Abstract
Objective
To determine whether implementation of provider-initiated HIV counseling would increase the proportion of tuberculosis patients that received HIV counseling and testing.
Design
Cluster-randomized trial with clinic as unit of randomization
Setting
Twenty, medium-sized primary care TB clinics in the Nelson Mandela Metropolitan Municipality, Port Elizabeth, Eastern Cape Province, South Africa
Subjects
A total of 754 adults (≥ 18 years) newly registered as tuberculosis patients the twenty study clinics
Intervention
Implementation of provider-initiated HIV counseling and testing.
Main outcome measures
Percentage of TB patients HIV counseled and tested.
Secondary
Percentage of patients HIV test positive and percentage of those that received cotrimoxazole and who were referred for HIV care.
Results
A total of 754 adults newly registered as tuberculosis patients were enrolled. In clinics randomly assigned to implement provider-initiated HIV counseling and testing, 20.7% (73/352) patients were counseled versus 7.7% (31/402) in the control clinics (p = 0.011), and 20.2 % (n = 71) versus 6.5% (n = 26) underwent HIV testing (p = 0.009). Of those patients counseled, 97% in the intervention clinics accepted testing versus 79% in control clinics (p =0.12). The proportion of patients identified as HIV-infected in intervention clinics was 8.5% versus 2.5% in control clinics (p=0.044). Fewer than 40% of patients with a positive HIV test were prescribed cotrimoxazole or referred for HIV care in either study arm.
Conclusions
Provider-initiated HIV counseling significantly increased the proportion of adult TB patients that received HIV counseling and testing, but the magnitude of the effect was small. Additional interventions to optimize HIV testing for TB patients urgently need to be evaluated.
Keywords: HIV, HIV counseling and testing, tuberculosis (TB), primary care clinics, South Africa, cluster randomized trial
BACKGROUND
Tuberculosis (TB) is the initial clinical manifestation of human immunodeficiency virus (HIV) disease for many people in sub-Saharan Africa, and a very high proportion of TB patients in this region are co-infected with HIV. HIV counseling and testing of TB patients is therefore an important method for identifying HIV-infected individuals, all of whom require HIV care and many of whom are indicated for antiretroviral therapy [1–6].
A variety of initiatives, including national HIV/AIDS programs, the Global Fund for AIDS, TB and Malaria, and the President’s Emergency Plan for AIDS Relief, are working toward the goal of providing antiretroviral therapy to millions of eligible HIV infected individuals in developing countries. It is imperative that individuals be given reasonable opportunities for appropriate HIV counseling and testing, as positive HIV test results are required to initiate care and treatment. In March 2005, it was estimated that of the 837,000 people in need of antiretroviral therapy in South Africa, only 104,600 were receiving it [7].
In the voluntary HIV counseling and testing model, health care workers ask patients if they want HIV counseling and testing. The burden of decision-making is placed on the individual patient, and to receive HIV counseling the patient must say ‘yes’. In June, 2004, UNAIDS and the World Health Organization (WHO) issued a policy statement promoting provider-initiated HIV counseling, which shifts the burden of decision making from the patient to the health care provider.
“…This includes HIV testing for all tuberculosis patients as part of their routine management…patients retain the right to refuse testing, i.e. to ‘opt out’ of a systematic offer of testing.”[8]
While an “opt out” strategy should increase the uptake of testing by TB patients, data supporting this approach in African settings have not been available. Therefore, we undertook a pragmatic, clinic-based trial to measure the impact of implementing the opt-out strategy on the uptake of HIV counseling and testing by TB patients in a typical TB clinic environment in South Africa, where space and staff are limited.
METHODS
Study Setting and Subjects
The study was performed in the Nelson Mandela Municipality Health District, in and around Port Elizabeth, Eastern Cape Province, South Africa. Twenty medium-sized clinics, representative of the geographic and demographic variability of the Municipality were selected from the District’s 44 primary health clinics based on the presence of a designated TB nurse and new registration of at least three TB patients per month. A baseline assessment of each clinic included a review of the clinic TB and HIV records and interviews with the TB and HIV nurses. The designated TB nurse in each study clinic was trained in the Health Department’s standard HIV counseling and testing course prior to the start of the study. No additional staff were provided to the clinics to assist with HIV counseling and testing, as the study was intended to evaluate the impact of the change in strategy in a real world setting, where resources are limited and staff are extremely busy.
