Abstract
Objectives
Development of imaging agents for pancreatic beta cell mass may provide tools for studying insulin-secreting beta cells and their relationship with diabetes mellitus. In this paper a new imaging agent, [18F](+)-2-oxiranyl-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline [18F](+)4, which displays properties targeting vesicular monoamine transporter 2 (VMAT2) binding sites of beta cells in the pancreas, was evaluated as a PET (positron emission tomography) agent for estimating beta cell mass in vivo. The hydrolyzable epoxide group of (+)4 may provide a mechanism for shifting biodistribution from liver to kidney thus, reducing the background signal.
Methods
Both 18F and 19F labeled (+) and (−) isomers of 4 were synthesized and evaluated. Organ distribution was carried out in normal rats. Uptake of [18F](+)4 in pancreas of normal rats was measured and correlated with blocking studies using competing drugs, (+)dihydrotetrabenazine, (+)-DTBZ or 9-fluoropropyl-(+)dihydro tetrabenazine (FP-(+)-DTBZ, (+)2).
Results
In vitro binding study of VMAT2 using rat brain striatum showed a Ki value of 0.08 and 0.15 nM for the (+)4 and (±)4, respectively. The in vivo biodistribution of [18F](+)4 in rats showed the highest uptake in the pancreas (2.68 %ID/g at 60 min post-injection). In vivo competition experiments with cold FP-(+)-DTBZ, (+)2, (3.5 mg/kg, 5 min iv pretreatment) led to a significant reduction of pancreas uptake (85 % blockade at 60 min). The inactive isomer [18F](−)4 showed significantly lower pancreas uptake (0.22 %ID/g at 30 min post-injection). Animal PET imaging studies of [18F](+)4 in normal rats demonstrated an avid pancreatic uptake in rats.
Conclusion
The preliminary results suggest that the epoxide, [18F](+)4, is highly selective in binding to VMAT2 and it has an excellent uptake in the pancreas of rats. The liver uptake was significantly reduced through the use of the epoxide group. Therefore, it may be potentially useful for imaging beta cell mass in the pancreas.
Keywords: Beta cell mass, vesicular monoamine transporter 2, diabetes, tetrabenazine and PET
1. Introduction
The pancreas is a dual-function organ (active as endocrine and exocrine organ). As such, parasympathetic and sympathetic neurons terminate in the pancreas and these neurons tightly control the functions [1, 2]. The endocrine tissue accounts for only a small percentage of the pancreatic mass (about 1 to 2 %); it is found scattered in the islets of Langerhans and comprised predominantly of beta cells. Beta cells produce insulin in response to metabolic demands, and loss of beta cells results in insulin deficiency and diabetes. In type 1 diabetes, common in juveniles, an autoimmune process dramatically disables beta cells. In type 2 diabetes, beta cell mass decreases as part of a slower, more insidious process that also involves peripheral insulin resistance and increased demand for insulin. Since there is a significant beta cell mass reserve, symptoms related to unstable glucose homeostasis are not obvious until beta cell mass has been reduced by more than 50–60 % [3]. Unfortunately, most studies measuring beta cell mass have relied on post-mortem examination of the pancreas because until recently it has been impossible to prospectively measure beta cell mass in vivo.
Although there is no cure for diabetes, several promising therapies for modifying the disease course are in clinical trials. These approaches mainly focus on preserving or replacing beta cell mass and include pancreas and/or islet cell transplantation [4–8] or islet cell regeneration from stem cells. The ability to monitor beta cell mass in vivo would greatly facilitate development of disease modifying therapies for diabetes mellitus [4–9]. Studies using labeled beta cell specific antibodies and antibody fragments as in vivo imaging agents have shown some promise but are not suitable for routine clinical use due to a relatively low cellular specificity (low pancreas accumulation vs. high liver and kidney accumulations) [10]. Additional beta cell mass ligands have been reported, but except for VMAT2 imaging agents, none have been successfully utilized to image diabetes in humans [1, 5–8, 10–17].
PET imaging of VMAT2 binding sites in the basal ganglia area of the brain using [11C](+)-dihydrotetrabenazine ([11C](+)-DTBZ) (Fig. 1) has been successfully applied in the diagnosis of Parkinson’s disease for the past decade [18–20]. Recently, high level of VMAT2 gene expression in the pancreas was detected [21–23]. The feasibility of using [11C](+)-DTBZ, a VMAT2 ligand, for PET imaging of the pancreas in monkeys and humans has also been reported [3, 21, 24, 25]. However, 11C has a very short half-life (t1/2 = 20 min). Analogs of DTBZ labeled with 18F, which has a longer half-life (t1/2 =110 min), would be more practical for a wide spread application. Previously, we had successfully tested a novel DTBZ derivative, an optically pure fluoropropoxyl- derivative (+)2) (FP-(+)-DTB) (Fig. 1) in animals [26, 27]. It displayed excellent binding affinities (Ki = 0.11 nM) for VMAT2. Using [18F](+)2, PET imaging of rat pancreas has been successfully reported [26–28].
Fig. 1.
Chemical structures of three ligands for VMAT2 binding: [11C](+)-DTBZ, [18F](+)2 and [18F](+)4.
Despite the success of using [18F](+)2 for PET imaging, it is not ideal for mapping beta cell mass in the pancreas. One of the major obstacles for pancreas imaging is the anatomical proximity of pancreas to liver, which contributes to a high background noise. The liver is a highly active organ, which traps a large number of lipophilic compounds. It subsequently secretes the sequestered lipophilic compounds through the bile or metabolizes the compounds through oxidative mechanisms leading to more water-soluble metabolites. Thus, they are readily excreted through kidney. The tetrabenazine derivatives, such as (±)-TBZ, (+)-DTBZ and (+)2, are neutral and inherently very lipophilic; therefore, normally liver excretion is the predominant route of excretion for these molecules. To further explore the feasibility of imaging beta cell mass, we have made an effort to shift the in vivo excretion pattern from the liver to the kidney, because the kidney is located distant from the target organ, the pancreas. We have designed a new “metabolizable” epoxide derivative of tetrabenazine (TBZ), which contains an epoxide ring at the C2 position of the tetrabenazine core structure (Fig. 1). By adding the epoxide group we can preserve the binding affinity to VMAT2 sites. It is hypothesized that the epoxide ring will be less stable in vivo. When the epoxide ring is opened, or “hydrolyzed”, in vivo, the resulting hydroxyl derivative will likely be more water-soluble. As such, the in vivo kinetics of the hydrolyzed epoxide tracer will show a lower liver accumulation and shift the metabolic path of the tracer towards kidney excretion. By lowering the liver uptake and retention we hope to improve the signal to noise ratio, and provide an enhanced contrast between the pancreas and the background (higher pancreas to liver ratio). The choice of selecting epoxide as an in vivo labile tracer, which may be “hydrolyzed” in vivo, is based on a large number of literature reports suggesting that mammalian epoxide hydrolases commonly present in all types of cells may likely hydrolyze the epoxide ring in vivo [29–31]. We report herein the synthesis and initial evaluation of the pancreas localization of [18F](+)-2-oxiranyl-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline, [18F](+)4, in normal rats.
2. Materials and Methods
2.1 General Methods
All reactions were performed under a dry nitrogen atmosphere. Dichloromethane (DCM) was distilled from CaH2. Reactions were monitored by TLC analysis (Analtech, Uniplate, silica gel HLF, 250 μm layer thickness). Reagents were purchased from Aldrich, Acros and Alfa Co. without further purification. Biotage Flash 40+ chromatography system and preparative thin layer chromatography were used to purify the crude reaction mixtures. NMR spectra were recorded in CDCl3 (unless otherwise noted) at 200 MHz (1H NMR) and 50 MHz (13C NMR) using a Bruker DPX instrument with XWIN-NMR software. Coupling constants are reported in hertz. Multiplicities are defined as: s (singlet), d (doublet), dd (doublet of doublet), t (triplet), br (broad), m (multiplet). Preparative thin layer chromatography (PTLC) was performed with Analtech Uniplate (20 × 20; 2000 μm m). Chromatography refers to flash chromatography on silica gel, unless otherwise indicated. Electrospray ionization mass spectra were recorded with LC MSD TOF, Agilent Technologies. The specific rotation was measured on a Perkin-Elmer 243B Polarimeter with Na light source at D line (589 nm) using CHCl3 as solvent. Chiral columns were obtained from Regis Technology Co., (http://www.registech.com/InfoPages/ChiralInfo).
Male rats weighing 200–250 g were used for the in vivo studies. The animal experiments were approved by the IACUC of the University of Pennsylvania in compliance with NIH guidelines. [3H](±)-TBZ and (±)-TBZ were kindly provided by NIH under the chemical synthesis and drug supply program.
