Abstract
Introduction
The aim of this study was to explore social and emotional functions in patients with medial frontal damage including the anterior cingulate cortex (ACC).
Methods
Three patients with medial frontal lobe lesions primarily involving the ACC performed tasks on motivational decision making, emotional facial expression recognition, and social cognition, including theory of mind (ToM). Their performance on these tasks was compared with age and education matched healthy controls.
Results
Patient performance on the motivational decision making and social situations tasks did not differ from controls. Selective emotional facial expression recognition impairment for fear was evident in one patient with a unilateral right ACC lesion (patient 3). ToM impairment was present in only one patient with a bilateral ACC lesion (patient 2). In contrast, the two patients with unilateral right ACC lesions had intact ToM (patients 1 and 3).
Conclusions
These findings suggest that medial frontal lobe lesions primarily involving the ACC do not appear to critically disrupt motivational decision making or social situation processing. The ACC plays a role in processing particular types of emotion (fear). Bilateral ACC damage impairs ToM processing, but unilateral damage to the right ACC is not sufficient to disrupt ToM.
The anterior cingulate cortex (ACC) has been extensively investigated in the neuroimaging literature which has highlighted its role in cognitive, emotional (see Bush, Luu & Posner, 2000 for a review), and autonomic functioning (Critchley, Mathias, Josephs, O'Doherty, Zanini, Dewar, Cipolotti, et al., 2003). Previous research has identified a distinction between dorsal and ventral ACC mediating cognitive and affective functions, respectively (Bush et al., 2000; Devinsky, Morrell, & Vogt, 1995). Few studies have focused on the role of the ACC in patients with circumscribed lesions. At present there is a paucity of studies systematically investigating social and emotional functions in patients with ACC lesions.
The ACC has been implicated in decision making cognition. Neuroimaging studies using various decision making paradigms have demonstrated ACC task-related activation (e.g., Bush, Vogt, Holmes, Dale, Greve, Jenike, et al., 2002; Elliott & Dolan, 1998; Paulus, Hozack, Frank, & Brown, 2002; Rogers, Owen, Middleton, Williams, Pickard, Sahakian, et al., 1999). The Iowa gambling task (Bechara, Damasio, Damsio, & Anderson, 1994) is commonly used to assess motivational decision making and also elicits ACC activation. For example, a PET study by Ernst, Bolla, Mouratidis, Contoreggi, Matochik, Kurian, et al. (2002) found activation of the right ACC in addition to bilateral dorsolateral prefrontal and orbitofrontal activation during performance of the gambling task. A recent functional magnetic resonance imaging (fMRI) study by Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter, et al. (2004) found that ACC activation (bilateral but greater on the left) was specifically associated with the processing of large reward magnitudes during the decision phase of a gambling task. Lesion studies have found that bilateral damage to the ventromedial prefrontal cortex causes impaired gambling task performance (e.g., Bechara et al., 1994; Bechara, Damasio, Tranel, & Anderson, 1998). In particular, a laterality effect has been proposed with the right ventromedial prefrontal cortex being more critical than the left (Clark, Manes, Antoun, Sahakian, & Robbins, 2003; Tranel, Bechara, & Denburg, 2002). These studies, however, do not document if the lesion included ACC damage. Interestingly, Manes, Sahakian, Clark, Rogers, Antoun, Aitken, et al. (2002) found that patients with focal dorsomedial (including the cingulate gyrus), dorsolateral, or large frontal lesions were impaired relative to controls on the gambling task, whereas patients with focal orbitofrontal lesions performed no differently to controls. A recent study by Clark et al. (2003) found that the volume of damage in the medial prefrontal cortex and middle and superior frontal gyrus on the right side was significantly correlated with gambling task performance. No details regarding ACC damage are included. To date there has been no previous study of decision making in patients with lesions primarily involving the ACC. Given that the Iowa gambling task is a well established measure of motivational decision making and previous research has identified ACC activation during performance of this task, it is somewhat surprising that no previous research has investigated whether lesions involving the ACC disrupt performance on this task. We aimed to explore how critical the ACC is in mediating Iowa gambling task performance.
Neuroimaging studies have demonstrated that the ACC plays a role in the subjective experience of emotion and the processing of emotional stimuli (e.g., Lane, Reiman, Bradley, Lang, Ahern, Davidson, et al., 1997; Lane, Reiman, Axelrod, Yun, Holmes, & Schwartz, 1998). In particular, ACC activation has been found during identification of a range of facial emotional expressions, including happiness (Dolan, Fletcher, Morris, Kapur, Deakin, & Frith, 1996; Phillips, Young, Scott, Calder, Andrew, Giampietro, et al., 1998), fear (Morris, Friston, Buchel, Frith, Young, Calder, et al., 1998), disgust (Wicker, Keysers, Plailly, Royet, Gallese, & Rizzolatti, 2003b), and anger (Blair, Morris, Frith, Perrett, & Dolan, 1999). Furthermore, viewing of individualised faces associated with romantic love (Bartels & Zeki, 2000) and grief (Gundel, O'Connor, Littrell, Fort, & Lane, 2003) has also elicited ACC activation. Electrical stimulation of the ACC can elicit fear, pleasure and agitation (Meyer, McElhaney, Martin, & McGraw, 1973). Changes in the volume and activity of the ACC have been documented in patients with depression, bipolar disorder and schizophrenia (Bouras, Kovari, Hof, Riederer, & Giannakopolous, 2001; Brody, Saxena, Mandelkern, Fairbanks, Ho, & Baxter, 2001). Lesion studies have also demonstrated a role of the ACC in emotion and emotional processing. For example, orbitofrontal damage including the ACC has been associated with emotional changes, such as apathy, aggression, and disinhibition (Eslinger & Damasio, 1985; Mazars, 1970). A recent study by Hornak et al. (2003) found that unilateral ACC lesions can cause impaired vocal and facial expression identification. Specifically, four patients with unilateral lesions to the ACC (two of whom also had orbital damage) had significant difficulty identifying anger and surprise, and three of these patients also had difficulty identifying happiness. Recognition of fear and disgust was also impaired in two patients (Hornak et al., 2003). Thus, accumulating evidence suggests that the ACC plays a role in emotional processing, although it is not specifically involved in a particular type of emotion.
