Abstract
Standardized, automated ligand binding assays facilitate evaluation of endocrine activities of environmental chemicals and identification of antagonists of nuclear receptor ligands. Many current assays rely on fluorescently labeled ligands which are significantly different from the native ligands. We describe a radiolabeled ligand competition scintillation proximity assay (SPA) for the androgen receptor (AR) using Ni-coated 384-well FlashPlates® and liganded AR-LBD protein. This highly reproducible, low cost assay is well-suited for automated HTS. Additionally, we show that this assay can be adapted to measure ligand affinities for other nuclear receptors (peroxisome proliferation activated receptor γ, thyroid receptors α and β).
Keywords: Scintillation Proximity Assay, androgen receptor, high-throughput screening, endocrine disrupting chemicals, nuclear receptors
INTRODUCTION
The androgen receptor (AR) mediates androgen functions, including maintenance of male secondary sexual characteristics and development of the prostate gland. Like other nuclear hormone receptors (NRs), AR is a transcription factor that becomes active upon binding to its natural ligand, dihydrotestosterone (DHT).1 Small molecules that inhibit ligand binding can modulate gene transcription regulated by AR. Environmental exposure to antiandrogens, such as DDT, can cause developmental abnormalities.2 On the other hand, antiandrogens (flutamide, bicalutamide) currently used to cure prostate cancer present side effects and drug resistance has been observed with these treatments which therefore provides a compelling need to discover new antiandrogens.
High-throughput screening (HTS) techniques are attractive for both of these needs. Two classes of AR assays have been developed: 1) cell-based transcription assays measuring the inhibition of AR transcriptional activity by small molecules and 2) biochemical competition assays measuring blockade of ligand binding AR by small molecules. Historically, biochemical assays have been limited by the lack of necessary amounts of pure and functional AR protein whose purification is complicated by low solubility and instability in the absence of androgen3–4. Utilizing a His6-tagged AR-LBD (Ligand Binding Domain) expressed in E. coli in the presence of DHT can overcome these problems.5
While measuring ligand binding by fluorescence polarization (FP) with commercially available fluorescently labeled ligands has become popular, this technique shows limitations in HTS.6 Both interference with the emission signal from the fluorescent ligand by tested compounds and perturbation of ligand binding and protein function by the fluorescent ligand can be problems. For a robust and broadly applicable biochemical method, radioligands are superior as they more closely mimic the natural ligand. However radioligands carry with them issues relating to safety and waste disposal. Among radiolabeled ligand binding assays developed for NRs, only scintillation proximity assays (SPAs) are truly HTS compatible.7–9 So far, few radiolabeled ligand binding assays have been described in the 96-well format for AR.10–11
Herein we report an AR ligand competition binding assay using SPA 384-well FlashPlates® and liganded AR-LBD protein expressed in E. coli. Additionally, we show that this assay can be used to measure ligand affinities for other NRs including the peroxisome proliferation activated receptor (PPARγ) and the thyroid receptors (TRα and TRβ).
MATERIALS AND METHODS
Materials
Chemicals and materials were purchased from vendors and used without purification: [1,2,4,5,6,7-3H(N)]-5α-Androstan-17β-ol-3-one ([3H]-DHT) (110 Ci/mmol) and [125I]-T3 (779 Ci/mmol) (PerkinElmer, Boston, MA); [3H]-Rosiglitazone (ARC, St. Louis, MO) (50 Ci/mmol); Uncoated 96-well polypropylene (3359) and 384-well polystyrene (3573) microplates (Corning Life Sciences, Acton, MA); 384 Ni-chelate HTS PLUS Flashplates® (PerkinElmer, Boston, MA).
