Abstract
We describe the design of an optical switch in the chaperonin GroEL that is opened and closed by its ATP- and cochaperonin GroES-driven conformational changes. The switch, based on a fluorophore and a quencher, is engineered into the single-ring variant of the chaperone, and shows dramatic modulation of its fluorescent intensity in response to the transition of the protein between its allosteric states. It, therefore, forms a sensitive probe for the dynamics of the allosteric transitions of this machine, both in the bulk and in single molecules.
Many proteins operate as molecular machines, using chemical energy to drive them through a functional cycle that involves multiple conformational states (1). The E. coli molecular chaperone GroEL is a machine that assists protein folding by undergoing allosteric transitions between protein substrate binding (T) and release (R) states (2). Fluorescence spectroscopy has been used for studying the functional cycle of GroEL in both ensemble (3, 4) and single-molecule experiments (5). However, it has been difficult to devise methods that allow probing the conformational dynamics of the chaperone itself at the single-molecule level. We designed an optical switch that is opened and closed by the conformational changes of GroEL, showing a dramatic modulation of its fluorescent intensity. This optical switch was demonstrated to be a sensitive probe of ATP-and cochaperone-driven allosteric transitions, both in the bulk and in individual molecules.
GroEL is made up of two homoheptameric rings, stacked back-to-back, with a cavity at each end (6), in which protein folding can take place. GroEL functions in conjunction with a heptameric ring-shaped cochaperonin, GroES, that caps the cavity of the so-called cis ring, thereby triggering dissociation of bound protein substrates into the cavity (7). The allosteric transitions of GroEL are induced by ATP binding that occurs with positive cooperativity within rings and negative cooperativity between rings (8). The intraring positive cooperativity is an outcome of a concerted switch of its rings between the T and R conformations. The structure of the GroEL-GroES-ADP7 complex has been determined at atomic resolution (9). A single-ring version of GroEL (SR1) displays similar positive cooperative behavior with respect to ATP binding (10) but, unlike GroEL, its complex with GroES is long-lived since the trigger for GroES dissociation is ATP binding to the opposite ring (11). In the rest of this Communication, we concentrate on SR1.
The switch we built into the structure of SR1 is based on the quenching of oxazine derivatives by tryptophan (Trp) (12). This electron transfer-based quenching reaction has been used previously to study folding dynamics in peptides and small proteins (13, 14). It requires the formation of a ground-state complex between an appropriate fluorophore (attached to the side chain of an amino acid residue) and the side chain of a tryptophan residue, and can thus operate only if the two are in close proximity to each other. The quenched state has low fluorescence intensity. A conformational change that increases the distance between the fluorophore and the quencher should, in principle, lead to a large increase in emission. The design of the optical switch that is described below was based on our knowledge of the structures of two conformations of GroEL (9).
The fluorophore we used in this work was the maleimide derivative of the oxazine dye Atto 655 (Atto-tec). The switch based on this dye requires inserting a cysteine residue (to be labeled) and a Trp residue at appropriate positions. To identify such positions, we first listed all pairs of amino acid residues (either within one subunit or in neighboring subunits) that are separated by a distance short enough to allow contact formation between the fluorophore and the Trp side chain in the T conformation, but too long for quenching to take place in the R’ (GroES-bound) conformation. All pairs in this list in which the interaction between the fluorophore and the Trp in the T conformation might be hindered by other amino acids were discarded. This analysis resulted in 51 suitable pairs that are listed in Table 1 of the Supporting Information. Pairs of mutations that were not likely, on the basis of the literature, to affect the protein’s stability and activity are highlighted in Table 1. One of these pairs, on which we focused, involves residue 44 in one subunit and residue 327 in the nearest-neighbor subunit in the counterclockwise direction. The mutations F44W and K327C were each characterized before and found to have only minor effect on the protein’s stability and activity (15, 16). In the T conformation, the distance between the CR atoms of these residues is ∼17 Å, allowing interaction of a dye molecule with the Trp side chain. In the R’ conformation, this distance grows to ∼46 Å, thereby preventing this interaction. A molecular graphics program was used to build a subset of the fluorophore’s rotamers into the two structures of GroEL with the above mutations, and test that the fluorophore is indeed in contact with the Trp side chain in the T conformation (Figure 1).
Figure 1.