Orbitofrontal damage including the ACC has been associated with deficits in social processing, such as diminished social awareness and disregard of social rules (e.g., Blair & Cipolotti, 2000; Eslinger & Damasio, 1985). Recently, Hornak et al. (2003) found that patients with surgical lesions of the orbitofrontal cortex including the ACC showed significantly lower scores on a social behaviour questionnaire completed by informants compared with patients with dorsolateral and other medial frontal lesions. The authors noted that the social changes of these patients were less dramatic than those previously reported in patients with large lesions of the ventromedial cortex resulting from head injury or stroke (e.g., Hornak, Rolls, & Wade, 1996; Rolls, Hornak, Wade, & McGrath, 1994). The role of the ACC in social processing deficits requires further investigation.
Previous neuroimaging studies have identified the medial frontal lobes, including the ACC, as integral in the mediation of ToM or “mentalising” (e.g., Baron-Cohen, Ring, Wheelwright, Bullmore, Brammer, Simmons, et al., 1999; Fletcher, Happe, Frith, Baker, Dolan, Frackowiak, et al., 1995; Vogeley, Bussfeld, Newen, Herrmann, Happe, Falkai, et al., 2001; Wicker, Perrett, Baron-Cohen, & Decety, 2003a). In particular, the anterior paracingulate cortex—approximately corresponding to Brodmann area (BA) 9/32—which is often considered to be part of the ACC (comprising BA 24, 25, and 33) has been specifically identified as the key region for mentalising (Gallagher & Frith, 2003; Gallagher, Jack, Roepstorff, & Frith, 2002; McCabe, Houser, Ryan, Smith, & Trouard, 2001; Shallice, 2001). Relatively few studies, however, have examined ToM function in patients with focal frontal lesions (e.g., Bird, Castelli, Malik, Frith, & Husain, 2004; Rowe, Bullock, Polkey, & Morris, 2001; Shamay-Tsoory, Tomer, Berger, & Aharon-Peretz, 2003; Stone, Baron-Cohen, & Knight, 1998; Stuss, Gallup, & Alexander, 2001). For example, Rowe et al. (2001) found impaired performance on a ToM task in patients with focal dorsolateral, medial frontal, and orbitofrontal lesions. Stuss et al. (2001) reported that patients with bifrontal and right frontal damage showed impaired mentalising, as assessed by a visual perspective task. Interestingly, on an additional task assessing the ability to infer deception, right ACC, right superior and inferior medial frontal damage correlated with number of errors. No significant correlation was found with left unilateral lesions. These findings, however, need to be interpreted with caution as it is implied that the bifrontal patients suffered head injury and therefore may have more diffuse anatomical damage. In a recent study, Bird et al. (2004) found intact ToM function in a patient with bilateral medial frontal damage, including the ACC. This finding raises the question of whether the medial frontal lobes are in fact necessary for ToM function. Further research is required to clarify this controversy.
The aim of this study is to provide a preliminary investigation of the role of the ACC in: (1) motivational decision making, as assessed by the Iowa gambling task; (2) emotional processing, as assessed using an emotional expression recognition task; and (3) social cognition, as assessed using social situations, joke interpretation, and advanced ToM tasks. Three patients with medial frontal lobe lesions primarily involving the ACC were examined in an attempt to further our understanding of the role of the ACC in these functions.
METHODS
Patients
Three patients were investigated in the Neuropsychology Department of the National Hospital for Neurology and Neurosurgery. Two of these patients (patients 1 and 2) have been shown to have autonomic functioning deficits (Critchley et al., 2003).
Patient 1 is a 28-year-old, left-handed female who had completed 15 years of education, including five years at university level. She underwent removal of a right prefrontal glioma. Magnetic resonance imaging (MRI) revealed a unilateral right ACC lesion extending anteriorly toward the frontal pole (BA 32, 24), posteriorly to mid cingulate (BA 24) and inferiorly to involve the genual region (BA 32, 25 see Figure 1a). There was also involvement of the anterior corpus callosum, anterior superior frontal gyrus, some of the supplementary motor area (medial BA 8) and some frontal white matter. See Table 1 for a summary of her cognitive scores.
Figure 1.
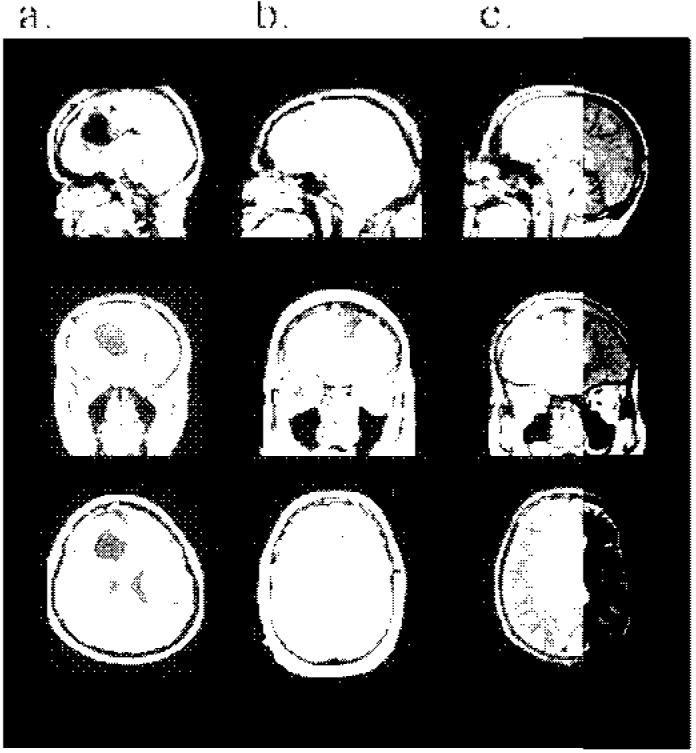
Sagittal, coronal and axial T1 weighted MRI images of the anterior cingulate cortex lesions of the three patients. (a) Patient 1, showing a postoperative unilateral right ACC lesion extending anteriorly toward the frontal pole (BA 32, 24), posteriorly to mid cingulate (BA 24), and inferiorly to involve the genual region (BA 32, 25). (b) Patient 2, showing a postoperative MRI of the location of surgical debulking of the bilateral tumour from a left dorsal approach. The tumour involved ACC bilaterally (BA 32, 24). (c) Patient 3, showing a haematoma involving the right ACC including dorsal and ventral regions (BA 32, 24) but sparing the subcallosal area (BA 25). (See http://www.fil.ion.ucl.ac.uk/-'hugo/ACCscans/SupplementaryACCscans.html for additional MRI slices showing the full extent of the lesion in each patient.)
