Abstract
Recent research examining Pavlovian appetitive conditioning has extended the associative properties of nicotine from the unconditioned stimulus or reward to include the role of a conditional stimulus (CS), capable of acquiring the ability to evoke a conditioned response. To date, published research has used pre-session extravascular injections to examine nicotine as a contextual CS in that appetitive Pavlovian drug discrimination task. Two studies in the current research examined whether a nicotine CS can function discretely, multiple times within a session using passive intravenous infusions. In Experiment 1, rats readily acquired a discrimination in conditioned responding between nicotine and saline infusions when nicotine was selectively paired with sucrose presentations. In Experiment 2, rats were either trained with nicotine paired with sucrose or explicitly unpaired with sucrose. The results showed that rats trained with explicitly unpaired nicotine and sucrose did not increase dipper entries after the infusions. Nicotine was required to be reliably paired with sucrose for control of conditioned responding to develop. Implications of these findings are discussed in relation to tobacco addiction, learning theory, and pharmacology.
Keywords: Pavlovian drug discrimination, appetitive conditioned response, IV nicotine, smoking cessation, drug stimulus
Chronic nicotine use is a major public health crisis. According to the World Health Organization, there are nearly 1.2 billion current smokers worldwide, and more than 4.9 million of them die each year of tobacco-related diseases (Esson & Leeder, 2004). While the smoking rates have remained stable in developed countries, middle and low-income countries have seen a steady increase in smoking rates. In these countries where people can least afford the economic and health costs of tobacco use, families in poverty spend up to 15% of their disposable income on tobacco products rather than on food, education, or healthcare. The United Nations as part of the Millennium Development Goals established in 2000, made a call to reduce poverty and promote health and human development worldwide (General Assembly, 2000). Cessation of tobacco use is inextricably linked to these goals because of the dramatic costs of tobacco consumption and control.
One of the objectives of the United States government is to reduce smoking rates to less than 12% of adults by 2010 (US Department of Health and Human Services, 2000). In 1997 there was a smoking prevalence of 24.7%. By 2004, this percentage had dropped to 20.9%. However, in 2006 approximately 20.8% of adults were current smokers suggesting that the decline in smoking in the United States may be stalling in recent years (CDC, 2007). Perhaps contributing to this stall is the co-occurrence of a decrease in state-funded cessation programs and an increase in tobacco company expenditures targeted at maintaining the customer base (CDC, 2007). Regardless, if the people who do receive therapy have a greater likelihood of cessation success, the population of tobacco users will decline. Among active smokers in the United States in 2000, approximately 70% wanted to quit using tobacco completely (CDC, 2002). Of these people, 41% stopped smoking for at least 1 full day during the preceding 12 months. Only 4.7% of smokers had maintained abstinence for 3–12 months. At this rate, the 2010 goal may be difficult to reach.
One way to reduce the prevalence of smoking is to improve the therapeutic techniques currently in practice. Improvements will come from diverse areas of research. These areas could inform therapies through studies concerning cultural factors, developmental effects, or genetic bases of vulnerability toward addiction. Learning theory informs another area of improvement. Indeed, a greater understanding of tobacco use based on Pavlovian conditioning has already enhanced behavioral therapy techniques. From this perspective, the unconditioned stimulus (US) effects, including the rewarding properties, of nicotine come to be associated with various non-drug stimuli that are repeatedly present during drug ingestion (Pavlov, 1927). These conditional stimuli (CSs) include environmental contexts as well as more proximal cues such as ashtrays and cigarettes. When tobacco users are later exposed to these CSs, the stimuli evoke drug-related physiological, behavioral, and subjective conditioned responses (CRs) that lead to craving and drug seeking (Bevins & Palmatier, 2004; Conklin & Tiffany, 2001; Conklin, 2006; Payne, Schare, Levis, & Colletti, 1991; Drummond, Tiffany, Glautier, & Remington, 1995; Poulos, Hinson, & Siegel, 1981; Shiffman et al., 2002; for similar discussion with opioids and cocaine, see Childress, Hole, Ehrman, Robbins, McLellan, & O’Brien, 1993). According to conditioning theory, repeated exposure to the CS without the nicotine US should decrease these drug-related CRs thus decreasing the likelihood of relapse (see Marlatt, 1990; Pavlov, 1927). Indeed, behavioral tobacco cessation therapies utilize cue exposure to help reduce the occurrence of craving in the presence of tobacco-related stimuli (see Conklin, 2006; Conklin & Tiffany, 2002).
Conceptualizing nicotine as a potent US that enters into conditioned associations with situational stimuli has advanced our understanding of nicotine addiction processes and has improved smoking cessation efforts. However, a likely contributor to the tenacity of nicotine dependence is an extension of the associative properties of nicotine. This extension includes nicotine’s interoceptive (subjective) stimulus, or CS, effects when reliably paired with another appetitive US (see Bevins & Palmatier, 2004). There has been a wealth of research in humans, monkeys, and rats showing that nicotine has interoceptive discriminative stimulus effects (e.g., Clements, Glautier, Stolerman, White, & Taylor, 1996; Morrison & Stephenson, 1969; Takada, Hagen, Cook, Goldberg, & Katz, 1988; for a review see Stolerman, 1999). Recently the pharmacological stimulus effects of nicotine have also been shown to serve as a contextual CS for a sucrose US in rats (Besheer, Palmatier, Metschke, & Bevins, 2004; Bevins & Palmatier, 2004; Bevins, Penrod, & Reichel, 2007; Murray & Bevins, 2007a,b; Palmatier & Bevins, 2007; Reichel, Linkugel, & Bevins, 2007; Wilkinson, Murray, Li, Wiltgen, Penrod, Berg, & Bevins, 2006). In that research, a subcutaneous (SC) injection of nicotine or saline was given before placement in a conditioning chamber. On nicotine sessions, liquid sucrose was delivered intermittently. On intermixed saline sessions sucrose was not available. Using head entries into the sucrose receptacle before the first sucrose delivery as a measure of conditioning (i.e., goal tracking; Boakes, 1977; Farwell & Ayres, 1979), nicotine readily served as a CS as evidenced by increased dipper entries on nicotine compared to saline sessions. Indeed, we now know some about the behavioral processes (Bevins et al., 2007; Besheer et al., 2004; Murray & Bevins, 2007b; Palmatier & Bevins, 2007; Wilkinson et al., 2006) and some about the receptor systems (Murray & Bevins, 2007a; Reichel et al., 2007) involved in the CS effects of nicotine.
While investigating various characteristics of the Pavlovian stimulus effects of nicotine, we have considered a variety of ways to extend that research to make the CS more similar to smoking in a human. Human smokers generally have a loading dose of nicotine in the mornings with frequent re-administrations that maintain desired blood levels throughout the day (Benowitz, 1996; Russell, 1989). Each repeated nicotine intake temporarily lifts blood levels above baseline and is often accompanied by enhanced mood and increased cognitive function (Parrott & Garnham, 1998; Parrott & Kaye, 1999; Warburton & Arnall, 1994). Indeed, these nicotine-induced enhancements can occur quite rapidly, after only a couple of puffs from a cigarette (Revell, 1988; Warburton & Arnall, 1994), and may be reinforcing repeated self-administration in humans (Parrott, 2006). The animal models of human smoking that are considered to have more face validity use IV nicotine rather than extravascular injections because of the faster increase in brain nicotine levels (see Benowitz, 1990; Matta et al., 2007). Intravenous administration, like inhalation, is not subject to the same process of drug absorption that occurs as a result of an extravascular injection (Benowitz, Porchet, & Jacob, 1990; Booze et al., 1999; Henningfield, Stapleton, Benowitz, Greyson, & London, 1993). Indeed, IV infusions of 0.03 mg/kg nicotine are common in rat models of nicotine self-administration (e.g., Corrigall & Coen, 1989; Corrigall & Coen, 1991; Donny, Caggiula, Knopf, & Brown, 1995; Donny, Caggiula, Mielke, Jacobs, Rose, & Sved, 1998). In those studies, rats have an operant requirement (e.g., multiple presses on a predetermined lever) to complete before an infusion. Rats will increase pressing on the lever associated with nicotine infusions relative to an inactive lever, indicating that the nicotine infusions are serving as a reinforcer (LeSage, Keyler, Collins, & Pentel, 2003; LeSage, Keyler, Shoeman, Raphael, Collins, & Pentel, 2002; Palmatier et al., 2006; Rauhut, Dwoskin, & Bardo, 2005; Rauhut, Mullins, Dwoskin, & Bardo, 2002). Presumably, this low yet reinforcing dose of nicotine is perceptible. If so, we hypothesized that a nicotine infusion would also function as an interoceptive CS for appetitive stimuli that occur in close temporal proximity.