Expression and Purification of Proteins
cAR-LBD (His6; residues 663-919) was expressed in E. Coli and purified in the presence of DHT using a modified version of published protocols.5 Briefly, (pKBU553) was transformed into OneShot BL21 Star (DE3) E. coli (Invitrogen) and streaked onto a LB agar Carbenicillin (100 μg/ml) plate. A single colony from this plate inoculated a seed culture (overnight, 37°C). 2 L of 2x LB + 1x Carbenicillin and 10 μM DHT were seeded at 0.1 OD and grown at 25°C with shaking until OD reached 0.6–0.8. Expression was induced with 60 μM (final concentration) isopropyl-β-D-thiogalactoside, and cultures were left to grow 14–16 h at 17°C. Cells were pelleted (20 min, 5000 g), transferred into a 50 mL conical tube, flash frozen (liquid N2), and stored at −80°C. To purify AR, cells were thawed at 4°C and resuspended in 30 mL of freshly prepared buffer 1 (50 mM Tris pH 7.5, 150 mM NaCl, 10 μM DHT, 0.1 mM phenylmethylsulfonyl fluoride, 10 mg/L Lysozyme, and Roche Complete EDTA free protease inhibitor cocktail tablet). Cells were lysed by sonication (4°C, 6 x 2 min cycles with 2 min breaks, 30% amplitude, Branson Digital Sonifier) and clarified by ultracentrifugation (2 x 30 min; 100,000 g; 4°C). Talon resin (1 ml per liter cell culture) was add to a 50 ml conical tube and washed twice with 15 ml freshly prepared buffer 2 (50 mM NaPO4 pH 8.0, 300 mM NaCl, 10% glycerol, 0.2 mM TCEP, 0.1 mM PMSF, 2 μM DHT). The protein supernatant was added to Talon resin (40 ml of supernatant for each conical tube) and rotated gently overnight at 4°C. The resin was pelleted by centrifuging for 20 min followed by washing five times with 10 ml buffer 3 (50 mM NaPO4 pH 8.0, 300 mM NaCl, 10% glycerol, 0.2 mM TCEP, 0.1 mM PMSF, 2 μM DHT, 10 mM imidazole). Additionally, resin was washed five times with 10 ml buffer 4 (50 mM NaPO4 pH 8.0, 300 mM NaCl, 10% glycerol, 0.2 mM TCEP, 0.1 mM PMSF, 2 μM DHT, 10 mM imidazole, 2 mM ATP, 10 mM MgCl2). Elution was carried out in fractions equal to or less then bed volume using buffer 5 (50 mM NaPO4 pH 8.0, 300 mM NaCl, 10% glycerol, 0.2 mM TCEP, 0.1 mM PMSF, 2 μM DHT, 250 mM imidazole, 100 mM KCl). Protein purity (>90 %) was assessed by SDS-PAGE and analytical size exclusion FPLC. Protein concentrations were measured by Bradford and BCA protein assays. Usually 6–8 mg of protein per liter of cell culture were obtained. The protein was dialyzed overnight against buffer 6 (50 mM HEPES pH 7.2, 150 mM Li2SO4, 10% glycerol, 0.2 mM TCEP, 20 μM DHT) and stored at −80°C in buffer 6.
hPPARγ was expressed and purified following the procedure above using the following modifications. Cultures were grown up and induced at 22°C for the same amount of time as above. Induction was obtained with 500 μM of isopropyl-β-D-thiogalactoside. Buffer 1 contained 20 mM Tris pH 7.5, 100 mM NaCl, 0.5 mM PMSF, 0.5% Triton X-100, and 10 mg/L Lysozyme. Buffer 2 contained 20 mM Tris pH 7.5, 100 mM NaCl, 1 mM imidazole, and 5 mM DTT. Buffer 3 contained 20 mM Tris pH 7.5, 100 mM NaCl, 5 mM DTT, and 1 mM imidazole and was used to wash the beads seven times instead of five. Buffer 4 was not necessary in the purification of hPPARγ. Buffer 5 contained 20 mM Tris pH 7.5, 100 mM NaCl, 5 mM DTT, and 250 mM imidazole. Buffer 6 contained 50 mM Tris pH 8.0, 25 mM KCl, 2 mM DTT, and 10% glycerol. PPARγ does not require any ligand to remain stable in buffer 6. The average yield was 15 mg per liter of cell culture.
hTRα and hTRβ were prepared using a published procedure.12
SPA Ligand Competition Binding Assay
All liquid handling was carried out using an automated liquid handling system (Biomek FX). To each well of a 384-well Ni-chelate coated Flashplate® (PerkinElmer) was added 50 μl of 5 μM NR-LBD in assay buffer. After 30–60 minute incubation the protein solution was discarded (followed eventually by washes with assay buffer). 25 μl of serial diluted small molecules in assay buffer containing 10% DMSO were added into each well followed by addition of 25 μl of a radioligand solution in assay buffer. The final assay solution contained 5% DMSO. The plates were sealed with clear tape (Millipore® tape multiscreen) and allowed to equilibrate for 1–24 hours at room temperature or 4°C. Radiocounts were measured using a TopCount Microplate Scintillation and Luminescence Counter (Packard Instrument Company). All data were analyzed using GraphPad Prism 4.03 (GraphPad Software, San Diego, CA); IC50 values were obtained by fitting data to equation (Sigmoidal dose-response (variable slope)): y = Bottom + (Top-Bottom)/(1+10^((LogIC50–x)*Hillslope)); x is the logarithm of concentration; y is the response. Two independent experiments, in triplicates, were carried out for each compound.
