Abstract
Sensory representations depend strongly on the descending regulation of perceptual processing. Generalization among similar stimuli is a fundamental cognitive process that defines the extent of the variance in physical stimulus properties that becomes categorized together and associated with a common contingency, thereby establishing units of meaning. The olfactory system provides an experimentally tractable model system in which to study the interactions of these physical and psychological factors within the framework of their underlying neurophysiological mechanisms. We here show that olfactory associative learning systematically regulates gradients of odor generalization. Specifically, increasing odor-reward pairings, odor concentration, or reward quality – each a determinant of associative learning – significantly transformed olfactory generalization gradients, each narrowing the range of variance in odor quality perceived as likely to share the learned contingency of a conditioned odor stimulus. However, differences in the qualitative features of these three transformations suggest that these different determinants of learning are not necessarily theoretically interchangeable. These results demonstrate that odor representations are substantially shaped by experience and descending influences.
Keywords: Generalization gradients, categorical representations, perceptual learning, sensory neurophysiology, top-down regulation, learning theory
Introduction
Inter-stimulus variance is inescapable. Even a nominally identical odor stimulus, delivered multiple times to the same animal under controlled conditions, will evoke different – albeit related – responses across any population of activated neurons. As it is essential both to recognize recurring stimuli despite such variability and to distinguish among genuinely different stimuli, a central problem in perceptual physiology arises: how are categorical representations of meaningful stimuli formed that can appropriately group or differentiate individual stimulus responses so as to correctly identify each of their sources and implications? In any given context, how much variance among stimulus representations is tolerated before they are interpreted as arising from different sources, thereby warranting different behavioral responses? Categorizing too great a range of stimulus variance into a common percept (lumping) effectively reduces the resolution of the sensory system; in contrast, treating highly similar stimuli as unrelated (splitting) is counterproductive, hindering recognition of the useful fact that things that smell very much like a Cortland apple are probably also apples.
Generalization is a fundamental cognitive process that governs the grouping of similar stimuli into functional categories perceived as likely to share contingency (Shepard, 1987). It differs from discrimination in that the emphasis is not on whether a set of stimuli can be distinguished from one another, but on whether the subject associates these stimuli with the same outcome or implications. The degree of generalization among stimuli is closely related to their similarity, declining as their neural representations become more dissimilar; indeed, behavioral generalization among experimentally presented stimuli is a key basis for the empirical assessment of perceptual similarity (Cleland, Morse, Yue, & Linster, 2002; Linster & Hasselmo, 1999; McLaren & Mackintosh, 2002). The resulting psychometric functions describing how increasingly dissimilar stimuli are progressively less likely to be grouped together are termed generalization gradients.
Generalization gradients are consistent in form, declining exponentially with increased psychometric distance from a consequential stimulus (Shepard, 1987). Among stimuli that vary along simple dimensions such as visual hue or auditory pitch, psychometric distances tend to directly reflect the underlying physical dissimilarities in wavelength or tone frequency. Higher-order stimuli such as shapes and phonemes require nonmetric multidimensional scaling in order to transform their psychometric similarities onto a single axis, but once this is achieved, the resulting generalization gradients adhere to the same principles evident with simpler stimuli, declining exponentially with dissimilarity along this synthetic, psychometric axis (Kruskal, 1962; Shepard, 1962a, 1962b, 1987, 2001). Despite this consistency in form, generalization gradients are dynamic; a number of psychological as well as physical factors affect generalization gradients and hence alter the breadth and form of perceptual categories. For example, sensory experience affects generalization across visual hues (Guttman & Kalish, 1956) and across auditory frequencies during development (Kerr, Ostapoff, & Rubel, 1979), whereas stimulus intensity affects olfactory quality generalization in bees (Bhagavan & Smith, 1997; Wright & Smith, 2004) and mice (Cleland & Narla, 2003). We here demonstrate that multiple different determinants of associative learning systematically regulate gradients of olfactory generalization in mice. Specifically, they each impart greater conditioning to the conditioned odor stimulus, as predicted, and also progressively narrow the generalization of contingency to more highly similar odorants, though not necessarily via identical transformations or mechanisms (see Discussion).
The olfactory system provides a unique opportunity to study the neural underpinnings of the cognitive process of generalization in a behaviorally and physiologically reduced model system. First, behaviorally validated trajectories of stimulus variance (typically homologous series of aliphatic odorants) provide “sequentially similar” sets of odors that are experimentally analogous to series of simple visual or auditory stimuli that vary in hue or pitch (Cleland et al., 2002). As with studies of auditory pitch, the ordering of these basic odor series enables the straightforward measurement of generalization among progressively dissimilar stimuli, bypassing the nonmetric multidimensional scaling techniques required to depict generalization gradients among higher-order stimuli (Kruskal, 1962; Shepard, 1962a, 1962b, 1987, 2001). Second, the perceptual similarity of odorants correlates both with similarities in their molecular structures and with the degree of overlap in the sensory neuron activation patterns that they evoke, enabling study of the physical representation and processing of odor similarity within neural circuitry (Mandairon et al., 2006; Yokoi, Mori, & Nakanishi, 1995). Third, lesion and pharmacological studies are increasingly indicating that olfactory generalization is strongly regulated within the olfactory bulb, a well-described, anatomically isolated, and experimentally accessible cortical network (Kiselycznyk, Zhang, & Linster, 2006; Linster & Cleland, 2002; Linster, Garcia, Hasselmo, & Baxter, 2001; Mandairon et al., 2006). These advantages enable a clean neurophysiological approach to a fundamentally cognitive question: how do factors such as learning and motivational state regulate the construction of olfactory perceptual categories?
We investigated olfactory learning and generalization among odor stimuli in mice using established techniques (Cleland et al., 2002; Cleland & Narla, 2003). Briefly, animals were conditioned to associate a given odorant (the conditioned stimulus; CS) with a 5 mg sucrose pellet reward (unconditioned stimulus; US) for which they had to dig in dishes of scented sand. After training, the strength of the CS-US association was measured by the time mice spent digging in a similarly scented dish containing no reward before giving up. Generalization gradients were measured by testing in this manner an ordered series of odorants structurally and perceptually similar to the CS. A second dish of unscented sand was present during both conditioning and testing as a control; however, at no time were multiple odorants presented simultaneously in generalization tasks.