The current study sought to test this hypothesis by assessing whether brief IV infusions of nicotine could serve as CSs for sucrose. As part of this assessment, we eliminated alternative accounts of the CR by verifying that the nicotine infusions are controlling behavior. We also established that the nicotine CS is mediated by centrally localized nicotinic acetylcholine receptors (nAChRs). Finally, we determined that the CR is susceptible to extinction when sucrose is no longer delivered and to rapid reacquisition upon representation of the sucrose US. Indeed, the finding that IV nicotine functions as an appetitive CS in the present studies has opened up a wealth of future research directions.
Experiment 1: Pavlovian nicotine discrimination
For Experiment 1 we used a discrimination procedure similar to previous nicotine CS research with SC nicotine (e.g., Besheer et al., 2004). On a given training day, a rat received either nicotine administration paired with sucrose deliveries or saline administration with no sucrose deliveries. The key difference of interest in the current experiment is that nicotine and saline were administered IV, multiple times within each session, and each nicotine infusion was followed by a single sucrose delivery. If nicotine functions as a CS in this situation, we expect it will come to control a centrally-mediated CR. We also expect that the CR will be susceptible to extinction like other Pavlovian CSs.
Methods
Subjects
Sixteen male Sprague-Dawley rats (338±4 g before start of study) were obtained from Harlan (Indianapolis, Indiana). Rats were housed individually in clear 48.3 × 26.7 × 20.3 cm (l × w × h) polycarbonate tubs lined with wood shavings. Water was continuously available in the home cage. Food (Harlan Teklad Rodent Diet) was restricted as described later. The colony was temperature and humidity controlled. All sessions were conducted during the light portion of a 12 hr light:dark cycle. Protocols were approved by the University of Nebraska-Lincoln Animal Care and Use Committee and followed the ‘Guide for the Care and Use of Laboratory Animals’ (National Research Council, 1996).
Apparatus
Eight conditioning chambers (ENV-008CT; Med Associates, Inc., Georgia, Vermont) measuring 30.5 × 24.1 × 21.0 cm (l × w × h) were used in this experiment. Each chamber was enclosed in a light and sound attenuating polyvinyl chloride cubicle fitted with a fan to provide airflow and mask noise. A houselight with two bulbs (28 V, 100 mA) was mounted on the back wall of the cubicle. It was centered side-to-side, 23.5 cm above the top of the conditioning chamber, and 5 cm below the ceiling of the cubicle. Chamber sidewalls were aluminum; the ceiling and front and back walls were clear polycarbonate. Chambers were equipped with a recessed receptacle (5.2 × 5.2 × 3.8 cm; l × w × d) on the right sidewall. A dipper arm raised a 0.1-ml cup of 26% sucrose solution (w/v) into the receptacle. An infrared emitter/detector unit, 1.2 cm into the receptacle and 3 cm from the floor, monitored head entries into the dipper. A second infrared emitter/detector unit bisected the chamber 14.5 cm from the sidewall containing the receptacle and was positioned 4 cm above the rod floor. This unit provided a measure of chamber activity. Each chamber had a computer-controlled variable-speed syringe pump (Med-Associates, PMH-100VS) that allowed solutions (nicotine or saline) to be delivered IV. Pumps were located outside the sound-attenuating cubicle. A spring leash hanging into the chamber from a swivel attached to a movable arm located outside the chamber was secured to the catheter. Tygon® tubing (AAQ04103; VWR, West Chester, PA) extended from a 5-ml syringe mounted on the syringe pump through the leash to attach to the catheter. A personal computer with Med Associates interface and software (Med-PC for Windows, version IV) controlled infusions and sucrose deliveries and recorded dipper entries and chamber activity.
Drugs
(−)-Nicotine hydrogen tartrate, hexamethonium bromide, and mecamylamine hydrochloride were purchased from Sigma (St. Louis, Missouri) or Tocris Cookson, Inc. (Ellisville, Missouri). Nicotine was mixed in 0.9% sterile saline and was adjusted to a pH of 7.0±0.2 using a dilute NaOH solution. Nicotine doses are reported in the base form; remaining drug doses are reported in salt form. Rats were assigned nicotine solutions based on their daily weights within a 20 g range. Nicotine was infused over 1 s at 0.03 mg/kg/infusion at a volume of 35.74 μl. Remaining drugs were mixed in 0.9% saline and injected SC at 1 ml/kg, 15 min before testing.
Preliminary Training
Rats were handled for at least 3 min per day for 3 days. Food was removed after handling on the last day. Dipper training began the following day. A 50-min session was conducted on each of 3 consecutive days with the session not starting until a rat’s first dipper entry. The probability of receiving sucrose decreased from 0.167 to 0.05 per 60 s over the 3 sessions (approximately 2.5 to 0.75 sucrose deliveries per minute). Rats received 20 g of food at the completion of the first 2 sessions; free access to food was returned following the third session.
Surgical Procedures
Surgical implantation of catheters occurred within 3 days of the last preliminary training session. Each rat was anesthetized with an intraperitoneal (IP) injection (1 ml/kg) of ketamine hydrochloride (100 mg/ml) followed by an IP injection (0.6 ml/kg) of xylazine hydrochloride (20 mg/ml) purchased from Midwest Veterinary Supply (Des Moines, Iowa). One end of a silastic catheter (CamCaths© IVSA28, Ely, Cambridgeshire, UK) was implanted into the external left jugular vein. The other end was positioned under the skin such that it exited just below the scapulae via a backmount through which the catheter was able to be accessed by a metal cannula. Buprenorphine hydrochloride (0.1 mg/kg) was injected SC immediately following surgery. For the evening and full day following surgery, buprenorphine (0.5 mg/kg) was available in the drinking water to mange post-surgical pain. The catheter was flushed twice a day for the duration of the experiment with 0.2 ml of sterile saline mixed with heparin (30 Units/ml; Midwest Veterinary Supply) except for the first 5 post-surgical flushes in which 0.1 ml of sterile heparinized saline was mixed with streptokinase (ca. 8000 Units/ml). Rats were allowed 5 days of recovery in their home cage with free access to food before the start of the experiments. Catheter patency was assessed with a 0.05 ml IV infusion of xylazine (20 mg/ml). This concentration produces clear motor ataxia within 5 s if the catheter is patent (cf. Bevins, 2005; Reichel, Linkugel, & Bevins, 2008; Weeks, 1972). Only rats with patent catheters were included in analyses of each phase in both experiments.