TABLE 1.
Summary of neuropsychological tests scores
| Patient I | Patient 2 | Patient 3 | |
|---|---|---|---|
| WAIS-RVIQ | 113 | 99 | 118 |
| WAIS-RPIQ | 112 | 113 | 104 |
| RMT words (%ile) | 50 (>75th) | 49 (> 75th) | 49 (90th) |
| RMT faces (%ile) | 49 (= 95th) | 50 (> 95th) | 41(25th) |
| RCFT copy (%ile) | 28 (< 1st) | 35 (= 84th) | 30 (18th) |
| RCFT recall (%ile) | 26 (= 73rd) | 30 (> 96th) | 21(76th) |
| Story immediate recall (%ile)a | 33 (25-50th) | 35 (50-75th) | 39 (50-75th) |
| Story delayed recall (%)a | 33 (= 50th) | 33 (50-75th) | 31(25-50th) |
| List learning total (%ile)b | 63 (75-90th) | 52 (25-50th) | 29 (< 10th) |
| Delay (%ile)b | 15 (>90th) | 12 (50-75th) | 8 (10-25th) |
| GNT (%ile) | 25 (= 90th) | 21 (= 50th) | 27 (>90th) |
| Object Decision (5% cut-off) | 19 (>5%) | 19 (> 5%) | 15 (5th) |
| TMTA s (%ile) | 18 | 37 | 25 |
| (80-90th) | (20-30th) | (50-75th) | |
| Digit Symbol (ss) | 14 | 7 | 8 |
| MCST categories | 6 | 6 | 6 |
| Proverbs | 8/8 | 6/8 | 7/8 |
| Stroop colour/word (%ile) | 112 (100) | 94 (15-17th) | 83 (<6th) |
| TMTB (s) (%) | 44 (>90th) | 86 (25-50th) | 107 (10-25th) |
| Hayling overall (ss) | 7 | 3 | 4 |
| Brixton (ss) | 10 | 10 | 7 |
| FAS total (%ile) | 49 (66th) | 24 (4th) | 33 (16th) |
| CET | 8 (abn) | 1 (wnl) | 4 (wnl) |
| BADS Six elements (ps) | 4 | 3 | 3 |
WAIS-R = Wechsler Adult Intelligence Scale Revised (Wechsler, 1981). RMT = Recognition Memory Test (Warrington, 1984). RCFT = Rey Complex Figure Test (Rey, 1941).
= Story recall, 30 minute delay from Assessment of Memory and Information Processing Battery (AMIPB: Coughlan & Hollows, 1985).
= List Learning Task from AMIPB.
GNT = Graded Naming Test (McKenna & Warrington, 1980). Object Decision from the Visual Object and Space Perception Battery (Warrington & James, 1991). TMTA = Trail Making Test Part A (Army Individual Test Battery, 1944). MCST = Modified Card Sorting Test (Nelson, 1976). TMTB = Trail Making Test, Part B (Army Individual Test Battery, 1944). FAS = controlled oral word association test (Benton, Hamsher, & Sivan, 1976). CET = Cognitive Estimates Test (Shallice & Evans, 1978). BADS = Behavioural Assessment of the Dysexecutive Syndrome (Wilson, Alderman, Burgess, Emslie, & Evans, 1996). nt = not tested; %ile = percentile; wnl = within normal limit. s = seconds; ss = scaled score; ps = profile score; abn = abnormal.
Patient 2 is a 39-year-old right-handed male who had completed 13 years of education. He underwent partial resection of an oligodendroglioma. The tumour involved the ACC bilaterally, including all of the left ACC (BA 32, 24) and spread diffusely through the left medial and lateral superior frontal gyri, right medial gyrus (BA 8), the gyrus rectus and orbitofrontal cortex (BA 11, 12), the medial thalamus and hypothalamus, insula cortex (BA 44), parahippocampal gyrus, and left hippocampus. Diffuse changes were also noted in the superior temporal gyrus (BA 22) and anterior temporal pole (BA 38). On the right, the lesion involved genual and dorsal ACC (BA 32, 24), orbitofrontal cortex (BA 11), the subcallosal gyrus (BA 25) and anterior insula cortex (BA 44). While these changes were apparent on close radiological examination of MR images, Figure 1b highlights the location of surgical debulking from a left dorsal approach. See Table 1 for a summary of his cognitive scores.
Patient 3 is a 48-year-old right-handed male who had completed 12 years of education. He sustained a subarachnoid haemorrhage in May 2002 associated with vasospasm. He subsequently underwent coiling of a right anterior communicating artery aneurysm. MRI showed a haematoma involving the right ACC including dorsal and ventral regions (BA 32, 24) but sparing the subcallosal area (BA 25, see Figure 1c). In addition there was bilateral involvement of the corpus callosum and white matter changes in the territory of the anterior cerebral artery distribution, the latter due to vasospasm. See Table 1 for a summary of his cognitive scores.
