Abstract
The ability of a series of molecular umbrellas, derived from cholic acid, L-lysine, spermidine and Cascade Blue, to cross fluid liposomal membranes made from 1-palmitoyl-2-oleyol-sn-glycero-3-phosphocholine (POPC)/1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG) (95/5, mol/mol) has been determined. In sharp contrast to the clasic “size/lipophilicity” rule of membrane transport, those molecular umbrellas that were larger in size and less lipophilic crossed these liposomal membranes more readily. The likely origin for this unusual behavior is briefly discussed.
This paper report the consequences of molecular umbrella size and lipophilicity on their ability to transport a small polar agent (Cascade Blue) across lipid bilayers. In contrast to predictions based on classic solution-diffusion theory, transport rates were found to increase with increasing umbrella size (i.e., 1b<2b<3, Chart 1). In addition, rate constants for the translocation step for 1a and 1b indicate that the less lipophilic umbrella (i.e., 1b) crosses lipid bilayers more readily. The likely origin for this unusual umbrella behavior is briefly discussed.
Chart 1.

One of the major goals in modern medicinal chemistry is to find ways of transporting polar drugs across hydrophobic barriers such as the plasma membrane of cells and the blood-brain-barrier.1-7 To date, most approaches have relied on the “size/lipophilicity rule”, which originates from the solution-diffusion model for bilayer transport. According to this model, the permeability coefficient (P) of a permeant is directly proportional to its water-lipid partition coefficient (K) and its diffusion coefficient (D), and inversely proportional to the thickness (x) of the bilayer; that is, P = (K × D)/x.1,6,7 Thus, an increase in lipophilicity (reflected by a higher K) or a decrease in the size of the permeant (reflected by a higher D) is expected to lead to increased permeability.
Our own approach to this problem has focused on the use of molecular umbrellas as transport vehicles.8 In essence, these molecules are composed of two or more facial amphiphiles (“walls”) attached to a central scaffold. A stylized illustration of a di-walled molecular umbrella is shown in Figure 1, where the shaded and unshaded rectangles represent a rigid lipophilic and hydrophilic face, respectively, and the oval represents the attached hydrophilic agent.
Figure 1.

Stylized illustration of a di-walled molecular umbrella binding to a lipid bilayer in an exposed (A) and shielded (B) state.
Our model for umbrella transport consists of initial adsorption to the membrane surface (A), followed by absorption into the interior of the membrane via the formation of a shielded conformation (B) (Figure 1). Subsequent translocation to the adjoining leaflet, 180° rotation, reversion to an adorbed state, and release from the other side of the membrane then allows for net transport. Recent studies of 1a, 1b, 2a and 2b indicate that A is their favored state when bound to lipid bilayers, and that a subpopulation exists for the sulfated umbrellas, which lies deeper within the membrane.9
In principle, molecular umbrellas that have more walls could exhibit higher transport rates due to a a greater shielding capacity. Alternatively, they could show lower transport rates due to a decreased diffusion coefficient. To distinguish between these two possibilities, and to probe the consequences of umbrella lipophilicity on membrane transport, we investigated the permeation behavior of a series of di-, tetra- and octa-walled conjugates (1a, 1b, 2a, 2b and 3), and a non-umbrella derivative of Cascade Blue (i.e., 4). The method used for synthesizing the largest of these umbrellas, 3, is described in the Supporting Information. Synthetic methods used to prepare 1a, 1b, 2a, 2b and 4 have previously been reported.9
Binding measurements via equilibrium dialysis showed that the sulfated molecular umbrellas have very little affinity toward lipid bilayers, but that the non-sulfated analogs are strongly bound. Thus, using a 100 μM umbrella solution and a 6.6 mM phospholipid dispersion, 85% of 1a and 97% of 2a became bound to the liposomes at 37°C, while under similar conditions, 1.21% of 1b, 4.71% of 2b, and 6.66% of 3 atttached themselves to the liposomes. The former values correspond to partition coefficients, K, of 9.5 × 104, and 5.4 × 105, while the latter correspond to 208, 835, and 1206, respectively.10
Liposomes (200 nm, extrusion) were prepared from 40 mg of a mixture of 1-palmitoyl-2-oleyol-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG) [i.e., POPC/POPG, 95/5, mol/mol] and 2 mL of phosphate buffered saline [10 mM phosphate, 150 mM NaCl, pH 7.4, PBS], which was ca. 180 μM in a given umbrella.8b After dialysis at 23°C for 48 h, to remove the umbrella from the external aqueous phase, the loss of umbrella that was still associated with the liposomes was monitored at 37°C during extended dialysis.