Training
Acquisition
Rats had 2-hr sessions once daily in which they received 10 infusions. On nicotine sessions, 4-s access to sucrose was given 30 s after each infusion. Sucrose was withheld on saline sessions. In order to prevent rats from timing infusions, four different MedPC programs for each session type were created. The average time to the first infusion was 11 min with a range of 8–14 min; the average time between infusions was also 11 min with a range of 8–14 min. In order to allow comparable measurement between nicotine (i.e., sucrose) and saline (i.e., no sucrose) sessions, the program types were matched for timing of infusions. Session types and programs were randomly interspersed with the restriction that no more than two nicotine or two saline sessions occurred in a row. The houselights were on for 1 min before each session; the light offset signaled the start of the session. The end of the session was also signaled by 1 min of chamber illumination.
Testing
After acquiring the discrimination, rats entered testing cycles. On the first two consecutive days of each three-day cycle, rats received one nicotine and one saline session as described earlier. If the rat met discrimination criteria, on day 3 a test session occurred in place of a training session. To meet criteria, a rat had to have higher conditioned responding (i.e., elevation scores; see later) on at least 7 of the 10 trials within the nicotine session compared to the corresponding trials within the saline session. If a rat did not meet the criteria, it continued to the next pair of nicotine and saline sessions. There were four tests. Rats received a SC injection of 5 mg/kg hexamethonium, 1 mg/kg mecamylamine, or 0.9% saline 15 min before chamber placement in a random order. A final test with 0.5 mg/kg mecamylamine was added after rats completed the other three tests. Test sessions were the same as nicotine sessions described previously (i.e., 10 nicotine infusions followed 30 s later by sucrose).
Extinction
Completion of the four test sessions was followed by another nicotine and saline training session. Rats (n=10) then received repeated nicotine sessions with no sucrose deliveries. Sucrose was reintroduced for each rat after that rat’s CR decreased by at least 50%.
Dependent Measures
The primary dependent measure was an elevation score: the number of dipper entries during the 30 s after the infusion minus the number of dipper entries in the 30-s interval before the infusion. The elevation score is a common measure in related Pavlovian conditioning research (e.g., Morris & Bouton, 2006; Murray, Li, Palmatier, & Bevins, 2007; Palmatier & Bevins, 2007; Simon & Setlow, 2006). A positive value indicates more dipper entries during the CS; 0 indicates no change from the interval before the CS. We also measured total dipper entries to examine changes across sessions, and as an index of activity, the number of infrared beam breaks in the chamber was also measured.
Data Analyses
Acquisition of the discrimination was examined using two-way repeated measures factorial analyses of variance (ANOVAs) with Drug (nicotine versus saline) as one factor and either Trial or Session (mean of the 10 trials) as the other factor. Responding on test sessions was examined using a one-way repeated measures ANOVA comparing Test Drug. Responding across trials within the test sessions was examined using a two-way repeated measures ANOVA with Test Drug as one factor and Trial as the other factor. Significant interactions were followed by pair-wise comparisons using Fisher’s Least Significant Difference (LSD) tests. Statistical significance was declared using a two-tailed rejection region of .05 for all tests.
Results
Acquisition
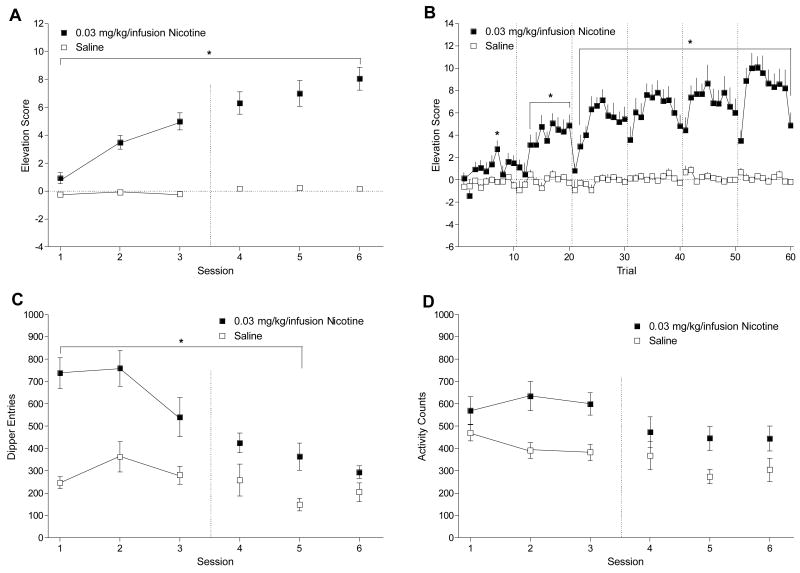
Figure 1 shows that rats readily acquired the nicotine-saline discrimination. Using the measure of mean elevation scores shown in Figure 1A, there were significant main effects of Drug, F(1, 15) = 113.85, p < .001, and Session, F(5, 75) = 25.49, p < .001, and a significant Drug × Session interaction, F(5, 75) = 17.32, p < .001, mean square error (MSE) = 2.70. Elevation scores were higher on nicotine than saline for all sessions, LSDmimimum mean difference (mmd) = 1.16. This pattern was also reflected across trials as shown in Figure 1B. There was a main effect of Drug, F(1, 15) = 113.85, p < .001, a main effect of Trial, F(59, 885) = 8.34, p < .001, and a Drug × Trial interaction, F(59, 885) = 6.19, p < .001, MSE = 9.25. Elevation scores were higher on nicotine than saline for Trials 7, 13–20, and 22–60, LSDmmd = 2.11. Importantly, the first trial within sessions was higher on nicotine than saline for sessions 4–6 (i.e., trials 31, 41, and 51) indicating that the interoceptive stimulus effects of nicotine and not the initial sucrose delivery served as the cue for session type. As goal tracking came under stimulus control, total dipper entries (Figure 1C) in the 2-hr nicotine sessions decreased. There were significant main effects of Drug, F(1, 15) = 64.68, p < .001, and Session, F(5, 75) = 15.59, p < .001, and a significant Drug × Session interaction, F(5, 75) = 4.93, p = .001, MSE = 36039.32. Total dipper entries were higher on nicotine than saline for sessions 1–5, LSDmmd = 134.24; there was no difference on session 6. For total chamber activity (Figure 1D), rats showed a general decrease in activity with repeated sessions. There was a main effect of Drug, F(1, 15) = 28.06, p < .001, indicating higher activity on nicotine than on saline. There was also a main effect of Session, F(5, 75) = 5.33, p < .001, MSE = 30192.77, denoting more activity in general on session 1 than on sessions 4, 5, and 6, LSDmmd = 86.88, but no Drug × Session interaction, F(5, 75) = 1.20, p = .319.
Figure 1.
Panel A shows the mean elevation scores (± 1 SEM) of acquisition for nicotine and saline sessions of Experiment 1. Panel B shows acquisition of the nicotine and saline discrimination (+ 1 SEM) across each trial. Panel C shows mean total dipper entries (± 1 SEM) across each session for acquisition. Panel D shows mean chamber activity counts (± 1 SEM) for each session. The vertical dashed line in Panels A, C, and D denote the start of testing. The dashed lines in Panel B separate the trials of each session. * denotes significant difference between nicotine and saline.