2.1.1 (±)-3-Isobutyl-9-(3-fluoropropoxy)-10-methoxy-3,4,6,7-tetrahydro-1H-pyrido[2,1-a]isoquinolin-2(11bH)-one, (±)3
To a solution of oxalyl chloride (160 mg, 1.3 mmol) in DCM (4 mL) was added a solution of DMSO in DCM (2 mL) dropwise at −78 °C. The mixture was stirred at this temperature for 5 min. Starting material (±)2, reported previously [26], (46 mg, 0.13 mmol) in DCM (4 mL) was added dropwise and the mixture was stirred at −78 °C for 30 min. Et3N was added dropwise and the resulting mixture was stirred at −78 °C for 10 min. The mixture was warmed to room temperature and water was added. The mixture was extracted with DCM. The combined organic layers was dried over Na2SO4, filtered, and concentrated to give the crude product, which was purified by PTLC (Hex:EA = 1:1) to give 37 mg (80.9%) of product, (±)3.
1H NMR 0.88 (d, J = 6.3 Hz, 3H), 0.90 (d, J = 6.3 Hz, 3H), 0.95–1.05 (m, 1H), 1.54–1.84 (m, 2H), 2.10–2.39 (m, 3H), 2.46–2.77 (m, 4H), 2.88 (d,d, J = 13.5, 3.0 Hz, 1H), 3.04–3.14 (m, 2H), 3.27 (d,d, J = 11.4, 6.1 Hz, 1H), 3.48 (d, J = 11.9 Hz, 1H), 3.79 (s, 6H), 4.52 (t, J = 5.7 Hz, 2H), 4.76 (t, J = 5.7 Hz, 2H), 6.55 (s, 1H), 6.64 (s, 1H).
The same procedure described above using (+)2 as the starting material gave (+)3 in 82.9% yield. (+)3: [α]D = +61.18° (CHCl3).
2.1.2 (±)-2-Oxiranyl-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline, (±)4
To a mixture of NaH (7.7 mg, 60% in mineral oil, 0.19 mmol) in dry DMSO (2 mL) was added Me3SOI (42 mg, 0.19 mmol) in portions. The mixture was stirred at room temperature for 20 min. Starting material 3 (35 mg, 0.1 mmol) in a mixed solvent (1.5 mL, THF: DMSO = 1:2) was added dropwise at room temperature. The resulting mixture was stirred at 50°C for 1 h. After the reaction mixture was cooled down, ice water was added and the mixture was extracted with EA. The combined organic layers was dried over Na2SO4, filtered, and concentrated to give the crude product, which was purified by PTLC (Hex:EA = 1:1) to give 25 mg (69%) of product.
1H NMR 0.87 (d, J = 6.4 Hz, 3H), 0.89 (d, J = 6.4 Hz, 3H), 0.92–0.95 (m, 2H), 1.52 (hept, J = 6.8 Hz, 1 H), 1.77 (d,d, J = 13.4, 2.8 Hz, 1H), 2.02–2.15 (m, 2 H), 2.18–2.38 (m, 3H), 2.50–2.68 (m, 3H), 2.94–3.16 (m, 4H), 3.47 (d, J = 11.5 Hz, 1H), 3.79 (s, 3H), 4.11 (t, J = 6.3 Hz, 2H), 4.64 (d,t, J = 47.1, 5.7 Hz, 2H), 6.57 (s, 1H), 6.62 (s, 1H).
The same procedure described above using (+)3 as the starting material gave (+)4 in 55.7% yield. (+)4: [α]D = +53.8° (CHCl3).
2.1.3 (±)-3-Isobutyl-9-hydroxy-10-methoxy-3,4,6,7-tetrahydro-1H-pyrido[2,1-a]isoquinolin-2(11bH)-one, (±)6
To a suspension of NaH (268 mg, 95%, 0.12 mol) and HMPA (14.9 g, 0.08 mmol) in dry p-xylene (34 mL) was added dropwise neat N-methylaniline (8.92 g, 0.08 mol) at 65 °C. The resulting mixture was stirred at 65 °C for 15 min. 5 (TBZ, 6.7 g, 0.02 mol) was added in portions. The reaction mixture was stirred at 65 °C for an additional 66 h. After the reaction mixture was cooled, ice cold HCl (5%, 55 mL) was added dropwise, and the mixture was extracted with ether (5 × 30 mL). The basic aqueous phase was neutralized with concentrated HCl and extracted with EA. The combined organic layers was dried over Na2SO4, filtered, and concentrated to give the crude product, which was purified by flash 40+ (Hex:EA = 9:1) to give 3.15 g (49%) of product.
1H NMR 0.90 (d, J = 6.1 Hz, 3H), 0.92 (d, J = 6.4 Hz, 3H), 0.98–1.09 (m, 1H), 1.41–1.86 (m, 2H), 2.33 (t, J = 11.6 Hz, 1H), 2.47–2.75 (m, 4H), 2.87 (d,d, J = 13.6, 3.1 Hz, 1H), 2.97–3.15 (m, 2H), 3.28 (d,d, J = 11.4, 6.1 Hz, 1H), 3.49 (d, J = 10.2 Hz, 1H), 3.82 (s, 3H), 5.60 (br. 1H), 6.52 (s, 1H), 6.67 (s, 1H).
2.1.4 (±)-2-Oxiranyl-3-isobutyl-9-hydroxy-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin, (±)7
To a mixture of NaH (0.97 g, 60% in mineral oil, 19.8 mmol) in dry DMSO (30 mL) was added The mixture was stirred at Me3SOI (4.36 g, 19.8 mmol) in portions. room temperature for 20 min. Starting material 6 (3 g, 9.9 mmol) in a mixed solvent (12 mL, THF:DMSO = 1:3) was added dropwise at room temperature. The resulting mixture was stirred at 50°C for 3 h. After the reaction mixture was cooled down, ice water was added and the mixture was extracted with EA. The combined organic layers was dried over Na2SO4, filtered, and concentrated to give the crude product, which was purified by flash 40+ (Hex:EA = 9:1) to give 2.05 g (65%) of product.
1H NMR 0.86 (d, J = 6.5 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H), 0.89–0.93 (m, 2H), 1.54 (hept, J = 6.7 Hz, 1 H), 1.75 (d,d, J = 13.5, 2.9 Hz, 1H), 2.09 (d,d, J = 13.4, 11.8 Hz, 1H), 2.22–2.36 (m, 2H), 2.47–2.62 (m, 3H), 2.91–3.11 (m, 4H), 3.46 (d, J = 9.9 Hz, 1H), 3.78 (s, 3H), 5.60 (br. 1H), 6.52 (s, 1H), 6.59 (s, 1H).
2.1.5 (±)-2-Oxiranyl-3-isobutyl-9-(3-hydroxypropyloxy-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin, (±)8
To a mixture of Starting material 7 (1.4 g, 4.4 mmol) and 3-bromopropan-1-ol (737 mg, 5.3 mmol) in DMF (30 mL) was added Cs2CO3 (2.88 g, 8.8 mmol). The resulting mixture was stirred at 110 °C overnight. After the reaction mixture was cooled down, ice water was added and the mixture was extracted with a mixed solvent (DCM:MeOH = 9:1). The combined organic layers was dried over Na2SO4, filtered, and concentrated to give the crude product, which was purified by flash 40+ (Hex:EA = 1:1) to give 1.03 g (62 %) of product. This racemic product was separated to two enantiomers by HPLC with a chiral column (Regis Co.).
1H NMR 0.87 (d, J = 6.5 Hz, 3H), 0.89 (d, J = 6.5 Hz, 3H), 0.90–0.95 (m, 2H), 1.56 (hept, J = 6.7 Hz, 1 H), 1.75 (d,d, J = 13.5, 2.9 Hz, 1H), 1.98–2.15 (m, 3 H), 2.22–2.38 (m, 2H), 2.50–2.67 (m, 3H), 2.86–3.11 (m, 4H), 3.47 (d, J = 10.7 Hz, 1H), 3.78 (s, 3H), 3.84 (t, J = 5.5 Hz, 2H), 4.14 (t, J = 5.9 Hz, 2H), 6.56 (s, 1H), 6.61 (s, 1H). 13C NMR 21.42, 23.32, 25.30, 28.73, 31.43, 34.21, 35.18, 40.25, 49.81, 51.61, 55.64, 58.93, 59.23, 59.87, 60.94, 67.96, 107.98, 113.19, 126.32, 129.79, 146.30, 147.30.
(+)8: [α]D = +50.99° (CHCl3); (−) 8: [α]D = −50.69° (CHCl3).
