2001). This could be due to differential expression of α4 and α6 in development; unlike α4 expression, peak α6 expression occurs well after birth (Azam et al., 2007). It is also possible that α6L9’S* receptors are comparatively insensitive to activation by choline at concentrations found in CSF.
In the mesostriatal and mesolimbic DA system, α4β2* nAChRs are expressed in DA neuron cell bodies, dendrites and axon terminals, as well as in cell bodies and axon terminals of midbrain and striatal GABAergic neurons. Conclusive evidence for α6* nAChR expression, however, is restricted to DA neuron cell bodies and axon terminals. Our present results show that, in midbrain, manipulating α6* nAChR sensitivity affects only DA neurons (Figure S11, Figure 8B, and D), whereas sensitized α4* receptors simultaneously increase the sensitivity of DA neurons and their inhibitory GABAergic inputs (Figure 6, 8A, and 8C). This circuit-level difference explains a distinction between the locomotor effects of nicotine in the two gain-of-function mouse strains. WT mice display a hypolocomotor response to nicotine, and α4L9’A mice recapitulate the WT response, only at much lower doses (Tapper et al., 2007). On the other hand, α6L9’S mice exhibit a sign change: psychomotor stimulation by nicotine (Figure 1F).
This cell-type difference in expression between α4 and α6 nAChR subunits may also lead to the behavioral differences between α4L9’A and α6L9’S mice in response to repeated nicotine injections. Repeated, selective activation of α4* nAChRs produces locomotor sensitization (Tapper et al., 2007; Tapper et al., 2004) whereas repeated activation of α6* nAChRs produces neither tolerance nor sensitization (Figure 1I). Locomotor sensitization may require nicotinic activation of GABAergic transmission, which is afforded by α4* nAChRs but not α6* nAChRs (Nashmi et al., 2007). Alternatively, the mechanism of sensitization could involve nAChR upregulation, to which α4β2* receptors are particularly prone (Nguyen et al., 2003) but to which α6* receptors are apparently resistant (Perry et al., 2007).
α6* nAChRs Preferentially Modulate the Mesolimbic vs. Mesostriatal DA System
The VTA and NAc (mesolimbic DA pathway) are key mediators of the addictive properties of nicotine (Corrigall et al., 1994), and a recent report using pharmacological blockade suggests that α6* nAChRs specifically mediate cholinergic modulation of DA release in NAc (Exley et al., 2007). Our results support this in two ways: 1) we observe greater expression of α6* currents in VTA versus SNc neurons (compare Figure 4E, 6D, and Figure S8A), and 2) DA release is more strongly controlled by α6* on VTA-derived terminals versus SNc-derived terminals (compare EC50 values in Figure 2G and H). Although we cannot completely distinguish the contribution of dorsal versus ventral striatum in our behavioral experiments, our DA release data suggest that the first α6* nAChRs significantly activated by nicotine are those in the mesolimbic pathway.
VTA β2* nAChRs are critical mediators of exploratory behavior in mice (Granon et al., 2003; Maskos et al., 2005). The VTA is important for curiosity or the response to novelty, perhaps by responding to cholinergic excitation to mediate the switch to burst firing in DA neurons. If VTA β2* nAChRs are necessary for normal responses to novelty, then it is not surprising that sensitized VTA β2* nAChRs such as α6L9’Sβ2* in mutant mice render the animals hypersensitive to novelty (Figure 1C and D). This phenotype is much smaller or absent in α4L9’A mice, reflecting the relative importance of selectively activating DA in eliciting this response.
α6L9’S Mice Reveal a Role for Cholinergic Transmission in Behavioral Hyperactivity
In midbrain, ACh released from mesopontine cholinergic terminals acts on DA neuron nAChRs in vivo (Mameli-Engvall et al., 2006) (Figure 8A). α6* nAChRs have the highest known sensitivity to ACh, making them excellent candidates to mediate the stimulatory action of endogenous ACh on DA neurons. The tonic activation of α6* nAChRs we observe in midbrain slices is likely due to endogenous ACh. Although we observe no difference in DA neuron baseline firing in vitro (Figure 4I and S8B), tonic midbrain α6* nAChR activation may be sufficient in vivo to contribute to behavioral phenotypes in α6L9’S mice. In striatum, tonic extracellular DA is controlled by presynaptic nAChRs via continuous, low-level ACh released from cholinergic interneurons (Zhou et al., 2001). This acts to maintain a high probability of DA release from the terminal during tonic firing (Rice and Cragg, 2004; Zhang and Sulzer, 2004). Sensitization of α6* nAChRs (in α6L9’S mice) likely modifies DA release at presynaptic terminals in addition to effects at DA neuron cell bodies; DA terminals with α6L9’S channels presumably have a decreased failure rate for single spike-induced release. This is supported by in vitro electrochemical studies of α6*-dependent DA release (Exley et al., 2007). Thus, genetic sensitization of DA neurons to ACh in midbrain and facilitation of DA release in striatum (Figure 8B) could easily account for the home cage hyperactivity and the sustained hyperactivity of α6L9’S mutant mice when placed in a novel environment. These phenotypes are reminiscent of DA transporter knock-down mice, which also show hyperactivity and impaired response habituation (Zhuang et al., 2001). Although α6*-regulated norepinephrine (NE) release does not contribute to nicotine psychomotor activation (Figure 1H), our results do not rule out a role for NE in α6L9’S spontaneous hyperactivity. Future studies on the mechanisms of α6L9’S mouse hyperactivity could be useful in understanding the causes of human hyperactivity such as that observed in ADHD.