Testing
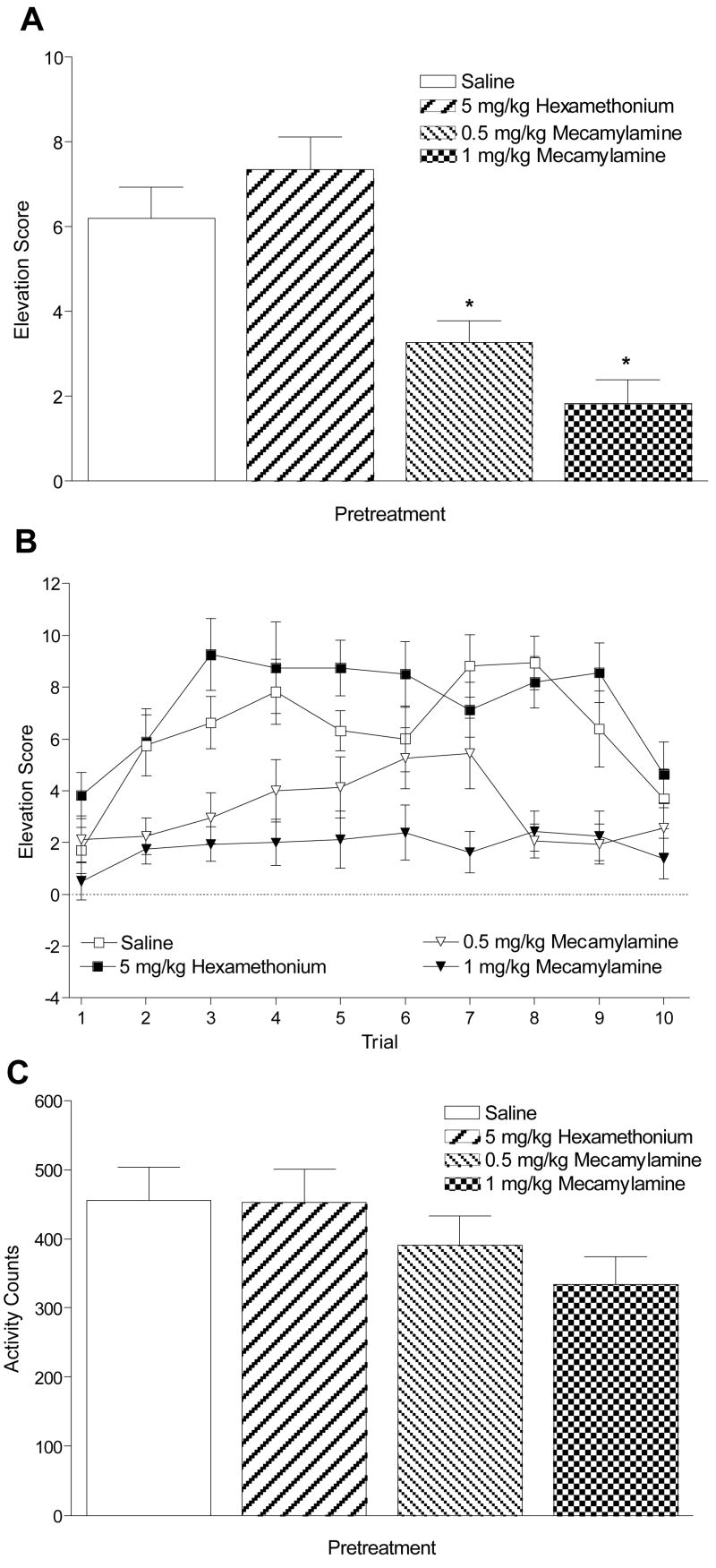
Pretreatment with mecamylamine, but not hexamethonium, blocked conditioned responding evoked by the IV nicotine CS, F(3, 45) = 17.15, p < .001, MSE = 6.06. Mean elevation scores (Figure 2A) were lower with 1 and 0.5 mg/kg mecamylamine pretreatment than saline pretreatment, LSDmmd = 1.76. Further examination showed this effect was consistent across trials (Figure 2B). There were main effects of Test Drug, F(3, 45) = 17.15, p < .001, and Trial, F(9, 135) = 7.82, p < .001, and a significant Test Drug × Trial interaction, F(27, 405) = 1.81, p = .009, MSE = 12.89. For trials 2–9, elevation scores were lower after pretreatment with 1 mg/kg mecamylamine than pretreatment with saline, and for trials 2–5 and 7–9, elevation scores were lower after pretreatment with 0.5 mg/kg mecamylamine than pretreatment with saline. There were higher elevation scores on trials 3 and 6 for 5 mg/kg hexamethonium pretreatment than saline pretreatment, LSDmmd = 2.49. There was no significant effect of Test Drug (Figure 2C) on chamber activity, F(3, 45) = 2.66, p = .06.
Figure 2.
Panel A shows mean elevation scores (+ 1 SEM) for the test sessions of Experiment 1. * denotes significant difference from saline pretreatment. Panel B shows mean elevation scores (± 1 SEM) for each trial of the test sessions. Significant differences from saline pretreatment are described in the text. Panel C shows mean (+ 1 SEM) total activity counts during test sessions.
Extinction
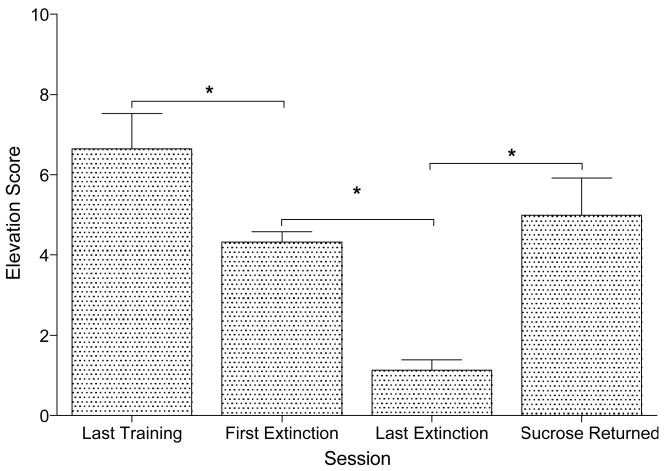
Removing sucrose deliveries from the nicotine sessions reduced the nicotine-evoked CR (Figure 3). Elevation scores for the rats’ first extinction session were lower than their last nicotine session before extinction, t(9) = 2.77, p = .022. Responding further decreased between the first and last extinction sessions (range of 3–7 sessions), t(9) = 6.69, p < .001. Responding recovered when sucrose was returned compared to responding on the last extinction session, t(9) = 4.04, p = .003.
Figure 3.
This figure shows mean elevation scores (+ 1 SEM) for the first and last extinction sessions of Experiment 1. The figure also shows the last nicotine training session before extinction and the sucrose session following extinction. * denotes significant difference between sessions.
Discussion
The interoceptive effects of a low dose of IV nicotine functioned as a CS for an appetitive outcome as shown by the differential responding on nicotine compared to saline sessions. This discrimination cannot be explained by the first sucrose delivery of the nicotine sessions being an indicator that subsequent infusions will be followed by sucrose. That is, differential responding was evoked by the first infusion of each session later in training, indicating that IV nicotine was responsible for the CR.
Consistent with the previous studies that used an extravascular injection of nicotine as the CS (e.g., Besheer et al., 2004), the CS effects of nicotine infusions were centrally mediated. Hexamethonium, an nAChR antagonist that only weakly penetrates the blood-brain barrier (Asghar & Roth, 1971), did not alter nicotine-evoked conditioned responding. Although only a single dose of hexamethonium was examined in the present study, this dose is often used for testing the central actions of nicotine (e.g., Besheer et al., 2004; Brazell et al., 1991; Loughlin et al., 2006; Stolerman et al., 1984). Mecamylamine, a nAChR antagonist that blocks both central and peripheral receptors (Papke, Sanberg, & Shytle, 2001), blocked the CR relative to saline pretreatment throughout the entire session.
Blockade of conditioned responding by mecamylamine pretreatment also suggests that peripheral effects of the infusions are not sufficiently perceptible to control conditioned responding. If the infusion alone (i.e., not the nicotine) was perceptible, then responding would likely have increased across trials during test sessions because the sucrose presentations would have been signaled. It should be noted that saline pretreatment reduced the first elevation score of the test session compared to training levels. We attribute this effect to external inhibition of the injection procedure. Because an injection had never previously occurred before a training session, the CR was slightly inhibited when the change in procedure occurred (see Pavlov, 1927). Notably, the injection procedure was the same for all test drugs including saline, making the findings across the test sessions comparable.