In case of AR binding assay, [3H]-DHT was used at a final concentration of 20 nM and the assay buffer contained 50 mM HEPES, 150 mM Li2SO4, 0.2 mM TCEP, 10% glycerol, 0.01% Triton X-100, pH 7.2. In case of hPPARγ assay, [3H]-Rosiglitazone was at 40 nM and the assay buffer contained 50 mM Tris pH 8.0, 25 mM KCl, 2 mM DTT, 10% glycerol, 0.01% Triton X-100, pH 7.2. In case of hTR assays, [125I]-T3 was at 1 nM and the assay buffer contained 50 mM HEPES, 100 mM NaCl, 1 mM DTT, 0.1% BSA, 10% glycerol, 0.01% Triton X-100, pH 7.2.
RESULTS AND DISCUSSION
Optimization of an AR SPA ligand competition assay
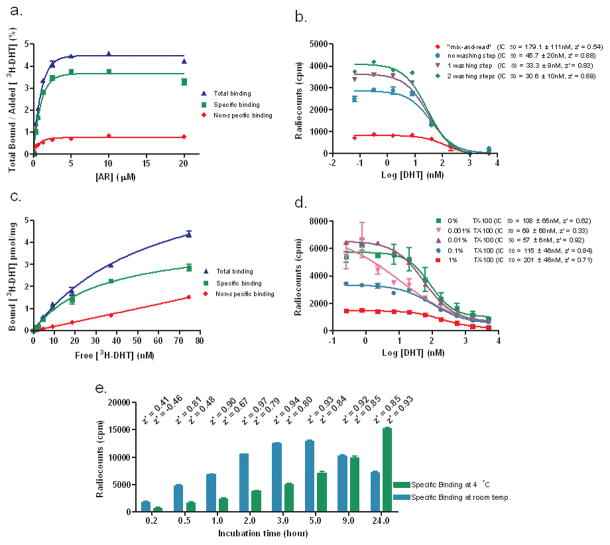
A number of assay parameters were optimized. First, we measured total binding (protein and radioligand), non-specific binding (NSB: protein, radioligand and excess of unlabeled ligand) and calculated specific binding (total – NSB) for different protein concentrations (Fig. 1a). A concentration of 20 nM [3H]-DHT was necessary to minimize background (data not shown). The percentage of bound [3H]-DHT in relationship with the input was low, saturating at 4.5%. Based on this result we used an AR concentration of 5 μM.
Figure 1.
Optimization of an AR SPA ligand competition assay. (a). Measurements of total binding (AR + 20 nM [3H]-DHT), non-specific binding (NSB: AR + 20 nM [3H]-DHT + 5 μM DHT) and specific binding (SB = total – NSB) for different AR concentrations after 2 washes. (b) Effect of washes: SB was measured for experiments carried out with 5 μM AR, serially diluted DHT in the presence of 20 nM [3H]-DHT. (c) Saturation binding plot: Measurements of total binding (5 μM AR), NSB (5 μM AR + 5 μM DHT) and SB for different [3H]-DHT concentrations. Bmax = 4.1 pmol/mg, Kd = 31.6 ± 9.3 nM. (d) Influence of Triton X-100 (TX-100): SB was measured for experiments carried out with 5 μM AR for incubation step, serially diluted DHT in the presence of 20 nM [3H]-DHT. (e) Incubation time course: measurements of SB after different incubation times at room temperature and 4°C. Corresponding z’ values are specified above columns.
