Abstract
When asked to identify two visual targets (T1 and T2) embedded in a sequence of distractors, observers will often fail to identify T2 when it appears within 200–500 ms of T1— an effect called the attentional blink. Recent work shows that attention does not blink when the task is to encode a sequence of consecutive targets, suggesting that distractor interference plays a causal role in the attentional blink. Here, however, we show that an attentional blink occurs even in the absence of distractors, with two letter targets separated by a blank interval. In addition, we found that the impairment for identification of the second of two targets separated by a blank interval is substantially attenuated when the inter-target interval is either filled with additional target items or when the second target is precued by an additional target. These findings show that the root cause of the blink lies in the difficulty of engaging attention twice within a short period of time for two temporally discrete target events.
The attentional blink refers to the finding of a severe impairment for detection or identification of the second of two masked visual targets that occurs when the targets are presented within less than 500 ms of each other (Raymond, Shapiro & Arnell, 1992). This effect has long been thought to reflect the time course of capacity-limited processing required to encode the first target in working memory (e.g., Bowman & Wyble, 2007; Broadbent & Broadbent, 1987; Chun & Potter, 1995; Dehaene, Sergent & Changeux, 2003; Shapiro, Raymond & Arnell, 1994; see also Duncan, 1980; Potter, 1976). However, recent work has posed a fundamental challenge to this line of reasoning by demonstrating that observers can in fact accurately report several target items provided that these items occur in direct succession, that is, without intervening distractors (Di Lollo, Kawahara, Ghorashi & Enns, 2005). If working memory encoding is a serial, severely capacity-limited process, then why is a third target in a sequence of three consecutive targets identified more accurately than the second of two targets separated by a single to-be-ignored distractor?
Distractor-based explanations of the attentional blink
The successive target advantage observed by Di Lollo et al. (2005) has been replicated in several experiments (Kawahara, Enns & Di Lollo, 2006; Kawahara, Kumada & Di Lollo, 2006; Nieuwenstein, 2006; Nieuwenstein & Potter, 2006; Olivers, Van der Stigchel & Hulleman, 2007), and it has inspired a new line of proposals regarding the cause of the attentional blink. Central to these accounts is the notion that the blink is instigated not by encoding a first target, but, rather by interference caused by the distractor immediately trailing T1. Two possibilities have been proposed regarding the mechanism through which this distractor might cause attention to blink. According to distractor-induced suppression accounts (Kessler et al., 2005; Olivers, 2007; Olivers & Nieuwenhuis, 2006; Olivers et al., 2007; Raymond et al., 1992), interference between T1 and a non-target stimulus initiates a suppressive mechanism that is aimed at inhibiting the distractor, but also often inadvertently affects selection of a trailing target. This view explains the successive target advantage with the assumption that the suppressive response is specific to situations in which encoding of T1 is interfered with by a non-target stimulus. Thus, when the item following T1 is another target, there is no reason for suppression to occur, and consequently a trailing target can be encoded without much difficulty.
The temporary loss of control account proposed by Di Lollo and colleagues envisions a different mechanism. According to this account, identification of T1 recruits processing resources that are also required to maintain a top-down attentional set. Consequently, control over target selection is temporarily compromised during identification of T1, allowing a trailing distractor item to disturb the attentional set. According to Di Lollo et al., this effectively results in a new attentional template that is set in the image of the post-T1 item. Thus, if the post-T1 item is another target, the attentional template remains unaltered, allowing a trailing target to be processed efficiently. However, if the post-T1 item is a distractor, the newly established attentional template no longer matches the target specification, resulting in difficulty for processing a trailing target until identification of T1 is completed and top-down control is regained.
An alternative to distractor-based explanations: The delayed reengagement account
An alternative account of the successive target advantage is also possible. In particular, it is possible to explain this finding in terms of the dynamics of processes of attentional selection (e.g., Nieuwenstein & Potter, 2006; Weichselgartner & Sperling, 1987). The central premise to this account is the assumption that target stimuli elicit the deployment of attentional processing resources, much like an exogenous cue commands the deployment of attention towards its location (cf. Bowman & Wyble, 2007; Chua, Goh & Hon, 2001; Nieuwenhuis, Gilzenrat, Holmes & Cohen, 2005; Weichselgartner & Sperling, 1987; see also Nakayama & Mackeben, 1989). Accordingly, a sequence of consecutive targets can be considered to yield a state of sustained attentional engagement during which each newly presented target benefits from the attentional resources deployed in response to a preceding target. However, when the sequence of targets is discontinued – for example due to the insertion of a distractor – there is a temporary discontinuation of the input that kept attention engaged, and, consequently, attention may be disengaged prior to the onset of the next target. The difficulty in encoding a trailing target would then stem from the difficulty of rapidly reengaging attention shortly after attention was disengaged following the selection of a first target item (Nieuwenstein, 2006; Nieuwenstein, Chun, Hooge & Van der Lubbe, 2005; Nieuwenstein & Potter, 2006). That is, the difficulty in identification of T2 might stem from a delay in initiating a second episode of attentional engagement (cf. Weichselgartner & Sperling, 1987).
This delayed reengagement account yields a counterintuitive prediction that is examined in the present study: Report of the second of two targets should suffer an attentional blink even when the two targets are separated by a blank interval. This is because a blank screen is similar to a distractor in that it does not provide a bottom-up signal that can keep attention engaged in the interval leading up to T2. In addition, this account predicts that any blink effect observed with an inter-target blank should be attenuated when the disengagement of attention is counteracted by providing continuous bottom-up target input (e.g., Nieuwenstein & Potter, 2006), or when the reengagement of attention is facilitated through precuing the second target (e.g., Nieuwenstein, 2006; Nieuwenstein et al., 2005). The aim of the present study was to examine these predictions.
Does attention blink following an inter-target blank?
Several studies reported data relevant to the issue of whether attention blinks for the second of two targets separated by a blank interval. These studies have yielded mixed results. In some cases, a blink effect was indeed seen for report of the second of two targets separated by a blank interval, although it was attenuated relative to a condition in which a distractor was presented in place of the blank (Chua, 2005; Ouimet & Jolicoeur, 2007; Raymond et al., 1992; Seiffert & Di Lollo, 1997; Visser, 2007). While this finding appears consistent with the possibility that a blank inter-target interval can yield a blink due to the disengagement of attention, the procedures used in these experiments also allow for alternative interpretations. In particular, the studies by Ouimet and Jolicoeur and by Visser both used designs in which there was a switch in perceptual set and task from T1 to T2, and it is possible that this switch contributed to the observed impairment in report of T2 (e.g., Potter, Chun, Banks & Muckenhoupt, 1998).