Finally, there was a non-significant trend for a decrease in chamber activity with mecamylamine dose that coincided with a more robust and significant decrease in elevation scores (i.e., conditioned responding). This data pattern suggests a potential relation between the conditioned response and locomotor activity during the mecamylamine tests. To assess this possibility, we conducted a multiple regression analysis using Drug Dose (saline, 0.5, and 1 mg/kg mecamylamine), Activity, and Rat Weight as the predictors for the Elevation Score. We included Weight as a predictor for two reasons. First, size of the rats might affect mobility in the in the chamber and hence alter dipper entries. Second, the size of the rat might affect absorption and distribution of mecamylamine or nicotine. Elevation scores were significantly predicted by the 3-factor model, r2 = .409, F(3, 44) = 10.14, p < .001. There was a significant contribution of Drug Dose, β = .54, p < .001, but Activity and Weight did not contribute to the model, βs ≤ .205, p ≥ .102. This model indicates a preferential impact of mecamylamine, not activity or body weight, on nicotine-evoked conditioned responding.
Finally, when repeated nicotine sessions were given without sucrose (i.e., extinction) conditioned responding decreased. This result extends research using SC injections of nicotine as a contextual CS (Besheer et al., 2004; Murray & Bevins, 2007b; Wilkinson et al., 2006) showing that the CR evoked by nicotine reflects a conditioned association between the IV nicotine infusion and the sucrose delivery. Indeed, an alternative account of the increased dipper entries with nicotine is that psychomotor stimulant effects of the nicotine somehow increased this behavior (e.g., Bevins & Palmatier, 2003; Shoaib & Stolerman, 1992). The reduction of the CR during extinction diminishes the feasibility of this account because nicotine is still being given in those sessions. In addition, re-introduction of sucrose after extinction resulted in reacquisition of the CR to nicotine in a single session. Taken together, these results support that the interoceptive stimulus effects of IV nicotine were serving as an appetitive CS.
Experiment 2: Importance of temporal contiguity
Experiment 2 was designed to extend the conditions under which the CR is observed and to provide a control to assess non-associative accounts of increased dipper entries in Experiment 1. As such, we trained one group with CS·US pairings as described previously and another group with explicitly unpaired CS and US presentations (e.g., Servatius, Brennan, Beck, Beldowicz, & Coyle-DiNorcia, 2001; Tiffany, Drobes, & Cepeda-Benito, 1992). If dipper entries increased after nicotine infusions in the Unpaired group, it would suggest that non-associative factors (see later) are responsible for the effects seen in Experiment 1.
Methods
Subjects, Apparatus, & Drugs
Fifteen male Sprague-Dawley rats (333±2 g before start of study) were housed and maintained as described earlier. The apparatus was unchanged, and nicotine was prepared and administered as described in Experiment 1.
Preliminary Training & Surgical Procedures
Rats were given the same preliminary training, surgical implantation of catheters, and recovery as described in Experiment 1.
Training
Acquisition
Rats were assigned to either the Paired group or the Unpaired group irrespective of preliminary training performance. Rats in the Paired group (n = 7) received 10 nicotine infusions followed 30 s later with 4-s access to sucrose. The average time to the first infusion was 11 min with a range of 8–14 min; the average time between infusions was also 11 min with a range of 8–14 min (cf. nicotine sessions of Experiment 1). Rats assigned to the Unpaired group (n = 8) received nicotine infusions explicitly unpaired with sucrose deliveries. The timing of infusions was identical to the Paired group. Sucrose deliveries were temporally spaced at least 4 min from nicotine infusions. Acquisition training continued for seven sessions.
Extinction
After acquisition, sucrose presentations were withheld for 11 consecutive sessions (i.e., extinction). Nicotine infusions were continued as described in acquisition.
Dependent Measures & Data Analyses
Dipper entries and activity were measured as described for Experiment 1. Acquisition and extinction were examined using two-way mixed groups ANOVAs with Group (Paired versus Unpaired) as the between-subject factor and Trial or Session (mean of the 10 trials) as the within-subjects factor. Significant interactions were followed by pair-wise comparisons using Fisher’s LSD tests. Statistical significance was declared using a two-tailed rejection region of .05 for all tests.
Results
Acquisition
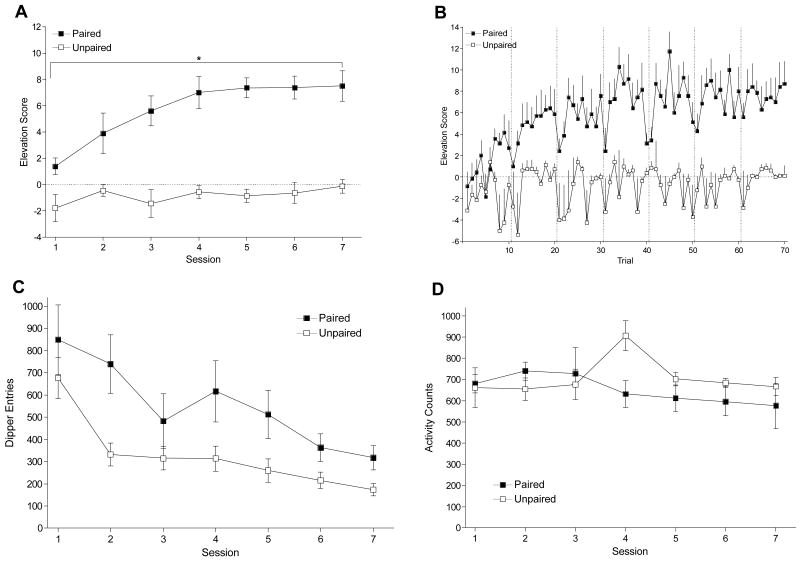
Figure 4A shows that the Paired group acquired a nicotine-specific CR; the Unpaired group did not selectively respond in the 30-s after the nicotine infusions. There were main effects of Group, F(1, 13) = 114.61, p < .001, and Session, F(6, 78) = 5.17, p < .001, and a significant Group × Session interaction, F(6, 78) = 2.68, p = .020, MSE = 5.45. Elevation scores were higher on all sessions for the Paired group compared to the Unpaired group, LSDmmd = 2.41. The same pattern was reflected across trials (Figure 4B). There was a main effect of Group, F(1, 13) = 109.19, p < .001, and of Trial, F(69, 897) = 50.24, p < .001, but no Group × Trial interaction, F(69, 897) = 1.15, p = .193. Elevation scores by trial were higher for the Paired group than the Unpaired group, Ms = 5.87±0.469 and −0.83±0.438, respectively. Similar to Experiment 1, total dipper entries (Figure 4C) decreased as goal tracking came under stimulus control. The Paired group also had more total dipper entries than the Unpaired group. There were main effects of Group, F(1, 13) = 6.54, p = .024, and Session, F(6, 78) = 13.85, p < .001, but no Group × Session interaction, F(6, 78) = 1.13, p = .351. There was no difference between groups on chamber activity (Figure 4D), Fs ≤ 2.18, ps ≥ .054.
Figure 4.
