Abstract
Background
As Earth warms, temperate and subpolar marine species will increasingly shift their geographic ranges poleward. The endemic shelf fauna of Antarctica is especially vulnerable to climate-mediated biological invasions because cold temperatures currently exclude the durophagous (shell-breaking) predators that structure shallow-benthic communities elsewhere.
Methodology/Principal Findings
We used the Eocene fossil record from Seymour Island, Antarctic Peninsula, to project specifically how global warming will reorganize the nearshore benthos of Antarctica. A long-term cooling trend, which began with a sharp temperature drop ∼41 Ma (million years ago), eliminated durophagous predators—teleosts (modern bony fish), decapod crustaceans (crabs and lobsters) and almost all neoselachian elasmobranchs (modern sharks and rays)—from Antarctic nearshore waters after the Eocene. Even prior to those extinctions, durophagous predators became less active as coastal sea temperatures declined from 41 Ma to the end of the Eocene, ∼33.5 Ma. In response, dense populations of suspension-feeding ophiuroids and crinoids abruptly appeared. Dense aggregations of brachiopods transcended the cooling event with no apparent change in predation pressure, nor were there changes in the frequency of shell-drilling predation on venerid bivalves.
Conclusions/Significance
Rapid warming in the Southern Ocean is now removing the physiological barriers to shell-breaking predators, and crabs are returning to the Antarctic Peninsula. Over the coming decades to centuries, we predict a rapid reversal of the Eocene trends. Increasing predation will reduce or eliminate extant dense populations of suspension-feeding echinoderms from nearshore habitats along the Peninsula while brachiopods will continue to form large populations, and the intensity of shell-drilling predation on infaunal bivalves will not change appreciably. In time the ecological effects of global warming could spread to other portions of the Antarctic coast. The differential responses of faunal components will reduce the endemic character of Antarctic subtidal communities, homogenizing them with nearshore communities at lower latitudes.
Introduction
Polar marine organisms are physiologically adapted to cold-water conditions. Being cold-stenothermal places them at great risk from the direct effects of global warming [1]. Warming seas are also promoting biological invasions of polar marine ecosystems, with potentially catastrophic consequences [2]–[4].
The endemic character of the Antarctic nearshore fauna and its uniquely truncated trophic structure [5]–[7] are products of climatic cooling that began in the Eocene and led to the growth of an ice sheet on Antarctica at the Eocene–Oligocene boundary ∼33.5 Ma (million years ago) [8], [9]. The Antarctic climate of the early Eocene was temperate by today's standards. Shallow-subtidal communities contained the functionally modern durophagous (shell-breaking) teleostean fish, decapod crustaceans, and neoselachian sharks and rays that are typical of Cenozoic nearshore faunas worldwide. The long-term cooling trend that led to the polar climate of today began with a cooling step ∼41 Ma during the middle Eocene. At that time coastal sea temperatures dropped by as much as 10°C over a period of several million years [10]. Durophagous predators persisted in Antarctica until at least the end of the Eocene, but they were far less active after 41 Ma [2], [11]. They became extinct as temperatures declined further, although the timing of those post-Eocene extinctions is uncertain.
Concomitant with the sharp reduction in activity and eventual loss of the durophagous predators, all of which are products of post-Paleozoic evolutionary radiations [12], [13], benthic communities in Antarctica regressed to an archaic state. The top predators of the living Antarctic benthos are now asteroids, nemertean worms, and other slow-moving invertebrates of a Paleozoic functional grade that cannot break hard-shelled prey. As early as the middle to late Eocene, declining predation pressure released epifaunal suspension-feeding invertebrates from predation pressure. They grew in dense populations, forming communities of a type common in nearshore environments during the Paleozoic [11].
We used the fossil record preserved in the La Meseta Formation at Seymour Island, Antarctic Peninsula, to track the response of ecologically significant taxonomic and functional groups to the Eocene cooling event and decline in predation pressure. Here we draw on those paleontological patterns to predict how the current episode of global warming will reverse ecological trends that began in the Eocene. Climate change is already facilitating predatory reinvasions of the Antarctic Peninsula [2], [4], and cascading trophic effects are likely to occur in nearshore communities on a decadal to centennial time scale.