Based on an average liposomal diameter of 200 nm (dynamic light scattering), the percent capture of the aqueous phase and umbrella is expected to be ca. 18%. As shown in Figure 2, capture of the weakly-bound persulfated molecular umbrellas, 1b, 2b and 3, was in good agreement with this value. Subsequent efflux obeyed first-order kinetics with experimental rate constants, kexp, of 0.0012, 0.0037 and 0.0065 h-1 for 1b, 2b and 3, respectively. Thus, observed transport rates increased with increasing umbrella size and were proportional to K.
Figure 2.
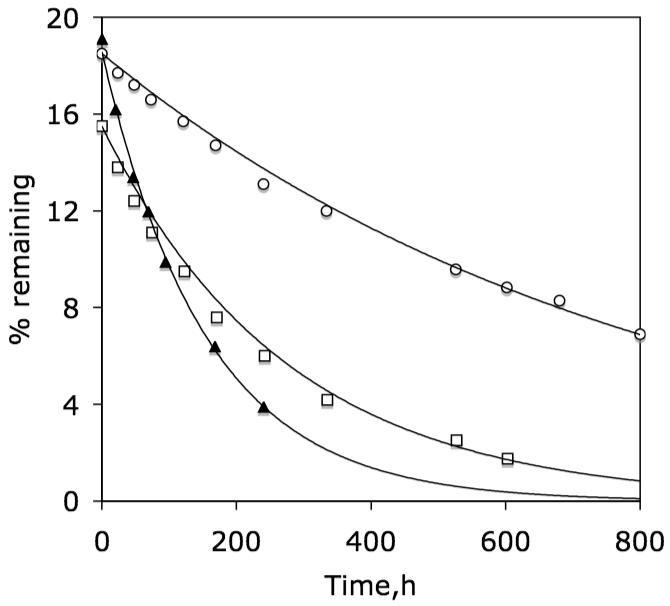
Plot of percent of 1b (◯), 2b (◻) and 3 (▴) that remains associated with liposomes made from POPC/POPG (95/5, mol/mol) as a function of dialysis time at 37°C after an initial dialysis period of 48 h at 23°C. Solid lines are first-order fits of the data.
These results are consistent with a steady state kinetic model in which the rate-determining step is the translocation of a weakly bound umbrella; that is, kexp= k1·K· L/(2·W), where k1 is the rate constant for translocation.10 For 1b, 2b and 3, this corresponds to k1 values of 0.097 h-1, 0.075 h-1 and 0.091 h-1. In the case of 4, no significant release was observed after 300 h (not shown).
In sharp contrast, the release of strongly bound 1a was biphasic in character while that of 2a could barely be detected (Figure 3). The biphasic release of 1a, together with a break point close to the “capture level”, indicates that the first stage is desorption of umbrellas bound to the exterior of the liposomes (O), and the slower second stage is the reversible translocation of umbrellas from the interior of the liposomes (I) to their exterior. These rates are consistent with the kinetic scheme, , where S is the umbrella that is released to the external aqueous phase, k1= 0.00047 h-1 and k2= 0.0060 h-1 (see Supporting Information). In the case of 2a, only the k2 value can be calculated from the initial rate (k2= 0.00037 h-1). The much slower desorption of 2a relative to 1a reflects its stronger affinity to lipid bilayers.