Newly registered adult (≥18 years) TB patients who remained in care for at least 14 days from registration, to allow time to receive HIV counseling and testing, were included in the study. TB was defined according to program criteria from the South African Department of Health, and pulmonary and extrapulmonary TB patients were eligible to participate. Patients were excluded if they were less than 18 years old or if they transferred out or died <14 days from registration.
The primary study outcome was the mean percentage of newly registered TB patients that received HIV counseling and testing in clinics randomized to use the opt-out strategy versus those that continued to use the opt-in approach (control). Secondary outcomes were the mean percentage of HIV test-positive patients and, of those, the mean percentage that were prescribed cotrimoxazole and/or were referred for HIV care.
Ethical approval of the study was obtained from the Department of Health of the Eastern Cape Province, the Johns Hopkins Bloomberg School of Public Health Committee on Human Research and the Faculty of Health Sciences Human Research Ethics and Bio-safety Committee of the University of Transkei, Eastern Cape Province. Waivers of informed consent of study participants were granted by the ethics committees.
Training
All twenty TB nurses received training from the study staff and a Health Department-contracted training nurse in the use of an HIV counseling and testing register prior to initiation of the trial. TB nurses who worked in the clinics allocated to the intervention arm were invited to attend a two-day course to learn the strategy of routine, provider-initiated counseling, also provided by study staff and the professional trainer from the Health Department. The TB nurses were trained to incorporate HIV counseling into the TB education given to all newly registered TB patients. The new strategy did not abbreviate the content of HIV counseling. The training encouraged the TB nurses to HIV counsel and test all new TB patients as soon as possible after registration. Treatment protocols were presented according to the national guidelines and included an introduction to The National Antiretroviral Treatment Guidelines for HIV/TB co-infected patients. The training included practical guidance and discussion of methods to enhance patient flow between TB and HIV treatment areas. The primary difference between the intervention training and the HIV counseling and testing modules as established by the South African Department of Health was the pointed emphasis placed on the interaction between HIV and TB. These modules highlighted the increased risk for TB for patients living with HIV, and underscored the need for integrated TB and HIV services. The practical modules, through which the intervention clinic nurses practiced HIV counseling for TB patients, included this information, and the trainers echoed this message as part of comprehensive care in a separate training module. Integrated care for TB and HIV was a central message throughout the intervention training.
Data Sources
There were no nationally standardized data collection tools for HIV test results in South Africa at the time of our study, so we designed a registry form for the clinics to document the dates when HIV counseling had been provided, when testing was carried out, and the results of the HIV tests. The TB Register, HIV Counseling Testing Register and patient clinical records were the sources of data to measure the study outcomes. Data were abstracted directly from these source documents onto data collection forms by study staff. When data were missing in the TB Register, the TB Treatment Card was checked or the TB nurse was asked to clarify information. Data were entered into a Windows Office Access database and transferred to STATA 8.0 [9] and SAS 9.1 [10] statistical software for analysis.
Statistical Methods
Based on Health Department estimates prior to the study, we assumed a 40 percent baseline rate of HIV counseling and testing for TB patients in the clinics, and that 20 newly registered tuberculosis patients per clinic would be available for recruitment over a 6 month period. We used the following Hayes and Bennett formula [11] to estimate the number of clinics to be randomized to each study arm:
where c is the number of clusters required, π1 and π0 are the population proportions with and without the intervention, respectively, n is the number of individuals in each group, and k is the coefficient of variation of true proportions between clusters within each group. Assuming 70 percent counseling uptake in the intervention arm and 40 percent counseling rate in the control arm (30 percent difference) and k= 0.30, we estimated that 10 clusters (clinics) of 20 patients each from each of the two study arms would be required to achieve 90 percent power with a 5 percent type I error rate. Sample size calculations were made intentionally conservative to allow for greater variability between clinics than anticipated.