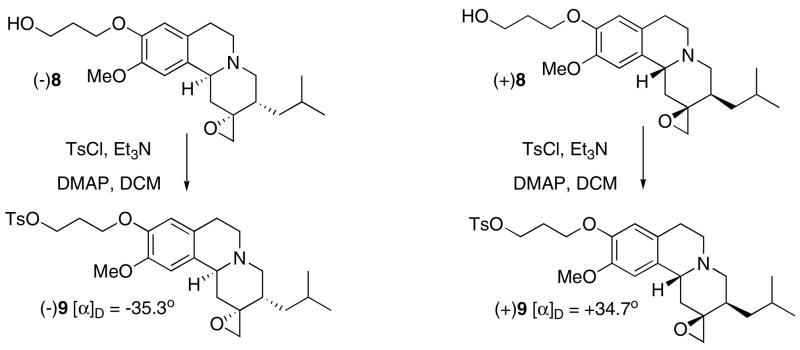
2.1.6 (+)-2-Oxiranyl-3-isobutyl-10-methoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-9-yloxy)propyl 4-methylbenzenesulfonate, (±)9
To a solution of (+)8 (196 mg, 0.52 mmol), obtained from chiral separation, in DCM (5 mL) was added Et3N (1 mL) followed by TsCl (199 mg 1.04 mmol) in DCM (5 mL) dropwise and DMAP (40 mg) at 0 °C. The mixture was stirred at room temperature for 3 h. Ice water was added and the mixture was extracted with EA. The combined organic layers was dried over Na2SO4, filtered, and concentrated to give the crude product, which was purified by flash 40+ (Hex:EA = 2:3) to give 235 mg (84.8 %) of product.
1H NMR 0.87 (d, J = 6.5 Hz, 3H), 0.89 (d, J = 6.5 Hz, 3H), 0.90–0.95 (m, 2H), 1.55 (hept, J = 6.7 Hz, 1 H), 1.76 (d,d, J = 13.5, 2.9 Hz, 1H), 1.93–2.18 (m, 3 H), 2.23–2.31 (m, 2H), 2.39 (s, 3H), 2.49–2.65 (m, 3H), 2.86–3.13 (m, 4H), 3.45 (d, J = 11.1 Hz, 1H), 3.73 (s, 3H), 3.94 (t, J = 6.0 Hz, 2H), 4.25 (t, J = 6.1 Hz, 2H), 6.51 (s, 1H), 6.54 (s, 1H), 7.25 (d, J = 8.2 Hz, 2H), 7.75 (d, J = 8.3 Hz, 2H). 13C NMR 19.97, 20.16, 22.07, 23.99, 27.31, 27.45, 32.92, 33.90, 38.96, 48.50, 50.34, 54.43, 57.62, 57.95, 58.58, 62.91, 65.74, 107.10, 112.20, 125.11, 126.16, 128.13, 128.57, 131.29, 143.00, 144.85, 146.05.
(+)9: [α]D = +34.66° (CHCl3).
The same procedure described above using (−)8 as the starting material gave (−)9 in 78.9% yield. (−)9: [α]D = −35.34° (CHCl3).
2.2 Radiochemistry
2.2.1 [18F](+)-2-Oxiranyl-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-2,3,4,6,7, 11b-hexahydro-1H-pyrido[2,1-a]isoquinoline, [18F](+)4
[18F]Fluoride was produced by a cyclotron using 18O(p,n)18F reaction and passed through a Sep-Pak Light QMA cartridge as an aqueous solution in [18O]-enriched water. The cartridge was dried by airflow, and the 18F activity was eluted with 1 mL of Kryptofix 222 (K222)/K2CO3 solution (11 mg of K222 and 2.6 mg of K2CO3 in CH3CN/H2O 0.93/0.07 mL). The solvent was removed at 120 °C under a nitrogen stream. The residue was azeotropically dried with 1 mL of anhydrous CH3CN twice at 120 °C under a nitrogen stream. A solution of tosylate precursor (+)9 (1 mg) in anhydrous DMSO (0.3 mL) was added to the reaction vessel containing the dried 18F activities. The mixture was heated at 120 °C for 4 min. Water (5 mL) was added and the mixture was passed through a preconditioned Waters OASIS HLB (3 mL) cartridge. The cartridge was washed with 10 mL of water and the labeled compound was eluted with 1 mL CH3CN. The eluted compound was purified by HPLC. [Phenonemex Gemini C18 semi-prep column (10 × 250 mm, 5 μm), CH3CN/ammoniun formate buffer (50 mM, pH = 4.0), 1/1, flow rate 3 mL/min for retention time (Rt) = 7.7 min]. The preparation time was approximately 70 min. The radiochemical yield (RCY) was 20–36 % (decay corrected) (EOS) (n = 8). To determine radiochemical purity (RCP) and specific activity (SA), analytical HPLC was used [Phenomenex Gemini C18 analytical column (4.6 × 250 mm, 5 μm), CH3CN/ammonium formate buffer (50 mM, pH = 4.0) 1/1; Flow rate 1 mL/min, UV 280 nm; Rt = 5.0 min. RCP was > 99%. SA was estimated by comparing UV peak intensity of purified [18F] labeled compound with reference non-radioactive compound 4 of known concentration. SA of [18F](+)4 was 48.1–292 GBq/μmol (1.3–7.9 Ci/μmol; n = 8) at the end of synthesis.
2.2.2 [18F](−)-2-Oxiranyl-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-2,3,4,6,7, 11b-hexahydro-1H-pyrido[2,1-a]isoquinoline, [18F](−)4
The same procedure was followed using the tosylate precursor (−)9.
The radiochemical yield (RCY) = 20–31 % (decay corrected) (n=2) RCP > 99%. SA of [18F](−)4 was 48.1–66.6 GBq/μmol (1.3–1.8 Ci/μmol; n = 2) at the end of synthesis.
2.2.3 [18F](−)-2-Hydroyxl-3-isobutyl-9-(3-fluoropropoxy)-10-methoxy-2,3,4,6,7, 11b-hexahydro-1H-pyrido[2,1-a]isoquinoline, [18F]2
Preparation of [18F]2 was achieved by an [18F]fluoride displacement of the corresponding mesylate derivatives as described previously [26, 27]. The product was purified by high-performance liquid chromatography. RCY was 20–37% (n = 5) (decay corrected)at the end of synthesis. RCP > 99%. The specific activity was 24.4–87.3 GBq/μmol (0.66–2.36 Ci/μmol; n = 6) at the end of synthesis.
2.3 Homogenate binding
Frozen rat brains were purchased from Pel-Freez (Invitrogen, http://www.invitrogen.com). The basal forebrain tissues containing the striatal region of the brains were dissected and pooled. The brain tissue homogenates were prepared in 50 mM Hepes, pH 7.5 and 0.32 M sucrose. The specific binding of [3H](±)-TBZ was determined following the procedures described [32]. The total volume for the assay was 0.2 mL. In competition experiments, compounds at concentrations up to 10−5 M were examined for their abilities to compete for the binding of [3H](±)-TBZ (1.0–1.5 nM). Incubations were carried out routinely at room temperature for 90 min. The samples were then filtered through glass fiber filters (No. 25 Schleicher and Schuell, Keene, NH) and the filters containing bound 3H ligand were dissolved in 7 ml Ecolite(+) overnight and the radioactivity was counted the next day in a scintillation counter (Beckman) with 65% counting efficiency. Nonspecific binding was determined in the presence of 10 μM (±)-TBZ. The results of the inhibition experiments were subjected to nonlinear regression values were calculated as analysis using equilibrium binding data analysis by which Ki reported previously {Kung, 2007 #20374}.
2.4 Biodistribution studies
Male Sprague-Dawley rats weighing 220–350 g were used for normal distribution and imaging studies. Rats were anesthesized with isoflurane and then injected via tail vein with 0.2 mL of a saline solution containing either [18F](+)4 or [18F](−)4 (20 μCi/dose). The rats were sacrificed under isoflurane anesthesia at indicated time-points post injection. Organs of interest were removed and weighed, and the radioactivity was counted with an automatic gamma counter. The percentage dose per organ was calculated by comparing tissue counts to suitably diluted aliquots of the injected material. The total activity of the blood was calculated under the assumption that it was 7% of the total body weight. The %ID/g of samples was calculated by comparing the sample counts with the count of the diluted initial dose.
To further confirm that the accumulation of [18F](+)4 in the pancreas was indeed due to the presence of VMAT2 binding sites, we pretreated the rats with (+)2, (3.5 mg/kg, iv), a selective VMAT2 ligand, at 5 min prior to tracer injection. At 60 min after the tracer (or tracer with added carrier) injection, the rats were sacrificed and organs or tissues were removed and counted as described above. A similar study in rats was carried out for the inactive isomer, [18F](−)4; the rats were pretreated with (+)-DTBZ (3.3 mg/kg, at 5 min prior) and the animals were dissected at 30 min post iv injection.
To evaluate the potential metabolites, a rat was injected with [18F](−)4 plasma and urine samples were obtained and the organ of interest was dissected (i.e. liver) at 30 min. After an iv injection of 37 MBq (1 mCi) 18F(+)4, the rat was sacrificed 30 min post-injection and the radioactive material was extracted with acetonitrile (0.5 mL)/methanol (0.1mL) from the samples of plasma, liver, and urine. Blood sample (1.5 mL) was collected via cardiac puncture. It was spun down at at 2300 rpm for 3 min and the plasma sample was saved. After adding acetonitrile/methanol mixture, The mixture was vortexed and centrifuged. The upper layer liquid was transferred into a new tube and blew down to ~200 μL L by a stream of nitrogen. Samples of liver were dissected and a small piece (about 1 g) was homogenized in phosphate buffer saline and transferred to a 1.7 mL microfuge tube. The sample tube was subjected to vortexing and then spun down. The same extraction procedure was carried out using acetonitrile and methanol. Urine was obtained directly from the bladder. The same extraction procedure was carried out as above.