In the present study, we varied three experimental parameters each broadly considered by contemporary learning theories to regulate the rate and/or asymptotic maximum of learning. In order to emphasize the parameters of interest within a simple framework, we modeled our predictions based on the original, trial-based Rescorla-Wagner relationship (Rescorla & Wagner, 1972), in which learning VA about stimulus A is incremented during training according to the equation
| (1) |
where αA represents CS salience (odor intensity), β and λ together reflect US properties (reward quality), and Vtotal represents the aggregate prediction of the US by all cues present during the conditioning trial, and ΔVA represents the change in associative strength predicted for each conditioning trial. Learning thereby increases with repeated iterations of CS-US pairings until it approaches an asymptotic level determined by the properties of the US. As this relationship does not incorporate time-derivative factors incorporated into subsequent real-time learning models (Brandon, Vogel, & Wagner, 2003; Sutton & Barto, 1990), known temporal determinants of learning such as training-testing latency and massed versus spaced training were not used as variables in this study. Similarly, elemental and componential theories of conditioning and generalization (Atkinson & Estes, 1963; Brandon et al., 2003; McLaren & Mackintosh, 2000, 2002) may more directly reflect the nature of physiological odor representations and offer independence from the limitations of discrete associative trials, but for simplicity are not engaged herein. The strengths and limitations of the Rescorla-Wagner model have been reviewed at length by Miller and colleagues (Miller, Barnet, & Grahame, 1995).
Method
Experiments
Four separate studies were performed according to established procedures (Cleland et al., 2002). First, a discrimination study was performed to demonstrate that 5 mg reward pellets with different proportions of sucrose and cellulose were differentially rewarding to mice and hence able to serve as unconditioned stimuli of systematically differing incentive values. Then, three generalization studies were performed: a variable-training study, a variable-salience study, and a variable-reward study.
Subjects
Four separate, age-matched cohorts of male CD-1 mice (outbred strain; Charles River Laboratories, Wilmington, MA) were used: 12 mice were used in the discrimination study, 23 in the variable-training study, 10 in the variable-salience study, and 17 in the variable-reward study. All mice were shaped (trained to dig for rewards in response to odor cues) from 5−8 weeks of age and employed in experiments between 9−18 weeks of age. Mice were maintained on a shifted 12L:12D cycle; all behavioral training was conducted during their dark cycle (9:00 am to 9:00 pm), during which mice are most active and tend to perform well (Hyman & Rawson, 2001). Water was continuously available; mice were food-deprived for 18 hours preceding each session to motivate them to obtain sucrose rewards. Mice were fed immediately following an experimental session, and were not deprived of food on two subsequent days. All procedures were performed under the auspices of a protocol approved by the Cornell University Institutional Animal Care and Use Committee.
Odor sets and dilutions
Multiple odor sets were used in each study to enable counterbalancing among subjects and ensure that results were not dependent on the use of specific odor sets. All mice in a cohort were tested using every odor set employed in the corresponding study; odor sets were presented sequentially in the order listed in Table 1. Each odor set in the three generalization studies consisted of a homologous series of 2−5 structurally similar, unbranched aliphatic odorants plus one structurally dissimilar odorant used as a control (Table 1C-E). Vapor pressures of pure odorants were estimated with the Hass-Newton equation as implemented in ACD/Boiling Point & Vapor Pressure Calculator (version 4.5; Advanced Chemistry Development, Toronto, Ontario, Canada); pure odorants were diluted in mineral oil to concentrations theoretically emitting vapor-phase partial pressures of 0.01 Pa in all studies except for the higher-intensity condition in the variable-salience study, in which dilutions were made to 1.0 Pa for both conditioning and test odorants. The corresponding vol/vol liquid dilutions in mineral oil are listed in Table 1. Solvent surface effects and other nonlinearities were neglected. These dilutions should be considered a reduction in the variance of odor concentrations rather than true gas-phase concentration matching as could be achieved by gas chromatographic measurements. Odorants were diluted ∼18 hours in advance of each experiment in order to ensure an even distribution of odorant within the mineral oil solvent.
Table 1.
Odorant sets with corresponding vol/vol dilutions in mineral oil
| Odorant | Dilution for 0.01 Pa |
|---|---|
| (A) Odor pairs used for shaping | |
| (+/−)-limonene | 2.0 × 10−5 |
| benzylamine | 3.0 × 10−5 |
| n-hexyl acetate | 2.3 × 10−5 |
| n-amyl acetate |
0.7 × 10−5 |
| (B) Odor pairs used for discrimination study | |
| n-octanal | 1.5 × 10−5 |
| n-butyl glycidyl ether | 1.9 × 10−5 |
| 1,8-cineole | 2.0 × 10−5 |
| 3-heptanone | 0.6 × 10−5 |
| n-butanoic acid | 1.3 × 10−5 |
| 2,3,5-trimethylpyrazine | 1.4 × 10−5 |
| n-amyl acetate | 0.7 × 10−5 |
| 5-methylfurfural |
3.0 × 10−5 |
| (C) Odor series: effect of CS-US pairing | |
| n-heptanoic acid | 4.6 × 10−4 |
| n-octanoic acid | 13.7 × 10−4 |
| neryl acetate | 16.4 × 10−4 |
| n-amyl acetate | 7.2 × 10−6 |
| n-butyl acetate | 2.2 × 10−6 |
| anisole | 5.2 × 10−6 |
| propanoic acid | 3.3 × 10−6 |
| n-butanoic acid | 12.7 × 10−6 |
| 3-heptanone | 6.5 × 10−6 |
| n-octanal | 14.7 × 10−6 |
| n-heptanal | 7.1 × 10−6 |
| trans-2-hexenyl acetate | 16.3 × 10−6 |
| n-butyl n-pentanoate | 5.7 × 10−5 |
| n-butyl n-hexanoate | 16.3 × 10−5 |
| citronellal | 16.6 × 10−5 |
| n-pentanol | 0.7 × 10−5 |
| n-hexanol | 2.5 × 10−5 |
| 2-furyl methyl ketone | 2.6 × 10−5 |
| ethyl n-butyrate | 1.8 × 10−6 |
| propyl n-butyrate | 5.2 × 10−6 |
| 2-hexanone | 1.8 × 10−6 |
| n-pentanoic acid | 4.5 × 10−5 |
| n-hexanoic acid | 14.9 × 10−5 |
| 5-methylfurfural |
3.0 × 10−5 |
| (D) Odor series: effect of CS salience | |
| acetic acid | 0.1 × 10−5 |
| propanoic acid | 0.3 × 10−5 |
| n-butanoic acid | 1.3 × 10−5 |
| n-pentanoic acid | 4.5 × 10−5 |
| n-hexanoic acid | 14.9 × 10−5 |
| methyl 2-furoate | 2.5 × 10−5 |