Secondly, we observed that performing the assay directly by mixing the protein along with the unlabeled and radiolabeled ligand (“mix-and-read”) led to a high background signal (higher than 50% of total binding), a narrow signal window 1000 cpm (Fig. 1b), high standard deviations (IC50 = 179.1 ± 111 nM), and a low z’ value (0.54). However, removal of the protein solution prior to the addition of unlabeled and radiolabeled ligand increased the signal substantially. The addition of consecutive wash-steps resulted in improved data (IC50 = 30.6 ± 10 nM) and assay quality (z’ = 0.89). Additionally, we reuse the protein solution and carried out the assay the next day without compromising the assay quality (data not shown).
Third, we determined a Kd of 31.6 nM of this specific ligand receptor interaction by measuring radiocounts for different [3H]-DHT concentrations after incubation with 5 μM AR (Fig. 1c). Thus the [3H]-DHT concentration (20 nM) used was lower than the calculated Kd, although it was 10 times higher than reported Kd for DHT. 10–11, 13 A Bmax of 4.1 pmoles of bound [3H]-DHT per mg of AR protein was calculated.
Fourth, we focused on the influence of Triton X-100 (TX-100) (Fig. 1d) or BSA (data not shown). No effect was observed in presence or absence of 0.1% BSA. The dose response curve obtained in absence of detergent showed high standard deviations among each triplicate and consequently a high variability of the IC50 (108.6 ± 65 nM). TX-100 concentrations of 0.01% increase the signal window, gave the best z’ value (0.92) and an IC50 value of 56.9 ± 6 nM.
Fifth, we analyzed the time dependency of the signal at room temperature and 4°C (Fig. 1e). Normally, we accumulated data after 5 hours but the assay could be read after one hour (z’ > 0.5). In both cases, the protein was stable at least for 24 hours.
Sixth, we changed the order of addition with no effect on the results (data not shown); thus, the radioligand can be safely added at the very last step of the assay.
Evaluation of an AR SPA ligand competition assay
To evaluate our AR ligand binding assay, we investigated several known competitors of DHT and applied this assay procedure to other nuclear receptors: PPARγ, TRα and TRβ (Table 1). The assay conditions were optimized using [3H]-Rosiglitazone in case of PPARγ and [125I]-T3 in case of TRα and TRβ (data not shown).
Table 1.
Summary of IC50 values measured in a SPA ligand competition assay for diverse nuclear receptors.
| AR | PPARγ | TRα | TRβ | |||
|---|---|---|---|---|---|---|
| IC50 (μM) | IC50 (μM) | IC50 (μM) | ||||
| DHT | 0.057 ± 0.006 | GW9662 | 0.23 ± 0.02 | T3* | 0.03 ± 0.02 | 0.14 ± 0.02 |
| Methyltrienolone (R1881) | 0.11 ± 0.02 | Rosiglitazone | 0.34 ± 0.08 | T4* | 1.4 ± 0.5 | 4.0 ± 2.0 |
| 17β-Estradiol | 3.2 ± 0.6 | Troglitazone | 3.70 ± 0.44 | GC1 | 1.6 ± 0.3 | 0.29 ± 0.11 |
| Cyproterone Acetate | 2.3 ± 0.4 | Lineolic Acid | 3.80 ± 0.49 | TRIAC* | 0.18 ± 0.1 | 0.07 ± 0.05 |
| Bicalutamide | 12.0 ± 2.6 | Arachidonic Acid | 4.20 ± 0.53 | T4Ac* | 10.0 ± 2.0 | 5.0 ± 2.0 |
| Progesterone | 5.0 ± 0.7 | T3Bz* | 12.0 ± 2.0 | 10.0 ± 4.0 | ||
| Hydroxyflutamide | 33.0 ± 8.0 | |||||
| Flutamide | 73.4 ± 29.3 | |||||
| Dexamethasone | 188.5 ± 100.0 | |||||
3,3',5-triiodo-L-thyronine sodium salt (T3); L-thyroxine (T4); 3,3’,5-triiodothyroacetic acid (TRIAC); 3,3’,5,5’-tetraiodothyroacetic acid (T4Ac); 4-(4'-Hydroxy-3'-iodophenoxy)-3,5-diiodo-benzoic acid (T3Bz).