Following baseline evaluations and training, the twenty study clinics were randomized in a 1:1 ratio to receive either the opt-out strategy (intervention clinics) or standard HIV counseling and testing (control clinics). Randomization was constrained so that the two study arms were nearly balanced in terms of the total number of clinic patients seen each month, the number of TB cases registered each month, the number of TB patients reported to have received HIV counseling and testing in the months prior to the intervention, and a summary score of the extent of TB/HIV collaboration as determined by study staff. A customized SAS macro was used to realize the desired constrained randomization [12].
The strategy was implemented at the clinic level so statistical analyses were also carried out at the cluster level. Chi-square tests adjusted for the intra-cluster correlation coefficient were used to evaluate differences across study arms, using ACluster software [13].
Role of the Funding Agencies
The funding agencies played no role in the protocol design, collection of data, analysis or preparation of the manuscript. The first and senior authors retain full control of all data and were responsible for the decision to submit for publication.
RESULTS
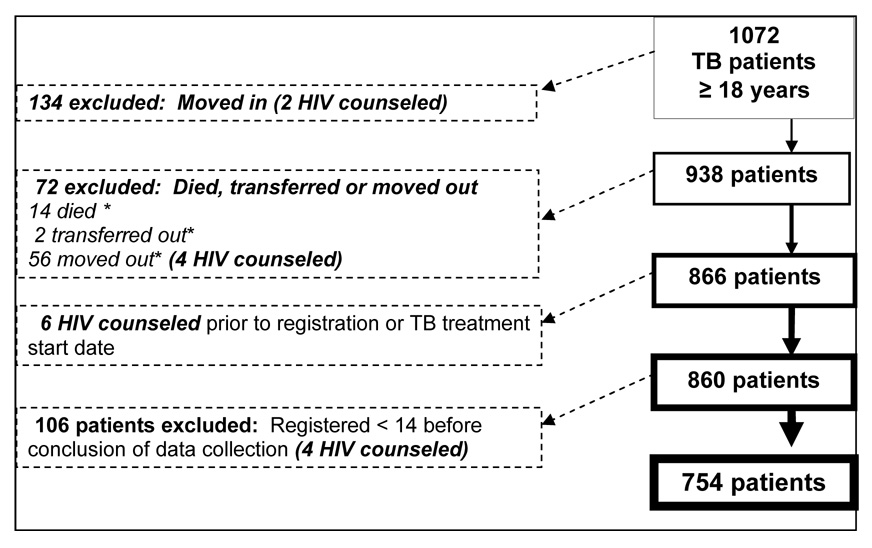
The 20 medium-sized clinics in both study arms were evenly distributed between populations that were primarily Xhosa and so-called coloured, who spoke Afrikaans. Between August 12 and November 10, 2005, 1072 adult TB patients registered in the study clinics, of whom 754 (70 percent) met the study criteria and were analyzed. Figure 1 shows the distribution of TB patients considered for trial and reasons for exclusion. A total of sixteen TB patients who received HIV counseling were excluded from the analysis because they did not fit study inclusion criteria. The numbers of TB patients and their mean age, sex, site of disease, sputum smear status and TB treatment history were comparable in the intervention and control clinics (Table 1). As would be expected, the majority of cases had sputum smear positive pulmonary disease. A significant proportion had a history of prior TB treatment. Male patients were older on average than female patients; the majority of patients were 30–40 years old.
FIGURE 1.
Summary of TB patients excluded from study analysis
* Died, transferred or moved out < 14 days after TB registration or TB Rx start, whichever was more inclusive
TABLE 1.