| ethyl acetate | 1.7 × 10−7 |
| propyl acetate | 6.3 × 10−7 |
| n-butyl acetate | 21.9 × 10−7 |
| n-amyl acetate | 72.3 × 10−7 |
| n-hexyl acetate | 227.4 × 10−7 |
| 2-pentanone | 5.4 × 10−7 |
| butanal | 1.8 × 10−7 |
| pentanal | 6.6 × 10−7 |
| hexanal | 22.1 × 10−7 |
| heptanal | 70.7 × 10−7 |
| octanal | 147.2 × 10−7 |
| methyl butyrate |
7.1 × 10−7 |
| (E) Odor series: effect of US reward value | |
| n-hexyl acetate | 22.7 × 10−6 |
| n-amyl acetate | 7.2 × 10−6 |
| n-butyl acetate | 2.2 × 10−6 |
| anisole | 5.2 × 10−6 |
| propanoic acid | 0.3 × 10−5 |
| n-butanoic acid | 1.3 × 10−5 |
| n-pentanoic acid | 4.5 × 10−5 |
| 5-methylfurfural | 3.0 × 10−5 |
| n-octanal | 14.7 × 10−6 |
| n-heptanal | 7.1 × 10−6 |
| n-hexanal | 2.2 × 10−6 |
| 3-heptanone | 6.5 × 10−6 |
| n-octanoic acid | 13.7 × 10−4 |
| n-heptanoic acid | 4.6 × 10−4 |
| n-hexanoic acid | 1.5 × 10−4 |
| neryl acetate | 16.4 × 10−4 |
| ethyl n-butyrate | 1.8 × 10−6 |
| propyl n-butyrate | 5.2 × 10−6 |
| n-butyl n-butyrate | 16.5 × 10−6 |
| 2-hexanone | 1.8 × 10−6 |
| n-butyl n-butyrate | 1.7 × 10−5 |
| n-butyl n-pentanoate | 5.7 × 10−5 |
| n-butyl n-hexanoate | 16.3 × 10−5 |
| citronellal | 16.6 × 10−5 |
Apparatus
All behavioral training took place in modified Plexiglas mouse cages (28×17×12 cm) divided into two subchambers (A and B) by a sliding, opaque Plexiglas board. Glass petri dishes (Pyrex, 60 mm diameter, 15 mm height) were used for placement of odorants and reward. At the beginning of each training session, separate dishes were prepared for the conditioned odorant stimulus (CS) and each of the test odorants (S#, D). Each dish was filled with ∼10 ml of white play sand (YardRight; Southdown, Inc., Easton, PA) and inoculated with 100 ul of diluted odorant. During conditioning trials, the reward, a 5 mg sucrose or sucrose/cellulose pellet (Noyes Precision Pellets; Research Diets, Inc., New Brunswick, NJ), was buried in the sand of the CS-scented dish. The sand and odorant in each dish were replaced after every trial.
Shaping
Mice were shaped between 5−8 weeks of age by being taught to retrieve a reward by digging in dishes of scented sand. Mice were placed in subchamber A of the modified cage apparatus, with the divider between the two subchambers closed. Two sand-filled dishes were then placed in subchamber B: one containing both a reward and a conditioning odorant (Table 1A), the other containing no reward and no odorant. Each trial began when the divider was removed, at which point the mouse entered subchamber B and was allowed to dig in both dishes until it retrieved the reward (i.e., self-correction was permitted). The mouse was then returned to subchamber A for the one-minute intertrial interval, during which the dishes were replaced for the next trial. To speed learning, the reward was placed on top of the sand in the odor-containing dish during the first few trials; after the mouse reliably retrieved the reward over several trials, the reward was buried more deeply in the sand. Dishes were moved around randomly within subchamber B on subsequent trials such that odor was the only reliable predictor of which dish contained a buried reward. Shaping was considered complete when a mouse would (1) reliably identify the reward-containing dish and retrieve deeply buried rewards, (2) dig in the odor-containing dish even in the absence of a reward (thus controlling for the possibility of mice directly detecting the reward), and (3) show no interest after training in digging in odors scented with dissimilar odorants, indicating that their reward associations were odor-specific. Mice generally reached criterion after two weeks’ time, or approximately five days of shaping.
Behavioral testing
In the first study, we used a motivated, forced choice simultaneous odor discrimination paradigm (Cleland et al., 2002) to validate the use of reward pellets with varying sucrose/cellulose ratios as reinforcers of systematically varying efficacy. Simultaneous discrimination tasks differ fundamentally from generalization tasks in that they reward animals for successfully distinguishing between two odor stimuli presented together. Mice were placed in subchamber A with the divider closed. Each trial began when the divider was removed, enabling the mouse to enter subchamber B which contained two sand-filled dishes. Each dish was scented with a distinctly different odorant (Table 1B) and contained a different reward (i.e., each contained a 5 mg reward pellet with a different sucrose/cellulose composition, or no reward at all); the available rewards were 100%, 75%, 50%, and 25% sucrose, balance cellulose. For each session, over the course of 15 identical trials presented in immediate succession, one of the two dissimilar odorants was repeatedly paired with a higher sucrose content reward and the other with a lower sucrose content reward or with no reward. Specifically, two sets of pairings were performed. In the first set, one odor was always paired with a 100% sucrose reward tablet whereas the other was paired with a 75%, 50%, or 25% sucrose tablet or with no reward (0%) in order to establish that mice preferred higher-sucrose to lower-sucrose reward tablets. In the second set of pairings, one odor was paired with one of the four types of reward tablet whereas the other was paired with no reward in order to establish that mice preferred any reward pellet, even the least-desirable (25% sucrose), to the prospect of no reward at all.
The dish in which a mouse dug first served as the dependent variable; each trial was scored as a preference either for the odorant associated with the higher-sucrose-content reward or for the odorant associated with the lower-sucrose reward. Data analyses were performed on the number of trials out of 14 (i.e., excluding the first trial) in which the higher-sucrose odorant was chosen. Mice were permitted to dig in only one of the two dishes and to retrieve only one of the two rewards each trial; i.e., self-correction was not permitted. The spatial locations of the dishes within subchamber B were varied randomly among trials. Different mice were trained using different reward contingencies for the same odorant pairs, odor-reward pairings were counterbalanced, and four different odor sets were used to repeat the experiment (Table 1); hence, the specific odors used as cues were not relevant to the results. As only 15 trials were performed in each discrimination task, these data probably reflect differences in learning rates rather than steady-state error probabilities.