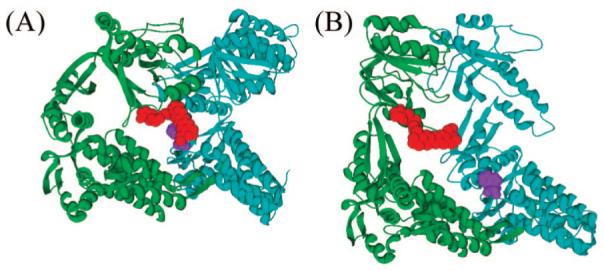
The optical switch in single-ring GroEL (SR1) molecules. (A) The fluorescence of the oxazine dye at position 327 (in red) is quenched by Trp44 (in violet) in the T conformation of GroEL. (B) In the R’ conformation, the quenching is removed. The structures shown in A and B are those of two adjacent subunits in the trans and cis rings, respectively, of the GroEL-GroES-ADP7 complex (9).
We generated SR1 molecules with the replacements F44W and K327C (SR1WC), using standard site-directed mutagenesis techniques, and labeled Cys327 with Atto655. Under the labeling conditions we used, wild-type cysteines are essentially unreactive (17). Care was taken so that less than one subunit in each SR1 molecule would be labeled on average. We also purified and labeled the Trp-lacking single mutant SR1-K327C (SR1C) as a control.
The addition of 1 mM ATP to 25 nM labeled SR1WC molecules generated a strong response, doubling the fluorescence intensity (Figure 2A, blue line). Addition of 50 nM GroES shortly after the addition of ATP (Figure 2A) essentially doubled the signal again. In contrast, the baseline fluorescence of labeled SR1C molecules (without ATP and GroES) was almost as high as the maximal fluorescence of SR1WC molecules in the presence of both additives (Figure 2A, gray line). Addition of ATP and GroES to the Trp-lacking molecules barely changed their fluorescence, thereby showing that the fluorescence changes observed in SR1WC molecules are related to the removal of Trp quenching. In the absence of GroES and as ATP was consumed with time, SR1WC molecules reverted to their initial conformation and the fluorescence returned essentially to its baseline (not shown). The optical switch, thus, operates as designed.
Figure 2.
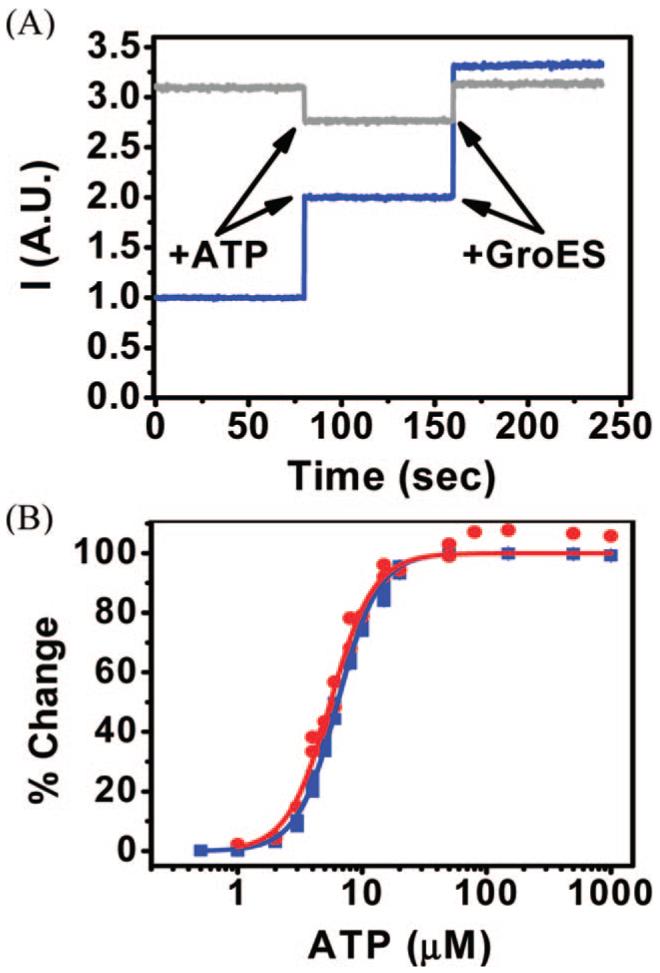
Response of the optical switch to conformational changes. (A) Response of SR1WC (blue) and SR1C (gray) to the addition of 1 mM ATP followed by addition of 50 nM GroES. (B) ATP titration curves of labeled SR1WC molecules obtained in the bulk (blue) and on a glass surface (red). Data were fitted to the Hill equation (lines).