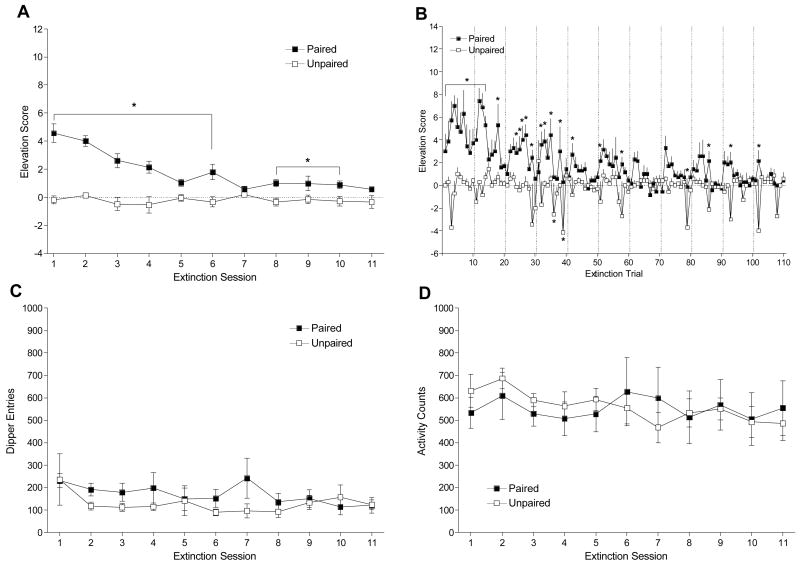
Panel A shows mean elevation scores (± 1 SEM) of each session of acquisition for Paired and Unpaired groups of Experiment 2. Panel B shows elevation scores (+ 1 SEM) across trials. Panel C shows mean total dipper entries (± 1 SEM) across each session. Panel D shows mean chamber activity counts (± 1 SEM) for each session. The vertical dashed lines in Panel B separate the trials of each session. * denotes significant difference between Paired and Unpaired groups.
Extinction
Figure 5A shows that removal of sucrose deliveries reduced the CR in the Paired group to the level of the Unpaired group. There were main effects of Group, F(1, 12) = 48.69, p < .001, and Session, F(10, 120) = 8.84, p < .001, and a significant Group × Session interaction, F(10, 120) = 8.58, p < .001, MSE = 0.79. The Paired group had higher elevation scores than the Unpaired group on extinction sessions 1 – 6 and 8 – 10, LSDmmd = 0.94. This pattern was also shown across trials (Figure 5B). There were main effects of Group, F(1, 12) = 48.69, p < .01, and Trial, F(109, 1308) = 2.00, p < .05, and a significant Group × Trial interaction, F(109, 1308) = 1.93, p < .05, MSE = 7.86. The Paired group had higher elevation scores than the Unpaired group for extinction trials 1–14, 18, 24–27, 29, 32–33, 35–36, 38–39, 42, 51, 58, 79, 86, 93, and 102, LSDmmd = 2.94. There was no difference between groups for total dipper entries (Figure 5C), Fs ≤ 1.36, ps ≥ .207, or chamber activity (Figure 5D), Fs ≤ 1.49, ps ≥ .152.
Figure 5.
Panel A shows mean elevation scores (± 1 SEM) of each session of extinction for Paired and Unpaired groups of Experiment 2. Panel B shows elevation scores (+ 1 SEM) across trials. Panel C shows mean total dipper entries (± 1 SEM) across each session. Panel D shows mean chamber activity counts (± 1 SEM) for each session. The vertical dashed lines in Panel B separate the trials of each session. * denotes significant difference between Paired and Unpaired groups.
Discussion
Only rats in the Paired group showed increased dipper entries immediately following nicotine infusions, indicating the importance of contiguity between the CS and US in acquisition of conditioned responding. Although the Unpaired group received equal nicotine presentations and sucrose deliveries, they were not temporally contiguous, and rats did not develop the CR. These findings from the Unpaired group eliminate non-associative accounts of the increased goal tracking seen in Experiment 1 and in the Paired group of this Experiment. One non-associative property of nicotine that has received a lot of recent attention is its reward-enhancing effects (e.g., Chaudhri et al., 2006, 2007; Donny et al., 2003; Olausson, Jentsch, & Taylor, 2003; Palmatier et al., 2006). In the current task, repeated sucrose presentations may be imbuing the goal receptacle with conditioned appetitive properties. If nicotine is responsible for enhancing the salience or appetitive quality of the goal receptacle, then each infusion would be followed by an increase in dipper entries. Because the groups received equal amounts of sucrose, goal receptacle quality would be enhanced to an equivalent degree, and elevation scores would have been similar in the two groups. This explanation, however, requires that rats in the Unpaired group access the sucrose so the goal receptacle acquires those similar appetitive properties. Elimination of such an account assumes that associative properties of nicotine are evoking the increase in dipper entries. We verified that rats were engaged in at least one dipper entry during each 4-s sucrose presentation. The Paired group retrieved 91.2% of their deliveries, and the Unpaired group retrieved 90.5% of their sucrose deliveries, confirming that conditioned appetitive properties of the goal receptacle were not driving dipper entries following nicotine infusions.
Finally, similar to Experiment 1, repeated nicotine presentations without sucrose resulted in a gradual decrease in conditioned responding across sessions in the Paired group. This decrease continued until it reached the response level of the Unpaired group. There was no change in dipper entries for the Unpaired group. Combined, these data support that the conditioned responding reflects a CS·US association.
General Discussion
The results of the two experiments in this report are consistent with the notion that the central nervous system (CNS) effects of a low dose of IV nicotine function as an appetitive CS. Nicotine is well-known to have profound effects in the periphery such as muscle control (e.g., Lembeck, 1999), vasodilation (e.g., Eguchi, Miyashita, Kitamura, & Kawasaki, 2007), and anti-inflammatory enhancement (e.g, Ulloa, 2005). However, blockade of peripheral nAChRs with hexamethonium did not alter the CR, whereas blockade of central and peripheral nAChRs blocked the CR, leaving the CNS to selectively mediate the nicotine CS. Notably, the present research also demonstrates a host of phenomena widely studied in more traditional Pavlovian conditioning tasks with discrete exteroceptive stimuli. This list includes an increase in conditioned responding with an increased number of trials (e.g., Kalish, 1954) and the importance of temporal contiguity (e.g., Murphy & Baker, 2004) during acquisition. In addition, there is a loss of conditioned responding with removal of the US (e.g., Ayres & DeCosta, 1971) followed by fast reacquisition of the CR upon US representation (e.g., Tomie, Hayden, & Biehl, 1980).
Indeed, even the striking pattern of within-session conditioned responding during acquisition fits within a framework of learning theory. Early-session infusions controlled lower levels of responding than infusions later in the session, followed by a downturn toward the end of the session. This inverted-U pattern of conditioned responding within sessions is similar to those studied in both Pavlovian and operant conditioning tasks (e.g., McSweeney, 1992; Servatious et al., 2001). A dual process theory of responding has been used to explain this pattern (see Groves & Thompson, 1970; McSweeney & Hinson, 1992). That account suggests that the first part of the pattern, the lower responding in the early portion of the acquisition sessions followed by increases across trials, may be the result of a response sensitization effect of the CS·US association. That is, repeated presentations of the stimuli enhance responsiveness. For example, in a study of the mouthing response to food in rat pups, initial presentations resulted in increased rates of responding (Swithers-Mulvey & Hall, 1992; see also McSweeney & Swindell, 1999). Similarly, in an operant task in which rats were trained to leverpress for food pellets there was an initial increase in response rates in the early portion of the sessions (McSweeney, 1992; see also McSweeney, Hinson, & Cannon, 1996).