The studies by Chua, Raymond et al., and Seiffert and Di Lollo did not involve a task switch (they all used the canonical dual-target RSVP task and compared blink magnitude between conditions in which the distractor immediately trailing T1 was absent versus present). Nevertheless, interpretation of the observed results is complicated because these studies used tasks in which the first target was a highly salient stimulus drawn from the same category as the distractors (i.e., T1 was a bright letter preceded by dim distractor letters). Under these conditions, distractors preceding T1 might interfere with selection and encoding, witness the fact that errors in identification of a salient T1 often involve reports of the item that directly preceded the target (Botella, Arend & Suero, 2004; Botella, Barriopedro & Suero, 2001; Botella, Garcia & Barriopedro, 1992; Chun, 1997; Vul, Nieuwenstein & Kanwisher, in press). According to Botella and colleagues, this effect may be accounted for by assuming that when the T1-defining feature is salient, and thus readily detected, it will often overlap in time with the representation activated by the item preceding T1. As a consequence, the T1-1 item engages in competition with T1 for selection, resulting in a prevalence of pre-T1 intrusion errors.
Studies that used conditions in which the potential for pre-T1 intrusions was low found no evidence for a blink when there was an inter-target blank. In these studies T1 was defined either by alphanumeric category or by being dimmer than preceding distractors. The results showed that a T2 that followed a short post-T1 blank interval (at an SOA of about 200 ms where the blink effect is typically most pronounced) was identified as accurately as a T2 that appeared 600 ms after T1, that is, well outside the temporal extent of the attentional blink (Breitmeyer, Ehrenstein, Pritchard, Crisan & Hiscock, 1999; Chua, 2005; Chun & Potter, 1995; Grandison, Ghirardelli & Egeth, 1997).
The present study
Previous work thus provides some indication that attention blinks even when two targets are separated by a blank interval. However, the procedures used in these studies do not allow for a definitive conclusion regarding the issue of whether this effect is due to distractor interference, switching in task and perceptual set, or, the possibility that the blank inter-target interval led to a disengagement of attention prior to the onset of T2. The goal of the present study was to provide a stricter test of whether attention blinks for the second of two targets separated by a blank interval. To this end, we used a task in which there was no switch in task or perceptual set from T1 and T2, and the potential for interference from distractors preceding T1 was low. In addition, we reduced the exposure duration of T2 to 58 ms to ensure that the T2 task would be sensitive enough to pick up on any disengagement effect1. The rationale for shortening T2's exposure duration derived from recent work showing that the main cause of T2 identification errors in the attentional blink lies in a delay in selection of T2 (e.g., Nieuwenstein, 2006; Nieuwenstein et al., 2005). Assuming that the main effect of the attentional blink is to delay selection, presenting T2 for a shorter duration means there is a greater chance that a delay incurred by T1 will be observed in T2 identification accuracy.
Experiment 1
A first goal of Experiment 1 was to examine whether the presence of inter-target distractors is a necessary condition for the occurrence of an attentional blink in a task in which the potential for interference between T1 and preceding distractors is low. To this end, we used the same task as that used by Chun and Potter (1995): Observers had to identify two letter targets embedded in a sequence of digit distractors presented at a rate of 10 items/second. To assess the role of inter-target distractors, the inter-target interval was either filled with distractors or left blank. A second goal of Experiment 1 was to probe the consequences of processing an unmasked T1 with a T2 task made more sensitive to processing delays by reducing the exposure duration of T2 from 100 to 58.3 ms.
Method
Participants
Ten volunteers from the subjects pool of the Department of Cognitive Psychology of the Vrije Universiteit participated in the experiment.
Apparatus and stimuli
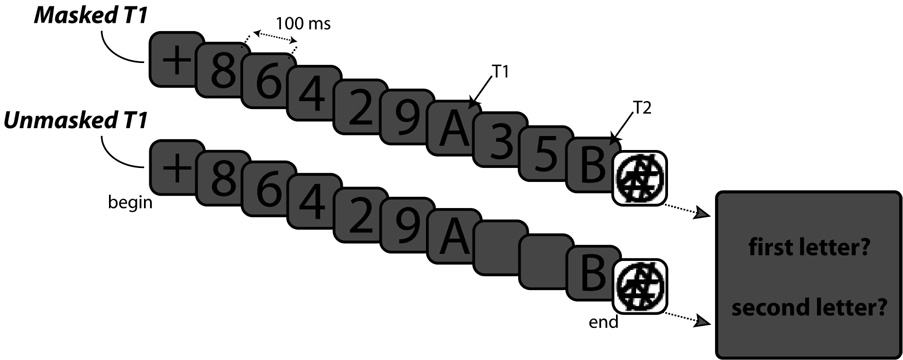
The stimuli were uppercase letters (excluding I, O, W, and M), and the digits 2–9, presented in a 20-point Helvetica font. The stimuli were presented in black on a dark gray background (RGB value 90 90 90). To mask T2, we used a pattern mask that consisted of a white square in which a circle, a pound sign, and some additional line segments were drawn in black (see Figure 1). This was done to ensure effective masking of T2 and to avoid variability in masking effects inherent in the standard procedure in which each target letter can be masked by any digit. The experiment was programmed in E-prime and the stimuli were presented on a 17-in. monitor that had a resolution of 1024 × 768 pixels and a refresh rate of 120 Hz.
Figure 1. Design Experiment 1.
Examples of the types of RSVP sequences used in the masked and unmasked T1 conditions. In these examples, two targets (T1 and T2, the letters A and B, respectively) are presented at lag 3, that is, with two distractor items intervening between them in the masked T1 condition. For the unmasked T1 condition, the interval separating the two targets was left blank and T2 could be presented for 100 ms or for 58.3 ms.
Design and procedure
The task was to identify two letters that were embedded in an RSVP sequence of digit distractors. Except for T2, each item was presented for 50 ms and followed by a 50-ms blank, yielding a presentation rate of 10 items/sec. The stimulus-onset asynchrony (SOA) of the two targets varied between 100 and 700 ms, in steps of 100 ms, excluding the 600-ms SOA. The interval separating the two targets was either filled with distractor items (the masked T1 condition where the item following T1 served as the T1 mask), or was left blank (the unmasked T1 condition). In the masked T1 condition, T2 was always presented for 100 ms. For the unmasked T1 condition, T2 was presented for 100 or 58.3 ms.
Observers began each trial by pressing the spacebar while they fixated a central fixation cross. Each sequence began with 5–8 digit distractors, followed by the two targets – with or without intervening items – and the pattern mask for T2, which was presented for 400 ms. After the offset of the mask for T2, observers were prompted to enter their responses. They were instructed to report the letters in the order in which they appeared, and they were allowed to guess if uncertain. The experiment consisted of 432 trials, divided into two blocks of 216 trials each. These trials included 24 replications for each of the 18 cells in the design (6 levels of SOA, crossed with 3 levels of masking/T2duration condition).