To the extracted solutions, 5 uL of the cold mixture ((+)4 and (+)4b) was added and injected into the HPLC (Phenomenex C18, acetonitrile/50mM ammonium formate buffer, (pH 4), 1 mL/min). The cold mixture of standards was added in order to identify the parent compound, [18F](+)4, along with its possible metabolite, [18F](+)4b.
2.5 Imaging studies
PET imaging of control rats (220–350 g) was achieved by using a small Animal PET scanner (A-PET) [33]. Each animal was anesthetized initially using 3 % isoflurane in 1.0 L/min oxygen, in an acrylic induction chamber. When fully anesthetized, the animal was placed on the A-PET bed with a nose cone used to maintain anesthesia at 1.5% isoflurane in 1.0 mL/min oxygen. Body temperature was maintained by placing a heating pad placed under the animal. A dose of 29.6–44.4 MBq (0.8–1.2 mCi) of [18F](+)4 (volume < 0.4 mL) was injected in the tail vein. Multiple dynamic scans (3 min/frame) were acquired for 3 hours. Images were reconstructed using a fully 3D iterative image reconstruction algorithm (3D-RAMLA) with system attenuation correction incorporated in the algorithm. Corrections were applied for scatter, randomness and attenuation. Regions of interest were drawn, guided by a detailed rat atlas and microCT images. Visual analysis was performed using coronal, transverse and sagittal reconstruction. Regions of interest were drawn over the pancreas as well as other organs or tissues. Mean counts per voxel per 37 KBq (μCi) injected dose in each region were calculated at various time intervals.
3. Results
3.1. Chemistry
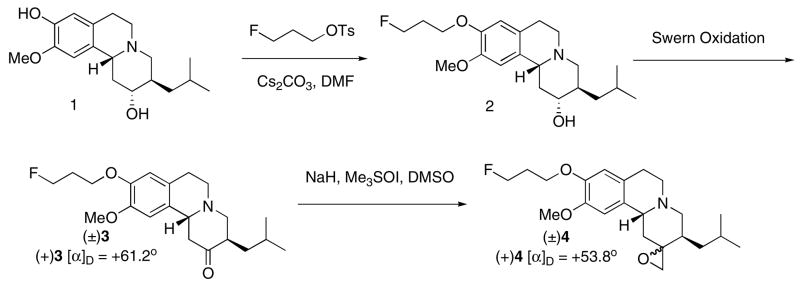
The new epoxide derivative of tetrabenazine was synthesized from the corresponding 9-fluoropropanoxy-(±)-DTBZ, (±)-2, [27] as shown in scheme 1. The Swern oxidation reaction converted 2 to ketone derivative 3. The C2-carbonyl group of 3 was converted to an epoxide (oxirane) by Me3SOI in good yield (69%).
Scheme 1.
In this epoxidation step, the less reactive oxosulfonium ylide- Me3SOI (compared to the sulfonium ylide- Me2S=CH2) gave exclusively the thermodynamically controlled product 4, in which the C2-O bond was syn to the isobutyl group on C3 (Scheme 2 and Fig. 5). A similar epoxidation reaction of benzo[a]-quinoline-2,2′-oxirane analogs has been reported [34]. The stereochemistry of the epoxide ring has been carefully studied and assigned by using C-13 NMR spectroscopy. The structural assignment of product 4 is supported according to this report [34].
Scheme 2.
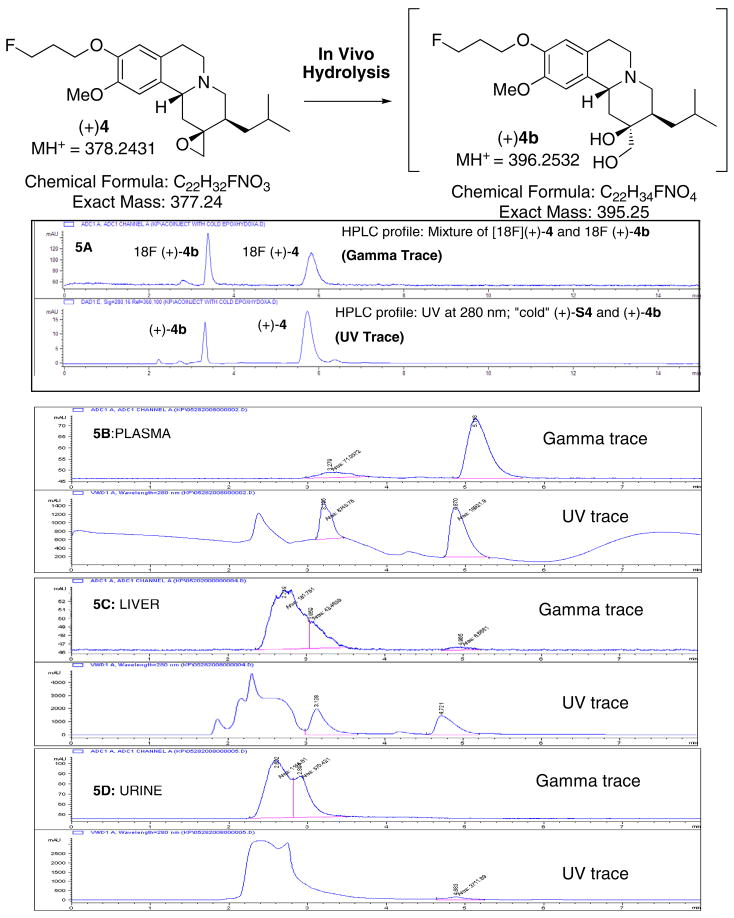
Fig. 5.
A proposed hydrolysis of epoxide (+)4 to (+)4b in vivo and in vitro is shown in this figure. It is likely that the epoxide ring of (+)4 was opened leading to the production of di-hydroxyl derivative, (+)4b. When “cold” (+)4 was treated with sodium hydroxide, it produced (+)4b. 5A: [18F](+)4 and [18F](+)4b were prepared and equal amounts of these two tracers were then mixed together (~1:1) and co-injected along with a cold mixture of (+)4 and (+)4b (~1:1) (HPLC condition: Phenomenex C18, acetonitrile/50 mM ammonium formate buffer, pH 4), 1 mL/min). After an injection of [18F](+)4 (37 MBq, 1 mCi) a rat was sacrificed and radioactive material was isolated from plasma, liver and urine samples. To the extracted samples, 5 μL of “cold mixture” was added and then injected into HPLC. The samples were eluted with the same solvent and column system (5B: plasma; 5C: liver; 5D: urine).
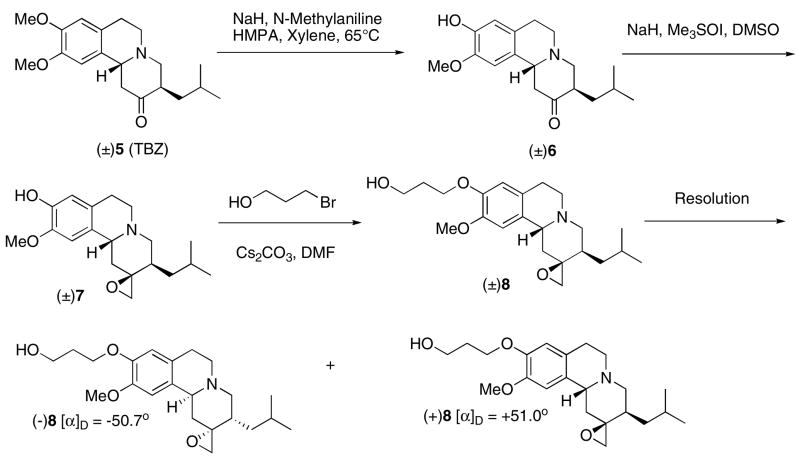
The HPLC analysis of racemic compound (±)4 on the chiral AD column showed two distinctive peaks with retention times at 7.03 and 15.3 min, respectively (Chiral AD column, Regis Co.; eluted with hexane: isopropanol = 9:1; 1 mL/min; 280 nm) (Fig. 2). By using the optically active (+)FP-DTBZ, (+)2, which was reported by our laboratory previously [26], the corresponding optically active (+)4 was synthesized by the same synthetic procedures. As expected, the resulting (+)4 showed only one peak on the chiral HPLC chromatogram (retention time at 7.2 min) (Fig. 2). The assignment of the stereochemistry of tetrabenazine epoxide derivative, 4, was consistent with the configuration reported previously [34]. To synthesize the precursor for the preparation of [18F](+)4 and (−)4, we explored a new synthetic route as shown in scheme 2. Selective demethylation of the 9-OMe group using a mixture of HMPA, N-methylaniline, xylene and sodium hydride was successfully carried out for synthesis of 1 [27, 35]. Compound 6 was obtained in 50% yield from TBZ (5). Epoxidation of 6 followed by alkylation with 3-bromopropan-1-ol afforded the racemic compound 8, which was separated into two enantiomers (+)8 and (−)8 by using HPLC separation on a chiral column (Regis Technology Co.). Tosylation of these enantiomers gave optically active precursors (+)9 and (−)9 (Scheme 3). Both precursors were successfully labeled to provide [18F](+)4 and [18F](−)4 for testing in rats (Scheme 4). The preparation using a nucleophilic fluorination reaction gave a radiochemical yield of 20–36% and 20–31% (decay corrected) for [18F](+)4 and [18F](−)4, respectively (radiochemical purity > 99%, specific activity > 48 GBq/μmol (1.3 Ci/μmol)). To test the stability of the epoxide, [18F](+)4 was dissolved in a 80/20 (v/v) saline/ethanol solution containing 80% saline and 20 ethanol. Upon standing at room temperature for 3 hours, no change in chemical purity was observed as determined by HPLC. It appears that the epoxide [18F](+)4 is stable under this condition.