In summary, in all patients there was marked vacuolar degeneration or surgical removal of genual regions of ACC (BA 32, 24). In patients 1 and 2 tumours originated in and spread from dorsal and genual ACC into neighbouring cortices as described. However, due to the diffuse infiltrating nature of gliomas, the functional impact of this spread is uncertain beyond the focus of ACC involvement.
Controls
For the decision making task, patient performance was compared to 18 age (X = 40 years, SD = 9 years, range = 3 1-54 years) and education-matched healthy controls (X= 17 years, SD = 2 years, range = 14-19 years). Patient performance on the emotional expression multimorph task was compared to 14 age (X = 38 years, SD = 11 years, range = 23-54 years) and education-matched healthy controls (X= 16 years, SD = 3 years, range = 10-19 years). Patient performance on the joke interpretation task was compared with eight age (X =29 years, SD = 1 year, range = 27-33 years) and education-matched healthy controls (X = 17 years, SD = 1 year, range = 15.5-18 years). Patient performance on the social situations and theory of mind tasks was compared with 15 age (X = 38.5 years, SD = 12 years, range = 24-60 years) and education-matched healthy controls (X = 15 years, SD = 3 years, range = 10-18 years).
Measures
Motivaional decision making task
A computerised version of the gambling task developed by Bechara and colleagues was used (Bechara, Tranel, & Damasio, 2000). This task aimed to simulate the decision made in real life in terms of reward, punishment, and the uncertainty of outcomes. Patients were presented with a total of 100 cards separated into four decks (A, B, C, and D) on a computer screen. Participants were required to select one card at a time from any of the four decks. Selecting a card from decks A or B resulted in a high reward (gain) but even higher punishment and if selected continuously results in a net loss. Selecting a card from decks C or D resulted in a low reward but even lower punishments and if played continuously resulted in a net gain. Participants have to learn to avoid the disadvantageous decks with the high reward but net loss decks (A and B) in favour of the low reward net gain decks (C and D). Card selections were recorded automatically on the computer.
Emotional facial expression multimorph task
A version of the emotional expression multimorph task was administered (Blair, Colledge, Murray, & Mitchell, 2001). The stimuli used are six different basic emotional facial expressions (happiness, sadness, anger, disgust, fear, and surprise) taken from the empirically validated Pictures of Facial Affect Series (Ekman & Friesen, 1976). Twenty-one morphed photographic images were used for each continuum. These were prepared by blending a prototypical expression (100% expression) in varying proportions with a neutral expression (0% expression). Participants viewed the computer screen as the neutral face gradually morphed through twenty 5% increment stages into one of six prototypical expressions. They were provided with a choice of the six emotions and asked to watch the expressions change and say out loud which emotion they thought was being shown as soon as they recognised it. Following a practise phase consisting of each of the six expressions, 18 test stimuli were presented in random order. The task was performed twice and each emotion presented six times. Accuracy of response and the number of stages required for successful facial expression recognition were recorded on the computer.
The task was scored according to a procedure described by Blair et al. (2001). Participant responses were scored according to the number of stages that were required before successful expression recognition. Each stage was converted into a score between 1 and 20, where one indicates successful recognition of the prototypical expression (stage 20) and 20 indicates recognition of a 5% morphed image (stage 1). A failure to recognise the emotion scored zero. A mean expression recognition score and the mean number of errors of recognition was obtained by collapsing the score for the six trials of each emotion.
Social cognition tasks
A modified battery of the social cognition tasks developed by Blair and Cipolotti (2000) was administered.
Social situations task
This task assessed the ability to judge the appropriateness of behaviours in different social contexts. Each patient read 20 short stories of social situations incorporating behaviour that was either normative or a violation. At various points in each story (marked with brackets, see Appendix 1), the subject was required to judge the appropriateness of behaviours, giving a score from A to D. “A” scores meant that the behaviour had been judged as normative. “B” to “D” scores meant that the behaviour was judged as a violation and indexed the extent of the violation (where “B” scores were mild and “D” scores were serious violations). In total, there were 17 normative situations and 20 violations. Three scores were generated. The first two indicate the number of normative situations (a maximum of 17) and violations (maximum of 20) correctly identified. The third refers the extent to which the subject judged the violations to be socially inappropriate. For each situation, the subject obtained a score of 0 to 3, matching their response of “A” to “D” (where “A” = 0, “B” = 1, “C” = 2 and “D” = 3). An example of a social situation story is presented in Appendix 1.
Joke interpretaion
This task assessed interpretation of humour. Each participant was presented with 15 single frame cartoons and asked to state whether the scenario was amusing and why. Responses were scored as correct if the answer referred directly to the thoughts, feelings and dispositions of the characters in the story (Channon & Crawford, 2000). For an example of one joke used see Appendix 2.
Advanced theory of mind (ToM) task
This task assessed the ability to represent the internal mental state of others. The patients were read 15 stories of naturalistic social situations and asked to interpret and justify the behaviour of the main protagonist. Three scores were derived from the subject's response. The first indexed the comprehension of the social situation. The other scores refer to the justification the subject used when interpreting the story characters' behaviour (i.e., reference to either the character's mental states or physical information). An example of a ToM story, with mental and physical justifications is shown in Appendix 3.
Analysis
Patient scores above or below two standard deviations from the control mean were considered as indicating significance.
RESULTS
Motivational decision making task
The performance of patients and controls on the decision making task is shown in Table 2. The number of selections from each deck (A, B, C, and D) is shown. There was no significant difference between the patients and controls on this task. However, patient 3 showed a tendency to select more cards from deck D (disadvantageous) than controls, while patient 2 showed a tendency to select less cards from deck B than controls (see Table 2).