Geology of the La Meseta Formation
The geologic setting, stratigraphy, depositional environment, and age of the Eocene La Meseta Formation at Seymour Island are reviewed in detail elsewhere [10] and summarized here. Seymour Island (64°15′S, 56°45′W) lies along a southwest–northeast axis, approximately 100 km southeast of the tip of the Antarctic Peninsula. The La Meseta Formation is the richest marine deposit in terms of macrofossils known from Antarctica, although there are numerous intervals in which fossils are rare or absent. The geology, geochemistry, and diverse composition of the fossil assemblages clearly indicate that the faunas inhabited a nearshore, shallow-water environment under fully marine conditions.
Sadler [14] divided the formation into seven discrete lithologic units, or Telms. (Telm is an acronym for “Tertiary Eocene La Meseta.”) Although there have been subsequent refinements of the stratigraphy [15], [16], for convenience we follow Ivany et al. [10] in retaining the Telm designations. The oldest unit, Telm 1, is represented by two small exposures at the margins of the La Meseta outcrop belt. The age of Telm 1 is poorly constrained. Telms 2–7, which are the focus of this paper, constitute the remainder of the La Meseta exposures. Telms 2–7 span most of the Eocene, from ∼54 Ma in the early Eocene to the Eocene–Oligocene boundary at ∼33.5 Ma. The pre-cooling interval, 55–41 Ma, is represented by Telms 2–5 and the lower two-thirds of Telm 6; the interval that includes the cooling event and its aftermath, 41–33.5 Ma, is represented by the upper third of Telm 6 and all of Telm 7 [10].
Methods
Field expeditions to Seymour Island in 1986, 1994, 2000, 2001, and 2003 provided the opportunity to amass substantial collections of fossil marine invertebrates from Telms 2–7. Sites within the La Meseta Formation were plotted on a GPS-based, 1∶10000 topographic map of the island prepared in 1995 by the United States Geological Survey (USGS 64056-T5-TM-010). The sites were assigned to stratigraphic intervals based on our own mapping work and other sources [14], [17]. Fossil collections were enumerated, measured, and treated statistically as described in the Results section.
Results
Echinoderms and Brachiopods
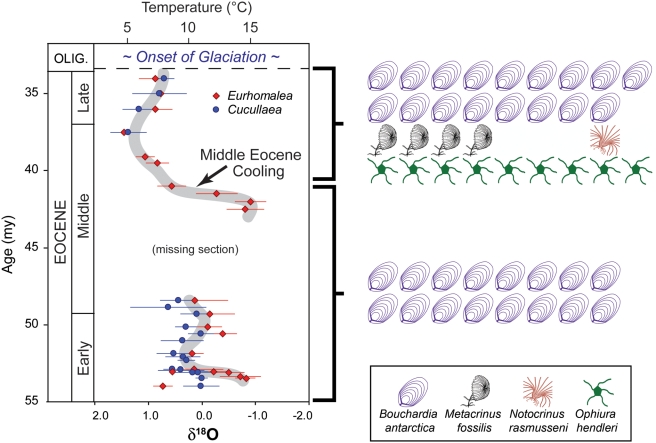
The stratigraphic distribution of autochthonous concentrations of articulated fossil echinoderms in the nearshore, shallow-marine facies that comprise the Eocene La Meseta Formation at Seymour Island shows that dense populations of ophiuroids (Ophiura hendleri) and crinoids (the stalked crinoid Metacrinus fossilis and the unstalked Notocrinus rasmusseni) flourished on soft substrata after the 41-Ma cooling event, but not before (Fig. 1; Table 1). Because ophiuroids and crinoids are vulnerable to both lethal and sublethal durophagy [18]–[20], their high abundances and low levels of sublethal damage (i.e., regenerating arms) are strong evidence for low predation pressure in these post-cooling populations [11].
Figure 1. Distribution of epifaunal suspension-feeders in Telms 2–7 of the La Meseta Formation at Seymour Island, below and above the cooling event at 41 Ma.
Graph on the left shows the Eocene paleotemperature curve derived from the La Meseta Formation at Seymour Island, based on mean oxygen-isotope values of shell material from two bivalve genera. Error bars represent standard deviations. Ages of horizons and the inferred presence of a middle Eocene unconformity are based on strontium-isotope stratigraphy (redrawn from [10]). Icons on the right denote dense, autochthonous or parautochthonous fossil concentrations that represent abundant paleopopulations of rhynchonelliform brachiopods, Bouchardia antarctica; stalked crinoids, Metacrinus fossilis; unstalked (comatulid) crinoids, Notocrinus rasmusseni; and ophiuroids, Ophiura hendleri. Paleontological data are based on surveys conducted in 1986, 1994, 2000, and 2001.