In α6L9’S mice, low-dose nicotine stimulates psychomotor activation similar to amphetamine in WT animals (Figure 1E). Nicotine thus recapitulates the spontaneous hyperactivity we observe, though with different kinetics. The difference in response magnitude and duration between responses to novelty and responses to nicotine may reflect different agonist concentration and desensitization kinetics. After ACh is released from nerve terminals, it is hydrolyzed by acetylcholinesterase (AChE), which has a turnover rate of 104/s (Wathey et al., 1979) and is abundant in DAergic areas. ACh may not reach concentrations, or remain long enough, to significantly desensitize α6* nAChRs, perhaps even those with mutant L9’S subunits. In contrast, nicotine is eliminated with a half life of ~7 min (Matta et al., 2007) - nearly 107 times longer – and can therefore desensitize receptors, especially β2* nAChRs (Mansvelder et al., 2002). Bolus injections of nicotine in α6L9’S mice potently activate mutant receptors, and the locomotor response decay kinetics could therefore be dominated by both receptor desensitization and metabolic breakdown of nicotine.
Therapeutic Targeting of DA Disorders with α6* nAChRs
The cholinergic system is targeted by several drugs used to treat neural disorders such as Alzheimer’s disease and Parkinson’s disease (PD). There is a well-documented inverse correlation between smoking and PD (Ritz et al., 2007), and other disorders that can be treated with DA drugs (ADHD, schizophrenia) are associated with a high incidence of smoking (de Leon et al., 1995; Pomerleau et al., 1995). These findings suggest the important role played by the cholinergic system in DA transmission. Our results show that a sensitized response in DA neurons to endogenous ACh may cause a behaviorally relevant state of excess DA.
Manipulations to decrease α6* nAChR function may, therefore, be a useful treatment for human disorders involving excess DA. This could be in the form of a competitive antagonist or via viral gene therapy designed to eliminate α6* activity. On the other hand, patients with PD (low DA) may be aided by α6* agonists or allosteric modulators to augment DA release from residual DA terminals (Quik and McIntosh, 2006). Our data clearly show that an α6-selective compound potently stimulates both DA release and locomotor activity (Figure 3). Further, the absence of sensitization or tolerance to repeated α6* activation (Figure 1I) suggests that clinical α6* agonists may have reduced abuse liability. Unlike L-DOPA or direct DA receptor agonists/antagonists, compounds manipulating DA neuron firing by targeting α6* nAChRs might avoid well known use-dependent side effects such as dyskinesias.
Experimental Procedures
Bacterial Artificial Chromosome Recombineering and Transgenesis
For a complete description of methods used for recombineering and transgenesis, see Supplemental Data. A bacterial artificial chromosome (BAC), RP24-149I12, containing the mouse α6 nicotinic receptor subunit gene (Chrna6) was obtained from the BACPAC Resource Center (BPRC) at Children's Hospital Oakland Research Institute (Oakland, CA). BAC recombineering was carried out using a Counter Selection BAC modification kit (Genebridges; Heidelberg, Germany). α6 nAChR Leucine 280 (Leu9’) was mutated to a serine using a two-step selection/counter selection protocol. First, a 15 bp stretch of α6 exon 5 containing the coding sequence for V278 through L282 was replaced with a cassette containing a tandem selection (neo)/counter selection (rpsL) marker. Neo was used to select positive recombinants, and an engineered NotI restriction site pair flanking the selection cassette was used to confirm the location of the exogenously inserted DNA within the BAC. In the second step, α6 exon 5 was restored using counter selection. Bacterial cells were placed under selective pressure (via streptomycin sensitivity gained by insertion of the rpsL marker) to lose the neo-rpsL cassette and replace it with non-selectable DNA engineered to insert the Leu9’ to Ser (L280S) mutation. The resultant strain harbored a BAC with no ectopic DNA in or around the α6 gene.