Comparison of intervention and control clinics by number of TB patients, demographics and TB disease characteristics
| Intervention Clinics | Control Clinics | P-value | |||||
|---|---|---|---|---|---|---|---|
| Total | Median per clinic | Range | Total | Median per clinic | Range | ||
| TB patients | 352 | 33 | 18–52 | 402 | 39.5 | 19–71 | 0.46 |
| Males (median age in years) | 194 (55%) | 36 | 18–72 | 238 (59%) | 35 | 18–79 | 0.39 |
| Females (median age in years) | 157 (45%) | 33 | 18–78 | 162 (41%) | 34 | 18–86 | 0.39 |
| Pulmonary TB | 317 (89%) | 29.5 | 16–48 | 330 (81%) | 32.5 | 14–62 | 0.02 |
| Sputum smear positive | 255 (72%) | 33 | 12–38 | 288 (72%) | 32 | 10–52 | 1.0 |
| Retreatment patients | 126 (35%) | 10.5 | 3–25 | 118 (26%) | 9.5 | 3-–9 | 0.07 |
Overall, 104/754 patients (13.8%) received HIV counseling and were tested. Significantly higher proportions of TB patients at intervention clinics were HIV counseled than in control clinics (73/352 [20.7%] versus 31/402 [7.7%], p = 0.011, Table 2). The proportion of patients who received HIV tests was also greater in the intervention arm, 71/352 (20.2%) versus 26/402 (6.5 %) than in control clinics (20.7 percent versus 7.7 percent, p = 0.009). The overall proportion of patients given counseling who accepted testing was higher in the intervention arm (97 percent) than in the control arm (79 percent), but the difference was not statistically significant (p = 0.12). Patients received counseling and were tested closer to their date of TB registration in the intervention arm, with a mean of 5.2 days (range 0 – 21 days) to test in the intervention arm versus 9.6 days (range 0 – 43 days) in the control arm (p = 0.12).
Table 2.
Number and percentage of TB patients HIV counseled and tested, HIV positive and prescribed cotrimoxazole and/or referred for HIV care
| Study Outcomes | Intervention n=352 | Control n = 402 | OR | p-value* | ||||
|---|---|---|---|---|---|---|---|---|
| Overall Number | Overall Mean % | Range (% per Clinic) | Overall Number | Overall Mean % | Range (% per Clinic) | |||
| Pre-test counseled | 73 | 20.7 | 3.5 – 66.7 | 31 | 7.7 | 1.5 – 15.8 | 3.1 | 0.011 |
| HIV tested | 71 | 20.2 | 1 – 18 | 26 | 6.5 | 0 – 6 | 3.7 | 0.009 |
| HIV test positive | 30 | 8.5 | 0 – 10 | 10 | 2.5 | 0 – 3 | 3.7 | 0.044 |
| HIV (+) Pts prescribed cotrimoxazole | 6 | 1.7 | 0 – 2 | 4 | 1.0 | 0 – 2 | 1.7 | 0.530 |
| HIV (+) Pts referred to clinic | 7 | 2.0 | 0 – 2 | 2 | 0.5 | 0 – 1 | 4.1 | 0.125 |
Chi-square test adjusted for intra-cluster correlation coefficient.
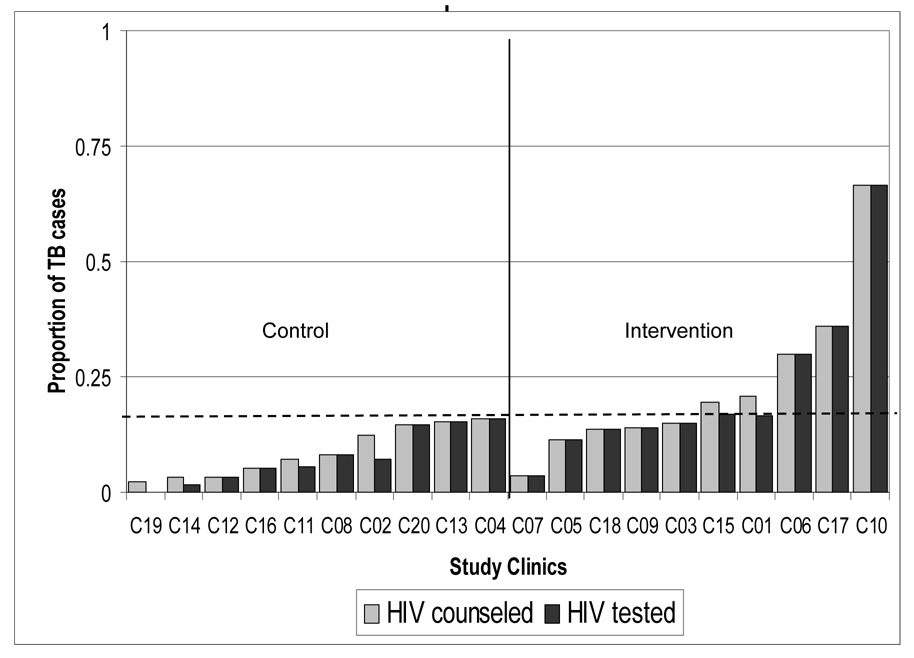
The proportion of patients tested was below the overall mean (13.8 percent) in all of the control clinics while five of the intervention clinics exceeded the study mean. Only one clinic, in the intervention arm, HIV counseled and tested more than half (66.7 percent) of their TB patients. Overall, the Opt-out strategy identified 8.5 percent of intervention clinic patients as HIV-infected, compared to 2.5 percent diagnosed with HIV infection in the control clinics (p = 0.044).