Table 1. Occurrences of fossil assemblages representing dense populations of epifaunal suspension-feeders in the Telm units of the La Meseta Formation.
| Echinoderms | Brachiopods | |||
| Telm | Ophiura | Metacrinus | Notocrinus | Bouchardia |
| 7 | 8 | 4 | 1 | 12 |
| Upper 6† | 1 | 0 | 0 | 5 |
| Lower 6‡ | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 7 |
| 4 | 0 | 0 | 0 | 6 |
| 3 | 0 | 0 | 0 | 3 |
| 2 | 0 | 0 | 0 | 0 |
| 1 | 0 | 0 | 0 | 2 |
Upper third of Telm 6, from ∼41 to ∼39 Ma.
Lower two-thirds of Telm 6, before the cooling event at ∼41 Ma.
An alternative hypothesis is that low-salinity conditions excluded dense ophiuroid and crinoid populations from the pre-cooling interval. This explanation can be eliminated for two reasons. First, other echinoderms, including asteroids and irregular echinoids, occur throughout the La Meseta Formation. Echinoderms have no excretory organs and as a result are strictly stenohaline; hyposaline conditions would have eliminated all echinoderms, not just dense populations of ophiuroids and crinoids. Second, geochemical data point to normal marine salinities during deposition of the fossiliferous intervals [10].
The presence of articulated asteroids from the basal Telm 1 to the uppermost Telm 7 of the La Meseta Formation provides a taphonomic control. Asteroids, like ophiuroids and crinoids, are susceptible to post-mortem disarticulation. If ophiuroids and crinoids had lived in dense populations prior to the cooling event, they would have been as readily preserved as the asteroids in the lower units.
Freed from the constraints of durophagy, dense populations of ophiuroids and sessile suspension-feeders cover vast areas of the soft-sediment seafloor in Antarctic shelf environments to this day [7], [21]. Ophionotus victoriae, the ophiuroid species that commonly forms modern dense populations, is a low-energy generalist, feeding on zooplankton in the water column as well as living and dead organic matter on the benthos [22]. Comatulid (unstalked) crinoids, the only crinoids which currently inhabit nearshore environments anywhere [18], are generally restricted in shallow Antarctic waters to rocky substrata and the stalks and branches of sessile invertebrates [7], but they have also been recorded occasionally in dense aggregations on unconsolidated substrata in <100 m depth [23]. The comatulids are suspension-feeders and, like the Antarctic ophiuroids, are adapted to surviving on low energy budgets [22]. Stalked crinoids were eliminated from Antarctic shallow waters after the Eocene, and today the genus Metacrinus, like all taxa of stalked crinoids worldwide, is confined to the deep sea [24].
In contrast to the ophiuroid and crinoid assemblages, spatially discrete concentrations of the rhynchonelliform brachiopod Bouchardia antarctica (Terebratellidae) occurred in the shallow-marine, soft-substratum paleoenvironments of the La Meseta Formation both before and after the 41-Ma temperature drop and associated decline in predation pressure (Fig. 1). The temporal distributions of brachiopod and echinoderm assemblages in Telms 2–7 differed significantly from each other across the cooling event (all echinoderm assemblages pooled; χ2 = 10.291, df = 1, P = 0.001). Incorporating data from Telm 1 (Table 1) does not alter the strength of the observed pattern (χ2 = 11.381, df = 1, P = 0.001).
Bouchardia antarctica is the only species in the formation found in dense brachiopod “nests,” which consist of tens to hundreds of specimens distributed over a square meter or less. Despite the possibility of centennial- to millennial-scale time-averaging, which has been observed in shell assemblages of a modern congener in Brazil, B. rosea [25], the abundance of articulated B. antarctica in good taphonomic condition in many of the Eocene brachiopod nests at Seymour Island suggests they formed dense living populations. Brachiopods are suspension-feeders, and B. rosea is epifaunal to semi-infaunal [26]; a similar life habit is inferred for B. antarctica. Conditions in the late Eocene following the cooling event may have favored dense populations of B. antarctica (Table 1), but brachiopod nests do not show the sudden post-cooling increase observed for dense echinoderm populations.