TABLE 2.
Decision making task
| Deck | Patient 1 | Patient 2 | Patient 3 | Controls (N = 18) |
|---|---|---|---|---|
| Mean (SD) | ||||
| A (−) | 12 | 24 | 21 | 17.28 (4.69) |
| B (−) | 23 | 16 | 36 | 32.50 (8.64) |
| C (+) | 25 | 31 | 28 | 19.110 (6.73) |
| D (+) | 40 | 29 | 15 | 30.0 (8.46) |
−, disadvantageous, net loss; +, advantageous deck, net gain.
Emotional facial expression multimorph task
Performance of patients and controls on the emotional facial expression multimorph task is displayed in Table 3. The mean number of stages until recognition and the mean number of errors are shown. Patient 1's performance did not significantly differ from controls across all emotions. However, there was a nonsignificant trend for her to require more stages to recognise fear, happiness, and surprise than controls (see Table 3). Patient 2 made more errors than controls in recognising surprise and there was a trend for him to require more stages than controls to recognise this emotion but this did not reach statistical significance (see Table 3). Patient 3 demonstrated a selective difficulty in recognising fear compared with controls. He required significantly more stages to recognise fear and also made significantly more errors than controls. He also made more errors in recognising disgust and there was a nonsignificant trend for him to require more stages to recognise this emotion (see Table 3).
TABLE 3.
Emotional facial expression multimorph task
| Emotion | Patient 1 | Patient 2 | Patient 3 | Controls (N = 14) |
|
|---|---|---|---|---|---|
| Mean (SD) | |||||
| Fear | |||||
| Mean ERS | 6.67 | 8 | 3.83a | 9.53 (1.92) | |
| Mean errors | 1 | 1 | 4a | 0.86 (0.95) | |
| Anger | |||||
| Mean ERS | 7.12 | 9.67 | 10.67 | 9.15 (2.09) | |
| Mean errors | 0 | 0 | 0 | 0.64 (0.93) | |
| Happiness | |||||
| Mean ERS | 10.33 | 12 | 14.33 | 12.88 (1.52) | |
| Mean errors | 0 | 0 | 0 | 0 | |
| Surprise | |||||
| Mean ERS | 9.67 | 6.67 | 10.33 | 10.68 (2.16) | |
| Mean errors | 0 | 2a | 0 | 0.14 (0.53) | |
| Disgust | |||||
| Mean ERS | 7.67 | 8.5 | 5.5 | 9.16 (3.21) | |
| Mean errors | 1 | 1 | 3 | 0.86 (1.56) | |
| Sadness | |||||
| Mean ERS | 8 | 9.67 | 9.83 | 8.49 (2.98) | |
| Mean errors | 0 | 0 | 0 | 1.07 (1.33) | |
Note: Higher score = earlier recognition of emotion.
± 2 standard deviations (SDs) from the control mean. ERS, expression recognition score.
Social cognition tasks
Social situations task
Performance of patients and controls on the social situations task is shown in Table 4. There was no significant difference between patients and controls on this task. However, there was a trend for the patients' judgements of appropriateness to be less severe than that of the controls. It is noteworthy that the controls show a large standard deviation for appropriateness judgements. The lack of significant difference between controls and patients may be due to this.
TABLE 4.
Social situations task
| Variable | Patient 1 | Patient 2 | Patient 3 | Controls (N= 15) | |
|---|---|---|---|---|---|
|
|
|||||
| Preop | Postop | Postop | Mean (SD) | ||
| Normative (max = 17) | 16 | 16 | 13 | 14 | 14.73 (1.58) |
| Violations(max=20) | 18 | 16 | 19 | 16 | 18.13(1.46) |
| Appropriate (max = 60) | 27 | 22 | 29 | 23 | 34.80 (7.49) |
Preop, preoperative; Postop, postoperative; Normative, number of normative situations identified; Violations, number of violations identified; Appropriate, total appropriateness score.
Joke interpretaion
Performance on the joke interpretation test is shown in Table 5. Performance of patient 1 and 3 was in keeping with controls. In contrast, Patient 2's interpretation ofjokes was poor. Similar to his performance on the ToM task, he tended to use physical interpretation rather than mental state interpretations (e.g., “he's tried to open the door and it's fallen over”, see Appendix 2).
TABLE 5.
Joke interpretation and theory of mind tasks
| Task | Patient I |
Patient 2 |
Patient 3 | Controls |
||
|---|---|---|---|---|---|---|
| Preop | Postop | Postop | Mean (SD) | |||
| Jokes | N= 8 | |||||
| (max = 15) | 12 | 12 | 7a | 14 | 13.14 (1.21) | |
| Theory of mind | N = 15 | |||||
| Comp total (max = 15) |
15 | 15 | 13 | 13 | 13.93 (0.26) | |
| Physical | 3 | 2 | 7a | 1 | 2.0 (1.13) | |
| Mental | 12 | 13 | 8a | 14 | 11.93 (1.09) | |
± 2 standard deviations from the control mean.
Preop, preoperative; Postop, postoperative; Comp total, comprehension total score; Physical, total physical state justifications; Mental, total mental state justifications.
Advanced theory of mind (ToM) task
Patients' and controls' performance on the advanced ToM task is shown in Table 5. None of the patients showed any difficulty with the control question, demonstrating that the ability to understand the story was intact. Performance of patients 1 and 3 was commensurate with controls. In contrast, patient 2's performance was impaired. Noticeably, he used a significantly higher number of physical justifications and fewer mental state justifications (see Table 5).
CONCLUSIONS
This study has provides preliminary evidence regarding the role of the ACC in social and emotional processing by investigating three patients with lesions primarily involving the ACC. The patients' performance on tasks assessing motivational decision making, emotional expression recognition, and social cognition including theory of mind, and the significance of these findings are discussed in turn.