For the homologous DHT competition assay, we measured an IC50 value of 56.9 nM. DHT and R1881 showed the highest affinities followed by miscellaneous steroid hormones. Different classes of PPARγ ligands were investigated. Most active were irreversible antagonist GW9662 and reversible agonist Rosiglitazone. The natural unsaturated fatty acids (linoleic and arachidonic acid) exhibited similar activities. Finally, we tested a panel of known T3 competitors. T3 and its analogue TRIAC showed the highest affinities for TRα and TRβ. The synthetic agonist GC1 and TRIAC exhibited high specificities for TRβ.
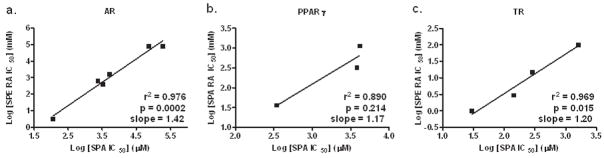
Using liganded NRs in these binding assays resulted in general higher IC50 values for competitors compared with the literature values. As calculated Kds gave us the same range of binding affinities (data not shown), relative binding affinities (RBAs) remain the best choice to draw comparisons with other binding assays. To show the relevance of our radioassay we plotted log values of measured IC50s against log values of reported binding affinities (Fig. 2). We found a statistically significant correlation for the AR (p = 0.0002, N = 6) and TR (p = 0.015, N = 4) receptors, whereas a much less significant correlation was observed for the PPARγ receptor (p = 0.214, N = 3).7, 13–14
Figure 2.
Correlation plots of IC50 values obtained in NR SPA ligand competition assay using FlashPlate® versus literature values. AR(a), PPARγ (b) and TR (c) SPA ligand competition assays using FlashPlate® vs. reported radioassays. 7, 13–14
Validation of an AR SPA ligand competition assay for HTS
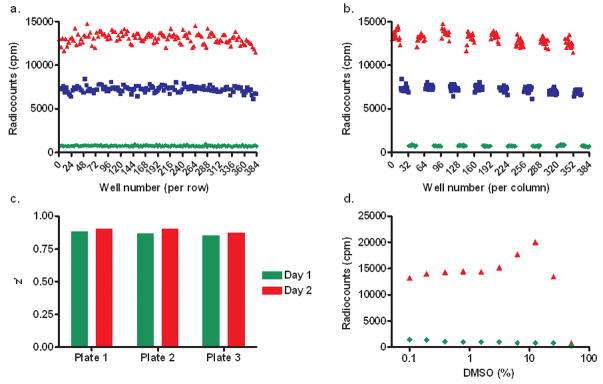
To investigate if the optimized AR SPA ligand competition assay can be automated for HTS, we followed validation protocols from the NIH-NCGC15. First, we carried out a plate uniformity assessment. Therefore, after pre-incubation with 5 μM AR solution, three different FlashPlates® were treated with DMSO (high signal), 50 nM DHT (medium signal), and 5 μM DHT (low signal) in the presence of 20 nM [3H]-DHT following the published plate layouts. Radiosignal measurements were read after 5 hours (Fig. 3a and b). Plotting the radiocounts against the well number by row we observed a linear relationship between radiocounts and well number. This behavior excluded the presence of drift or edge effects. Additionally, we plotted radiocounts against the well number by column. The clustering of values indicated no major variation of the measured signal depending on the geographic position. The experiment was repeated twice and z’ values were found between 0.85 and 0.92 for all plates confirming the integrity of independent assays (Fig. 3c). Finally, we noticed that DMSO concentrations were tolerated up to 5% without a change in signal (Fig. 3d).
Figure 3.
HTS validation. Plate geographic effects investigation across rows (a), across columns (b). DHT was assayed at different concentrations in the presence 20 nM [3H]-DHT (▲ DMSO, ■ [DHT] = 50 nM, ◆ [DHT] = 5 μM). (c) Assay reproducibility: comparison of z’ values determined by using two independent assays performed in triplicates. (d) Assay stability: influence of DMSO content on radiocounts.
In summary, we describe a ligand competition assay for androgen receptor using 384-well FlashPlates® and purified liganded AR-LBD. The “mix-and-read” process leads to very low accuracy and z’ values. We recommend the removal of unbound AR-LBD prior to the addition of small molecule and [3H]-DHT. This process allows protein recycling. Additionally, we were able to confirm the robustness of signal from 1 to 24 hours allowing the detection of slow binders. Drift experiments showed excellent homogeneity and reproducibility. All z’ values measured in the optimized conditions are higher than 0.85 whereas fluorescence polarization methods tend to perform around 0.6. Although absolute measured IC50s of known binders vary from reported values, we observed a strong correlation between IC50s determined by both methods, indicating this method provides reliable measurement of relative binding affinities. The addition of the radioligand as the last protocol step followed by sealing with clear tape decreases significantly the risk of contamination. Finally, the cost per data point was relatively low in comparison with other NR binding assays due to the 384-well format and in-house production and recycling of proteins. Overall, we are convinced that this assay can be fully automated and used for HTS purpose.