Morphometric Analysis of Brachiopod Shells
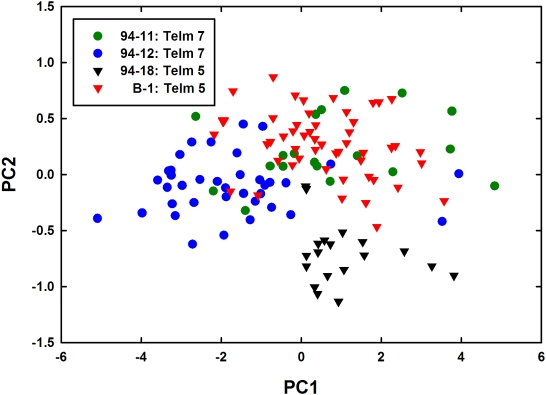
If predation pressure on brachiopods did not change appreciably across the 41-Ma cooling event, then the defensive attributes of their shells should not have changed either. Shell morphologies of Bouchardia antarctica from two sites in Telm 5 (B1 and 94-18; pre-cooling) and two in Telm 7 (94-11 and 94-12; post-cooling) were compared using Principal Components Analysis (PCA). Only well-preserved, undistorted, articulated valve-pairs were used. Four linear shell metrics, which could be measured precisely in the fossil specimens, were recorded for each individual: length of the pedicle valve, length of the brachial valve, width of the brachial valve, and shell height. Shell height, the maximum thickness of the valve-pair in the closed position, is a measure of shell inflation. Shell heights of gaped specimens were calculated by regressing shell height on pedicle valve length for closed specimens from the same site. Each variable was measured to the nearest 0.01 mm using digital calipers. Total sample sizes were n = 69 for Telm 5 and n = 56 for Telm 7.
We used PCA to examine the proportion of the total variance in shell morphology distributed among sites and time intervals (Telms). The data were 1/x-transformed prior to analysis to satisfy the requirement that samples be drawn from a multivariate, normal distribution. (The commonly used logarithmic transformation did not normalize the data.) Significant eigenvector loadings were identified using Pearson correlation analysis between the independent variables and their corresponding loadings for each principal component (PC).
Two PCs extracted from the correlation matrix explained 97.7% of the variability in the data set (Fig. 2, Table 2). PC1, which accounted for 92.8% of the total variance, yielded highly negative loadings for all independent variables, suggesting that it represents variability in shell size; specimens with high PC1 scores had small shells, whereas those with low scores had large shells. PC2 accounted for 4.8% of the total variance; it yielded a significantly positive loading for shell height and a significantly negative loading for shell width. We interpret PC2 as describing variability in shell shape, essentially independent of shell size. Specimens with low PC2 scores had wide, flat shells, whereas those with high PC2 scores had inflated, narrow shells.
Figure 2. Morphometric analysis of Bouchardia antarctica shells from before and after the 41-Ma cooling event.
Shell-morphology scores for the first two principal components explain 97.7% of the among-site variability. PC1 describes variability in size and PC2 represents variability in shape. Sites B-1 and 94-18 predate the cooling event, whereas sites 94-11 and 94-12 postdate the event.
Table 2. Eigenvector loadings and eigenvalues for PCA of four linear shell measurements of Bouchardia.
| Results | Original variable | PC1 | PC2 |
| Eigenvector loadings | Pedicle valve length | −0.513* | 0.083 |
| Brachial valve length | −0.511* | −0.013 | |
| Shell height | −0.489* | 0.664* | |
| Shell width | −0.486* | −0.743* | |
| Eigenvalues (%) | 92.8 | 4.8 |
Loadings denoted with an asterisk (*) are significantly correlated with the original independent variables (Pearson correlations; P≤0.0010; Bonferroni-corrected αadj = 0.00625 for 8 tests).
A nested Analysis of Variance (ANOVA) was performed on the PC2 scores, with Telm as the fixed factor and Site nested within Telm. There was no significant effect of Telm (F = 0.28, df = 1, 121; P = 0.649). There was, however, a significant Site effect (F = 91.87; df = 2, 121; P<0.0005). A nested ANOVA on the PC1 scores also yielded a significant Site effect (F = 16.47; df = 2, 121; P<0.0005) but no significant Telm effect (F = 1.19, df = 1, 121; P = 0.390). In summary, the morphometric analysis showed no consistent changes in shell shape or size associated with either the temperature drop or declining predation pressure.