In the three subsequent studies, we used an odor generalization paradigm to measure the degree to which mice generalized between test odorants (Cleland et al., 2002; Cleland & Narla, 2003; Linster & Hasselmo, 1999). In this paradigm, mice were first placed in subchamber A with the divider closed. During conditioning trials, two sand-filled dishes were then placed in subchamber B: one containing both a sucrose reward and a conditioned odorant CS (Table 1), the other containing no reward and no odorant. Each trial began when the divider was removed, at which point the mouse entered subchamber B and was allowed to dig in both dishes until it retrieved the reward. The mouse was then returned to subchamber A for the one-minute intertrial interval, during which the divider was replaced and dishes were prepared for the next trial. During each of the subsequent test trials, one dish was scented either with the CS odorant or with one of a series of similar or dissimilar test odorants (Table 1), whereas the other contained no odorant, and neither dish contained any reward. The amount of time that a mouse spent digging in the scented sand served as the dependent variable. The duration of test trials was 1 minute, whereas conditioning trials ended after mice recovered the sucrose reward (up to a maximum of 1 minute). Intertrial intervals were 1 minute long, and test trials began directly after the completion of the conditioning trials.
In the variable-training study, mice were divided into three training groups; each mouse was trained on an odorant CS over three, six, or twelve conditioning trials. For each of the eight odor sets, one aliphatic odorant was selected as the conditioned odorant (CS); this conditioned odorant, a structurally similar odorant (S1) and a control odorant (D) served as test odorants (Table 1). Subsequently, three unrewarded test trials (in which mice were offered a choice between a dish scented with a test odorant and an unscented dish) were performed in a pseudorandom order. In order to control for handling, all mice were placed in the testing chamber a total of fifteen times; for mice given twelve conditioning trials this included the twelve conditioning trials plus three test trials, while mice given six or three conditioning trials were exposed to subchamber B six or nine times (respectively) prior to the first conditioning trial, but were presented with no odorant or reward during those dummy trials. To control for accumulated experience, mice were assigned to different training groups on subsequent odor sets; hence, each mouse received approximately the same amount of training over the course of the study.
The variable-salience study is a novel re-analysis of previously published data (Cleland & Narla, 2003). The methods employed are substantially similar to those described in the variable-training study, except that the variable of interest was odor concentration; for each of the odor sets used, half of the mice were trained and tested on odorants presented at 0.01 Pa while the other half were trained and tested at 1.0 Pa.
In the variable-reward study, mice were divided into two training groups. Each mouse was trained on a conditioned odorant over six conditioning trials; mice in one group were rewarded with a 100% sucrose reward tablet (better reward) whereas mice in the other group were rewarded with a 25% sucrose tablet (lesser reward). For each of the six odor sets used, one aliphatic odorant was selected as the conditioned odorant (CS); this conditioned odorant, two sequentially similar odorants (S1, S2) and a structurally dissimilar control odorant (D) all served as test odorants. Subsequently, two unrewarded test trials (in which the mouse was offered a choice between a dish scented with a test odorant and an unscented dish), two more rewarded trials using the CS odor, and two more unrewarded test trials were performed. The four test trials were performed in a pseudorandom order; furthermore, mice were assigned to different training groups on subsequent odor sets; hence, each mouse received approximately the same assortment of rewards over the course of the study.
In all generalization studies, during one-minute test trials, total digging times in the dish containing each test odorant were recorded using a stopwatch. Each mouse encountered each test odorant only once. The experimenter was blind to test odorant identities during performance of these experiments.
Data analysis
In the discrimination task, the degrees of preference for higher-proportion sucrose rewards were assessed by one-factor ANOVA with degree of preference as main effect as well as by linear regression (R2). Individual comparisons were also made with two-tailed one-sample t-tests compared to chance (i.e., 7 of 14 trials correct). For initial assessments of CS conditioning, one-way analyses of variance were performed; to further assess the effects of training, for which there were three groups, post hoc multiple comparisons tests using Tukey's honestly significant difference (HSD) criterion were also performed.
In the generalization tasks, repeated-measures analyses of variance using Wilks’ lambda criterion were first performed on generalization gradients of digging times with odorant (CS, S#, D) as a repeated-measures main effect and the learning variable of interest (number of conditioning trials, CS odorant concentration, or US reward value) as a between-subjects main effect. Post hoc multiple comparisons analyses were subsequently performed on the variable-training data (which included three between-subjects factors) using Tukey's HSD criterion. For figure annotation, and for elaborations in the Results in which comparisons of responses to individual odorants were made between experimental groups, independent samples t-tests were used to generate p-values (two-tailed; equal variances not assumed).
All statistical analyses were performed using SPSS statistical software. The criterion for significance was set at α = 0.05. Error bars depict standard errors of the mean.
Results
Validation of independent variables
The three independent variables in this study were the number of iterations of CS-US pairing, the CS salience (α), and the US quality (β, λ). The pairing iterations were explicitly represented by the number of conditioning trials preceding testing. Variation of the CS salience was accomplished by varying odorant stimulus concentrations. Stimulus intensity has long been recognized as a factor in CS salience, sometimes explicitly segregated from other (diagnostic) factors by using the term intensive salience (Tversky, 1977). To avoid artifacts owing to uncontrolled diagnostic salience, all studies were performed multiple times with different odor sets (Table 1), and counterbalanced so that each animal was tested on every odorant and under every experimental condition manipulated within a study (see Method).
Variation of the US quality, a factor predicted to shape learning through changing motivational incentive, was accomplished by rewarding mice with pellets consisting of different proportions of sucrose from 100% (best reward) to 25% (least reward). The non-nutritive carbohydrate cellulose was selected as filler so that nutritive and hedonic contributions to reward quality perception (Sclafani & Ackroff, 2004, 2006; Sheffield & Roby, 1950) would correlate. While sucrose concentrations in liquid rewards have been successfully used to systematically vary reward quality (Sclafani & Ackroff, 2003), the use of pellets in variable-reward contexts (Phillips, Willner, & Muscat, 1991) is uncommon. Therefore, to validate this technique, we subjected a cohort of mice to an odor discrimination task. Mice were presented with two sand-filled dishes scented with dissimilar odorants (Table 1; note that this procedure differs from generalization tasks in which only one of the two dishes was scented). Over 15 identical trials performed in immediate succession, one of the two odorants was repeatedly paired with a higher-sucrose-content reward and the other with a lower-sucrose content reward. The dish in which mice first dug was scored in each trial.