The proportion of tested patients with positive HIV test results was similar in the intervention and control arms (36 percent versus 43 percent). The numbers of patients with a positive HIV test who were prescribed cotrimoxazole (n = 6/31 and 4/11) or referred for HIV care (n = 7/31 and 2/11) were very small and did not differ significantly between study arms.
DISCUSSION
This study was designed to evaluate the impact of implementing a strategy of routine, provider-initiated (Opt-out) HIV counseling on the uptake of counseling and testing of newly registered TB patients in primary care clinics in the Eastern Cape Province of South Africa. The results show that the use of an Opt-out strategy was associated with significantly higher HIV counseling and testing rates and that the time to testing tended to be faster in the Opt-out study arm than in the control arm, but the overall proportion of those counseled and tested following training in the Opt-out approach remained unacceptably low at only 21 percent.
The major purpose of HIV testing of TB patients is to ensure that HIV care and treatment are rendered in a timely fashion. In this study, the number of HIV-infected TB patients who were prescribed cotrimoxazole and/or referred for HIV care was small and did not differ between arms. The South African Tuberculosis Control guidelines in place at the time of this study were last updated in 2000, and clinical and treatment guidelines for HIV/AIDS were not included in them[14, 15]. However, all TB nurses had received specific training about current HIV/AIDS guidelines and should have been aware of the recommendations regarding cotrimoxazole use and referral for HIV care. Thus, while the trial demonstrates the positive impact that training in an Opt-out strategy can have on the uptake of testing, it underscores the difficulty of integrating HIV and TB care in the challenging world of clinical practice in resource poor settings.
This trial was carried out without an influx of additional personnel or resources, in a primary care setting that was already overburdened with a large clinical workload. Although HIV testing for TB patients is recommended by global and South African guidelines, implementation of this mandate into practice depends on the capacity of local health facilities to incorporate additional and time-consuming clinical tasks into their already overstretched workloads. Reducing the effort required by both health workers and patients for providing HIV testing by offering the Opt-out approach makes it more likely that testing will be provided, but this study illustrates that although instituting a strategy of provider-initiated counseling can increase rates of HIV counseling and testing, it is clearly not sufficient. Additional interventions and resources will surely be required to attain high levels of HIV testing referral into HIV care for TB patients in settings such as ours. In order to better understand the reasons for the low uptake of HIV counseling by the staff in the clinics, we have undertaken a qualitative study of staff and administrators, the results of which are being analyzed. The issues of stigma connected to both TB and HIV, experienced by both patients and staff, have been well-documented in several studies and undoubtedly confound an already complex task [16, 17]
Pragmatic studies carried out in primary care settings, often have modest results. In Brazil, a cross-sectional study designed to assess the rate of HIV screening of TB patients in primary care clinics found that approximately 23 percent of the patients had been screened, and that perceived risk by the health worker determined who was tested [18]. A cluster randomized trial that trained clinic TB nurses in Free State Province, South Africa, to use an algorithm for the diagnosis and management of respiratory diseases (including TB), had voluntary HIV counseling and testing rates of only 9.7 percent in the intervention and 7.3 percent in the control study arms [19].