Shell-Breaking and Shell-Drilling Predation
None of the hundreds of B. antarctica collected from pre- and post-cooling horizons displayed evidence of sublethal shell breaks (repaired cracks). The absence of sublethal damage could indicate either very low or very high frequencies of lethal predation [11], [27]. In aggregate, however, the distributional, morphometric, and shell-repair data argue that Bouchardia populations did not experience significant shell-breaking predation before or after the 41-Ma cooling event.
Brachiopods, like bivalves, are potentially susceptible to predation by shell-drilling gastropods. We compared evidence of drilling predation between nests of Bouchardia and dense aggregations of venerid bivalves, Eurhomalea spp. Eurhomalea is the most abundant genus of infaunal bivalves in the La Meseta Formation. Of 93 well-preserved, articulated pairs of Bouchardia valves recovered from 5 dense aggregations before the 41-Ma cooling event, and 116 pairs from 5 dense aggregations after the event, none (0%) had a borehole. In contrast, naticid boreholes occurred in 4.9% of well-preserved, articulated valve-pairs of Eurhomalea spp. (Veneridae) in dense fossil aggregations from before the cooling event, and in 5.7% of Eurhomalea valve-pairs in dense aggregations from after 41 Ma (Table 3; χ2 = 0.796, df = 1, P = 0.372). Almost all the boreholes in Eurhomalea from both intervals—154 out of 157, or 98%—were complete, indicating that the preponderance of attacks were lethal both before and after the cooling event. Inclusion of disarticulated valves in the calculations yielded similar results, with drilling frequencies of 5.9% before the cooling event and 6.0% after 41 Ma (Table 3; χ2 = 0.408, df = 1, P = 0.523). Combining articulated and disarticulated Eurhomalea valves, 183 of 188 drillholes, or 97.3%, were lethal. No valve-pair or disarticulated valve had more than one borehole.
Table 3. Frequencies of naticid drillholes in Eurhomalea spp. bivalves from the La Meseta Formation.
| Valves analyzed | Interval | N | Complete | Incomplete | Drilled (%) |
| Paired | Post-cooling | 1750 | 96 | 3 | 5.66 |
| Pre-cooling | 1246 | 61 | 0 | 4.90 | |
| Paired plus disarticulated | Post-cooling | 1830 | 106 | 4 | 6.01 |
| Pre-cooling | 1329 | 77 | 1 | 5.87 |
Bivalves were analyzed from 9 pre-cooling sites and 8 post-cooling sites. For the samples of paired plus disarticulated valves, the number of disarticulated valves was divided by 2 to calculate the number of individuals, N.
Declining temperatures in the Eocene thus did not appreciably alter the frequency of shell-drilling predation by infaunal naticid gastropods on the infaunal Eurhomalea. The epifaunal/semi-infaunal Bouchardia were not affected by drilling gastropods either before or after the cooling event. The dense aggregations of Eurhomalea and Bouchardia were found separately, so the living populations may have occupied different microhabitats. Our results are nevertheless consistent with the observed low levels of drilling predation in modern Brazilian populations of B. rosea, with higher frequencies in co-occurring venerids and other infaunal bivalves [26]. Thus, predatory gastropods may have been physically segregated from B. antarctica on a small spatial scale, or they may have co-occurred but avoided eating them.
The numerically dominant gastropods of the La Meseta Formation are semi-infaunal species in the families Naticidae and Struthiolariidae. Like Bouchardia, the naticids show little morphological variation and no taxonomic change across the 41-Ma cooling event. The struthiolariids exhibit a species-replacement sequence in which they become smaller and thinner-shelled upsection, but apparently not in association with the cooling event [17].
Rhynchonelliform brachiopods at all latitudes, including Bouchardia, are low-energy, sessile suspension-feeders that today survive under both oligotrophic and eutrophic conditions [28]–[30]. Fossil evidence suggests they were unaffected or weakly affected by post-Paleozoic secular trends in shell-drilling and shell-breaking predation [31], [32]. B. antarctica apparently tolerated a broad range of ecological conditions over Eocene time, including substantial variations in productivity and the activity of predators [10], [11], which would explain why dense aggregations are found throughout the La Meseta Formation. Today, aggregations of the rhynchonelliform Liothyrella uva are prominent constituents of the nearshore benthos off the Antarctic Peninsula, but unlike Bouchardia the aggregations occur on rocky bottoms rather than soft substrata [7], [33].
