Abstract
Two experiments were conducted to investigate the possibility of faster forgetting by PDAPP mice (a well-established model of Alzheimer’s disease as reported by Games and colleagues in an earlier paper). Experiment 1, using mice aged 13–16 mo, confirmed the presence of a deficit in a spatial reference memory task in the water maze by hemizygous PDAPP mice relative to littermate controls. However, after overtraining to a criterion of equivalent navigational performance, a series of memory retention tests revealed faster forgetting in the PDAPP group. Very limited retraining was sufficient to reinstate good memory in both groups, indicating that their faster forgetting may be due to retrieval failure rather than trace decay. In Experiment 2, 6-mo-old PDAPP and controls were required to learn each of a series of spatial locations to criterion with their memory assessed 10 min after learning each location. No memory deficit was apparent in the PDAPP mice initially, but a deficit built up through the series of locations suggestive of increased sensitivity to interference. Faster forgetting and increased interference may each reflect a difficulty in accessing memory traces. This interpretation of one aspect of the cognitive deficit in human mutant APP mice has parallels to deficits observed in patients with Alzheimer’s disease, further supporting the validity of transgenic models of the disease.
Considerable progress has been made in modeling selected aspects of Alzheimer’s disease (AD) by exploiting human neurogenetics in transgenic animals that overexpress mutant human genes related to familial AD (Hardy and Selkoe 2002). The identification of several gene mutations that are predictive of, or risk factors for, AD led directly to the engineering of a number of analytically useful lines of mice (Price et al. 1998; Kobayashi and Chen 2005). One of these, based on a familial mutation at codon 717 of the amyloid precursor protein (Goate et al. 1991), is the PDAPP line in which a human minigene with this mutation is overexpressed on a murine background under the control of a platelet-derived growth factor promoter (Games et al. 1995). PDAPP mice show a phenotype that includes an age-related deposition of diffuse plaques beginning at around 8 mo of age in hemizygous animals (Games et al. 1995; Masliah et al. 1996); an age-related up-regulation of Aβ levels (Johnson-Wood et al. 1997); a decrease in the magnitude of evoked field potentials in hippocampal brain slices (Hsia et al. 1999; Larson et al. 1999) possibly related to glucose hypometabolism (Dodart et al. 1999); a smaller hippocampal volume, particularly in the dentate gyrus (Redwine et al. 2003), but no cell loss (Irizarry et al. 1997); and numerous other changes. Behavioral studies have indicated further important components of the phenotype in separate lines of PDAPP mice including both age-related and age-independent deficits in spatial learning (Chen et al. 2000; Dodart et al. 2000). These and other important lines of transgenic mice carrying human mutations that occur in familial Alzheimer’s disease show learning deficits in a variety of other cognitive tasks (Hsiao et al. 1996; Chapman et al. 1999; Kobayashi and Chen 2005; Billings et al. 2007; Eriksen and Janus 2007).
Revealing a “learning deficit” is often only the first step in the analysis of a behavioral phenotype. Such a deficit can occur for several reasons—a failure to encode or store information effectively into long-term memory, a failure to consolidate memory traces, or a deficit in retrieving them. One or a combination of these memory processes may be compromised by a specific transgenic modification, and identifying which is affected may be relevant to the development of novel therapeutics. The focus of this study was to establish whether a previously established deficit in spatial learning and memory in PDAPP mice is solely due to a deficit in encoding or storage, as may often be assumed, or might also be due to faster forgetting that arises from a failure of consolidation or retrieval. This issue has been studied in patients where dissociation has been observed between equivalent rates of forgetting for recognition tasks but faster forgetting in recall (Christensen et al. 1998). We had previously shown that our line of PDAPP mice show normal forgetting in an object recognition task (Chen et al. 2000), but had not studied memory recall as occurs during probe tests in the water maze (but see Dodart et al. 2002 for a suggestion of faster forgetting of recognition memory). Analytically suitable behavioral protocols were therefore used to identify faster forgetting and the possible underlying reasons for it. The study was conducted with both middle-aged (16 mo—Experiment 1) and young (5–6 mo—Experiment 2) PDAPP mice, together with age-matched control littermates.