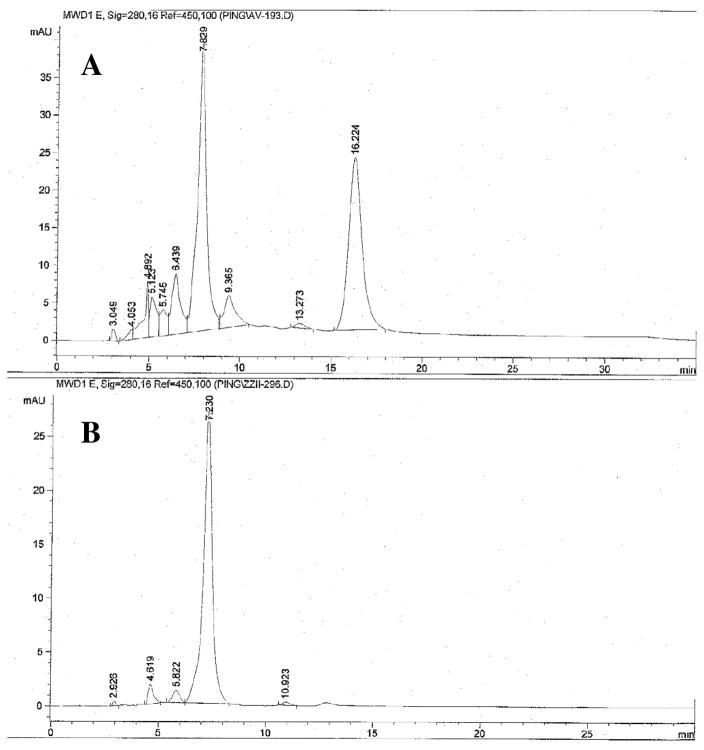
Fig. 2.
HPLC chiral column separation profiles of racemic (±)4 and its corresponding active isomer (+)4. The profiles were obtained by using a AD chiral column (Regis Technology Co., http://www.registech.com/InfoPages/ChiralInfo/ChiralApplications.html) and eluted with 9:1 hexane and isopropanol.
Scheme 3.
Scheme 4.
3.2. In vitro binding studies
To evaluate the binding affinity of the new TBZ derivatives, we use in vitro for VMAT2 binding sites. The racemic epoxide (±)4 binding assay to determine the Ki and the resolved enantiomer (+)4 were subjected to a competition binding study with [3H](±)-TBZ in rat striatal homogenates. The active enantiomer, (+)4, was more potent than the mixture of enantiomer, (±)4, showing Ki values of 0.08 ± 0.01 and 0.15 ± 0.01 nM, respectively (Table 1). It is clear that the epoxide ring at the C2 position of tetrabenazine ring does not affect the binding affinity to VMAT2 binding sites.
Table 1.
Inhibition constants (Ki, mean ± SEM) of epoxide derivatives of tetrabenazine on [3H](±)-TBZ binding to VMAT2 in rat striatal homogenates*
| Compound | Ki (nM) |
|---|---|
| (±)4 | 0.15 ± 0.01 |
| (+)4 | 0.08 ± 0.01 |
| (±)-TBZ, (±)5 | 1.3 ± 0.1 |
Kd value of 8.1 nM for [3H](±)-TBZ was used for the calculation based on the Ki value reported previously [41]. The results are the mean ± SEM of three independent measurements done in duplicates.
Preliminary in vitro binding studies (in vitro autoradiography) of [18F](+)4 and (−)4 using rat brain sections were also performed (data not shown). As expected only the active isomer, [18F](+)4, showed intense labeling of the striatum, olfactory tubercle, substantia nigra and dorsal raphe, areas of the brain which contain a high density of monoamine neurons. While the inactive isomer, [18F](−)4, showed no specific binding in any brain region, only the background activity was observed. The regional brain distribution pattern of [18F](+)4 is consistent with VMAT2 binding in the brain reported previously [26].
3.3. In vivo biodistribution and blocking studies in rats
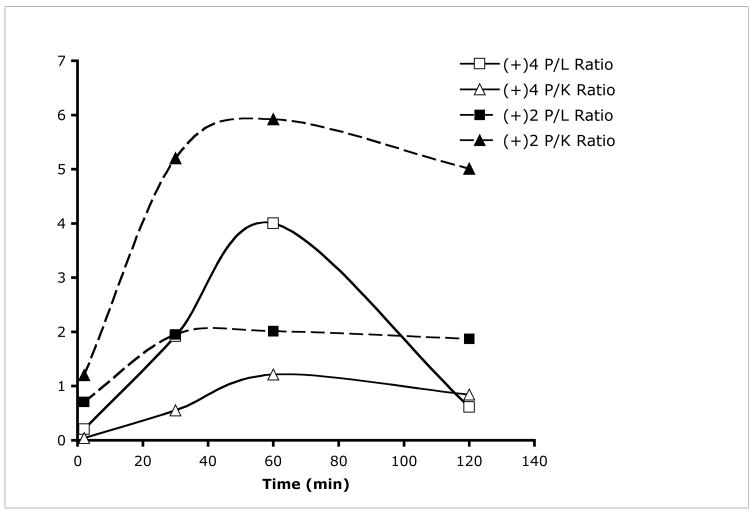
Initial biodistribution study of [18F](+)4 as a new radiotracer targeting VMAT2 binding sites for imaging beta cell mass was tested in rats. In addition, the inactive isomer, [18F](−)4, was also tested in rats. In vivo biodistribution study of [18F](+)4 in rats showed that the pancreas displayed the highest uptake (2.68 %ID/g at 60 min post-injection) (Table 2). The liver, an organ adjacent to the pancreas, showed a lower uptake (0.67 %ID/g) as compared to that in the pancreas. The washout of radioactivity from the pancreas was relatively slow with 1.3 %ID/g remaining at 2 h post iv injection. The lower liver uptake may be important because of its proximity to pancreas; as such the contrast between the pancreas and the liver will be enhanced. Kinetically, the biodistribution pattern of [18F](+)4 favors the imaging of pancreas. Figure 3 shows the pancreas to liver (P/L) and pancreas to kidney (P/K) ratios for [18F](+)4 and [18F](+)2 at different time points. Due to the change of excretion pattern from liver to kidney for [18F](+)2, there is a significant difference between [18F](+)4 and [18F](+)2 in the dynamics of P/L and P/K ratios. At 60 min after injection, when the pancreas uptake peaked for [18F](+)4, a significantly higher P/L ratio (4:1) was obtained; the previously reported [18F](+)2 showed a lower P/L ratio (2:1). It is important to note that altering of the in vivo metabolism from liver to kidney for [18F](+)4, as compared to that of [18F](+)2, is likely to be responsible for this distinctive change.
Table 2.
In vivo biodistribution of [18F](+)4 in control Sprague-Dawley rats (%ID/g, avg ± SD). For comparison, the biodistribution data of [18F](+)2 in rats reported previously [28] were added.