Discussion
Sea-surface temperatures off the western Antarctic Peninsula (WAP) have risen by 1°C in the last 50 years, making that area one of the fastest-warming regions of the World Ocean [34]. Up to this point, brachyuran and anomuran crabs (and other reptant decapods) have been excluded from Antarctic shelf environments by an unusual physiological constraint: they are unable to down-regulate the magnesium ions they naturally take up from seawater by diffusion. Lower temperatures decrease their scope for aerobic activity, and the added narcotic effect of Mg2+ is lethal at temperatures below ∼1°C [35]. Predatory crabs are now reinvading WAP: their larvae cross the Polar Front in warm-core rings and in the ballast-water released by ships [36]. Furthermore, adult populations of lithodids (anomuran king crabs) have recently been discovered in the deeper waters of the continental slope off the WAP, which are slightly warmer at 1–2°C [4].
Continued warming of shallow waters and break-up of the ice shelves will likely increase water-column productivity and prolong the growing season for incoming crab larvae [37], [38]. Warmer sea temperatures will also permit lithodids from the slope to move onto the shelf and establish viable predatory populations. Durophagous fish from lower latitudes may be able to invade as well [2].
Running the Eocene cooling event in rapid-reverse, we predict the trajectories of three taxonomic and functional components of the benthic fauna. First, increasing predation pressure associated with climatic warming and introduction of exotic taxa over the next decades to centuries will reduce or eliminate the dense populations of epifaunal ophiuroids and crinoids that currently flourish in Antarctic shelf environments. Second, epifaunal brachiopods, in contrast, will not decline precipitously in the face of increasing durophagy. Supporting this second prediction, rhynchonelliform brachiopods are currently prominent constituents of the rocky-subtidal epifauna at subpolar, temperate and tropical latitudes in the Southern Hemisphere [29], [33]. Third, the intensity of shell-drilling predation on infaunal bivalves will not change appreciably. These trophic effects will occur first off the WAP and could eventually spread to other coastal areas of Antarctica.
Even if rising temperatures were to eliminate Ophionotus victoriae from subtidal habitats in Antarctica prior to any predation effects, its taxonomic and ecological equivalent from the subantarctic, O. hexactis [22], would likely expand poleward. Subantarctic brachiopods such as Magellania spp. potentially could replace Liothyrella uva [30], [39]. The differential effects of increasing durophagous predation would play out on replacement taxa in an analogous fashion. The effects of ocean acidification on ophiuroids and other faunal elements remain uncertain [1], [40], so it would be premature to speculate about how declining pH might affect predator–prey relationships in the Antarctic benthos.
The Antarctic shelf fauna expanded and contracted during the Pleistocene glacial cycles [41]. It is possible that durophagous predators appeared during warm periods, but this is by no means certain given the poor Pleistocene record in Antarctica. Today's situation differs from the Pleistocene interglacials and the mid-Holocene climatic optimum in its artificially rapid rate of change [1], [34] and the fact that humans are now introducing durophagous predators from as far away as the sub-Arctic [4]. Mitigating the direct introduction of exotic predators will require strengthening the Antarctic Treaty to tighten controls on rapidly expanding ship traffic in Antarctica. The Treaty, however, cannot address global warming. Without a comprehensive, international commitment to control greenhouse-gas emissions, climate change will promote biotic invasions and destroy the endemic character of the Antarctic shelf fauna, homogenizing it taxonomically and functionally with nearshore faunas elsewhere.