Sucrose proportion had a significant effect on reward preference (ANOVA; F(3,44) = 13.267; p < 0.001); specifically, mice preferred higher-sucrose-content pellets in direct proportion to sucrose concentration (R2 = 0.472; p < 0.001; Figure 1A), reflecting the comparable linearity of reward value observed in liquid sucrose dilution series (Sclafani & Ackroff, 2003). Furthermore, mice strongly preferred any reward pellet tested to no reward (one-sample t-tests; p < 0.001 in all cases; Figure 1B).
Figure 1.
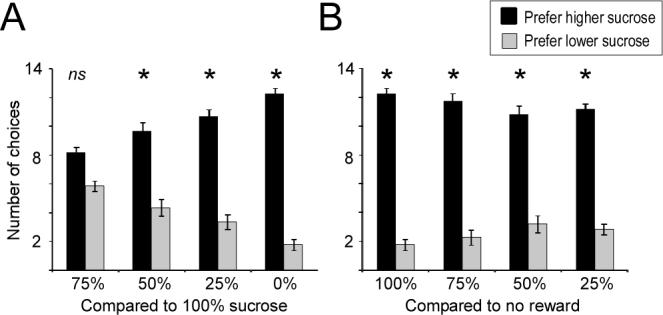
Odor discrimination task revealing reward preferences. (A) Preference for rewards of higher quality when each reward is reliably cued by an easily discriminable odor. When offered a choice between a 100% sucrose pellet or an otherwise identical pellet containing some proportion of cellulose, mice preferred the higher-sucrose pellets in direct proportion to the difference in sucrose content (R2 = 0.472; p < 0.001). Asterisks denote significant preference for the 100% sucrose pellet (one-sample t-test, comparison vs. chance, p < 0.01); ns: not significant. (B) Preference for different rewards over no reward. When offered a choice between a sucrose/cellulose pellet and no reward, mice strongly preferred even the least-favored reward tested (25% sucrose/75% cellulose) to no reward. Asterisks denote significant preference for the reward (one-sample t-test, comparison vs. chance, p < 0.001). Percentage values on the abscissa refer to sucrose content; 0% connotes no reward. Ordinates depict the number of trials in which the mouse chose the scented dish containing the designated reward pellet. Trials were scored beginning with the second trial, after the mouse first had the opportunity to learn the odor-reward association; hence fourteen trials were scored for each mouse.
Conditioned responses to CS odors follow associative learning rules
Increases in the values of each of the three parameters – CS-US pairings, CS salience, and US value – are predicted to increase associative learning levels. Indeed, we found that our three experimental manipulations each exerted a significant effect on associative learning, as predicted by learning theory and measured by digging times in response to presentation of the conditioned odorant in the absence of reward.
First, increasing the number of pairings of the odor CS with reward significantly strengthened the response to the subsequent unrewarded presentation of that odor (main effect of training; F(2,181) = 17.436; p < 0.001). Post hoc analysis using Tukey's honestly significant difference (HSD) criterion demonstrated that all three pairwise comparisons were also significant (p < 0.05; Figure 2A). In the second study, the salience of the odor CS was varied by adjusting odor concentrations 100-fold, from 0.01 Pa to 1.0 Pa. As theoretically predicted, this greater salience significantly enhanced associative learning (main effect of intensity, F(1,57) = 5.281, p < 0.05; Figure 2B). Finally, the reward value of the US was varied by rewarding mice with tablets of either 100% sucrose or 25% sucrose/75% cellulose, as described and validated above, in otherwise identical associative learning protocols. As predicted, this manipulation of US value significantly affected associative learning (main effect of reward, F(1,88) = 8.724, p < 0.01; Figure 2C). The effects of these three parameters on odor generalization gradients were then also assessed.
Figure 2.
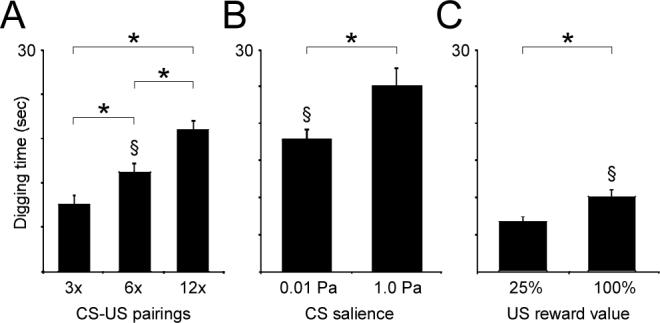
Multiple determinants contribute to associative learning. (A) Effect of the number of CS-US pairings (three, six, or twelve conditioning trials) on associative learning, measured as digging times in response to presentation of the odor CS. Mice receiving more training responded correspondingly more strongly to presentation of the CS. Asterisks denote significant differences in digging times (p < 0.05). (B) Effect of CS salience (odor intensity) on associative learning. Odorants presented at 100x greater concentration evoked significantly greater learning in an otherwise identical conditioning paradigm. Asterisks denote significant differences in digging times (p < 0.05). (C) Effect of US value (perceived reward quality) on associative learning. Otherwise identical paradigms in which 100% sucrose reward pellets were used during conditioning resulted in significantly greater learning than when 25% sucrose/75% cellulose reward pellets were used. Asterisks denote significant differences in digging times (p < 0.01). Section symbols depict the groups among the different experimental cohorts that were conditioned using similar parameters (see Replicability section within Results).
Effect of the number of CS-US pairings on generalization gradients
The number of pairings of the odor CS with reward had a significant effect on the odor generalization gradient, as measured by digging times in response to presentation of a series of test odorants in the absence of reward (Wilks’ lambda, interaction of odorant and training; F(4,360)=5.633, p < 0.001). Post hoc multiple comparisons testing further demonstrated that six pairings of the CS with reward produced significantly different effects on the generalization gradient than did three pairings (Tukey's HSD; p < 0.05), and twelve pairings significantly different effects than six (p < 0.05).
Further testing showed that whereas increased CS-US pairings progressively increased digging times in response to all odors, the effect on the CS itself was proportionately stronger than it was on other, less similar odorants. Multiple comparisons analysis (Tukey's HSD) demonstrated that digging times in response to the odor CS significantly increased between three and six pairings, and again between six and twelve pairings, whereas digging in response to odors S or D increased significantly only when directly comparing three pairings with twelve pairings (p < 0.05 in all cases; Figure 3A). This characteristic pattern has also been observed in studies of the effect of increased CS-US pairings on the visual generalization of hue (Guttman & Kalish, 1956).
Figure 3.