Figure 3.

Plot of percent of 1a (◯) and 2a (◻) that remains associated with liposomes made from POPC/POPG (95/5, mol/mol) as a function of dialysis time at 37°C after an initial dialysis period of 48 h at 23°C.
The fact that the observed transport rates of 1b, 2b, and 3 are proportional to K indicates that membrane partitioning is a dominant factor. The similarity of their k1 values further implies that a common rate-limiting step exists, which is most probably the detachment/partial dehydration of a “wet” fluorophore from the aqueous phase (Figure 1, structure A).11 Because sulfated umbrella walls should be more highly hydrated than hydroxylated walls, detachment should be easier, which would account for the greater translocation rate (i.e., higher k1 value) for the less lipophilic 1b relative to 1a.
The results presented in this paper highlight the fact that the crossing of lipid bilayers is a multistep process consisting of adsorption, dehydration, diffusion, rehydration, and desorption. Depending on the nature of the molecule to be transported, as reflected by the number, type and distribution of polar groups, its shape, and its hydrophilic/lipophilic balance (HLB), any one of these steps can become rate-limiting. These considerations should weigh heavily in optimizing the design of drugs targeted to cross biological membranes.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of General Medical Sciences (R01GM051814).
REFERENCES
- (1).Stein WD. Transport and Diffusion Across Cell Membranes. Academic Press; San Diego, CA: 1986. [Google Scholar]
- (2).Takeuchi T, Kosuge M, Tadokoro A, Sugiura Y, Nishi M, Kawata M, Sakai N, Matile S, Futaki S. ACS Chem. Biol. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- (3).Rothbard JB, Jessop TC, Lewis RS, Murray BA, Wender PA. J. Am. Chem. Soc. 2004;126:9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- (4).Potocky TB, Menon AK, Gellman SH. J. Biol. Chem. 2003;278:50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- (5).Fuchs SM, Raines RT. Biochemistry. 2003;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Pidgeon C, Ong S, Liu H, Qiu X, Pidgeon M, Dantzig AH, Munroe J, Homback WJ, Kasher JS, Glunz L, Szczerba T. J. Med. Chem. 1995;38:590–594. doi: 10.1021/jm00004a004. [DOI] [PubMed] [Google Scholar]
- (7).Boguslavsky V, Hruby VJ, O’Brien DF, Misicka A, Lipkowski A. J. Peptide Res. 2003;61:287–297. doi: 10.1034/j.1399-3011.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- (8) (a).Janout V, Staina IV, Bandyopadhyay P, Regen SL. J. Am. Chem. Soc. 2001;123:9926–9927. doi: 10.1021/ja016265a. [DOI] [PubMed] [Google Scholar]; (b) Jing B, Janout V, Herold BC, Klotman ME, Heald T, Regen SL. J. Am. Chem. Soc. 2004;126:15930–15931. doi: 10.1021/ja044400o. [DOI] [PubMed] [Google Scholar]; (c) Janout V, Regen SL. J. Am. Chem. Soc. 2005;127:22–23. doi: 10.1021/ja044257z. [DOI] [PubMed] [Google Scholar]; (d) Janout V, Jing B, Regen SL. J. Am. Chem. Soc. 2005;127:15862–15870. doi: 10.1021/ja053930x. [DOI] [PubMed] [Google Scholar]
- (9).Kondo M, Mehiri M, Regen SL. J. Am. Chem. Soc. 2008;130:13771–13777. doi: 10.1021/ja804929m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Binding data were analyzed using the nonsaturable partitioning model.7 If Co is the equilibrium concentration of the conjugate in solution as measured in the absence of liposomes, and C is the concentration in the presence of liposomes, then the partition coefficient is calculated as Here, L /2 is the concentration of the lipids in the external half of the bilayer with a weighted average molecular weight of 649 and W is the concentration of water, 55.5 M.7
- (11).For evidence against umbrella transport occurring through pores, see reference 8b.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