The response rates in both types of studies reached a peak level, and then declined. Similarly, in the current research the decrease in responding on the later trials may be an effect of CS·US habituation due to decreased strength of the motivated goal-tracking behavior after repeated contact with the sucrose goal (McSweeney & Swindell, 1999). Alternatively, the later trial responding can be explained pharmacologically. As each successive infusion accumulates into the background brain nicotine level during the session, the perceptible change brought about by each infusion may be decreasing, resulting in decreased responding. This account, however, cannot explain the response pattern of early trials. After 22 hrs since finishing the previous session, brain nicotine levels in the rats would be nearly nothing. Therefore, the first several infusions should be highly perceptible and hence evoke a strong CR. Interestingly, the different subtypes of nAChRs responsible for the behavioral effects of nicotine differentially respond to nicotine administration (e.g., McGehee, Heath, Gelber, Devay, & Role, 1995; Pidoplichko, DeBiasi, Williams, & Dani, 1997) and may help explain the response pattern in early trials. Following activation, some receptors are desensitized to further effects of nicotine much more readily than others (e.g., Fenster, Rains, Noerager, Quick, & Lester, 1997; Wooltorton, Pidoplichko, Broide, & Dani, 2003). The effects of those receptors that experience prolonged desensitization following activation may predominate during the first couple infusions; however, those effects would not be available for conditioning in later trials. Perhaps the CR in the current research is selectively mediated by receptors that do not remain in a desensitized state during chronic nicotine exposure. Of course, more research will be required to determine the actual processes responsible for the pattern of conditioned responding.
Upon examination of the extinction across trials, a second series of intriguing within-session patterns is visible in the Paired group. There is an initial burst in conditioned responding early in sessions that drops off in later parts of the sessions. These early session increases might reflect spontaneous recovery of the CR (Bouton, 1993; Pavlov, 1927; Rescorla, 1997, 2004). The subsequent responding within each session then gradually tapers off across trials when the US does not appear. Between sessions, the degree of this apparent spontaneous recovery gradually diminishes with continued CS alone exposure. Notably, spontaneous recovery is regarded as one of the ‘threats to extinction’ of drug use (see Conklin & Tiffany, 2002). Therefore, spontaneous recovery of a CR evoked by a nicotine CS may also contribute to tobacco addiction.
Perhaps the most exciting aspects of the present research are the empirical and theoretical advances for future studies. For example, having a single nicotine infusion paired with a single sucrose delivery permits trial-by-trial measures of the nicotine CS-US association. How responding changes across a session and manipulations of the intertrial and interstimulus intervals could offer a clearer picture of the associability of the infusions. Within-session manipulations would also be possible, allowing examinations of higher-order associations such as the addition of positive or negative features that indicate when the nicotine·sucrose association is active. Because IV nicotine is used in animal models of self-administration, and because the IV route of administration is generally considered to have more face validity than extravascular routes of administration (see Benowitz, 1990; Matta et al., 2007), we can also better examine the possibility that a stimulus such as nicotine may be able to serve multiple roles at the same time (i.e., as a reinforcer and as a CS). The ability of nicotine to ‘multitask’ has been shown by Caggiula and colleagues who have demonstrated that nicotine can be a primary reinforcer while simultaneously enhancing the reinforcing effects of other stimuli (e.g., Chaudhri et al., 2006; Chaudhri et al., 2007; Palmatier et al., 2006). As such, it is of interest to determine whether the CS effects of nicotine can modify the rate of IV nicotine self-administration when the CS properties are manipulated. If so, these multifaceted characteristics of nicotine can be incorporated into models of tobacco dependence and relapse. It may be the case that the limited efficacy of behavioral therapy as a smoking cessation technique could be due to the need to consider this CS property of nicotine along with other aspects of the individual’s learning history. These therapies rely on repeated, non-reinforced exposure of drug-related stimuli (see Conklin, 2006; Conklin & Tiffany, 2002). If the stimulus properties of nicotine are capable of forming compound CSs with the environmental stimuli that are extinguished during cue-exposure therapy, it may be a step in explaining the partial effectiveness of these therapies. Using IV nicotine as a CS would relatively easily allow for examination of cue competition between nicotine and other stimuli. Overall, this research could significantly enhance our understanding of how the nicotine in tobacco may modulate behavior, and subsequently enhance the success of cessation treatments.
Acknowledgments
We thank Chia Li, Jessica D. Linkugel, Rachel D. Penrod, Carmela M. Reichel, and Jamie L. Wilkinson for their assistance with surgeries. We also thank Steven B. Harrod for his thoughtful comments on an earlier version of this report. The research and Rick A. Bevins were supported by United States Public Health Service grants DA018114. MED-PC programs used in the present article are available in a modified version upon request.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
References
- Asghar K, Roth LJ. Entry and distribution of hexamethonium in the central nervous system. Biochemical Pharmacology. 1971;20:2787–2795. doi: 10.1016/0006-2952(71)90189-4. [DOI] [PubMed] [Google Scholar]
- Ayers JJB, DeCosta MJ. The truly random control as an extinction procedure. Psychonomic Science. 1971;24:31–33. [Google Scholar]
- Benowitz NL. Pharmacokinetic considerations in understanding nicotine dependence. In: Bock G, Marsh J, editors. The biology of nicotine dependence. Chichester, New York: Wiley; 1990. pp. 186–209. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: Addiction and therapeutics. Annual Review of Pharmacology and Toxicology. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P. Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott S, Russell MAH, Stolerman IP, editors. Nicotine Psychopharmacology: Molecular, Cellular, and Behavioural Aspects. Oxford UK: Oxford University Press; 1990. pp. 112–157. [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA. The reference-dose place conditioning procedure yields a graded dose-effect function. International Journal of Comparative Psychology. 2005;18:101–111. [Google Scholar]
- Bevins RA, Palmatier MI. Nicotine-conditioned locomotor sensitization in rats: assessment of the US-preexposure effect. Behavioural Brain Research. 2003;14:365–374. doi: 10.1016/s0166-4328(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Reviews. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Penrod RD, Reichel CM. Nicotine does not produce state-dependent effects on learning in a Pavlovian appetitive goal-tracking task in rats. Behavioural Brain Research. 2007;177:134–141. doi: 10.1016/j.bbr.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. New Jersey: Erlbaum; 1977. pp. 67–97. [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: Gender differences and gonadal hormones. Pharmacology, Biochemistry and Behavior. 1999;64:827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Brazell MP, Mitchell SN, Gray JA. Effect of acute administration of nicotine on in vivo release of noradrenaline in the hippocampus of freely moving rats: a dose-response and antagonist study. Neuropharmacology. 1991;30:823–833. doi: 10.1016/0028-3908(91)90116-s. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarette smoking among adults---United States, 2000. MMWR. 2002;51(29):642–645. [PubMed] [Google Scholar]
- CDC. Cigarette smoking among adults---United States, 2006. MMWR. 2007;56(44):1157–1161. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology. 2006;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Research Monograph. 1993;137:73–95. [PubMed] [Google Scholar]
- Clements K, Glautier S, Stolerman IP, White JAW, Taylor C. Classical conditioning in humans: Nicotine as CS and alcohol as US. Human Psychopharmacology. 1996;11:85–95. [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Experimental and Clinical Psychopharmacology. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Experimental and Clinical Psychopharmacology. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology. 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology. 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Cue exposure in understanding and treating addictive behaviours. In: Drummond DC, Tiffany ST, Glautier S, Remington B, editors. Addictive behavior: Cue exposure theory and practice. Oxford, England: John Wiley & Sons; 1995. pp. 1–17. [Google Scholar]
- Eguchi S, Miyashita S, Kitamura Y, Kawasaki H. Alpha3beta4-nicotinic receptors mediate adrenergic nerve- and peptidergic (CGRP) nerve-dependent vasodilation induced by nicotine in rat mesenteric arteries. British Journal of Pharmacology. 2007;151:1216–1223. doi: 10.1038/sj.bjp.0707331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esson CM, Leeder SR. The Millennium Development Goals and Tobacco Control: An Opportunity for Global Partnership. France: World Health Organization; 2004. [Google Scholar]
- Farwell BJ, Ayres JJB. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (“goal-tracking”) in rats. Learning and Motivation. 1979;10:295–312. [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RAJ. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. The Journal of Neuroscience. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- General Assembly. United Nations Millennium Declaration. Resolution 55/2. 2000 Retrieved February 21, 2008, from http://www.un.org/millennium/declaration/ares552e.pdf.