Results
In both experiments, PDAPP animals swam normally and showed swim speeds that were generally indistinguishable from those of wild-type (WT) controls. We did not use a formal SHIRPA test to screen for behavioral abnormalities (Rogers et al. 1997), but did not see any obvious behavioral abnormalities during routine handling or in the animals’ home cage.
Experiment 1: Learning and forgetting in 16-mo-old PDAPP mice
Cue task
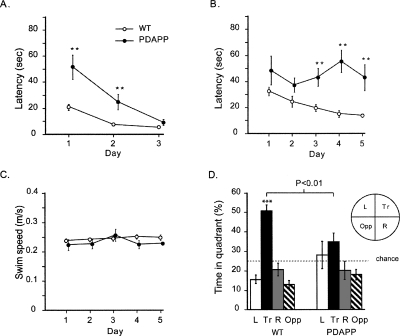
PDAPP mice were initially impaired with respect to their latency to approach a visible platform, but reached the same level of performance as WT by day 3 (Group × Day interaction, F(1.38,27.53) = 12.08, p < 0.005) (Fig. 1A) (A Greenhouse-Geisser correction to the degrees of freedom was used for all instances of multiple within-subjects measures.) This suggested no persistent sensorimotor or motivational abnormalities. Both groups averaged escape times of <10 sec on day 3 of training and did not differ (F(1,20) = 3.04, p > 0.10). PDAPP mice displayed a nonsignificant trend toward a slower swim speed than controls (F(1,20) = 3.60, 0.10 > p > 0.05). There was therefore, little indication that sensory or motor functions required in the water maze are affected in middle-aged PDAPP mice.
Figure 1.
Experiment 1: Cue task and spatial reference memory in 16-mo-old mice. (A) Cue task. Escape latency as a function of days showing the transitory nature of the PDAPP deficit at 16 mo of age. (B) Spatial learning. Escape latency as a function of days showing the severe deficit in learning by PDAPP mice. (C) Swim speed during spatial learning. (D) First probe test (PT1) performed 10 min after training completion, revealing a deficit in spatial reference memory in PDAPP mice. Proportion of time spent searching each of the four quadrants of the pool during 60 sec of swimming in the absence of the hidden platform. (L) Left; (Tr) training; (R) right; (OPP) opposite quadrant. All data plotted ±1 SEM. (**) p < 0.01; (***) p < 0.001.
Spatial learning
The PDAPP mice were impaired in a standard spatial reference memory place-navigation task (F(1,20) = 47.25, p < 0.001) (Fig. 1B). They showed no detectable improvement over the 5 d of training, while the WT littermates displayed a steady decline in escape latency such that, by day 5, they were escaping to the platform significantly more quickly than the PDAPP group (F(1,20) = 17.37, p < 0.001). The Group × Day interaction did not, however, quite reach significance (0.1 > p > 0.05). The poorer performance of the PDAPP mice is unlikely to be due to effects on motor function as no groups’ difference in swim speed was observed during this training (F < 1) (Fig. 1C). Detailed inspection of acquisition revealed that the PDAPP mice did show a within-day improvement in escape latency. However, while the WT mice tended to retain their improvement to trial 1 of the next day of training, PDAPP mice did not show such a “carry-over” effect. For example, the PDAPP mice were significantly slower to escape on the first trial of days 4 and 5 of training (p < 0.005)—an early indication of faster forgetting that could be due to a memory consolidation deficit.
The analysis of probe test 1, 10 min after training (PT1; platform absent with the mice swimming for 60 sec), revealed that the WT mice swam persistently in the quadrant where the hidden platform was normally located, while the PDAPP mice did not (Group × Quadrant interaction, F(2.39,47.79) = 4.18, p < 0.025) (Fig. 1D). In addition, the PDAPP mice spent significantly less time in the training quadrant than WT mice (F(1,20) = 9.35, p < 0.01), and WT mice performed significantly better than chance (t = 8.99, p < 0.01; chance = 25%), whereas PDAPP animals did not. Thus, in keeping with previous studies, PDAPP mice displayed a deficit in spatial learning.