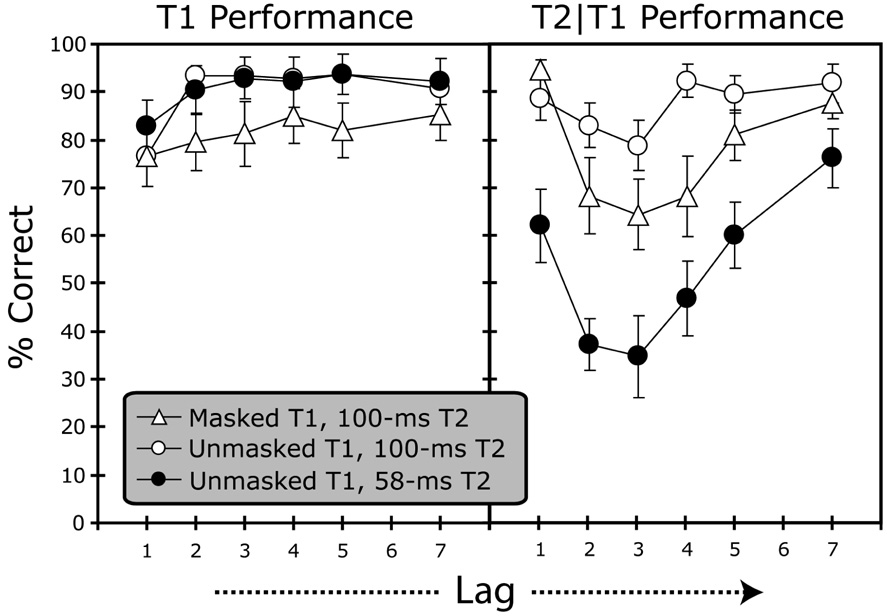
Results
Accuracy of target identification was scored with no regard to order of report. The left panel in Figure 2 shows the results for T1 identification. On average, T1 was correctly identified on 81.7% of the trials in the condition in which T1 was masked. For trials with an unmasked T1, accuracy for T1 identification was 90.1 and 90.7% for the conditions with a 100 and 58-ms T2, respectively. The results for T2 identification are shown in the right panel in Figure 2. A comparison of the masked and unmasked T1 conditions with a 100-ms T2 duration reveal the cost of masking T1: When T1 was masked, a typical U-shaped attentional blink curve was observed for T2 performance, with accurate performance at lag 1 (“lag-1 sparing”), followed by impaired identification at lags 2–4, and subsequent recovery across lags 5–7. When T1 was unmasked, T2 showed a weak effect of lag that failed to reach significance, F(1.87, 16.71; degrees of freedom corrected using Greenhouse-Geisser adjustment) = 2.49, p = .12. In striking contrast, the results for trials with a 58-ms T2 duration revealed a substantial attentional blink, with recovery taking up to lag 7 (i.e., an SOA of 700 ms). Thus, provided that the probe task is sensitive enough, a substantial attentional blink can be observed even when the two targets are separated by a blank screen.
Figure 2. Results Experiment 1.
Results for T1 identification performance and for T2 identification for trials on which T1 was correctly identified (T2|T1). Error bars show standard errors of the mean.
Experiment 2
The results from Experiment 1 show that the ostensibly simple task of identifying an unmasked letter interferes with perception of a trailing target across a period of about 500 ms. The aim of Experiment 2 was to confirm that the attentional blink seen in the blank conditions of Experiment 1 indeed stems from selection of T1, and not from some extraneous factor related to the lack of visual stimulation during the blank interval preceding T2. To examine this possibility, we manipulated whether T1 was present versus absent. The rationale was that the difference between T2 performance for trials with versus without T1 would provide an uncontaminated measure of T1-related interference.
Method
Participants
Fourteen members of the VU-subject pool volunteered to participate in the experiment, none of whom had had participated in Experiment 1.
Apparatus and stimuli
Experiment 2 was conducted using the same equipment and stimuli as Experiment 1.
Design and procedure
The trial sequences were constructed in the same way as those in the unmasked T1, 58-ms T2 condition of Experiment 1. The novel manipulation in Experiment 2 was that we omitted T1 from the sequence on 50% of the trials, replacing T1 by a digit distractor. The T1-present and the T1-absent conditions were randomly intermixed, with 30 replications for each of the 12 cells in the design (T1 present versus T1 absent crossed with 6 SOAs). At the end of each trial, the number of letters presented on that trial was indicated to avoid confusion about the number of responses required. The experiment consisted of two blocks of 180 trials each, and observers could take a break between blocks.
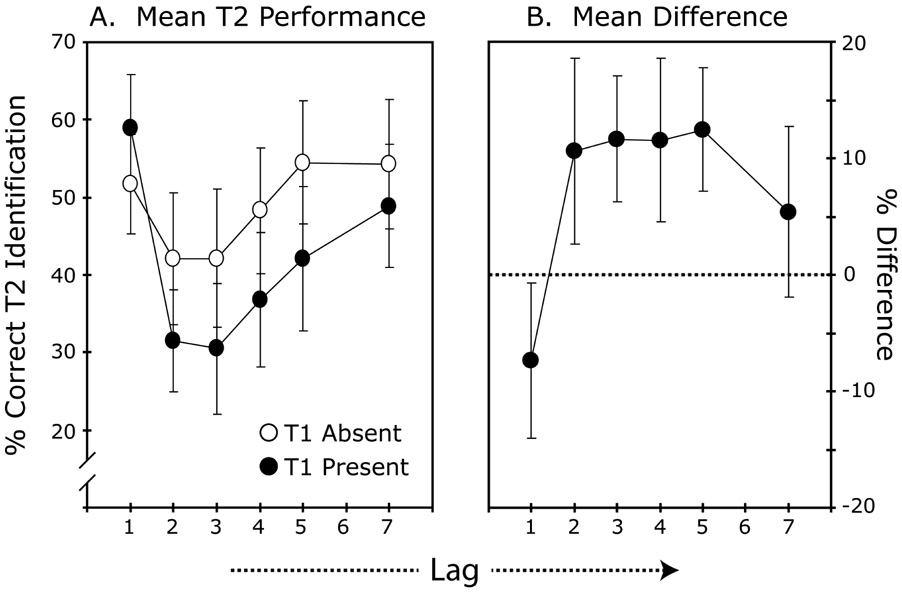
Results
When present, T1 was correctly identified on 96.5% of the trials. The effect of T1 presence versus absence on T2 performance interacted with lag, F(3.32, 43.11) = 6.79, p = .001 (see Figure 3a for mean T2 performance and Figure 3b for the mean difference in T2 performance between the T1-present and T1-absent conditions). Further analyses revealed that there was a significant effect of lag in the T1-absent condition, F(2.82, 36.71) = 4.35, p = .01. However, this effect was much less pronounced than that observed for the T1-present condition. Pair-wise comparisons between the two conditions at each lag indicated that T2 performance was better in the T1 present condition at lag 1, t(13) = 2.37, p = .03. However, at lags 2–5, performance was significantly worse when T1 was present, all p’s < .013. At lag 6, the difference was not significant, p = .14.