| Biodistribution of [18F](+)4 in rats
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2min n=3 | 30min n=3 | 60min n=7 | 120min n=7 | 240min n=4 | ||||||
| Blood | 0.27 | ± 0.05 | 0.15 | ± 0.01 | 0.10 | ± 0.01 | 0.04 | ± 0.00 | 0.02 | ± 0.00 |
| Heart | 0.48 | ± 0.09 | 0.11 | ± 0.02 | 0.07 | ± 0.01 | 0.02 | ± 0.00 | 0.01 | ± 0.00 |
| Muscle | 1.00 | ± 0.01 | 0.61 | ± 0.01 | 0.04 | ± 0.01 | 0.02 | ± 0.00 | 0.01 | ± 0.00 |
| Lung | 0.79 | ± 0.04 | 0.48 | ± 0.15 | 0.14 | ± 0.04 | 0.05 | ± 0.01 | 0.03 | ± 0.00 |
| Kidney | 4.60 | ± 1.10 | 3.59 | ± 0.80 | 2.21 | ± 0.53 | 1.58 | ± 0.17 | 2.01 | ± 0.30 |
| Pancreas | 1.64 | ± 0.48 | 1.96 | ± 0.19 | 2.68 | ± 0.28 | 1.33 | ± 0.30 | 0.81 | ± 0.16 |
| Spleen | 1.36 | ± 0.48 | 0.26 | ± 0.05 | 0.16 | ± 0.04 | 0.08 | ± 0.01 | 0.04 | ± 0.01 |
| Liver | 3.28 | ± 0.49 | 1.02 | ± 0.12 | 0.67 | ± 0.10 | 0.54 | ± 0.06 | 0.54 | ± 0.11 |
| Skin | 0.21 | ± 0.05 | 0.14 | ± 0.01 | 0.09 | ± 0.01 | 0.04 | ± 0.01 | 0.02 | ± 0.01 |
| Brain | 0.95 | ± 0.30 | 0.30 | ± 0.01 | 0.32 | ± 0.06 | 0.24 | ± 0.03 | 0.28 | ± 0.04 |
| Bone | 0.29 | ± 0.04 | n/a | n/a | 0.23 | ± 0.03 | 0.22 | ± 0.04 | 0.27 | ± 0.05 |
| Biodistribution of [18F](+)2 in rats (%ID/g, avg ± SD) reported previously [28]
| |||||
|---|---|---|---|---|---|
| 2min | 30min | 60min | 120min | ||
| Blood | 0.26 ± 0.03 | 0.23 ± 0.06 | 0.18 ± 0.02 | 0.12 ± 0.03 | |
| Heart | 0.71 ± 0.07 | 0.41 ± 0.03 | 0.30 ± 0.10 | 0.22 ± 0.01 | |
| Muscle | 0.14 ± 0.03 | 0.17 ± 0.02 | 0.13 ± 0.01 | 0.10 ± 0.01 | |
| Lung | 0.94 ± 0.04 | 0.69 ± 0.03 | 0.54 ± 0.14 | 0.35 ± 0.03 | |
| Kidney | 2.10 ± 0.17 | 1.06 ± 0.06 | 0.84 ± 0.09 | 0.55 ± 0.06 | |
| Pancreas | 2.51 ± 0.22 | 5.50 ± 0.97 | 4.98 ± 0.24 | 2.76 ± 0.61 | |
| Spleen | 1.46 ± 0.15 | 1.03 ± 0.18 | 0.83 ± 0.18 | 0.48 ± 0.04 | |
| Liver | 3.53 ± 0.56 | 2.82 ± 0.24 | 2.47 ± 0.30 | 1.47 ± 0.13 | |
| Skin | 0.23 ± 0.05 | 0.28 ± 0.01 | 0.23 ± 0.04 | 0.16 ± 0.02 | |
| Brain | 0.71 ± 0.05 | 0.62 ± 0.08 | 0.42 ± 0.04 | 0.35 ± 0.02 | |
Fig. 3.
Comparison of pancreas to liver (P/L) and pancreas to kidney (P/K) ratios for [18F](+)4 and [18F](+)2 at different time points. The ratios were calculated based on %ID/g of each organ listed in Table 2. At 60 minutes after injection a significantly higher P/L ratio (4:1) for [18F](+)4 was obtained, while the previously reported [18F](+)2, showed a lower P/L ratio (2:1). It is important to note that the shifting the in vivo metabolism from liver to kidney for [18F](+)4, as compared to that of [18F](+)2, may contribute to this distinctive change.
To confirm that the pancreatic uptake of [18F](+)4 is specifically due to VMAT2 binding, we performed blocking studies. In one experiment, the pancreas uptake was blocked with a competing dose of “cold” (+)2 (3.5 mg/Kg, iv, 5 min pretreatment). There was a significant blocking of pancreas uptake (85% reduction at 60 min) (Table 3). The result of pancreas and blocking studies suggest that the [18F](+)4 displayed a lower pancreas uptake in normal rats (peak uptake of 2.19 %ID/g at 60 min) as compared to that of [18F](+)2 (peak uptake 4.9 %ID/g at 60 min). However, the blocking study using “cold” (+)2, (3.5 mg/Kg, iv, 5 min pretreatment) suggested that [18F](+)2 may have a lower peak pancreas uptake (2.19 %ID/g). However, [18F](+)2 displayed a significant level of blocking (pancreas uptake was reduced to 0.32 %ID/g, which represented a 85% reduction at 60 min) (Table 3). Comparatively, [18F](+)4 showed a higher peak pancreas uptake (4.9 %ID/g) in the control rats, but it displayed a higher level of residual pancreas uptake after blocking. The pancreas uptake was reduced to 1.11 %ID/g for [18F](+)4, which represented a 78% reduction at 60 min, but this value is still three times higher than that of [18F](+)2 (0.32 %ID/g after the blocking; Table 3). The results imply that lower pancreas uptake for [18F](+)2 may also lead to a lower non-specific binding; therefore, the blocking studies showed a higher level of reduction of VMAT2 binding, which leads to a lower pancreas uptake (Table 3). Thus, in addition to shifting the highest uptake organ from the liver to the kidneys, the new VMAT2 ligand, [18F](+)4, may show “cleaner” pancreas images due to its lower background.
Table 3.
Blocking studies of pancreatic uptake in rats (%ID/g ± SD, Control n = 4; Blocking n = 5).
| A: Comparison of [18F](+)4 and [18F](+)2 (the active isomers for VMAT2 binding in the pancreas) at 60 min after tracer injection in rats with pretreatment of (+)2 (3.5 mg/kg, iv, 5 min prior). | ||||
|---|---|---|---|---|
| [18F](+)4 | [18F](+)2** | |||
| Control | Pretreatment | Control | Pretreatment | |
| Blood | 0.27 ± 0.05 | 0.06 ± 0.01 | 0.20 ± 0.02 | 0.26 ± 0.04 |
| Pancreas | 2.19 ± 0.37 | 0.32 ± 0.07* | 4.93 ± 0.49 | 1.11 ± 0.07* |
| B: [18F](−)4 (the inactive isomer) at 30 min after tracer injection in rats with pretreatment of (+)DTBZ (3.3 mg/kg, iv, 5 min prior) | ||
|---|---|---|
| Control | Pretreatment | |
| Blood | 0.07 ± 0.00 | 0.12 ± 0.03 |
| Pancreas | 0.22 ± 0.02 | 0.22 ± 0.04 |
p<0.05;
data reported previously [28].
In a similar biodistribution study in rats, [18F](−)4 (an optically inactive isomer for VMAT2 binding) showed a ten-fold lower specific pancreas uptake (0.22 %ID/g at 30 min post-injection). The pancreas uptake of the inactive isomer, [18F](−)4, was relatively low and non-displaceable by iv pretreatment of (+)DTBZ (3.3 mg/kg, iv, 5 min prior treatment) (Table 3). We choose to use (+)DTBZ for the blocking study because its kinetics are faster and at 30 min the pancreas uptake of the inactive isomer, [18F](−)4, in the rats were the highest. The results suggest that the binding of [18F](+)4 is highly selective to VMAT2 binding sites in the pancreas; the inactive isomer, [18F](−)4, showed very low uptake in the pancreas due to its lack of affinity to the VMAT2 binding sites. As expected, the inactive isomer [18F](−)4, also showed low brain uptake and no selective accumulation in the striatum area of the brain (data not shown).
3.4. PET imaging studies in rats
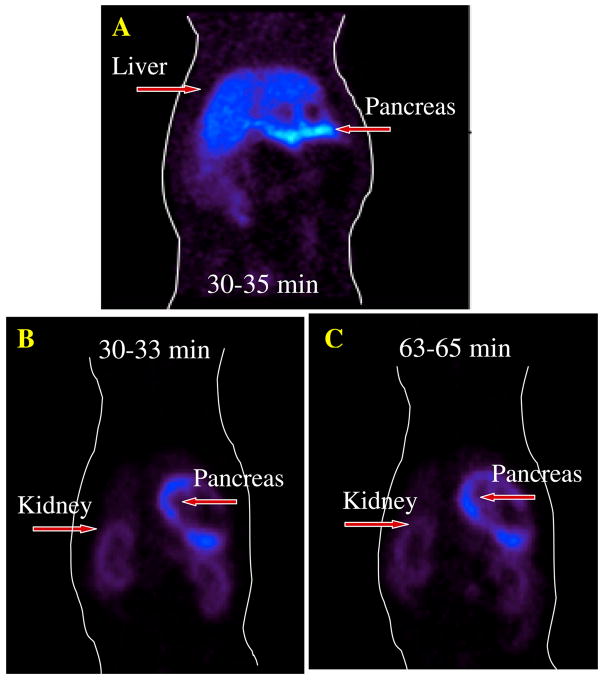
Small animal PET imaging was performed on Sprague Dawley rats after an iv injection of [18F](+)4. The pancreas was clearly visualized. Pancreatic uptake was greater than that of any other organ in a coronal view (Fig. 4A). As expected, the liver uptake and retention was lower than that of the kidneys, confirming the data obtained from dissection experiments (Table 2). It appeared that [18F](+)4 may have been metabolized in vivo to the corresponding C-2 hydroxymethylene derivative (Fig. 5). The added hydrophilicity of the C-2 hydroxymethylene derivative may direct the removal of the metabolized tracer from the blood by the kidneys. As such, the kidney uptake and retention is higher than that of liver. Uptake and retention of [18F](+)4 in the pancreas appears to peak around 60 min post iv injection, followed by a slight washout. Due to lower accumulations of radioactivity in the muscle or liver, the higher pancreas uptake provides an excellent signal to noise ratio. As compared to previously reported [18F](+)2, there is a significant reduction of the liver activity (Fig. 4B and 4C). Thus, the biodistribution and PET imaging in rats consistently demonstrated high pancreas uptake and retention than the surrounding tissues making [18F](+)4 a potential agent for imaging VMAT2 binding sites of beta cells in the pancreas.