The observation that the fluorescence intensity of labeled SR1WC, in the presence of ATP and GroES, is similar to the baseline fluorescence of the Trp-lacking mutant suggests that the SR1-GroES complex is in a conformation in which the interaction between Trp44 and the label is not possible. On the other hand, the fluorescence intensity attained with a saturating concentration of ATP is only about half of the value when GroES is also present. This suggests that ATP-bound SR1 molecules are in dynamic equilibrium with conformations in which the quenched state can be formed. This intriguing observation awaits further study.
ATP titration experiments with the labeled double mutant revealed a gradual increase in fluorescence with a sigmoidal dependence on ATP concentration (Figure 2B, blue points), indicating a cooperative behavior. Fitting the data to the Hill equation yielded a Hill coefficient of 2.65 ± (0.05, similar to a previously published value for SR1 based on its ATPase activity (2.87 ± (0.16) (10), thus showing that the labeled protein is fully functional.
In order to test the applicability of the design for single-molecule experiments, we deposited labeled SR1WC molecules on a glass slide and observed them under a fluorescence microscope. First, a dense molecular layer was formed, and a titration curve was obtained by registering fluorescence changes upon gradual addition of ATP. The surface titration curve was similar to the bulk titration curve (Figure 2B, red), indicating that the deposition of the protein on the surface does not significantly change its activity. Washing the surface following ATP addition led to a return of the fluorescence intensity to baseline levels, establishing reversibility of the optical switch.
A sparse layer of protein molecules was then formed, and fluorescence changes were monitored on the single-molecule level. Figure 3 shows a typical microscope field before (A) and after (B) addition of 1 mM ATP. A strong increase in the fluorescence of many of the molecules is readily observed. The temporal trajectory of the fluorescence of one of these (circles in Figure 3A,B) is shown in Figure 3c.
Figure 3.
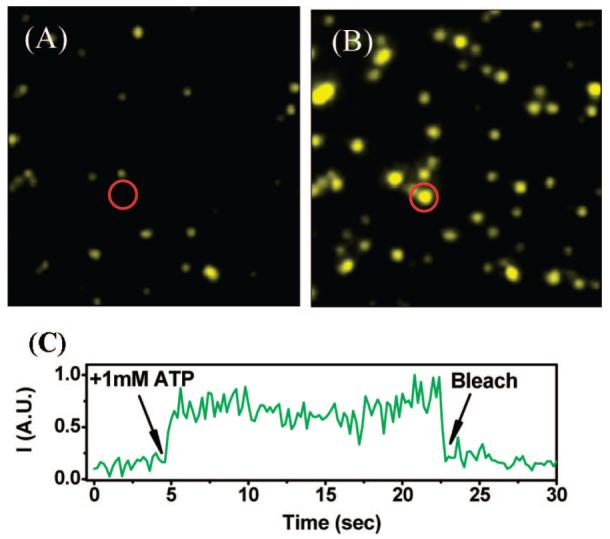
Activity of the optical switch in labeled SR1WC molecules deposited on a glass surface, monitored at the single molecule level. Changes in fluorescence after addition of (A) buffer only and (B) 1 mM ATP. (C) Temporal trajectory of the molecule marked with a red circle in (A) and (B).
We have shown here how an optical switch can be incorporated into the structure of GroEL to report on its structural changes. This switch has several advantages over other fluorescence-based methods for probing conformational transitions. First, it provides a very large dynamic range, as the signal is large in one conformation and essentially null in the other. Second, labeling with only one dye is required in contrast with FRET which usually necessitates labeling with two different dyes. The optical switch built into SR1 now provides us with a convenient and powerful route for studying its dynamics on the single-molecule level, which we hope to report soon.
Supplementary Material
ACKNOWLEDGMENT
This research was made possible by the historic generosity of the Perlman Family, and financial support from the NIH (GM080515 to G.H.) and the ISF (67/05 to A.H.).