Figure 3. Results Experiment 2.
A. T2 identification performance for the T1-present and T1-absent conditions, plotted across lags. Results for the T1-present condition were pooled across only those trials on which T1 was correctly identified. Error bars show the standard errors of the means. B. Mean difference in T2 performance between the T1-absent and the T1-present trials with error bars showing the 95% confidence intervals.
The observed pattern of differences between T1-present and T1-absent trials reveals that T2 identification benefited from the presence of a preceding target at lag 1. In this case, performance was significantly better in T1-present trials than in T1-absent trials in which T2 was preceded by a distractor. This result corroborates the notion that target detection elicits a transient attentional response that facilitates the encoding of information across a window of about 100 ms following target onset (Bowman & Wyble, 2007; Nieuwenhuis et al., 2005; Olivers, 2007).
A surprising result was the finding that the T1-absent trials also revealed a significant lag effect, with T2 performance showing an attenuated attentional blink. This effect may have been due to the fact that the omission of T1 led to a violation of the expectancy of having to encode two targets. Since observers knew that the first target would be followed by a blank, it could be that they were temporarily distracted when they noticed the blank without having seen a preceding target. Alternatively, one could argue that the blink effect seen on T1-absent trials was due to the fact that the distractor that replaced T1 was inadvertently encoded on some trials because the lack of a backward mask enhanced the salience of this item. To differentiate between these two possibilities, we ran an additional experiment in which the T1-absent and T1-present trials were presented in separate blocks of trials. In this case, any blink effect observed for T1-absent trials could only be due to the inadvertent encoding of the unmasked distractor that appeared instead of T1. Indeed, the results for ten participants showed a blink effect for trials on which T1 was absent: Performance for T2 identification was 72% correct for lag-3 trials and 88% correct for lag-7 trials (F[1, 9] = 12.3, p = .006). When T1 was present, T2 was identified on 43% of the lag-3 trials and on 83% of the lag-7 trials. The interaction of condition (T1 absent versus T1 present) and lag (3 versus 7) was significant, F(1, 9) = 16.8, p = .002. Thus, a salient, unmasked distractor causes interference with encoding a trailing target, but this effect is much less pronounced than that observed following an unmasked target. This pattern of results is consistent with previous work showing that salient, non-target items can cause an attentional blink due to attentional capture and the inadvertent encoding of the distractor item (e.g., Maki & Mebane, 2006; Most, Chun, Widders & Zald, 2005).
Discussion of Experiment 1 and Experiment 2
The results from the first two experiments of this study show that masking of T1 is not necessary for the occurrence of an attentional blink. This finding poses a significant challenge to a large number of theories that ascribe the attentional blink to a mechanism that is instigated by non-target stimuli presented in the interval separating the two targets (Di Lollo et al., 2005; Kawahara et al., 2006; Kessler et al., 2005; Olivers, 2007; Olivers et al., 2007; Olivers & Nieuwenhuis, 2006; Raymond et al., 1992; Shapiro et al., 1994; Shapiro, Arnell & Raymond, 1997). This is not to say that the presence of distractors has no effect: The results from Experiment 1 replicate the findings from several experiments in showing that the blink magnitude is larger when T1 and T2 are separated by distractors (or a pattern mask) than when the targets are separated by a blank screen (Breitmeyer et al., 1999; Chua, 2005; Chun & Potter, 1995; Grandison et al., 1997; Ouimet & Jolicoeur, 2007; Raymond et al., 1992; Seiffert & Di Lollo, 1997; Visser, 2007). Thus, the main conclusion to be drawn from these results is that the root cause of the attentional blink lies in the difficulty in encoding two temporally discrete stimuli, and that the role of distractor interference is auxiliary in that it exacerbates the effect (for discussion of the possible mechanisms through which distractor interference may increase the blink magnitude, see Bowman & Wyble, 2007; Olivers, 2007; Wyble, Bowman & Nieuwenstein, under review).
Experiment 3
The results from Experiment 1 and Experiment 2 show that a substantial attentional blink occurs for the second of two targets separated by a blank screen. According to the delayed reengagement account, the impairment in T2 identification is due to the fact that the discontinuity in target input imposed by the inter-target blank leads to the disengagement of attention, with the subsequent problem for selecting T2 being due to the difficulty of rapidly reengaging attention when T2 is detected. This account entails that the blink effect should be attenuated if a signal triggering reengagement is presented prior to the onset of the actual target. In Experiment 3, we tested this prediction by examining whether the blink effect is reduced when T2 is preceded by an additional target. Previous studies show that this type of precuing manipulation is very powerful in RSVP tasks (e.g., Kawahara et al., 2006; Nieuwenstein, 2006; Nieuwenstein et al., 2005; Olivers et al., 2007). Under these conditions, the blink effect can be eradicated when the stimulus preceding T2 captures attention because it has a target-defining characteristic (e.g., color). The question addressed in Experiment 3 is whether this effect generalizes to the present conditions. Is the attentional blink seen for the second of two targets separated by a blank screen attenuated when the second target is directly preceded by another letter target?
Method
Participants
Twelve volunteers participated in the experiment in return for monetary compensation. None had participated in any of the other experiments in this article.
Apparatus and stimuli
Stimuli and equipment were the same as in the preceding experiments.
Design and procedure
The task and presentation conditions were the same as that used in Experiment 1 and Experiment 2: Observers had to report letter targets embedded in an RSVP sequence of digit distractors presented at a rate of 10 items per second. In the blank condition, there were two target letters. These targets were separated by a blank interval of 50, 250, or 650 ms, yielding SOAs of 100, 300 and 700 ms. In the cued condition, T2 could appear at an SOA of 300 or 700 ms, and it was directly preceded by another letter that also had to be reported. This letter was presented 100 ms prior to T2, for a duration of 50 ms (similar to the 50 on – 50 off presentation of items in the RSVP sequence). In all conditions, T2 was presented for 58.3 ms and followed a pattern mask. Observers were instructed to identify letters. The instructions informed them of the fact that any trial could contain two or three letters. At the end of each trial, the number of targets on that trial was indicated. Observers were asked to report the letters in the correct order and they were allowed to guess.