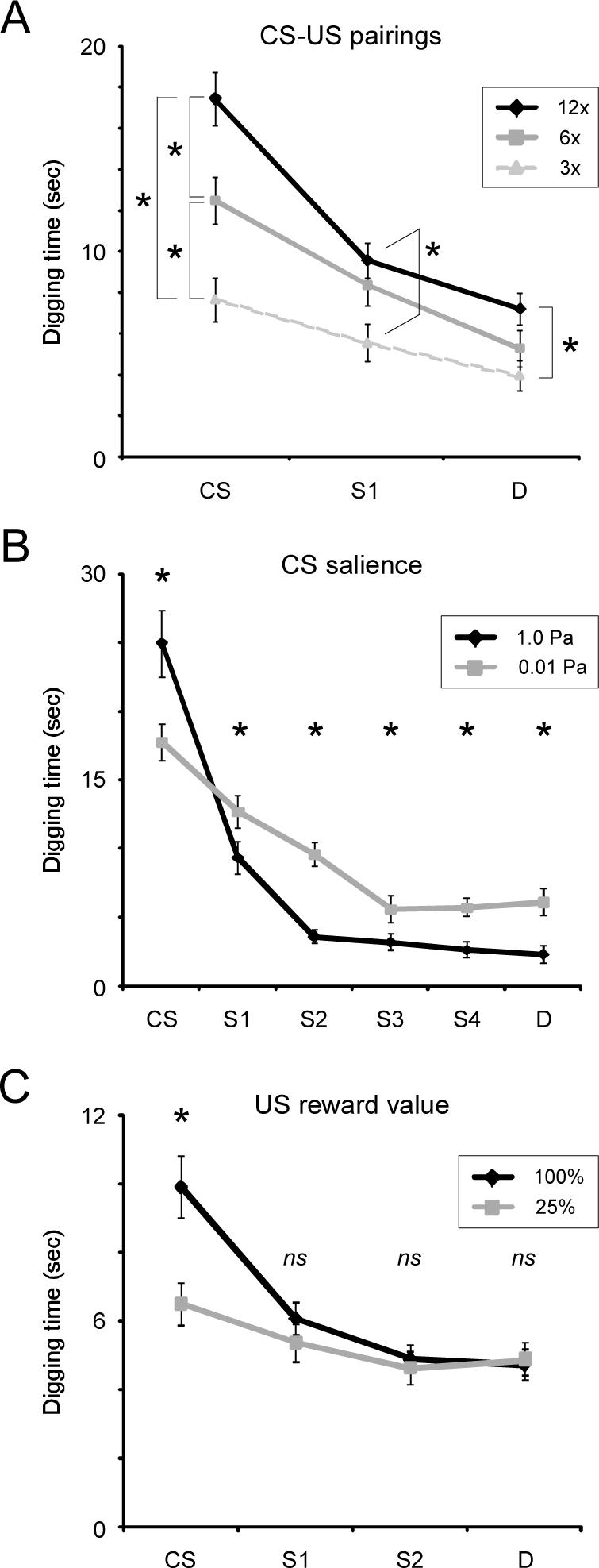
Generalization gradients are regulated by determinants of associative learning. (A) Effect of the number of CS-US pairings. Mice receiving larger numbers of conditioning trials exhibited progressively stronger responses to all test odors, though the effect was greatest for the CS itself, resulting in an overall sharpening of generalization gradients with greater learning. That is, learning deriving from additional CS-US pairing generalized broadly, including even very dissimilar odorants. Asterisks denote significant differences between individual points (p < 0.05). (B) Effect of CS salience (odor intensity). Mice presented with higher-intensity odor CSs during conditioning generalized less to similar odorants than did mice conditioned with lower-intensity odorants. Increased CS salience dramatically sharpened the generalization gradient, such that whereas responsiveness to the CS was increased, responsiveness to similar and dissimilar odorants was reduced. Asterisks denote significant differences (p < 0.05); note that the gradients cross between the odorants CS and S1. These results are based on a reanalysis of previously published data (Cleland & Narla, 2003). (C) Effect of US reward value. Mice receiving US reward pellets with higher perceived value during CS-US pairing (i.e., with a proportional sucrose content of 100% rather than 25%) generalized less to similar odorants than did mice trained with the lesser reward. Learning derived from superior reward value generalized very narrowly, having a statistically significant effect only on responses to the CS itself. Asterisks denote significant differences between individual points (p < 0.05); ns: not significant.
Effect of CS salience (intensity) on generalization gradients
The intensity of the odor CS had a significant effect on the odor generalization gradient (Wilks’ lambda, interaction of odorant and intensity; F(5,53)=5.738, p < 0.001), although this effect differed qualitatively from the corresponding effect of increased CS-US pairings. Specifically, higher CS intensity significantly increased digging times in response to the odor CS (p < 0.05) while significantly decreasing responsiveness to all other odors (p < 0.05 in all cases; Figure 3B).
Effect of US value on generalization gradients
The reward value of the US had a significant effect on the odor generalization gradient (Wilks’ lambda, interaction of odorant and reward; F(3,86)=4.084, p < 0.01), which again differed qualitatively from the effects of the other two learning determinants studied. Specifically, higher US value significantly increased digging times in response to the odor CS (p < 0.01), but neither increased nor decreased responsiveness to any other odorant tested (p > 0.05 in all cases; Figure 3C).
Replicability
Olfactory generalization gradients are reasonably replicable over time and between subjects. Cleland and Narla (2003) explicitly repeated a set of generalization trials at the beginning and end of their study of odor concentration effects, demonstrating that experience accumulated over the course of a comparable study does not progressively affect odor generalization gradients. Furthermore, the three generalization studies performed herein were performed with three different cohorts of mice over an extended period, yet their respective results under comparable conditions are reasonably consistent (Figure 2, section symbols; also compare the corresponding trajectories in Figure 3A-C). Specifically, mice trained using the set of experimental parameters common to all three studies (i.e., trained for ∼6 trials with odorants at 0.01 Pa and rewarded with 100% sucrose pellets) responded to the CS for 14 +/− 4 seconds during testing, and to the dissimilar odorant for 6 +/− 1 seconds. Mean digging times in all test odorants substantially exceeded the 200−300 ms required for odor discrimination in mice (Abraham et al., 2004), indicating that the observed differences in generalization gradients do not primarily reflect changes in the detection or physical sampling of odorants.