LITERATURE CITED
- (1).Chiu W, Baker ML, Almo SC. Structural biology of cellular machines. Trends Cell Biol. 2006;16:144–150. doi: 10.1016/j.tcb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- (2).Horovitz A, Willison KR. Allosteric regulation of chaperonins. Curr. Opin. Struct. Biol. 2005;15:646–651. doi: 10.1016/j.sbi.2005.10.001. [DOI] [PubMed] [Google Scholar]
- (3).Rye HS. Application of fluorescence resonance energy transfer to the GroEL-GroES chaperonin reaction. Methods. 2001;24:278–288. doi: 10.1006/meth.2001.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kim SY, Semyonov AN, Twieg RJ, Horwich AL, Frydman J, Moerner WE. Probing the sequence of conformationally induced polarity changes in the molecular chaperonin GroEL with fluorescence spectroscopy. J. Phys. Chem. B. 2005;109:24517–24525. doi: 10.1021/jp0534232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Taguchi H, Ueno T, Tadakuma H, Yoshida M, Funatsu T. Single-molecule observation of protein-protein interactions in the chaperonin system. Nat. Biotechnol. 2001;19:861–865. doi: 10.1038/nbt0901-861. [DOI] [PubMed] [Google Scholar]
- (6).Braig K, Otwinowski Z, Hegde R, Boisvert DC, Joachimiak A, Horwich AL, Sigler PB. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature. 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- (7).Xu Z, Sigler PB. GroEL/GroES: structure and function of a two-stroke folding machine. J. Struct. Biol. 1998;124:129–141. doi: 10.1006/jsbi.1998.4060. [DOI] [PubMed] [Google Scholar]
- (8).Yifrach O, Horovitz A. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry. 1995;34:5303–5308. doi: 10.1021/bi00016a001. [DOI] [PubMed] [Google Scholar]
- (9).Xu Z, Horwich AL, Sigler PB. The crystal structure of the asymmetric GroEL-GroES-(ADP)7 chaperonin complex. Nature. 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- (10).Inobe T, Makio T, Takasu-Ishikawa E, Terada TP, Kuwajima K. Nucleotide binding to the chaperonin GroEL: non-cooperative binding of ATP analogs and ADP, and cooperative effect of ATP. Biochim. Biophys. Acta. 2001;1545:160–173. doi: 10.1016/s0167-4838(00)00274-0. [DOI] [PubMed] [Google Scholar]
- (11).Rye HS, Burston SG, Fenton WA, Beechem JM, Xu Z, Sigler PB, Horwich AL. Distinct actions of cis and trans ATP within the double ring of the chaperonin GroEL. Nature. 1997;388:792–798. doi: 10.1038/42047. [DOI] [PubMed] [Google Scholar]
- (12).Marme N, Knemeyer JP, Sauer M, Wolfrum J. Inter- and intramolecular fluorescence quenching of organic dyes by tryptophan. Bioconjugate Chem. 2003;14:1133–1139. doi: 10.1021/bc0341324. [DOI] [PubMed] [Google Scholar]
- (13).Neuweiler H, Schulz A, Bohmer M, Enderlein J, Sauer M. Measurement of submicrosecond intramolecular contact formation in peptides at the single-molecule level. J. Am. Chem. Soc. 2003;125:5324–5330. doi: 10.1021/ja034040p. [DOI] [PubMed] [Google Scholar]
- (14).Neuweiler H, Doose S, Sauer M. A microscopic view of miniprotein folding: enhanced folding efficiency through formation of an intermediate. Proc. Natl. Acad. Sci. U.S.A. 2005;102:16650–16655. doi: 10.1073/pnas.0507351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Yifrach O, Horovitz A. Transient kinetic analysis of adenosine 5′-triphosphate binding-induced conformational changes in the allosteric chaperonin GroEL. Biochemistry. 1998;37:7083–7088. doi: 10.1021/bi980370o. [DOI] [PubMed] [Google Scholar]
- (16).Shiseki K, Murai N, Motojima F, Hisabori T, Yoshida M, Taguchi H. Synchronized domain-opening motion of GroEL is essential for communication between the two rings. J. Biol. Chem. 2001;276:11335–11338. doi: 10.1074/jbc.M010348200. [DOI] [PubMed] [Google Scholar]
- (17).Yamasaki R, Hoshino M, Wazawa T, Ishii Y, Yanagida T, Kawata Y, Higurashi T, Sakai K, Nagai J, Goto Y. Single molecualr observation of the interaction of GroEL with substrate proteins. J. Mol. Biol. 1999;292:965–972. doi: 10.1006/jmbi.1999.3129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