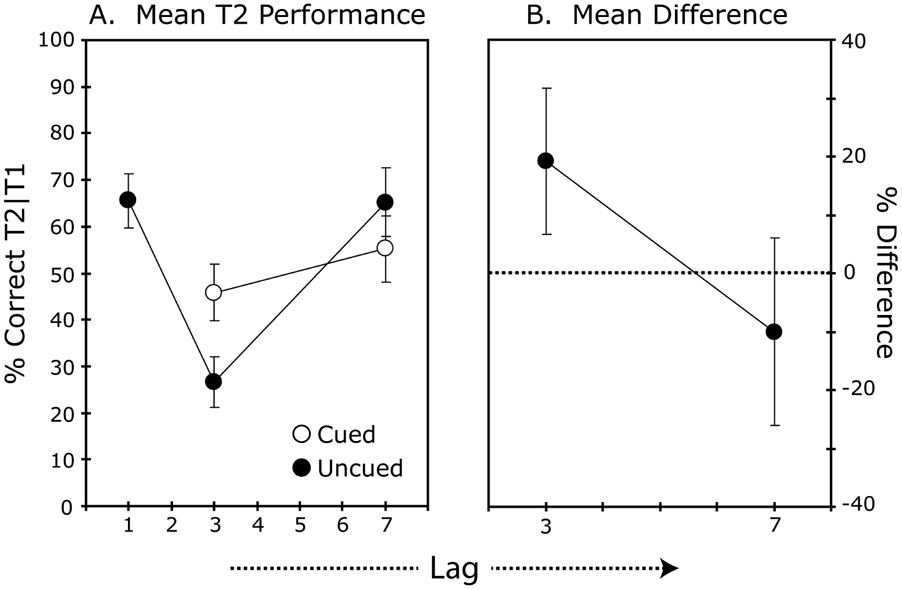
Results
On average, T1 was correctly identified in 90.6% of the trials. For the cued condition, the letter that preceded T2 was identified in 92.0% of the trials, with no difference between trials with an SOA of 300 or 700 ms. Thus, identification of this letter – which was presented for 50 ms and followed by a 50-ms blank before the onset of the trailing T2 – did not suffer an attentional blink, replicating the finding observed with an unmasked T1 and a 100-ms duration T2 in Experiment 1. The results for identification of T2 on trials in which T1 was correctly identified are shown in Figure 4a (Figure 4b shows the mean difference between performance for cued and uncued T2s at the SOAs of 300 and 700 ms). The blank condition showed the familiar attentional blink pattern. Compared to this condition, the cued condition showed a substantial improvement in T2 performance at an SOA of 300 ms (M = 26 versus M = 46% correct, t(11) = 3.4, p = .006) and a slight decrement at an SOA of 700 ms (M = 65 versus M = 55% correct, t(11) = 1.3, p = .20). This resulted in a significant interaction of SOA and Cuing, F(1, 11) = 15.10, p = .003. Thus, cuing produced a substantial benefit for report of targets presented during, but not outside of the attentional blink, corroborating the claim that the effect of the attentional blink is to delay the reengagement of attention when a new target stimulus is encountered (Nieuwenstein, 2006; Nieuwenstein et al., 2005).
Figure 4. Results Experiment 3.
A. Mean T2 identification accuracy is plotted across stimulus onset asynchrony (SOA) for trials on which T1 was correctly identified (T2|T1). Error bars show the standard errors of the means. B. Mean difference in T2 performance between the blank and cued conditions with error bars showing the 95% confidence intervals.
Experiment 4
The results from Experiment 3 show that the attentional blink is attenuated when attention is captured just prior to the onset of a second target. This confirms our hypothesis that the mechanism through which the blink affects encoding of T2 involves a delay in allocating attention to that target. In Experiment 4, we examined whether the blink is likewise attenuated for report of targets in a sequence of successive target stimuli beginning with T1. If the delay in reengaging attention is due to an earlier disengagement of attention during the blank inter-target interval, this effect should be counteracted when target input is continuous rather than temporally discrete. To examine this hypothesis, we used the same paradigm as in the preceding experiments and we compared two conditions. In the discrete targets condition, each trial contained two letter targets that were separated by a blank interval of varying duration. In the continuous targets condition, the inter-target interval was filled with additional to-be-reported letters, which appeared at the same rate as the distractor sequence preceding the letters (i.e., 10 items per second).
Method
Participants
Sixteen volunteers from the subject-pool of the Department of Cognitive Psychology of the Vrije Universiteit participated in the experiment.
Apparatus and stimuli
The stimuli and equipment were the same as in the preceding experiments. The specifications were different for the second target – i.e., the last target letter in both the discrete and continuous targets conditions. This letter was presented in a larger font (size 30), and in white so as to ensure that its onset would be salient even in the continuous targets condition where this critical target appeared shortly after a preceding letter. We reasoned that making T2 larger and white would reduce effects due to differences in onset salience of T2, which could counteract the predicted benefit from presenting a continuous sequence of targets because onset salience is bound to be higher in the discrete targets condition where T2 appeared after a longer blank interval than in the continuous targets condition. The T2 mask was scaled to match the size of T2, and its contrast was inverted.
Design and procedure
The task was to identify letters that followed after an RSVP sequence of 5–8 digit distractors. Except for T2, each item was presented for 50 ms, and followed by a 50-ms blank, yielding a presentation rate of 10 items/sec. T2 was presented for 58.3 ms and was immediately replaced by the pattern mask which was presented for 400 ms. The interval separating the two targets was either filled with additional letters that had to be reported (the continuous targets condition), or it was left blank (the discrete targets condition). The two critical targets were separated by 1, 2, 3, or 6 letters in the continuous targets condition. In the condition with discrete targets, the two targets appeared at corresponding SOAs of 200, 300, 400 and 700 ms, but now the inter-stimulus interval was left blank.
Observers began each trial by pressing the spacebar while they fixated a central fixation cross. Each sequence began with 5–8 digit distractors, followed by the two targets – with or without intervening letters – and the pattern mask for T2. After the offset of the mask for T2, observers were informed of the number of letters present on that trial, and they could type in their responses in a dialogue box on the screen. They were not forced to guess. The experiment consisted of 192 trials, divided into four blocks of 48 trials each. These trials included 24 replications for each of the 8 cells in the design (4 levels of SOA, crossed with 2 levels of condition – discrete versus continuous targets). The different trial types were randomly intermixed within each block. The experiment began with 16 practice trials, and observers could take a break after each block in the experiment.