Acknowledgments
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC), St. Jude Children’s Research Hospital (SJCRH), and the Department of Defense, Prostate Cancer Research Program (PC060344-W81XWH-07-1-0073).
References
- 1.Chang C. Androgens and androgen receptor: mechanisms, functions, and clinical applications. Kluwer Academic Publishers; Norwell: 2002. [Google Scholar]
- 2.Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7(3):248–64. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- 3.Liao M, Wilson EM. Production and purification of histidine-tagged dihydrotestosterone-bound full-length human androgen receptor. Methods Mol Biol. 2001;176:67–79. doi: 10.1385/1-59259-115-9:67. [DOI] [PubMed] [Google Scholar]
- 4.Juzumiene D, Chang CY, Fan D, Hartney T, Norris JD, McDonnell DP. Single-step purification of full-length human androgen receptor. Nucl Recept Signal. 2005;3:e001. doi: 10.1621/nrs.03001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matias PM, Donner P, Coelho R, Thomaz M, Peixoto C, Macedo S, Otto N, Joschko S, Scholz P, Wegg A, Basler S, Schafer M, Egner U, Carrondo MA. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. Implications for pathogenic gene mutations. J Biol Chem. 2000;75(4):26164–71. doi: 10.1074/jbc.M004571200. [DOI] [PubMed] [Google Scholar]
- 6.Turek-Etienne TC, Small EC, Soh SC, Xin TA, Gaitonde PV, Barrabee EB, Hart RF, Bryant RW. Evaluation of fluorescent compound interference in 4 fluorescence polarization assays: 2 kinases, 1 protease, and 1 phosphatase. J Biomol Screen. 2003;8(2):176–84. doi: 10.1177/1087057103252304. [DOI] [PubMed] [Google Scholar]
- 7.Nichols JS, Parks DJ, Consler TG, Blanchard SG. Development of a scintillation proximity assay for peroxisome proliferator-activated receptor gamma ligand binding domain. Anal Biochem. 1998;257(2):112–9. doi: 10.1006/abio.1997.2557. [DOI] [PubMed] [Google Scholar]
- 8.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc Natl Acad Sci USA. 1999;96:266–71. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allan GF, Hutchins A, Clancy J. An ultrahigh-throughput screening assay for estrogen receptor ligands. Anal Biochem. 1999;275(2):243–7. doi: 10.1006/abio.1999.4335. [DOI] [PubMed] [Google Scholar]
- 10.Bauer ER, Bitsch N, Brunn H, Sauerwein H, Meyer HH. Development of an immuno-immobilized androgen receptor assay (IRA) and its application for the characterization of the receptor binding affinity of different pesticides. Chemosphere. 2002;46(7):1107–15. doi: 10.1016/s0045-6535(01)00145-x. [DOI] [PubMed] [Google Scholar]
- 11.Freyberger A, Ahr HJ. Development and standardization of a simple binding assay for the detection of compounds with affinity for the androgen receptor. Toxicology. 2004;195(2–3):113–26. doi: 10.1016/j.tox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Arnold LA, Estébanez-Perpiñá E, Togashi M, Shelat A, Ocasio CA, McReynolds AC, Nguyen P, Baxter JD, Fletterick RJ, Webb P, Guy RK. A high-throughput screening method to identify small molecule inhibitors of thyroid hormone receptor coactivator binding. Sci STKE. 2006;341:l3. doi: 10.1126/stke.3412006pl3. [DOI] [PubMed] [Google Scholar]
- 13.Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, Xie Q, Perkins R, Owens W, Sheehan DM. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003;16(10):1338–58. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- 14.Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5(6):299–306. doi: 10.1016/s1074-5521(98)90168-5. [DOI] [PubMed] [Google Scholar]
- 15.NIH. http://www.ncgc.nih.gov/guidance/section2.html#two-day_plate.