Fig. 4.
Comparison of in vivo PET images of normal male rats (29.6–44.4 MBq; 0.8–1.2 mCi, iv); A: [18F](+)2 (data acquired at 30–35 min) B: [18F](+)4, (data acquired at 30–33 min); C: [18F](+)4 (data acquired at 63–65 min). Both agents showed excellent pancreas uptake. The epoxide derivative, (+)4, showed a lower liver uptake (images B and C) as compared to the image A, suggesting a shift of the liver uptake and retention to the kidneys. The background activity (i.e. liver) is lower when using the epoxide derivative, [18F](+)4.
To further confirm the identity of the metabolites we have performed an in vitro hydrolysis experiment, in which “cold” (+)4 was treated with a sodium hydroxide/ethanol solution to hydrolyze the epoxide ring (Fig. 5A). The reaction mixture was injected into HPLC and showed two peaks- one corresponding to the starting material, (+)4; while the other appearing to be the ring-opened hydroxylated derivative. The LC-Mass (Agilent Technologies, model LC/MSD/TOF) showed two fractions with the exact mass as expected (MH+ for (+)4 at 378.2431 and MH+ for the hydrolyzed material at 396.2532, respectively).
Preliminary HPLC analysis of metabolites in the plasma, liver and urine at 30 min after iv injection of [18F](+)4 in a rat suggested that there were at least two acetonitrile extractable components- one was consistent with the parent [18F](+)4 and the other corresponded to the same retention time of the hydrolyzed [18F](+)4b (Fig. 5B, 5C and 5D). The urine samples showed only the hydrolyzed metabolite and multiple more water-soluble peaks. The data lend additional support for our contention that the new epoxide tracer, [18F](+)4, was metabolized in vivo to a more water-soluble dihydroxyl derivative, [18F](+)4b (Fig. 5A). As such, the majority of the in vivo excretion shifted from the liver to the kidney. A lower liver uptake and retention provided a better pancreas signal.
4. Discussion
Recently, there has been a dramatic increase in the number of patients having diabetes mellitus in the US. There is an urgent need to develop effective drug treatment for diabetes. A strategy towards a preventive treatment prior to the emergence of diabetes would also be very important. When diabetic symptoms (such as inadequate fluctuation of blood glucose level - hyperglycemia) emerge there is already a significant loss of insulin-secreting beta cells (> 65%). Therefore, developing an in vivo imaging tool to monitor the loss of beta cell mass prior to the development of diabetic symptoms is not only scientifically interesting but also critical for advancing new drug treatments aiming at preserving the beta cell mass.
There are several formidable challenges in seeking imaging agents that target beta cells. The pancreas consists of endocrine and exocrine cells; beta cells are only about 1–2% of the total cell mass. Using the same analogy for neuro-receptor PET imaging, the number of neurons containing the neuro-receptors may also be overwhelmed by the glial cells in the brain [36, 37]. The total number of neurons containing CNS receptors may also be a small fraction of total number of brain cells. Nevertheless, PET imaging of CNS receptors has been successfully tested in humans [38–40]. Therefore, the number of VMAT2 binding sites of beta cells may be sufficient for developing a PET based imaging tool for studying beta cell mass in the pancreas.
The novel VMAT2 ligands, tetrabenazine derivatives ([18F](+)4 and [18F](+)4b) reported in this paper show different preference in their in vivo biodistribution. The epoxide containing VMAT2 ligands may reduce the liver uptake and shift the excretion from the liver to the kidney. Since the pancreas is close to the liver; this metabolism pattern has effectively led to a reduction of background noise from surrounding organs (see Fig. 3 and Fig. 4). The new VMAT2 ligands are likely bound to the beta cells. Although, the possible correlation between VMAT2 ligand binding of beta cells in the pancreas and total beta cell mass has been reported, but their quantitative relationship has not been fully established [21, 23]. We, and other researchers in this exciting field, are generating additional data on the relationship between VMAT2 binding and beta cell mass. These new advances will provide solid scientific justification for using VMAT2 as a biomarker for beta cell mass in the future.
5. Conclusion
Preliminary results suggest that [18F](+)4, a new VMAT2 binding ligand, may be a useful marker for beta cell mass. It is likely that the presence of a hydrolyzable epoxide group shifted the in vivo uptake and retention from liver to kidney. This new excretion pattern may contribute to a higher pancreas to liver ratio. Improved PET images of the pancreas in normal rats may be obtained using this radiotracer. Beta cell imaging could be enhanced by further reduction of non-specific binding to other none insulin secreting cells in the pancreas. Identification of better animal models of diabetes may also facilitate research into developing new VMAT2 ligands for PET imaging beta cell mass.
Acknowledgments
The authors thank Dr. Paul Harris for his helpful discussion and Drs. Carita C. Huang and Ann-Marie Chacko for their editorial assistance.
Abbreviations
- (+)FP-DTBZ
(+)2, 9-fluoropropyl-(+)dihydrotetrabenazine
- (+)-DTBZ
(+)dihydrotetrabenazine
- (±)-TBZ
(±)tetrabenazine
- VMAT2
vesicular monoamine transporter 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li H, Waites CL, Staal RG, Dobryy Y, Park J, Sulzer DL, Edwards RH. Sorting of vesicular monoamine transporter 2 to the regulated secretory pathway confers the somatodendritic exocytosis of monoamines. Neuron. 2005;48:619–33. doi: 10.1016/j.neuron.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 2.Anlauf M, Eissele R, Schafer MK, Eiden LE, Arnold R, Pauser U, Kloppel G, Weihe E. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J Histochem Cytochem. 2003;51:1027–40. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- 3.Souza F, Freeby M, Hultman K, Simpson N, Herron A, Witkowsky P, Liu E, Maffei A, Harris PE. Current progress in non-invasive imaging of Beta cell mass of the endocrine pancreas. Curr Med Chem. 2006;13:2761–73. doi: 10.2174/092986706778521940. [DOI] [PubMed] [Google Scholar]
- 4.Bertuzzi F, Marzorati S, Secchi A. Islet cell transplantation. Curr Mol Med. 2006;6:369–74. doi: 10.2174/156652406777435453. [DOI] [PubMed] [Google Scholar]
- 5.Briones RM, Miranda JM, Mellado-Gil JM, Castro MJ, Gonzalez-Molina M, Cuesta-Munoz AL, Alonso A, Frutos MA. Differential analysis of donor characteristics for pancreas and islet transplantation. Transplant Proc. 2006;38:2579–81. doi: 10.1016/j.transproceed.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 6.Frank AM, Barker CF, Markmann JF. Comparison of whole organ pancreas and isolated islet transplantation for type 1 diabetes. Adv Surg. 2005;39:137–63. doi: 10.1016/j.yasu.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Iwanaga Y, Matsumoto S, Okitsu T, Noguchi H, Nagata H, Yonekawa Y, Yamada Y, Fukuda K, Tsukiyama K, Tanaka K. Living donor islet transplantation, the alternative approach to overcome the obstacles limiting transplant. Ann N Y Acad Sci. 2006;1079:335–9. doi: 10.1196/annals.1375.051. [DOI] [PubMed] [Google Scholar]
- 8.Pileggi A, Cobianchi L, Inverardi L, Ricordi C. Overcoming the challenges now limiting islet transplantation: a sequential, integrated approach. Ann N Y Acad Sci. 2006;1079:383–98. doi: 10.1196/annals.1375.059. [DOI] [PubMed] [Google Scholar]
- 9.Sherr J, Sosenko J, Skyler JS, Herold KC. Prevention of type 1 diabetes: the time has come. Nat Clin Pract Endocrinol Metab. 2008 doi: 10.1038/ncpendmet0832. [DOI] [PubMed] [Google Scholar]
- 10.Hampe CS, Wallen AR, Schlosser M, Ziegler M, Sweet IR. Quantitative evaluation of a monoclonal antibody and its fragment as potential markers for pancreatic beta cell mass. Exp Clin Endocrinol Diabetes. 2005;113:381–7. doi: 10.1055/s-2005-865716. [DOI] [PubMed] [Google Scholar]