Results
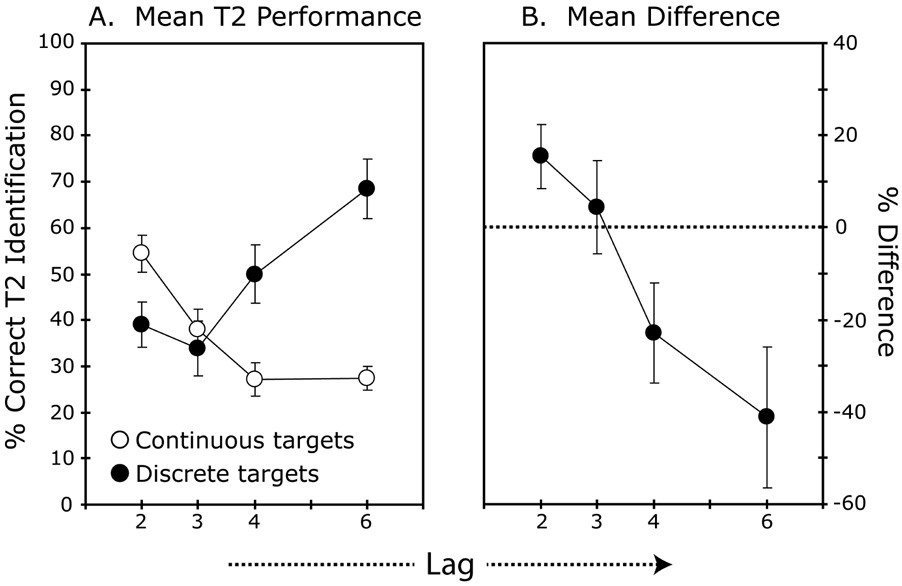
Analyses of T1 identification accuracy revealed a significant main effect of condition, F(1, 15) = 85.21, p < .001. Identification performance for T1 was close to ceiling in the discrete condition (M = 97.1%), and substantially worse in the continuous condition (M = 68.7%). The results for T2 performance are shown in Figure 5a (Figure 5b shows the mean difference between the two conditions). These are the results for all trials (the same pattern of results was seen when only trials with a correct T1 response were included). There were significant main effects of lag and condition, F(1.8, 27.4) = 6.54, p = .006, and, F(1, 15) = 7.4, p = .016, respectively, as well as a significant interaction of lag and condition, F(3, 45) = 44.34, p < .001. As can be seen in Figure 5a, T2 performance showed a substantial attentional blink when the two targets were temporally discrete events. However, when the inter-target interval was filled with additional to-be-reported items, this blink effect was strongly attenuated. At lag 2, T2 identification was more accurate in the condition with three successive targets than in the condition with two targets separated by a blank, M = 54 versus 39% correct, respectively, t(15) = 4.73, p < .001 (for those trials with a correct T1 response, average performance in the continuous and discrete conditions was 49 versus 38% correct, respectively p = .01). At lag 3, there was no difference between the two conditions, but at lags 4 and 6 performance was significantly worse with continuous targets, both p’s < .001. Thus, the requirement to encode multiple target items led to a rapid fall-off in accuracy for the last, backward masked target in the continuous targets condition. Nevertheless, performance for identifying the third target in a sequence of three consecutive targets was still better than identification of the second of two targets separated only by a blank screen. Moreover, even when we only analyzed trials with a correct T1 response (67% correct in the continuous targets condition, 97.1% correct in the discrete targets condition), we still observed attenuation of the attentional blink in the condition with continuous targets.
Figure 5. Results Experiment 4.
A. Mean T2 identification accuracy plotted across stimulus onset asynchrony (SOA). Error bars show the standard errors of the means. B. Mean difference in T2 performance between the conditions with continuous and discrete targets. Error bars show the 95% confidence intervals.
Taken together, these results strongly oppose the notion that the attentional blink for a second target is due to the depletion of working memory resources in encoding preceding items – a notion that predicts that a more difficult T1 task will cause a greater deficit in T2 report (e.g., Chun & Potter, 1995; Seiffert & Di Lollo, 1997). Instead, they provide compelling support for the hypothesis that the root cause of the attentional blink lies in the temporary discontinuation of target input in the interval separating two target items.
General Discussion
Although the attentional blink has evolved to become a standard paradigm for studying visual attention, theoretical interpretation of the phenomenon has remained a matter of intensive dispute for nearly two decades. Various accounts have been proposed that focus primarily on the role of the T1 mask. These accounts can be categorized as target-based and distractor-based explanations. Target-based explanations ascribe the attentional blink to the capacity limits of working memory processes, with the role of the T1 mask being to increase the amount of time and resources needed to encode T1 (Bowman & Wyble, 2007; Broadbent & Broadbent, 1987; Chun & Potter, 1995; Dehaene et al., 2003; Jolicoeur & Dell’Acqua, 1998; Seiffert & Di Lollo, 1997; Shapiro et al., 1997; Visser, 2007). Distractor-based accounts on the other hand assume that the attentional blink reflects a mechanism initiated not by encoding a first target, but, rather by the disruptive effect of distractor interference (Di Lollo et al., 2005; Kessler et al., 2005; Olivers, 2007; Raymond et al., 1992).
The present study shows that neither type of account provides a complete explanation of the attentional blink. In Experiment 1 and Experiment 2, we found that masking interference is not a necessary condition for the occurrence of an attentional blink. Simply encoding an unmasked, highly familiar stimulus (a letter) was found to obstruct perception of a trailing target across a period of at least 500 ms. Clearly, this finding poses a fundamental problem for distractor-based accounts which do not include any mechanism through which an unmasked target could interfere with perception of a trailing target. In addition, the sheer magnitude of interference observed following an unmasked T1 is also likely to surprise proponents of the limited-capacity view who have suggested that encoding an unmasked and familiar stimulus such as a letter is completed within about 100–200 ms (Chun & Potter, 1995; Visser, 2007). More problematic for capacity-limited accounts are the results from Experiment 3 and Experiment 4. Here, we found that encoding more target stimuli actually leads to attenuation of the attentional blink: A second target suffered a pronounced blink when it was separated by a blank interval from a preceding target, but this effect was reduced when the second target was precued by another target or when it was part of a sequence of successive targets that began with T1 (see also Di Lollo et al., 2005; Nieuwenstein, 2006; Nieuwenstein & Potter, 2006; Olivers et al., 2007). The latter finding is particularly important in this regard: With a sequence of successive targets, the processing demands for encoding the first target are high due to the fact that this target is masked by another target – that is, an item that has a high degree of conceptual and featural similarity to the leading target. Nevertheless, identification of a trailing letter was easier in this case than in a condition in which the leading target was left unmasked because only two letters had to be identified that were separated by a blank screen. Clearly, this result poses a significant problem to the notion that the cause of the attentional blink lies in the depletion of working memory resources by a first target item.
A new perspective: Competitive regulation of attention
Taken together, the present results make clear that a new perspective is needed to explain the conditions under which attention does and does not blink. Why is it that attention blinks for the second of two targets separated by a blank screen? And why is this effect reduced when the second target is cued, or when it is part of a sequence of successive target items that began with T1? Descriptively, these results suggest that the attentional blink stems from the difficulty of rapidly reengaging attention shortly after it was disengaged following selection of a preceding target stimulus. In this view, the process of reengagement is facilitated through cuing, and disengagement is counteracted by presenting sequences of successive target items.