Acknowledgments
We thank K. C. Burmeister, J. Evans, C. D. Thomann, and W. J. Zinsmeister for assistance in the field and laboratory; and A. Clarke, E. M. Harper, L. S. Peck, S. Thatje, and an anonymous reviewer for advice and comments on the manuscript. This is Contribution No. 1 from the Institute for Adaptation to Global Change at the Florida Institute of Technology, and Contribution No. 296 from Dauphin Island Sea Lab.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the Office of Polar Programs, US National Science Foundation (grants OPP-9908828 and ANT-0245563 to RBA, OPP-9908856 to DBB, and OPP-0125409 to LCI). NSF had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barnes DKA, Peck LS. Vulnerability of Antarctic shelf biodiversity to predicted regional warming. Clim Res. 2008;37:149–163. [Google Scholar]
- 2.Aronson RB, Thatje S, Clarke A, Peck LS, Blake DB, et al. Climate change and invasibility of the Antarctic benthos. Ann Rev Ecol Evol Syst. 2007;38:129–154. [Google Scholar]
- 3.Vermeij GJ, Roopnarine PD. The coming Arctic invasion. Science. 2008;321:780–781. doi: 10.1126/science.1160852. [DOI] [PubMed] [Google Scholar]
- 4.Thatje S, Anger K, Calcagno JA, Lovrich GA, Pörtner H-O. Challenging the cold: crabs reconquer the Antarctic. Ecology. 2005;86:619–625. [Google Scholar]
- 5.Dayton PK, Robilliard GA, Paine RT, Dayton LB. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecol Monogr. 1974;44:105–128. [Google Scholar]
- 6.Clarke A, Aronson RB, Crame JA, Gili J-M, Blake DB. Evolution and diversity of the benthic fauna of the Southern Ocean continental shelf. Antarct Sci. 2004;16:559–568. [Google Scholar]
- 7.Gili J-M, Arntz WE, Palanques A, Orejas C, Clarke A, et al. A unique assemblage of epibenthic sessile suspension feeders with archaic features in the high-Antarctic. Deep-Sea Res II. 2006;53:1029–1052. [Google Scholar]
- 8.Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. [DOI] [PubMed] [Google Scholar]
- 9.Lear CH, Bailey TR, Pearson PN, Coxall HK, Rosenthal Y. Cooling and ice growth across the Eocene–Oligocene transition. Geology. 2008;36:251–254. [Google Scholar]
- 10.Ivany LC, Lohmann KC, Hasiuk F, Blake DB, Glass A, et al. Eocene climate record of a high southern latitude continental shelf: Seymour Island, Antarctica. Geol Soc Am Bull. 2008;120:659–678. [Google Scholar]
- 11.Aronson RB, Blake DB, Oji T. Retrograde community structure in the late Eocene of Antarctica. Geology. 1997;25:903–906. [Google Scholar]
- 12.Vermeij GJ. The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology. 1977;3:245–258. [Google Scholar]
- 13.Harper EM. The Mesozoic marine revolution. In: Kelley PH, Kowalewski M, Hanson TA, editors. Predator-prey interactions in the fossil record. New York: Kluwer/Plenum; 2003. pp. 433–455. [Google Scholar]
- 14.Sadler PM. Geometry and stratification of uppermost Cretaceous and Paleogene units on Seymour Island, northern Antarctic Penunsula. In: Feldmann RM, Woodburne MO, editors. Geology and paleontology of Seymour Island, Antarctic Peninsula. Boulder: Geological Society of America Memoir 169; 1988. pp. 303–320. [Google Scholar]
- 15.Porębski SJ. Facies architecture in a tectonically-controlled incised-valley estuary: La Meseta Formation (Eocene) of Seymour Island, Antarctic Peninsula. Stud Geol Polon. 1995;107:7–97. [Google Scholar]
- 16.Marenssi SA, Santillana SN, Rinaldi CA. Stratigraphy of the La Meseta Formation (Eocene), Marambio (Seymour) Island, Antarctica. In: Casadío S, editor. Paleógeno de América del Sur y de la Península Antártica. Buenos Aires. Revista de la Asociación Paleontológica Argentina, Publicación Especial 5; 1998. pp. 137–146. [Google Scholar]
- 17.Stilwell JD, Zinsmeister WJ. Molluscan systematics and biogeography: Lower Tertiary La Meseta Formation, Seymour Island, Antarctica. Washington, D.C.: American Geophysical Union, Antarctic Research Series 55; 1992. p. 192. [Google Scholar]
- 18.Meyer DL, Macurda DB., Jr Adaptive radiation of the comatulid crinoids. Paleobiology. 1977;3:74–82. [Google Scholar]
- 19.Aronson RB. Biology of a scale-independent predator-prey interaction. Mar Ecol Prog Ser. 1992;89:1–13. [Google Scholar]