Discussion
We here show that olfactory associative learning not only increases the strength of conditioned responses to the odor CS, as broadly predicted by theories of learning, but also exerts consistent effects upon olfactory generalization gradients. Specifically, increased learning about an odor CS results in progressively sharper generalization gradients, such that the range of variance in odor quality perceived as likely to share the learned contingency of the CS (i.e., to predict the US) is correspondingly narrowed. This sharpening effect was observed irrespective of the method used to increase the rate or level of associative learning. However, beyond this basic commonality, the three learning variables tested appeared to effect qualitatively distinct transformations of olfactory generalization gradients, though as they were tested in separate experiments this cannot be statistically confirmed. Specifically, in their respective experiments, increasing CS salience significantly reduced responses to dissimilar odorants such as D, increasing the number of conditioning trials significantly increased responses to all odors tested including the arbitrarily dissimilar odorants D, and increasing reward value did not affect the responses to D or to other dissimilar odorants. In other words, whereas increasing CS-US pairings appeared to evoke a degree of nonselective learning despite a bias favoring the CS, increasing reward value appeared to only enhance learning with respect to a narrow gradient of generalization surrounding the CS. If confirmed, these results will require extension of contemporary theories of learning to consider the effects of these determinants on the strengths and shapes of conditioned stimulus representations.
Why should associative learning sharpen olfactory generalization gradients? The underlying effect is unlikely to be sharpening per se, but rather the gradual adaptation of the generalization gradient to reflect the actual pattern of CS contingency as approximated over a period of accumulated sensory experience. That is, learning may serve to optimize the shapes of categorical odor representations until their predictions of the meanings of novel (but similar) odorants reflect reality (Rosenthal, Fusi, & Hochstein, 2001). In the present studies, for example, generalization gradients would be predicted to sharpen until they approximated the actual distribution of variance in CS representations evoked across repeated experimental trials – a quite narrow distribution, given that the same monomolecular odorant stimulus was repeatedly used in any given set of trials under controlled conditions. In contrast, learning might broaden generalization gradients from their a priori state if substantial variations in scent quality were all found to predict the same food source or other consequence. This hypothesis of course does not explain the qualitatively different forms of sharpening or broadening that different determinants of learning appear to exert on generalization gradients. The underlying principle of progressively learning the distribution of CS quality variance as a basis for stimulus categorization has been directly observed in human visual studies of categorical learning (Rosenthal et al., 2001); it is also embedded in the error minimization principle of elemental learning models (McLaren & Mackintosh, 2000) and explicitly modeled in theories of concept learning and categorization (Nosofsky, 1986; Shepard, 1986; Stewart & Brown, 2005). Elucidating these learning and classification phenomena in the present olfactory generalization paradigm enables extension of these psychometric analyses to incorporate their underlying neurophysiological mechanisms.
The physiology of olfactory generalization
The olfactory system exhibits singular advantages for the study of stimulus generalization and other properties of perceptual learning. Among these, one of the most important is the emerging capacity to map these cognitive processes onto the activity of particular neuronal circuits such that physiological stimulus representations and their neural transformations can be studied in concert with their respective influences on sensory perception. A number of innate features of the olfactory system support this capacity. First, olfactory perceptual similarity shares a metric space with the receptive fields of primary sensory neurons; there is no need for computational transformations to achieve, for example, positional invariance. This implies both that behaviorally meaningful odor objects have consistent, potentially stationary primary representations, and that overlap in these primary representations corresponds to perceptual similarity in these objects. Second, the axons of primary olfactory sensory neurons expressing the same odorant receptor proteins, and hence exhibiting similar receptive fields, converge together to form bundles of neuropil (glomeruli) on the surface of the olfactory bulb (Mombaerts et al., 1996), the activity of which can be collectively visualized using optical imaging techniques (Leon & Johnson, 2003; Rubin & Katz, 1999). In principle, therefore, the entire primary olfactory representation can be physiologically measured. Third, the cortical architecture of the olfactory bulb is well-described, and its relative morphological segregation from the rest of the telencephalon facilitates its study as a sensory signal processor. Indeed, recent computational models of the bulbar neural network have proposed mechanisms for the neural representation of odor similarity (Cleland, Johnson, Leon, & Linster, 2007; Cleland & Sethupathy, 2006) that have directly linked established cellular neuromodulatory effects within the olfactory bulb to changes in a non-rewarded form of olfactory generalization (Mandairon et al., 2006). Similar behavioral effects have been demonstrated in mouse models of human dementia (Bath et al., 2008), indicating that a flattening of olfactory generalization gradients may be a consequence of a generally reduced learning capacity in relevant brain regions.
Higher-order olfactory representations in other parts of the brain exhibit properties that may derive from the associative, adaptive sensory processes described herein. In the piriform cortex, cross-habituation studies reveal configural odor representations insensitive to the elemental similarities that are evident in the more peripheral representations of the same odorants within the olfactory bulb (McNamara, Magidson, Linster, Wilson, & Cleland, 2008; Wilson, Kadohisa, & Fletcher, 2006). Further downstream, neuronal activity in the orbitofrontal cortex reflects the expected contingency and cross-modal associations of odor stimuli more than it does odor quality per se (Rolls, 2001, 2005; van Duuren et al., 2007). The construction of these increasingly configural, associative odor representations likely depends on layers of learning-regulated sensory processing implemented as peripherally as the first sensory synapse (Brennan & Keverne, 1997).
Conclusions
The construction of meaningful odor representations out of the primary sensory sampling of the olfactory environment depends strongly on learning, motivation, expectation, and other psychological factors as well as on the physical properties of sampled stimuli. Whether to group together a given range of variance in physical odor quality and categorize it as a single “odor,” or to segregate this range into multiple distinct odors with potentially different meanings, depends in large part on prior learning and the motivations associated with the task at hand. For example, the same set of similar odorants may be perceptually grouped together in a nonrewarded cross-habituation task and yet be strongly differentiated in a motivated discrimination task (Cleland et al., 2002; Linster, Johnson, Morse, Yue, & Leon, 2002). The emerging principle is that perceptually meaningful odors are not passively detected, but rather that sensory input is continuously and dynamically modulated by a learned recognition of which patterns are meaningful and which discriminations, however subtle, are important.