Studies that demonstrated high uptake of counseling and testing in TB patients have been carried out in hospitals (Malawi) [6], in vertical TB programs (Malawi) [1, 2], during interventions with extra staff dedicated to the process (ProTEST, Côte d’Ivoire and Democratic Republic of Congo) [4, 20, 21], or by study staff (Haiti and Thailand) [3, 5]. Recently, TB programs in Rwanda, Malawi and Guyana[22] have reported substantial increases in HIV testing of TB patients when national initiatives that provided additional training and resources were coupled with use of the provider initiated strategy[22, 23].
Our simple and pragmatic cluster randomization design does not permit us to assess a number of qualitative factors that could influence the success of provider-initiated HIV testing as a public health strategy. Randomization based on baseline data permitted us to distribute the diversity of the study clinics evenly between the two study arms. If there were factors that influenced the uptake of HIV counseling and testing, the evidence from our evaluations indicates that they were not differentially represented in one study arm versus the other. A more intensive intervention or an intervention more specifically tailored to the needs of individual study clinics may have resulted in greater numbers of TB patients receiving HIV counseling and testing. However, our intention was to test an intervention that was reproducible in the very real environment of staff shortages, limited privacy and time constraints.
It is possible that our intervention would have been more effective if it had been a Health Department initiative aided by researchers rather than a research project supported by the Health Department. The nurses in the Municipality knew that the interventions were not health department initiatives. Given the high work load, it is perhaps not surprising that an outside intervention was not a high priority for the nurses. Researchers from other pragmatic South African studies of interventions in primary care settings have concluded that the involvement of supervisors and management are essential to the success of interventions [19, 24]. Although we had the full support of the highest levels of supervision, the concept of provider-initiated HIV counseling and testing was still novel and controversial when this study was begun.
Training in the implementation of routine, provider-initiated HIV counseling increased the proportion of adult TB patients that received HIV counseling and testing by 3-fold, but the absolute magnitude of the effect was small. Given the multitude of restraints that hindered the success of HIV counseling of TB patients, we believe that additional interventions will be necessary to optimize HIV testing and care for TB patients in similar settings in sub-Saharan Africa. Provider-initiated HIV counseling and testing is an important step to ensure that TB patients with HIV-related illnesses are diagnosed and referred into HIV care, but a greater investment in primary care health resources is required to improve comprehensive care of TB and HIV in those areas of the world suffering from the collision of these two epidemics.
FIGURE 2.
Proportion of TB patients that received HIV counseling and testing by control and intervention clinics
---- Study mean proportion of TB patients HIV counseled (0.138)
Acknowledgments
This publication was made possible through support provided by the Office of Health, Infectious Diseases, and Nutrition, Global Health Bureau, U.S. Agency for International Development, under the Global Research Activity Cooperative Agreement (Award No. GHS-A-00-03-00019-00). The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the U.S. Agency for International Development or the United States Government.
Funding: United States Agency for International Development and NIH Grant AI 01637
REFERENCES
- 1.Chimzizi R, Gausi F, Bwanali A, et al. Voluntary counselling, HIV testing and adjunctive cotrimoxazole are associated with improved TB treatment outcomes under routine conditions in Thyolo District, Malawi. Int J Tuberc Lung Dis. 2004;8:579–585. [PubMed] [Google Scholar]
- 2.Chimzizi RB, Harries AD, Manda E, Khonyongwa A, Salaniponi FM. Counselling, HIV testing and adjunctive cotrimoxazole for TB patients in Malawi: from research to routine implementation. Int J Tuberc Lung Dis. 2004;8:938–944. [PubMed] [Google Scholar]
- 3.Desormeaux J, Johnson MP, Coberly JS, et al. Widespread HIV counseling and testing linked to a community-based tuberculosis control program in a high-risk population. Bull Pan Am Health Organ. 1996;30:1–8. [PubMed] [Google Scholar]
- 4.Hausler H. Lessons learned from ProTEST TB/HIV pilot districts in South Africa. ProTEST lessons learned workshop. Powerpoint presentation. 2003 February 3; [Google Scholar]
- 5.Suggaravetsiri P, Yanai H, Chongsuvivatwong V, Naimpasan O, Akarasewi P. Integrated counseling and screening for tuberculosis and HIV among household contacts of tuberculosis patients in an endemic area of HIV infection: Chiang Rai, Thailand. Int J Tuberc Lung Dis. 2003;7:S424–S431. [PubMed] [Google Scholar]
- 6.Zachariah R, Spielmann MP, Harries AD, Salaniponi FL. Voluntary counselling, HIV testing and sexual behaviour among patients with tuberculosis in a rural district of Malawi. Int J Tuberc Lung Dis. 2003;7:65–71. [PubMed] [Google Scholar]
- 7.WHO. South Africa: Summary country profile for HIV/AIDS treatment scale-up. Treat 3 million by 2005. http://www.who.int/3by5/support/june2005_zaf.pdf:3.