- 20.Oji T. Is predation intensity reduced with increasing depth? Evidence from the west Atlantic stalked crinoid Endoxocrinus parrae (Gervais) and implications for the Mesozoic marine revolution. Paleobiology. 1996;22:339–351. [Google Scholar]
- 21.Raguá-Gil JM, Gutt J, Clarke A, Arntz WE. Antarctic shallow-water mega-epibenthos: Shaped by circumpolar dispersion or local conditions? Mar Biol. 2004;144:29–40. [Google Scholar]
- 22.McClintock JB. Trophic biology of Antarctic shallow-water echinoderms. Mar Ecol Prog Ser. 1994;111:191–202. [Google Scholar]
- 23.Dearborn JH. Foods and feeding characteristics of Antarctic asteroids and ophiuroids. In: Llano GA, editor. Adaptations within Antarctic ecosystems. Washington, D.C.: Smithsonian Institution; 1977. pp. 293–326. [Google Scholar]
- 24.Zinsmeister WJ, Feldmann RM. Cenozoic high latitude heterochroneity of Southern Hemisphere marine faunas. Science. 1984;224:281–283. doi: 10.1126/science.224.4646.281. [DOI] [PubMed] [Google Scholar]
- 25.Carroll M, Kowalewski M, Simões MG, Goodfriend GA. Quantitative estimates of time-averaging in terebratulid brachiopod shell accumulations from a modern tropical shelf. Paleobiology. 2003;29:381–402. [Google Scholar]
- 26.Simões MG, Rodrigues SC, Kowalewski M. Comparative analysis of drilling frequencies in Recent brachiopod-mollusk associations from the southern Brazilian shelf. Palaios. 2007;22:143–154 (2007). [Google Scholar]
- 27.Vermeij GJ. Unsuccessful predation and evolution. Am Nat. 1982;120:701–720. [Google Scholar]
- 28.Rhodes MC, Thompson RJ. Comparative physiology of suspension-feeding in living brachiopods and bivalves: evolutionary implications. Paleobiology. 1993;19:322–334. [Google Scholar]
- 29.Kowalewski M, Simões MG, Carroll M, Rodland DL. Abundant brachiopods on a tropical, upwelling-influenced shelf (southeast Brazilian Bight, South Atlantic). Palaios. 2002;17:277–286. [Google Scholar]
- 30.Peck LS. Brachiopods and climate change. Trans R Soc Edinb Earth Env Sci. 2007;98:1–6. [Google Scholar]
- 31.Leighton L. Predation on brachiopods. In: Kelley PH, Kowalewski M, Hanson TA, editors. Predator-prey interactions in the fossil record. New York: Kluwer/Plenum; 2003. pp. 215–237. [Google Scholar]
- 32.Kowalewski M, Hoffmeister AP, Baumiller TK, Bambach RK. Secondary evolutionary escalation between brachiopods and enemies of other prey. Science. 2005;308:1774–1777. doi: 10.1126/science.1113408. [DOI] [PubMed] [Google Scholar]
- 33.Foster MW. Recent Antarctic and sub-Antarctic brachiopods. Washington, D.C.: American Geophysical Union, Antarctic Research Series 22; 1974. p. 189. [Google Scholar]
- 34.Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, et al. Climate change and the marine ecosystem of the western Antarctic Peninsula. Phil Trans R Soc Lond B. 2007;362:149–166. doi: 10.1098/rstb.2006.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frederich M, Sartoris FJ, Pörtner H-O. Distribution patterns of decapod crustaceans in polar areas: a result of magnesium regulation? Polar Biol. 2001;24:719–723. [Google Scholar]
- 36.Thatje S, Fuentes V. First record of anomuran and brachyuran larvae (Crustacea: Decapoda) from Antarctic waters. Polar Biol. 2003;26:279–282. [Google Scholar]
- 37.Smetacek V, Nicol S. Polar ocean ecosystems in a changing world. Nature. 2005;437:362–368. doi: 10.1038/nature04161. [DOI] [PubMed] [Google Scholar]
- 38.Doney SC. Plankton in a warmer world. Nature. 2006;444:695–696. doi: 10.1038/444695a. [DOI] [PubMed] [Google Scholar]
- 39.McCammon HM. The ecology of Magellania venosa, an articulate brachiopod. J Paleontol. 1973;47:266–278. [Google Scholar]
- 40.Wood HL, Spicer JI, Widdicombe S. Ocean acidification may increase calcification rates, but at a cost. Proc R Soc Lond B. 2008;275:1767–1773. doi: 10.1098/rspb.2008.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thatje S, Hillenbrand C-D, Mackensen A, Larter R. Life hung by a thread: Endurance of Antarctic fauna in glacial periods. Ecology. 2008;89:682–692. doi: 10.1890/07-0498.1. [DOI] [PubMed] [Google Scholar]