Injection-grade α6L9’S BAC DNA was prepared via double CsCl banding (Lofstrand Labs; Gaithersberg, MD). To produce transgenic animals, BAC DNA was injected into the male pronucleus of recently fertilized FVB/N embryos and implanted into pseudopregnant Swiss-Webster surrogates. Transgenic founders were identified using tail biopsy DNA and PCR primers designed to detect the L9’S mutation. Founders were crossed to C57BL/6J (Jackson Labs; Bar Harbor, ME) to obtain germline transmission and to establish a colony, and transgenic mice were continually backcrossed to C57BL/6J.
Locomotor Activity
Locomotor activity was assayed similar to (Fonck et al., 2005), and is described in Supplemental Data.
Neurotransmitter Release from Striatal Synaptosomes
DA and GABA release from striatal synaptosomes was carried out similar to (Grady et al., 2002; Nashmi et al., 2007), and is described in Supplemental Data.
Patch-Clamp Electrophysiology
Methods for patch-clamp recordings are completely detailed in Supplemental Data. Transgenic and non-transgenic mice (midbrain, P17–P25; locus coeruleus, P21–P28; striatum, P42–P56) were anesthetized followed by cardiac perfusion with oxygenated (95% O2/5% CO2) ice-cold glycerol-based artificial CSF (gACSF). Coronal slices (midbrain, striatum: 250 µm; pons: 200 µm) were cut and allowed to recover for at least 1 h at 32°C in regular, oxygenated artificial CSF (ACSF). Somatic recordings from visually identified midbrain, pontine, or striatal neurons were obtained using patch electrodes filled with internal pipette solution containing: 135 mM potassium gluconate, 5 mM EGTA, 0.5 mM CaCl2, 2 mM MgCl2, 10 mM HEPES, 2 mM Mg-ATP, and 0.1 mM GTP. Recordings were taken at 32°C with an Axopatch 1D amplifier, a 16-bit Digidata 1322A A/D converter, and pCLAMP9.2 software (all Molecular Devices Axon; Sunnyvale, CA). For nicotine application, a drug-filled micropipette was used to locally apply nicotine using pressure application. A piezoelectric translator rapidly moved the nicotine-filled pipette close to and away from the cell to avoid nAChR desensitization.
Immunohistochemistry and Spectral Confocal Imaging
Coronal brain slices cut for patch-clamp recording were processed similar to (Lerchner et al., 2007) and stained according to (Drenan et al., 2005). Spectral confocal imaging was similar to (Drenan et al., 2008). See Supplemental Data for more details.
Supplementary Material
Acknowledgment
We thank members of the Lester lab for helpful discussions. Special thanks to B. Drenan. Thanks to C. Wageman, E. Myers, C. Xiao, A. Tapper, R. Nashmi, C. Fonck, J. Schwarz, J. Jankowsky, M. Liu, P. Deshpande, S. Benazouz, and C. Zhou. This work was supported by H.H.M.I. (N.H., J.M.Miwa, S.B.) and grants from NIH (DA09121, DA17279, and NS11756 to H.A.L.; DA19375 to H.A.L. and M.J.M.; DA03194 to M.J.M.; DA12242 to M.J.M. and P.W.; MH53631 and GM48677 to J.M. McIntosh), the Moore Foundation, the Croll Research Foundation (to J.M. Miwa) and the California Tobacco Related Disease Research Program (TRDRP; 12RT-0245 to H.A.L.). R.M.D. was supported by postdoctoral fellowships from TRDRP (15FT-0030) and NIH (DA021492 and NS007251).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H]norepinephrine release from mouse hippocampal synaptosomes. Mol Pharmacol. 2006;70:967–976. doi: 10.1124/mol.106.024513. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Lovette ME, Fowler KW, Arrington S, Reeves L, Caldwell WS, Lippiello PM. RJR-2403: a nicotinic agonist with CNS selectivity I. In vitro characterization. J Pharmacol Exp Ther. 1996;279:1413–1421. [PubMed] [Google Scholar]
- Bhatti BS, Strachan JP, Breining SR, Miller CH, Tahiri P, Crooks PA, Deo N, Day CS, Caldwell WS. Synthesis of 2-(pyridin-3-yl)-1-azabicyclo[3.2.2]nonane, 2-(pyridin-3-yl)-1-azabicyclo[2.2.2]octane, and 2-(pyridin-3-yl)-1-azabicyclo[3.2.1]octane, a class of potent nicotinic acetylcholine receptor-ligands. J Org Chem. 2008;73:3497–3507. doi: 10.1021/jo800028q. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, et al. The β3 nicotinic receptor subunit: a component of α-Conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM. Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry. 1995;152:453–455. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Involvement of Ih in dopamine modulation of tonic firing in striatal cholinergic interneurons. J Neurosci. 2007;27:3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Doupnik CA, Boyle MP, Muglia LJ, Huettner JE, Linder ME, Blumer KJ. Palmitoylation regulates plasma membrane-nuclear shuttling of R7BP, a novel membrane anchor for the RGS7 family. J Cell Biol. 2005;169:623–633. doi: 10.1083/jcb.200502007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Nashmi R, Imoukhuede P, Just H, McKinney S, Lester HA. Subcellular trafficking, pentameric assembly, and subunit stoichiometry of neuronal nicotinic acetylcholine receptors containing fluorescently labeled α6 and β3 subunits. Mol Pharmacol. 2008;73:27–41. doi: 10.1124/mol.107.039180. [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. α6-Containing Nicotinic Acetylcholine Receptors Dominate the Nicotine Control of Dopamine Neurotransmission in Nucleus Accumbens. Neuropsychopharmacology. 2007;21:21. doi: 10.1038/sj.npp.1301617. [DOI] [PubMed] [Google Scholar]
- Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, et al. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive α4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd edn. New York, NY: Elsevier, Inc; 2008. [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol Pharmacol. 2005a;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Zanardi A, Gaimarri A, Champtiaux N, Changeux JP, Whiteaker P, Marks MJ, Clementi F, Zoli M. Heterogeneity and selective targeting of neuronal nicotinic acetylcholine receptor (nAChR) subtypes expressed on retinal afferents of the superior colliculus and lateral geniculate nucleus: identification of a new native nAChR subtype α3β2(α5 or β3) enriched in retinocollicular afferents. Mol Pharmacol. 2005b;68:1162–1171. doi: 10.1124/mol.105.015925. [DOI] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [3H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Granon S, Faure P, Changeux JP. Executive and social behaviors under nicotinic receptor regulation. Proc Natl Acad Sci U S A. 2003;100:9596–9601. doi: 10.1073/pnas.1533498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S. Differential inhibition by α-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem. 1998;70:1069–1076. doi: 10.1046/j.1471-4159.1998.70031069.x. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. α-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, et al. Point mutant mice with hypersensitive α4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BL, Corrigall WA. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience. 2000;96:735–742. doi: 10.1016/s0306-4522(99)00607-7. [DOI] [PubMed] [Google Scholar]
- Le Novere N, Zoli M, Changeux JP. Neuronal nicotinic receptor α6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci. 1996;8:2428–2439. doi: 10.1111/j.1460-9568.1996.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl− channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Bencherif M, Gray JA, Peters S, Grigoryan G, Hodges H, Collins AC. RJR-2403: a nicotinic agonist with CNS selectivity II. In vivo characterization. J Pharmacol Exp Ther. 1996;279:1422–1429. [PubMed] [Google Scholar]
- Lu Y, Grady S, Marks MJ, Picciotto M, Changeux JP, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptosomes. J Pharmacol Exp Ther. 1998;287:648–657. [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, Kuryatov A, Garrett JE, Marks MJ, Whiteaker P. Analogs of α-Conotoxin MII are selective for α6-containing nicotinic acetylcholine receptors. Mol Pharmacol. 2004;65:944–952. doi: 10.1124/mol.65.4.944. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, et al. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, McIntosh JM, Pezzullo JC, Kellar KJ. Chronic nicotine differentially regulates α6- and β3-containing nicotinic cholinergic receptors in rat brain. J Pharmacol Exp Ther. 2007;322:306–315. doi: 10.1124/jpet.107.121228. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Quik M, McIntosh JM. Striatal α6* nicotinic acetylcholine receptors: potential targets for Parkinson's disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux JP. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL. α4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci. 2008;28:519–528. doi: 10.1523/JNEUROSCI.3666-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Marks MJ, Lester HA. Nicotine responses in hypersensitive and knockout α4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics. 2007;31:422–428. doi: 10.1152/physiolgenomics.00063.2007. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Wathey JC, Nass MM, Lester HA. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979;27:145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. [125I]-α-Conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000;57:913–925. [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Liang Y, Dani JA. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat Neurosci. 2001;4:1224–1229. doi: 10.1038/nn769. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci. 2002;22:8785–8789. doi: 10.1523/JNEUROSCI.22-20-08785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.