- 8.UNAIDS/WHO. Policy Statement on HIV Testing. 2004 June; http://data.unaids.org/una-docs/hivtestingpolicy_en.pdf.
- 9.Stata Statistical Software: Release 8. College Station, TX: StataCorp LP; 2003. [Google Scholar]
- 10.Statistical Analysis System. Cary, North Carolina: SAS Institute Inc; 2003. [Google Scholar]
- 11.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol. 1999;28:319–326. doi: 10.1093/ije/28.2.319. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhary MA, Moulton LH. A SAS macro for constrained randomization of group-randomized designs. Comput Methods Programs Biomed. 2006;83:205–210. doi: 10.1016/j.cmpb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.WHO. ACluster: Design and analysis of cluster randomization trials. 2.0 ed 2000. [Google Scholar]
- 14.Hausler H. Tuberculosis & HIV/AIDS: clinical guidelines. South Africa: National Department of Health; 2000. p. 40. [Google Scholar]
- 15.Balt E, Edginton M, Lotter J, Preller A, Uys M. Tuberculosis: A training manual for health workers. 1998
- 16.Thomas BE, Ramachandran R, Anitha S, Swaminathan S. Feasibility of routine HIV testing among TB patients through a voluntary counselling and testing centre. Int J Tuberc Lung Dis. 2007;11:1296–1301. [PubMed] [Google Scholar]
- 17.Daftary A, Padayatchi N, Padilla M. HIV testing and disclosure: a qualitative analysis of TB patients in South Africa. AIDS Care. 2007;19:572–577. doi: 10.1080/09540120701203931. [DOI] [PubMed] [Google Scholar]
- 18.DeRiemer K, Soares EC, Dias SM, Cavalcante SC. HIV testing among tuberculosis patients in the era of antiretroviral therapy: a population-based study in Brazil. Int J Tuberc Lung Dis. 2000;4:519–527. [PubMed] [Google Scholar]
- 19.Fairall LR, Zwarenstein M, Bateman ED, et al. Effect of educational outreach to nurses on tuberculosis case detection and primary care of respiratory illness: pragmatic cluster randomised controlled trial. Bmj. 2005;331:750–754. doi: 10.1136/bmj.331.7519.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abouya L, Coulibaly IM, Wiktor SZ, et al. The Cote d'Ivoire national HIV counseling and testing program for tuberculosis patients: implementation and analysis of epidemiologic data. Aids. 1998;12:505–512. doi: 10.1097/00002030-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Van Rei A. Counseling and testing TB patients for HIV: Evaluation of three implementation models in Kinshasa, DRC. Int J Tuberc Lung Dis. 2006 Accepted for publication. [PubMed] [Google Scholar]
- 22.WHO. Global tuberculosis control: surveillance, planning and financing. 2007
- 23.Persaud S, Mohanlall J, Bateganya M, et al. HIV Counseling, Testing, and Care of Tuberculosis Patients at Chest Clinics -- Guyana, 2005–2006. Morbidity and Mortality Weekly Report. 2006;55:849–851. [PubMed] [Google Scholar]
- 24.Lewin S, Dick J, Zwarenstein M, Lombard CJ. Staff training and ambulatory tuberculosis treatment outcomes: a cluster randomized controlled trial in South Africa. Bull World Health Organ. 2005;83:250–259. [PMC free article] [PubMed] [Google Scholar]