Training to criterion
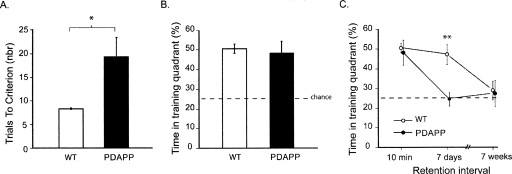
The next and novel steps of the study were then carried out to identify whether one component of this apparent “learning deficit” included an increase in the rate of forgetting. To examine this, it was first necessary to train all mice to an equivalent level of performance. All mice were therefore continued in the spatial navigation task until each one met a strict criterion of performance (eight successive trials with an escape latency <20 sec or 40 trials were completed). As expected, the PDAPP mice required significantly more trials than controls (F(1,20) = 15.98, p < 0.001), but five out of seven mice in this group eventually reached criterion (Fig. 2A). The second probe test (PT2) conducted 10 min after reaching criterion (or 40 trials) revealed that the PDAPP mice now searched in the training quadrant as well as WT mice, with both groups being highly significantly above chance (t = 3.68, p < 0.01) (Fig. 2B). The two PDAPP mice that failed to reach criterion also searched appropriately in the correct quadrant, indicating that overtraining is sufficient to enable them to learn a single platform location (even though they could not quite reach the stringent performance criterion). At this point of training, both groups had reached an equivalent level of search performance and, by inference, of memory trace strength. Reaching this criterion set the stage for the next phase of testing.
Figure 2.
Experiment 1: Faster forgetting in 16-mo-old PDAPP. (A) Training to criterion. Number of trials to the strict training criterion of eight trials averaging <20 sec, revealing a deficit in PDAPP mice. (B) Probe test. Proportion of time spent searching in PT2 in the training quadrant 10 min after reaching criterion. Note equivalence of PDAPP and WT mice. (C) Forgetting of spatial memory. Time spent in the training quadrant in each of three successive retention tests (PTs 2–4) conducted 10 min, 7 d, or 7 wk after the animals reached criterion. Note faster forgetting in PDAPP mice but both groups reaching chance eventually. All data plotted ±1 SEM. (**) p < 0.01.
Forgetting
Three successive “probe” or memory retention tests (PTs 2–4) were then compared—without intervening training—to examine forgetting. The first of these, PT2, was the test just described during which the PDAPP and WT group, showed equivalent memory at a 10-min retention interval. However, by PT3, conducted 7 d later, the PDAPP group had forgotten the platform location, whereas the WT mice continued to search appropriately (Fig. 2C) (Groups × Retention Interval interaction, F(1,20) = 4.91, p < 0.05; Groups difference at 7 d, p < 0.01). PT4 was conducted 7 wk after reaching criterion as a check that all animals would eventually display forgetting to chance levels of performance.
Memory reactivation
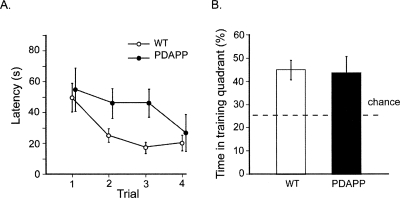
Faster forgetting may be due to a decline in trace strength over time (a deficit in consolidation) or an inability to recall information (a retrieval deficit). These processes are notoriously difficult to dissociate, but to partially distinguish these possibilities, all animals were given very limited retraining to their previous platform location after PT4. This served mainly as a “reminder” of the presence and location of the platform, and would only be enough to reinstate a latent memory trace that had somehow become inaccessible (de Hoz et al. 2004). If memory trace strength had truly declined to zero, it should take a large number of retraining trials to bring the animals back to the same level of performance as displayed in Figure 2B (particularly the PDAPP group). However, the results showed that after 7 wk of “forgetting,” only four retraining trials were sufficient to restore the performance of both PDAPP and WT mice to their previous levels of good search performance (Fig. 3). While the reacquisition rate of the WT mice showed a nonsignificant trend toward being slightly faster (F(1,20) = 3.11, 0.10 > p > 0.05) (Fig. 3A), both groups reached an equivalent and rapid escape latency by trial 4 at which point they did not differ (F < 1). A probe test conducted 10 min later (PT5) revealed that both groups spent as much time in the training quadrant (Fig. 3B) as they had immediately after reaching the earlier criterion in PT2 (Fig. 2B). The groups did not differ from each other (F < 1), but they were both significantly above chance (p < 0.01).