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug and Alcohol Dependence. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Kalish HI. Strength of fear as a function of the number of acquisition and extinction trials. Journal of Experimental Psychology. 1954;47:1–9. doi: 10.1037/h0053732. [DOI] [PubMed] [Google Scholar]
- Lembeck F. Epibatidine: high potency and broad spectrum activity on neuronal and neuromuscular nicotinic acetylcholine receptors. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1999;359:378–385. doi: 10.1007/pl00005364. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology. 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacology, Biochemistry, & Behavior. 2002;72:279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. The Journal of Comparative Neurology. 2006;497:575–588. doi: 10.1002/cne.20999. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addictive Behaviors. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJS, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McSweeney FK. Rate of reinforcement and session duration as determinants of within-session patterns of responding. Animal Learning & Behavior. 1992;20:160–169. [Google Scholar]
- McSweeney FK, Hinson JM. Patterns of responding within sessions. Journal of the Experimental Analysis of Behavior. 1992;58:19–36. doi: 10.1901/jeab.1992.58-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney FK, Hinson JM, Cannon CB. Sensitization-habituation may occur during operant conditioning. Psychological Bulletin. 1996;120:256–271. [Google Scholar]
- McSweeney FK, Swindell S. General-process theories of motivation revisited: The role of habituation. Psychological Bulletin. 1999;125:437–457. [Google Scholar]
- Morris WR, Bouton ME. Effect of unconditioned stimulus magnitude on the emergence of conditioned responding. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:371–385. doi: 10.1037/0097-7403.32.4.371. [DOI] [PubMed] [Google Scholar]
- Morrison CF, Stephenson JA. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia. 1969;15:351–360. doi: 10.1007/BF00403710. [DOI] [PubMed] [Google Scholar]
- Murphy RA, Baker AG. A role for CSyUS contingency in Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:229–239. doi: 10.1037/0097-7403.30.3.229. [DOI] [PubMed] [Google Scholar]
- Murray JE, Li C, Palmatier MI, Bevins RA. The interoceptive Pavlovian stimulus effects of caffeine. Pharmacology, Biochemistry and Behavior. 2007;86:838–846. doi: 10.1016/j.pbb.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. Behavioral and neuropharmacological characterization of nicotine as a conditional stimulus. European Journal of Pharmacology. 2007a;561:91–104. doi: 10.1016/j.ejphar.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Bevins RA. The conditional stimulus effects of nicotine vary as a function of training dose. Behavioural Pharmacology. 2007b;18(8):707–716. doi: 10.1097/FBP.0b013e3282f14ec6. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA. Facilitation by drug states does not depend on acquired excitatory strength. Behavioural Brain Research. 2007;176:292–301. doi: 10.1016/j.bbr.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. Journal of Pharmacology and Experimental Therapeutics. 2001;297:646–656. [PubMed] [Google Scholar]
- Parrott AC. Nicotine psychobiology: how chronic-dose prospective studies can illuminate some of the theoretical issues from acute-dose research. Psychopharmacology. 2006;184:567–576. doi: 10.1007/s00213-005-0294-y. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Garnham NJ. Comparative mood states and cognitive skills of cigarette smokers, deprived smokers, and non-smokers. Human Psychopharmacology. 1998;13:367–376. [Google Scholar]
- Parrott AC, Kaye FJ. Daily uplifts, hassles, stresses and cognitive failures: in cigarette smokers, abstaining smokers, and non-smokers. Behavioural Pharmacology. 1999;10:639–646. doi: 10.1097/00008877-199911000-00010. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relavent cues: Effects on desire to smoke and topographical components of smoking behavior. Addictive Behaviors. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Poulos CX, Hinson RE, Siegel S. The role of Pavlovian processes in drug tolerance and dependence: Implications for treatment. Addictive Behaviors. 1981;6:205–211. doi: 10.1016/0306-4603(81)90018-6. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine & Tobacco Research. 2005;7:901–907. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Mullins SN, Dwoskin LP, Bardo MT. Reboxetine: attenuation of intravenous nicotine self-administration in rats. The Journal of Pharmacology and Experimental Therapeutics. 2002;303:664–672. doi: 10.1124/jpet.303.2.664. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Nicotine as a conditioned stimulus: Impact of attention deficit/hyperactivity disorder medication. Experimental and Clinical Psychopharmacology. 2007;15:501–509. doi: 10.1037/1064-1297.15.5.501. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Linkugel JD, Bevins RA. Bupropion differentially impacts acquisition of methamphetamine self-administration and sucrose-maintained behavior. Pharmacology, Biochemistry and Behavior. 2008;89:463–472. doi: 10.1016/j.pbb.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery after Pavlovian conditioning with multiple outcomes. Animal Learning & Behavior. 1997;25:99–107. [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learning & Memory. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Revell AD. Smoking and performance – a puff-by-puff analysis. Psychopharmacology. 1988;96:563–565. doi: 10.1007/BF02180043. [DOI] [PubMed] [Google Scholar]
- Russell MA. Subjective and behavioural effects of nicotine in humans: some sources of individual variation. Progress in Brain Research. 1989;79:289–302. doi: 10.1016/s0079-6123(08)62488-7. [DOI] [PubMed] [Google Scholar]
- Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K. Stress facilitates acquisition of the classically conditioned eyeblink response at both long and short interstimulus intervals. Learning and Motivation. 2001;32:178–192. [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. MK801 attenuates behavioural adaptation to chronic nicotine administration in rats. British Journal of Pharmacology. 1992;105:514–515. doi: 10.1111/j.1476-5381.1992.tb09010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiology of Learning and Memory. 2006;86:305–310. doi: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Inter-species consistency in the behavioural pharmacology of nicotine dependence. Behavioural Pharmacology. 1999;10:559–580. doi: 10.1097/00008877-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology. 1984;84:413–419. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- Swithers-Mulvey SE, Hall WG. Control of ingestion by oral habituation in rat pups. Behavioral Neuroscience. 1992;106:710–717. doi: 10.1037//0735-7044.106.4.710. [DOI] [PubMed] [Google Scholar]
- Takada K, Hagen TJ, Cook JM, Goldberg SR, Katz JL. Discriminative stimulus effects of intravenous nicotine in squirrel monkeys. Pharmacology, Biochemistry, and Behavior. 1988;30:243–247. doi: 10.1016/0091-3057(88)90452-2. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ, Cepeda-Benito A. Contribution of associative and nonassociative processes to the development of morphine tolerance. Psychopharmacology. 1992;109:185–190. doi: 10.1007/BF02245498. [DOI] [PubMed] [Google Scholar]
- Tomie A, Hayden M, Biehl D. Effects of response elimination procedures upon the subsequent reacquisition of autoshaping. Animal Learning & Behavior. 1980;8:237–244. [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nature Reviews Drug Discovery. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2. Washington DC: US Department of Health and Human Services; [Google Scholar]
- Warburton DM, Arnall C. Improvements in performance without nicotine withdrawal. Psychopharmacology. 1994;115:539–542. doi: 10.1007/BF02245578. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Long-term intravenous infusion. In: Myers RD, editor. Methods in Psychobiology. New York, NY: Academic Press; 1972. pp. 155–168. [Google Scholar]
- Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, et al. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as function of number of conditioning trials and unpaired sucrose deliveries. Behavioural Pharmacology. 2006;17:161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]
- Wooltorton JRA, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. The Journal of Neuroscience. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]