Motivational decision making
The performance of the patients on the gambling task did not significantly differ from that of controls. This suggests that the ACC may not play a critical role in the mediation of motivational decision making. Previous neuroimaging studies have demonstrated ACC activation during performance of decision making tasks (e.g., Bush et al., 2002; Elliott, Rees, & Dolan, 1999; Paulus et al., 2002), including the gambling task (Ernst et al., 2002; Rogers et al., 2004). Previous lesion studies have highlighted the role of the ventromedial cortex, but have made no reference to the ACC. Two recent lesion studies have found that damage to the cingulate gyrus (Manes et al., 2002) and middle prefrontal cortex (Clark et al., 2003) impairs gambling task performance. This is the first study to date that has examined gambling task performance in patients with ACC lesions. Our findings suggest that the ACC is not critically involved in this function. Interestingly, our findings are in keeping with those of Hornak, O'Doherty, Bramham, Rolls, Morris, Bullock, et al. (2004) who recently reported that patients with unilateral medial prefrontal lesions including the ACC were not impaired on a reversal learning task involving the reward or loss of imaginary money. In contrast, a reversal learning impairment was evident in patients with bilateral orbital/medial prefrontal lesions. Although patient 2 in our series had bilateral orbitofrontal and ACC damage, he did not show impaired performance on the gambling task. This may be due to task differences, and further research is required to explore this function in patients with focal frontal lesions.
Emotional processing
Only one of our three patients (patient 3) showed a selective impairment on the emotional facial expression multimorph task. Specifically, patient 3 had a selective difficulty in recognising fear. In addition, he also made more errors in recognising disgust and there was a nonsignificant trend for him to require more stages to recognise this emotion. Patient 2 made more errors than controls in recognising surprise and there was a trend for him to require more stages than controls to recognise this emotion. The performance of patient 1 was in keeping with controls, although there was a nonsignificant trend for her to require more stages to recognise fear, happiness and, surprise.
These findings are in accordance with previous neuroimaging and neuropsychological research which suggests that the ACC plays a role in emotional processing, although it does not mediate a specific emotion. As discussed in the introduction, ACC lesions have been associated with a range of difficulties in emotional processing. Our finding that fear recognition was impaired in one patient supports previous neuroimaging and lesion studies. In particular, activation of the ACC in fear processing has been documented in several neuroimaging studies (e.g., Morris et al., 1998; Pissiota, Frans, Michelgard, Appel, Langstrom, Flaten, et al., 2003). A recent neuropsychological study reported impaired processing of both fear and surprise in two patients with orbitofrontal damage including the ACC (Hornak et al., 2003). In a recent fMRI study, surprised facial expression were shown to activate the amygdale and ventral medial prefrontal cortex, including the ACC (BA 32), especially when associated with uncertain valence (Kim, Somerville, Johnstone, Alexander, & Whalen, 2003). This was taken to support the view that the medial prefrontal cortex is sensitive to both the magnitude of valenced outcome and in mediating advantageous choices (e.g., Bechara, Damasio, Damasio, & Lee, 1999; O'Doherty, Kringelback, Rolls, Hornak, & Andrews, 2001). Overall, these findings suggest that the ACC may play a role in the processing of a range of emotional expressions, particularly suprise and fear. Further research is required to explore the ACC's role in this function.
Social cognition
Social situations
There was no significant difference between patients and controls on the social situations task. However, there was a trend for the patients' judgements of appropriateness to be less severe than that of the controls. It is noteworthy that the controls show a large standard deviation for appropriateness judgements. The lack of significant difference between controls and patients may be due to this. Previous studies have found impaired performance on this task in patients with orbitofrontal damage (e.g., Blair & Cipolotti, 2000). Our findings suggest that the ACC is not crucial for intact performance on this task. More extensive damage to orbitofrontal regions may be required before impairments are evident on this task.
Theory of mind
To the best of our knowledge, this is the first study to date to examine ToM function in patients with lesions primarily involving the ACC. ToM impairment was evident only in patient 2, with a bilateral ACC lesion. He performed poorly on two tests used to measure theory of mind, namely the advanced ToM and joke interpretation tasks. In particular, he tended to use physical rather than mental interpretations on both these tasks. In contrast, the performance of Patient 1 and 3 on the advanced ToM and joke interpretation was in keeping with controls. Both of these patients had unilateral right ACC lesions. Patient 2's impairment in ToM tasks can not be accounted for by a more generalised executive deficit. Indeed, patient 2 failed only 3 out of 10 executive tests, in keeping with patient 3 who did not show a ToM impairment. This evidence is in keeping with recent literature indicating a dissociation between ToM and executive functioning (e.g., Fine, Lumsden, & Blair, 2001; Lough, Gregory, & Hodges, 2001; Rowe et al., 2001).
Previous neuroimaging studies have identified the anterior paracingulate cortex, typically considered part of the ACC, as a critical structure for mentalising (e.g., Baron Cohen et al., 1999; Fletcher et al., 1995; Gallagher & Frith, 2003; Shallice, 2001; Vogeley et al., 2001; Wicker et al., 2003a). Lesion studies, however, have been less definitive in providing a distinct neuroanatomical correlate (e.g., Rowe et al., 2001; Stuss et al., 2001). Indeed, a recent study by Bird et al. (2004) raises the question of whether the medial frontal cortex is necessary for ToM. In our study we have provided evidence that focal lesions of the right ACC are not sufficient to disrupt ToM functioning. Only bilateral damage to the ACC, more prominent on the left was found to be associated with a ToM deficit. We can offer at least two explanations or these findings. First, we can suggest that the left ACC may be more critical than the right in mentalising. Our two patients with selective right ACC lesions showed no deficit on ToM tasks. However, this conclusion can be regarded only as tentative as the current neuroimaging and neuropsychological literature regarding a possible laterality effect in ToM is inconclusive. For example, Calarge, Andreason, and O'Leary (2003) found greater activation in the left frontal lobe including the left ACC in a PET study of ToM function. Vogeley et al. (2001) specifically emphasised the role of the right ACC and Gallagher et al. (2002) found bilateral anterior paracingulate activation. Neuropsychological studies have emphasised the role of the left frontal lobe in mentalising (e.g., Channon & Crawford, 2000), although no specific reference to the ACC was made. Some studies have found greater ToM impairment patients with right frontal lesions (Shamay-Tsoory et al., 2003; Stuss et al., 2001) or no laterality effect (Rowe et al., 2001). Interestingly, Bird et al. (2004) found intact ToM in a patient with bilateral medial frontal damage that included the rostral and ventral areas of the ACC, but spared the dorsal regions bilaterally. Clearly, further research is required to explore a potential laterality effect in the mediation of ToM.