The reasons why these manipulations are effective in counteracting the attentional blink may be further understood in the context of a recently proposed computational model of the attentional blink called the simultaneous type – serial token model (STST; Bowman & Wyble, 2007). According to this model, visual stimuli are rapidly recognized through feedforward activation of corresponding representations in ventral visual processing areas. However, under conditions of brief and masked exposure, the resulting information is equally rapidly forgotten unless it is selected for consolidation in working memory (see also Chun & Potter, 1995; Potter, 1976). The transition between a fleeting trace of perceptual input and a durable, consciously accessible memory representation is held to rely on an attentional enhancement mechanism called the blaster. This mechanism is triggered by potentially relevant stimuli and it results in a brief window of enhancement of representations activated in the first stage of processing (cf. Bowman & Wyble, 2007; Nieuwenhuis et al., 2005; Olivers, 2007; Weichselgartner & Sperling, 1987; see also Nakayama & Mackeben, 1989). Thus, once the blaster is triggered, attention is engaged. This entails that Stage-1 representations – called visual types – may engage a tokenization process through which an episodic memory representation – a token – can be formed in working memory (for further details, see Bowman & Wyble, 2007; Wyble et al., under review).
In accounting for the attentional blink, the STST model further assumes that working memory consolidation temporarily inhibits the further deployment of attention so as to prevent new inputs from exciting attention and interfering with the establishment of a distinctive episodic memory representation for the leading target. Thus, once tokenization is underway, attention is disengaged by inhibition of the blaster mechanism. This sequence of events may occur whenever an item captures attention, whether it be because the item matches top-down goals or because the item captures attention in a stimulus-driven fashion (e.g., Maki & Mebane, 2006; Most et al., 2005). In both cases, attentional capture increases the chance that tokenization occurs (see e.g., Experiment 2), and this could result in an attentional blink because tokenization inhibits the blaster.
An explanation of the successive target advantage can be found in an extension of the STST model proposed by Wyble et al. (under review). This version of the model differs from STST in assuming that inhibition of the blaster can be countermanded by a continual sequence of targets: Each newly presented target continues to excite the blaster, and, consequently the inhibitory effect of working memory encoding may be superseded by the continued excitation of the blaster. This implementation entails that there is a competitive regulation of attention allocation, such that the blaster can be triggered by new target input, even when it is inhibited by ongoing working memory processing. With this conjecture, the model is capable of explaining several key findings from research on the attentional blink, including the present results. To start, the model blinks because working memory consolidation inhibits the deployment of attention. This effect may be more pronounced when T1 encoding is more difficult (e.g., due to masking; see Breitmeyer et al., 1999; Chun & Potter, 1995; Grandison et al., 1997; Seiffert & Di Lollo, 1997), or when the T1 task involves a demanding working memory operation (e.g., memory search, mental rehearsal, or a same-different judgment based on a comparison to a memorized standard; Akyürek, Hommel & Jolicoeur, 2007; Ouimet & Jolicoeur, 2007; Visser, 2007). The mechanism through which this leads to a blink effect is through inhibition of the blaster. This entails that observers will fail to encode a trailing T2 because more time (and target input) is needed to elicit a second episode of transient attention – hence precuing T2 alleviates the blink effect. However, with a sequence of consecutive target items, the inhibitory effect of working memory processing is overruled by the continuous drive of the blaster. This results in a sustained mode of attention allocation that benefits the encoding of consecutive target items, modulo constraints imposed by competition amongst items represented in Stage 1 (e.g., Potter, Staub & O’Connor, 2002) and the limitations of working memory capacity (e.g., Alvarez & Cavanagh, 2004; Awh, Barton & Vogel, in press).
Conclusions
The starting point for the present study was the finding that observers can accurately report three consecutive targets, while they will often miss the second of only two targets separated by a distractor. This effect has been taken to suggest that the attentional blink is instigated by distractor interference. However, our results show that an attentional blink occurs even when two targets are separated by a blank interval, indicating that the root cause of the attentional blink lies in the difficulty of attending and encoding temporally discrete target events – not in the disruptive effects of distractors, and not in the depletion of processing resources by a first target item.
A second implication of the present study is that it shows that a simple modification of the standard paradigm – presenting T2 for a slightly shorter duration with an effective visual mask – yields a task that produces a robust and typical attentional blink under conditions where the first target is not masked and there is little potential for distractor interference (in an experiment not reported in this study, we found a robust attentional blink with a skeletal presentation of only the two letter targets and the T2 mask). An important advantage of this simplified paradigm is that it allows for a relatively straightforward interpretation of any observed blink effects. Using an unmasked T1, any observed effects on T2 can be ascribed directly to T1 processing and one no longer needs to take into account the myriad of effects – conceptual masking, low-level masking, filter reconfiguration, competition for central resources, suppression – that may or may not arise because of the mask.
Acknowledgment
This work was supported by NIMH grant MH47432. We thank Brad Wyble and Chris Olivers for valuable discussion and comments.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/xhp/
Previous studies that examined whether attention blinks for the second of two targets separated by a blank interval all used T2 tasks that required detection or identification of a letter that was presented for 90–100 ms. It is therefore possible that these studies failed to observe an attentional blink because the task used to probe this effect was not sensitive enough.