Acknowledgments
We gratefully acknowledge the assistance of Sonica Bhatia, Timothy Miller, and Charles Wiltrout in gathering behavioral data. We also thank Ralph R. Miller, Brian H. Smith, and Christiane Linster for particularly fruitful discussions. Supported by NIDCD grant DC007725 and the Hughes Scholars Program at Cornell University.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44(5):865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Atkinson RC, Estes WK. Stimulus sampling theory. In: Luce RD, Bush RR, Galanter E, editors. Handbook of mathematical psychology. Vol. 2. Wiley; New York: 1963. pp. 121–268. [Google Scholar]
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen Z-Y, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28(10):2383–2393. doi: 10.1523/JNEUROSCI.4387-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavan S, Smith BH. Olfactory conditioning in the honey bee, Apis mellifera: effects of odor intensity. Physiol Behav. 1997;61(1):107–117. doi: 10.1016/s0031-9384(96)00357-5. [DOI] [PubMed] [Google Scholar]
- Brandon SE, Vogel EH, Wagner AR. Stimulus representation in SOP: I. Theoretical rationalization and some implications. Behavioural Processes. 2003;62(1−3):5–25. doi: 10.1016/s0376-6357(03)00016-0. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51(4):457–481. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Johnson BA, Leon M, Linster C. Relational representation in the olfactory system. Proc Natl Acad Sci U S A. 2007;104(6):1953–1958. doi: 10.1073/pnas.0608564104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Narla VA. Intensity modulation of olfactory acuity. Behav Neurosci. 2003;117(6):1434–1440. doi: 10.1037/0735-7044.117.6.1434. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 2006;7:7. doi: 10.1186/1471-2202-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N, Kalish HI. Discriminability and stimulus generalization. J Exp Psychol. 1956;51(1):79–88. doi: 10.1037/h0046219. [DOI] [PubMed] [Google Scholar]
- Hyman S, Rawson NE. Preliminary results of olfactory testing in rats without deprivation. Lab Anim (NY) 2001;30(1):38–39. [PubMed] [Google Scholar]
- Kerr LM, Ostapoff EM, Rubel EW. Influence of acoustic experience on the ontogeny of frequency generalization gradients in the chicken. J Exp Psychol Anim Behav Process. 1979;5(2):97–115. doi: 10.1037//0097-7403.5.2.97. [DOI] [PubMed] [Google Scholar]
- Kiselycznyk CL, Zhang S, Linster C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn Mem. 2006;13(5):575–579. doi: 10.1101/lm.285706. [DOI] [PubMed] [Google Scholar]
- Kruskal JB. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika. 1962;29(1):1–27. [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Brain Res Rev. 2003;42(1):23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Linster C, Cleland TA. Cholinergic modulation of sensory representations in the olfactory bulb. Neural Netw. 2002;15(4−6):709–717. doi: 10.1016/s0893-6080(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001;115(4):826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 1999;66(3):497–502. doi: 10.1016/s0031-9384(98)00324-2. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22(16):6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24(11):3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- McLaren IP, Mackintosh NJ. An elemental model of associative learning: I. Latent inhibition and perceptual learning. Animal Learning and Behavior. 2000;28(3):211–246. [Google Scholar]
- McLaren IP, Mackintosh NJ. Associative learning and elemental representation: II. Generalization and discrimination. Anim Learn Behav. 2002;30(3):177–200. doi: 10.3758/bf03192828. [DOI] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15(3):117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Barnet RC, Grahame NJ. Assessment of the Rescorla-Wagner model. Psychol Bull. 1995;117(3):363–386. doi: 10.1037/0033-2909.117.3.363. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87(4):675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM. Attention, similarity, and the identification-categorization relationship. J Exp Psychol Gen. 1986;115(1):39–61. doi: 10.1037//0096-3445.115.1.39. [DOI] [PubMed] [Google Scholar]
- Phillips G, Willner P, Muscat R. Suppression or facilitation of operant behaviour by raclopride dependent on concentration of sucrose reward. Psychopharmacology (Berl) 1991;105(2):239–246. doi: 10.1007/BF02244316. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations of the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WT, editors. Classical conditioning. II. Current research and theory. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Rolls ET. The rules of formation of the olfactory representations found in the orbitofrontal cortex olfactory areas in primates. Chem Senses. 2001;26(5):595–604. doi: 10.1093/chemse/26.5.595. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Rosenthal O, Fusi S, Hochstein S. Forming classes by stimulus frequency: behavior and theory. Proc Natl Acad Sci U S A. 2001;98(7):4265–4270. doi: 10.1073/pnas.071525998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23(3):499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Physiol Behav. 2003;79(4−5):663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82(1):89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiol Behav. 2006;88(1−2):88–94. doi: 10.1016/j.physbeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Sheffield FD, Roby TB. Reward value of a non-nutritive sweet-taste. J Comp Physiol Psychol. 1950;43(6):471–481. doi: 10.1037/h0061365. [DOI] [PubMed] [Google Scholar]
- Shepard RN. The analysis of proximities: multidimensional scaling with an unknown distance function. I. Psychometrika. 1962a;27:125–140. [Google Scholar]
- Shepard RN. The analysis of proximities: multidimensional scaling with an unknown distance function. II. Psychometrika. 1962b;27:219–246. [Google Scholar]
- Shepard RN. Discrimination and generalization in identification and classification: Comment on Nosofsky. Journal of Experimental Psychology: General. 1986;115(1):58–61. doi: 10.1037//0096-3445.115.1.39. [DOI] [PubMed] [Google Scholar]
- Shepard RN. Toward a universal law of generalization for psychological science. Science. 1987;237(4820):1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Shepard RN. Perceptual-cognitive universals as reflections of the world. Behav Brain Sci. 2001;24(4):581–601. discussion 652−571. [PubMed] [Google Scholar]
- Stewart N, Brown GDA. Similarity and dissimilarity as evidence in perceptual categorization. Journal of Mathematical Psychology. 2005;49(5):403–409. [Google Scholar]
- Sutton RS, Barto AG. Time-derivative models of Pavlovian reinforcement. In: Gabriel M, Moore J, editors. Learning and computational neuroscience: foundations of adaptive networks. MIT Press; Cambridge, MA: 1990. pp. 497–537. [Google Scholar]
- Tversky A. Features of similarity. Psychol Rev. 1977;84(4):327–352. [Google Scholar]
- van Duuren E, Escamez FA, Joosten RN, Visser R, Mulder AB, Pennartz CM. Neural coding of reward magnitude in the orbitofrontal cortex of the rat during a five-odor olfactory discrimination task. Learn Mem. 2007;14(6):446–456. doi: 10.1101/lm.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Kadohisa M, Fletcher ML. Cortical contributions to olfaction: plasticity and perception. Semin Cell Dev Biol. 2006;17(4):462–470. doi: 10.1016/j.semcdb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Wright GA, Smith BH. Variation in complex olfactory stimuli and its influence on odour recognition. Proc Biol Sci. 2004;271(1535):147–152. doi: 10.1098/rspb.2003.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92(8):3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]