Second, we can suggest that the ACC plays a role in mentalising only when there is additional damage to other components of the ToM network, specifically the temporal lobe. Accumulated evidence from neuroimaging studies suggests that the neural system underlying ToM is widely distributed. Although the medial frontal regions have received much research attention, the temporal poles and the posterior superior temporal sulcus have also been implicated (Frith & Frith, 2003; Gallagher & Frith, 2003; Samson, Aperly, Chiavarino, & Humphreys, 2004; Siegal & Varley, 2002). For example, left temporal activation has been demonstrated during ToM task performance in autistic (e.g., Frith, 2001) and healthy participants (e.g., Goel, Grafman, Sadato, & Hallett, 1995; Vogeley et al., 2001). A recent study by Samson et al. (2004) found impaired false belief reasoning in three patients with lesions of the left temporoparietal junction. Our patient with a mentalising deficit had not only a bilateral ACC lesion more prominent on the left but also a lesion involving the left temporal lobe. In summary, the intact performance of patients 1 and 3 on ToM tasks suggests that a unilateral right ACC lesion is not sufficient to disrupt ToM. It is only in the face of more extensive damage to the neural network underlying ToM that impairments are manifested, as in the case of patient 2.
In summary, we have provided a preliminary exploration of social and emotional functions in three patients with medial frontal lobe lesions involving the ACC. Our findings suggest that first, the ACC does not appear to be critically involved in motivational decision making, as assessed by the Iowa gambling task, or social situation processing. Second, the ACC plays a role in processing specific emotions, particularly fear. Third, unilateral damage to the right ACC is not sufficient to disrupt ToM. It appears that impairments are only evident in the face of more extensive damage to the neural network underlying this function.
APPENDIX 1
Example of a story from the Social Situations task
Scale used:
Fairly normal behaviour in that situation (A)
Rather strange behaviour in that situation (B)
Very eccentric behaviour in that situation (C)
Shocking behaviour in that situation (D)
Keith, aged 25, was a file clerk who worked in an office in the city. At noon he took his lunch to a small park and sat on a sunny bench to eat. Often he tore part of a sandwich into bits, scattering it on the groundfor pigeons (). One day when he came to his favourite bench a baby carriage was parked beside it. Keith noticed that a young woman was swinging an older child nearby. The baby in the carriage began to cry but the mother did not hear this because the swing was creaking. Now, Keith had learnt that when his baby nephew screamed, sometimes this meant that a pin in his nappy had opened. Rather than bother the mother in the park, Keith quickly checked the baby's clothing to see whether he could feel an open pin ().
APPENDIX 2
Example of a joke from the joke interpretation task
APPENDIX 3
Example of an advanced theory of mind story with physical and mental justifications
Simon is a big liar. Simon's brother Jim knows this, he knows that Simon never tells the truth! Now yesterday Simon stole Jim's ping-pong bat, and Jim knows that Simon has hidden it somewhere, though he can't find it. He's very cross. So he finds Simon and he says, ‘Where is my ping-pong bat? You must have hidden it either in the cupboard or under your bed, because I've looked everywhere else. Where is it, in the cupboard or under your bed?’ Simon tells him that the bat is under the bed.
The participant is asked:
Qi. Was it true, what Simon told Jim? (comprehension question)
Q2. Where will Jim look for his ping-pong bat?
Q3. Why will Jim look there for his bat?
An example of a mental states justification for this story is ‘Because Jim knows that Simon lies so he should look in the other location’. An example of a justification involving physical information is “Because it will be in the opposite place to wherever Simon says’.
Contributor Information
Tim Shallice, Institute of Cognitive Neuroscience, London, UK.
Lisa Cipolotti, Department of Neuropsychology, National Hospital for Neurology and Neurosurgery, London, UK.