- 11.Markmann JF, Deng S, Desai NM, Huang X, Velidedeoglu E, Frank A, Liu C, Brayman KL, Lian MM, Wolf B, Bell E, Vitamaniuk M, Doliba N, Matschinsky F, Markmann E, Barker CF, Naji A. The use of non-heart-beating donors for isolated pancreatic islet transplantation. Transplantation. 2003;75:1423–9. doi: 10.1097/01.TP.0000061119.32575.F4. [DOI] [PubMed] [Google Scholar]
- 12.Sweet IR, Cook DL, Lernmark A, Greenbaum CJ, Krohn KA. Non-invasive imaging of beta cell mass: a quantitative analysis. Diabetes Technol Ther. 2004;6:652–9. doi: 10.1089/dia.2004.6.652. [DOI] [PubMed] [Google Scholar]
- 13.Schneider S, Feilen PJ, Schreckenberger M, Schwanstecher M, Schwanstecher C, Buchholz HG, Thews O, Oberholzer K, Korobeynikov A, Bauman A, Comagic S, Piel M, Schirrmacher E, Shiue CY, Alavi AA, Bartenstein P, Rosch F, Weber MM, Klein HH, Schirrmacher R. In vitro and in vivo evaluation of novel glibenclamide derivatives as imaging agents for the non-invasive assessment of the pancreatic islet cell mass in animals and humans. Exp Clin Endocrinol Diabetes. 2005;113:388–95. doi: 10.1055/s-2005-865711. [DOI] [PubMed] [Google Scholar]
- 14.Sweet IR, Cook DL, Lernmark A, Greenbaum CJ, Wallen AR, Marcum ES, Stekhova SA, Krohn KA. Systematic screening of potential beta-cell imaging agents. Biochem Biophys Res Commun. 2004;314:976–83. doi: 10.1016/j.bbrc.2003.12.182. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen AM, Andersson P, Saglik G, Andersson E, Kolby L, Erickson JD, Forssell-Aronsson E, Wangberg B, Ahlman H, Nilsson O. Differential expression of vesicular monoamine transporter (VMAT) 1 and 2 in gastrointestinal endocrine tumours. J Pathol. 2001;195:463–72. doi: 10.1002/path.973. [DOI] [PubMed] [Google Scholar]
- 16.Weihe E, Schafer MK, Erickson JD, Eiden LE. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci. 1994;5:149–64. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- 17.Anlauf M, Schafer MK, Schwark T, von Wurmb-Schwark N, Brand V, Sipos B, Horny HP, Parwaresch R, Hartschuh W, Eiden LE, Kloppel G, Weihe E. Vesicular monoamine transporter 2 (VMAT2) expression in hematopoietic cells and in patients with systemic mastocytosis. J Histochem Cytochem. 2006;54:201–13. doi: 10.1369/jhc.5A6739.2005. [DOI] [PubMed] [Google Scholar]
- 18.Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006;26:1198–212. doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 19.Kilbourn MR. In vivo radiotracers for vesicular neurotransmitter transporters. Nucl Med Biol. 1997;24:615–9. doi: 10.1016/s0969-8051(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 20.Koeppe RA, Frey KA, Vander Borght TM, Karlamangla A, Jewett DM, Lee LC, Kilbourn MR, Kuhl DE. Kinetic evaluation of and [11C]dihydrotetrabenazine by dynamic PET: measurement of vesicular monoamine transporter. J Cereb Blood Flow Metab. 1996;16:1288–99. doi: 10.1097/00004647-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 21.Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A. VMAT2 gene expression and function as it applies to imaging beta-cell mass. J Mol Med. 2008;86:5–16. doi: 10.1007/s00109-007-0242-x. [DOI] [PubMed] [Google Scholar]
- 22.Maffei A, Liu Z, Witkowski P, Moschella F, Del Pozzo G, Liu E, Herold K, Winchester RJ, Hardy MA, Harris PE. Identification of tissue-restricted transcripts in human islets. Endocrinology. 2004;145:4513–21. doi: 10.1210/en.2004-0691. [DOI] [PubMed] [Google Scholar]
- 23.Raffo A, Hancock K, Polito T, Xie Y, Andan G, Witkowski P, Hardy M, Barba P, Ferrara C, Maffei A, Freeby M, Goland R, Leibel RL, Sweet IR, Harris PE. Role of vesicular monoamine transporter type 2 in rodent insulin secretion and glucose metabolism revealed by its specific antagonist tetrabenazine. J Endocrinol. 2008;198:41–9. doi: 10.1677/JOE-07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson NR, Souza F, Witkowski P, Maffei A, Raffo A, Herron A, Kilbourn M, Jurewicz A, Herold K, Liu E, Hardy MA, Van Heertum R, Harris PE. Visualizing pancreatic beta-cell mass with [(11)C]DTBZ. Nucl Med Biol. 2006;33:855–64. doi: 10.1016/j.nucmedbio.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Souza F, Simpson N, Raffo A, Saxena C, Maffei A, Hardy M, Kilbourn M, Goland R, Leibel R, Mann JJ, Van Heertum R, Harris PE. Longitudinal noninvasive PET-based beta cell mass estimates in a spontaneous diabetes rat model. J Clin Invest. 2006;116:1506–13. doi: 10.1172/JCI27645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung MP, Hou C, Goswami R, Ponde DE, Kilbourn MR, Kung HF. Characterization of optically resolved 9-fluoropropyl-dihydrotetrabenazine as a potential PET Imaging agent targeting vesicular monoamine transporters. Nucl Med Biol. 2007;34:239–46. doi: 10.1016/j.nucmedbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goswami R, Ponde DE, Kung MP, Hou C, Kilbourn MR, Kung HF. Fluoroalkyl derivatives of dihydrotetrabenazine as positron emission tomography imaging agents targeting vesicular monoamine transporters. Nucl Med Biol. 2006;33:685–94. doi: 10.1016/j.nucmedbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Kung MP, Hou C, Lieberman BP, Oya S, Ponde DE, Blankemeyer E, Skovronsky D, Kilbourn MR, Kung HF. In Vivo Imaging of {beta}-Cell Mass in Rats Using 18F-FP-(+)-DTBZ: A Potential PET Ligand for Studying Diabetes Mellitus. J Nucl Med. 2008;49:1171–76. doi: 10.2967/jnumed.108.051680. [DOI] [PubMed] [Google Scholar]
- 29.Morisseau C, Hammock BD. Gerry Brooks and epoxide hydrolases: four decades to a pharmaceutical. Pest Manag Sci. 2008;64:594–609. doi: 10.1002/ps.1583. [DOI] [PubMed] [Google Scholar]
- 30.Morisseau C, Newman JW, Wheelock CE, Hill T, III, Morin D, Buckpitt AR, Hammock BD. Development of metabolically stable inhibitors of Mammalian microsomal epoxide hydrolase. Chem Res Toxicol. 2008;21:951–7. doi: 10.1021/tx700446u. [DOI] [PubMed] [Google Scholar]
- 31.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–37. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 32.Scherman D, Raisman R, Ploska A, Agid Y. [3H]Dihydrotetrabenazine, a new in vitro monoaminergic probe for human brain. J Neurochem. 1988;50:1131–36. doi: 10.1111/j.1471-4159.1988.tb10583.x. [DOI] [PubMed] [Google Scholar]
- 33.Surti S, Karp JS, Adam LE, Muehllenher G. Design and development of a GSO-based animal PET scanner (A-PET) Rockville, MD: 2001. [Google Scholar]
- 34.Davis R, Kluge AF, Maddox ML, Sparacino ML. Synthesis of spiro[1,3,4,6,7,11b-hexahydro-2H-benzo[a]quinolizine-2,2′-oxiranes] and spiro[1,3,4,6,7,11b-hexahydro-2H-benzo[a]quinolizine-2,5′ oxazolidin-2′-ones] and the use of carbon-13 nuclear magnetic resonance spectroscopy in the assignment of stereochemistry to epoxides. J Org Chem. 1983;48:255–59. [Google Scholar]
- 35.Kilbourn MR, Lee LC, Heeg MJ, Jewett DM. Absolute configuration of (+)alpha-dihydrotetrabenazine, an active metabolite of tetrabenazine. Chirality. 1997;9:59–62. doi: 10.1002/(SICI)1520-636X(1997)9:1<59::AID-CHIR11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill Companies; 2000. [Google Scholar]
- 37.Kung MP, Kung HF. Mass effect of injected dose in small rodent imaging by SPECT and PET. Nucl Med Biol. 2005;32:673–8. doi: 10.1016/j.nucmedbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Eckelman WC, Kilbourn MR, Mathis CA. Discussion of targeting proteins in vivo: in vitro guidelines. Nucl Med Biol. 2006;33:449–51. doi: 10.1016/j.nucmedbio.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Eckelman WC, Mathis CA. Molecular targets. Nucl Med Biol. 2006;33:1. doi: 10.1016/j.nucmedbio.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Eckelman WC, Frank JA, Brechbiel M. Theory and practice of imaging saturable binding sites. Invest Radiol. 2002;37:101–6. doi: 10.1097/00004424-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Kilbourn M, Sherman P. In vivo binding of (+)-[alpha]-[3H]dihydrotetrabenazine to the vesicular monoamine transporter of rat brain: bolus vs. equilibrium studies. Eur J Pharmacol. 1997;331:161–68. doi: 10.1016/s0014-2999(97)01054-6. [DOI] [PubMed] [Google Scholar]