References
- Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- Akyürek EG, Hommel B, Jolicoeur P. Direct evidence for a role of working memory in the attentional blink. Memory and Cognition. 2007;35:621–627. doi: 10.3758/bf03193300. [DOI] [PubMed] [Google Scholar]
- Awh E, Barton B, Vogel EK. Visual working memory represents a fixed number of items, regardless of complexity. Psychological Science. doi: 10.1111/j.1467-9280.2007.01949.x. (in press). [DOI] [PubMed] [Google Scholar]
- Botella J, Arend I, Suero M. Illusory conjunctions in the time domain and the resulting time course of the attentional blink. The Spanish Journal of Psychology. 2004;7:63–68. doi: 10.1017/s1138741600004753. [DOI] [PubMed] [Google Scholar]
- Botella J, Barriopedro MI, Suero M. A model of the formation of illusory conjunctions in the time domain. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:1452–1467. doi: 10.1037//0096-1523.27.6.1452. [DOI] [PubMed] [Google Scholar]
- Botella J, Garcia ML, Barriopedro MI. Intrusion patterns in rapid serial visual presentation tasks with two response dimensions. Perception & Psychophysics. 1992;52:547–552. doi: 10.3758/bf03206716. [DOI] [PubMed] [Google Scholar]
- Bowman H, Wyble B. The simultaneous type, serial token model of temporal attention and working memory. Psychological Review. 2007;114:38–70. doi: 10.1037/0033-295X.114.1.38. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Breitmeyer BG, Ehrenstein A, Pritchard K, Hiscock M, Crisan J. The role of location specificity and masking mechanisms in the attentional blink. Perception & Psychophysics. 1999;61:798–809. doi: 10.3758/bf03206898. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Broadbent MH. From detection to identification: Response to multiple targets in rapid serial visual presentation. Perception & Psychophysics. 1987;42:105–113. doi: 10.3758/bf03210498. [DOI] [PubMed] [Google Scholar]
- Chua FK. The effect of target contrast on the attentional blink. Perception & Psychophysics. 2005;67:770–788. doi: 10.3758/bf03193532. [DOI] [PubMed] [Google Scholar]
- Chua FK, Goh J, Hon N. Nature of the codes extracted during the attentional blink. Journal of Experimental Psychology: Human Perception & Performance. 2001;27:1229–1242. [PubMed] [Google Scholar]
- Chun MM. Temporal binding errors are redistributed by the attentional blink. Perception & Psychophysics. 1997a;59:1191–1199. doi: 10.3758/bf03214207. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proceedings of the National Academy of Sciences. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara JI, Shahab Ghorashi SM, Enns JT. The attentional blink: Resource depletion or temporary loss of control? Psychological Research. 2005;69:191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Duncan J. The locus of interference in the perception of simultaneous stimuli. Psychological Review. 1980;87:272–300. [PubMed] [Google Scholar]
- Grandison TD, Ghirardelli TG, Egeth HE. Beyond similarity: Masking of the target is sufficient to cause the attentional blink. Perception & Psychophysics. 1997;59:266–274. doi: 10.3758/bf03211894. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P, Dell'Acqua R. The demonstration of short-term consolidation. Cognitive Psychology. 1998;36:138–202. doi: 10.1006/cogp.1998.0684. [DOI] [PubMed] [Google Scholar]
- Kawahara JI, Enns JT, Di Lollo V. The attentional blink is not a unitary phenomenon. Psychological Research – Psychologische Forschung. 2006;70:405–413. doi: 10.1007/s00426-005-0007-5. [DOI] [PubMed] [Google Scholar]
- Kawahara JI, Kumada T, Di Lollo V. The attentional blink is governed by a temporary loss of control. Psychonomic Bulletin and Review. 2006;13:886–890. doi: 10.3758/bf03194014. [DOI] [PubMed] [Google Scholar]
- Kessler K, Schmitz F, Gross J, Hommel B, Shapiro K, Schnitzler A. Target consolidation under high temporal processing demands as revealed by MEG. Neuroimage. 2005;26:1030–1041. doi: 10.1016/j.neuroimage.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Maki WS, Mebane MW. Attentional capture triggers an attentional blink. Psychonomic Bulletin and Review. 2006;13:125–131. doi: 10.3758/bf03193823. [DOI] [PubMed] [Google Scholar]
- Most SB, Chun MM, Widders DM, Zald DH. Attentional rubbernecking: Cognitive control and personality in emotion-induced blindness. Psychonomic Bulletin and Review. 2005;12:654–661. doi: 10.3758/bf03196754. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual-attention. Vision Research. 1989;29:1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Gilzenrat MS, Holmes BD, Cohen JD. The role of the locus coeruleus in mediating the attentional blink: A neurocomputational theory. Journal of Exprimental Psychology: General. 2005;34:291–307. doi: 10.1037/0096-3445.134.3.291. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR. Top-down controlled, delayed selection in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:973–985. doi: 10.1037/0096-1523.32.4.973. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Chun MM, Hooge ITC, Van der Lubbe RHJ. Delayed attentional engagement in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:1463–1475. doi: 10.1037/0096-1523.31.6.1463. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC. Temporal limits of selection and memory encoding: A comparison of whole versus partial report in rapid serial visual presentation. Psychological Science. 2006;17:471–475. doi: 10.1111/j.1467-9280.2006.01730.x. [DOI] [PubMed] [Google Scholar]
- Ouimet C, Jolicoeur P. Beyond task-1 difficulty: The duration of T1 encoding modulates the attentional blink. Visual Cognition. (in press). [Google Scholar]
- Olivers CNL. The time course of attention: It is better than we thought. Current Directions in Psychological Science. 2007;16:11–15. [Google Scholar]
- Olivers CNL, Nieuwenhuis S. The beneficial effects of additional task load, visual distraction, and positive affect on the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:364–379. doi: 10.1037/0096-1523.32.2.364. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Van der Stigchel S, Hulleman J. Spreading the sparing: against a capacity-limited account of the attentional blink. Psychological Research. 2007;71:126–139. doi: 10.1007/s00426-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Potter MC. Short-term conceptual memory for pictures. Journal of Experimental Psychology: Human Learning and Memory. 1976;2:509–522. [PubMed] [Google Scholar]
- Potter MC, Chun MM, Banks BS, Muckenhoupt M. Two attentional deficits in serial target search: The visual attentional blink and an amodal task-switch deficit. Journal of Experimental Psychology: Learning, Memory and Cognition. 1998;24:979–992. doi: 10.1037//0278-7393.24.4.979. [DOI] [PubMed] [Google Scholar]
- Potter MC, Staub A, O'Connor DH. The time course of competition for attention: Attention is initially labile. Journal of Experimental Psychology: Human Perception and Performance. 2002;28:1149–1162. doi: 10.1037//0096-1523.28.5.1149. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Seiffert AE, Di Lollo V. Low-level masking in the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:1061–1073. [Google Scholar]
- Shapiro KL, Arnell KM, Raymond JE. The attentional blink. Trends in Cognitive Sciences. 1997;1(8):291–296. doi: 10.1016/S1364-6613(97)01094-2. [DOI] [PubMed] [Google Scholar]
- Shapiro KL, Raymond JE, Arnell KM. Attention to visual pattern information produces the attentional blink in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:357–371. doi: 10.1037//0096-1523.20.2.357. [DOI] [PubMed] [Google Scholar]
- Visser TAW. Masking T1 difficulty: Processing time and the attentional blink. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:285–297. doi: 10.1037/0096-1523.33.2.285. [DOI] [PubMed] [Google Scholar]
- Vul E, Nieuwenstein MR, Kanwisher N. Temporal selection is diffused, delayed, and suppressed during the attentional blink. Psychological Science. doi: 10.1111/j.1467-9280.2008.02046.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichselgartner E, Sperling G. Dynamics of controlled and automatic visual attention. Science. 1987 November 6;238:778–780. doi: 10.1126/science.3672124. [DOI] [PubMed] [Google Scholar]
- Wyble B, Bowman H, Nieuwenstein MR. The attentional blink and episodic distinctiveness: Sparing at a cost. doi: 10.1037/a0013902. (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]