REFERENCES
- Army Individual Test Battery . Manual and directions for scoring. War Department, Adjutant General's Office; Washington, DC: 1944. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social intelligence in the normal and autistic brain: An fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd ed. AJA Associates; Iowa City, IA: 1976. [Google Scholar]
- Bird C, Castelli F, Malik O, Frith U, Husain M. The impact of extensive medial frontal lobe damage on ‘Theory of Mind’ and cognition. Brain. 2004;127:914–928. doi: 10.1093/brain/awh108. [DOI] [PubMed] [Google Scholar]
- Blair RJK, Cipolotti L. Impaired response reversal: A case of ‘acquired sociopathy’. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJK, Morris JF, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expression of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopolous P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol (Berl) 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biological Psychiatry. 2001;50:171–78. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Science. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: A role in reward based decision making. Proceedings of the National Academy of Sciences USA. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge C, Andreasen NC, O'Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. American Journal of Psychiatry. 2003;160:1954–1964. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- Channon S, Crawford A. The effects of anterior lesions on performance on a story comprehension test: Let anterior impairment on a theory of mind-type task. Neuropsychologia. 2000;38:1006–1017. doi: 10.1016/s0028-3932(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision making impairments following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. 2003. [DOI] [PubMed] [Google Scholar]
- Coughlan AK, Hollows AK. The Adult Memory and Information Processing Battery (AMIPB) A. K. Coughlan, St. James's University Hospital; Leeds, UK: 1985. [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, et al. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Fletcher P, Morris J, Kapur N, Deakin JF, Frith CD. Neural activation during covert processing of positive emotional facial expressions. Neuroimage. 1996;4:194–200. doi: 10.1006/nimg.1996.0070. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Elliott R, Dolan RJ. Activation of different anterior cingulate foci in association with hypothesis testing and response selection. Neuroimage. 1998;8:17–29. doi: 10.1006/nimg.1998.0344. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rees G, Dolan RJ. Ventromedial prefrontal cortex mediates guessing. Neuropsychologia. 1999;37:403–411. doi: 10.1016/s0028-3932(98)00107-9. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal ablation: Patient EVR. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. Decision making in a risk taking task: A PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind’ and executive functions in a patient with early left amygdala damage. Brain. 2001;124:287–298. doi: 10.1093/brain/124.2.287. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, et al. Other minds in the brain: A functional imaging study of ‘theory of mind’ in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;20:969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalising. Philosophical Transactions of the Royal Society London. B: Biological Sciences. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H, Frith C. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher H, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance. Neuroimage. 2002;16(1):847–82. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato M, Hallett M. Modeling others minds. Neuroreport. 1995;6:1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Gundel H, O'Connor MF, Littrell L, Fort C, Lane RD. Functional neuroanatomy of grief: An FMRI study. 2003. American Journal of Psychiatry. 2003;160:1946–53. doi: 10.1176/appi.ajp.160.11.1946. [DOI] [PubMed] [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–78. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression changes in patients with emotional and behavior changes following ventral frontal damage. Neuropsychologia. 1996;34:274–261. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–1712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone J, Alexander AL, Whalen PJ. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport. 2003;14:2317–2322. doi: 10.1097/00001756-200312190-00006. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, et al. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997;35:1437–44. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lough S, Gregory C, Hodges JR. Dissociation of social cognition and executive function in frontal variant frontotemporal dementia. Neurocase. 2001;7:123–130. doi: 10.1093/neucas/7.2.123. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mazars G. Criteria for identifying cingulate epilepsies. Epilepsia. 1970;11:41–47. doi: 10.1111/j.1528-1157.1970.tb03865.x. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two person reciprocal exchange. Proceedings of the National Academy of Sciences USA. 2001;98:11832–11835. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna P, Warrington EK. Graded Naming Test. NFER-Nelson Publishing Co. Ltd.; Windsor, UK: 1983. [Google Scholar]
- Meyer G, McElhaney M, Martin W, McGraw CP. Stereotactic cingulotomy with results of acute stimulation and serial psychological testing. In: Laitinen L,V, Livingston KE, editors. Surgical approaches in psychiatry. MPT; Lancaster, UK: 1973. pp. 39–58. [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Nelson HE. A modified card sorting test sensitive to frontal lobe deficits. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelback ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG. Error rate and outcome predictability affect neural activation in prefrontal cortex and anterior cingulate during decision making. Neuroimage. 2002;15:836–846. doi: 10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. Proceedings of the Royal Society of London. B: Biological Sciences. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA, et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. European Journal of Neuroscience. 2003;18:1325–1331. doi: 10.1046/j.1460-9568.2003.02855.x. [DOI] [PubMed] [Google Scholar]
- Rey A. L 'examen clinique en psychologie. 2nd ed. Presses universitaires de France; Paris: 1964. [Google Scholar]
- Rogers RD, Owen AM, Middleton HH, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large unlikely rewards activated inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;19:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision making cognition. Biological Psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe A, Bullock PR, Polkey CE, Morris RG. ‘Theory of mind’ impairments and their relationship to executive functioning following frontal lobe excisions. Brain. 2001;124:600–616. doi: 10.1093/brain/124.3.600. [DOI] [PubMed] [Google Scholar]
- Samson D, Aperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else's belief. Nature Neuroscience. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Shallice T. ‘Theory of mind’ and the prefrontal cortex. Brain. 2001;124:247–248. doi: 10.1093/brain/124.2.247. [DOI] [PubMed] [Google Scholar]
- Shallice T, Evans M. The involvement of the frontal lobes in cognitive estimation. Cortex. 1978;14:294–303. doi: 10.1016/s0010-9452(78)80055-0. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S, Tomer R, Berger BD, Aharon-Peretz J. Characterisation of empathy deficits following prefrontal brain damage: The role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15:324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Siegal M, Varley R. Neural systems involved in ‘theory of mind’. Nature Review Neuroscience. 2002;3:463–471. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neurosciences. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup GG, Jr., Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124:279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg AL. Asymmetric functional role of right and left ventromedial prefrontal cortices in social conduct, decision making and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, et al. Mind reading: Neural mechanisms of theory of mind and self-perspective. NeuroImage. 2001;14:170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Recognition Memory Test. NFER-Nelson Publishing Co. Ltd.; Windsor, UK: 1984. [Google Scholar]
- Warrington EK, James M. The Visual Object and Space Perception Battery. Thames Valley Test Co.; Bury St Edmunds, UK: 1991. [Google Scholar]
- Wechsler DA. Wechsler Adult Intelligence Test—Revised. The Psychological Corporation; London: 1981. [Google Scholar]
- Wicker B, Perrett DI, Baron-Cohen S, Decety J. Being the target of another's emotion: A PET study. Neuropsychologia. 2003a;41:139–146. doi: 10.1016/s0028-3932(02)00144-6. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron. 2003b;3:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. Behavioural Assessment of the Dysexecutive Syndrome (BADS) Thames Valley Test Co.; Bury St Edmunds, UK: 1996. [Google Scholar]