Figure 3.
Experiment 1: Reactivation of memory in 16-mo-old mice. (A) Reinstatement of spatial memory. Escape latency over the course of four retraining trials conducted after the 7-wk retention test of Figure 2. (B) Final memory test. Final probe test (PT5) performed 10 min after retraining. The PDAPP and WT mice spend an equivalent above-chance time searching in the training quadrant (Tr). All data plotted ±1 SEM.
Immunocytochemistry
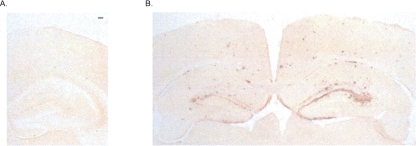
At the end of this behavioral training, the animals were killed, their brains were removed, and sections were cut on a cryostat. Analysis of the brains of these now 18-mo-old animals revealed heavy diffuse amyloid plaque deposition in the PDAPP group (Fig. 4B). This served primarily to confirm group identity, and we made no attempt to quantify this.
Figure 4.
Immunocytochemistry on fixed brain sections using 3D6 antibody to reveal plaques. (A) The absence of plaques in young 6-mo-old PDAPP mice. Scale bar, 100 μm. (B) Diffuse amyloid plaque deposition in the hippocampus and cortex in 16-mo-old PDAPP mice.
Experiment 2: Memory interference prior to amyloid plaque deposition?
Chen et al. (2000) showed that PDAPP mice express an age-dependent spatial learning deficit when trained in a behavioral protocol that requires them to reach criterion on each of a series of spatial locations in the same water maze across sessions. Such a “serial spatial learning” protocol is likely to increase interference through successive reversals, and so challenge memory retrieval in a more demanding way than when all training is exclusively to a single location. In Experiment 2, we therefore focused on a specific issue: Could detectable differences be observed in probe tests in young PDAPP mice trained at 5–6 mo of age, even though their performance in navigating to a hidden platform may be indistinguishable from that of WT mice. Our logic in using younger animals is because recent studies have implicated alterations in neural function associated with soluble oligomeric β-amyloid species prior to overt amyloid plaque deposition (Lambert et al. 1998; Klein et al. 2001; Walsh et al. 2005; Lesne et al. 2006). Our previous study (Chen et al. 2000) failed to detect a memory deficit in young PDAPP mice using a trials-to-criterion measure (defined in relation to escape latency during training), but the ability to retrieve information effectively may be more sensitively identified using a post-criterion probe test.
Cue task
Training began with the cued task as in Experiment 1, but we extended the training to a full 5 d to check whether the PDAPP animals would reach a common asymptote of performance as that of WT mice. As shown in Figure 5A, a similar trend was apparent like that in Figure 1A, with the much younger PDAPP mice also displaying slightly slower escape latencies initially. However, overall, this group did not differ from the WT group (F(1,32) = 2.95, p > 0.05), with both groups showing an efficient decrease in escape latency across days (F(2.133,68.263) = 102.03, p < 0.001) and no interaction between groups across days (F(92.133,68.263) = 2.68, p > 0.05). Performance on days 4 and 5, not tested in Experiment 1, was equivalent across groups.
Figure 5.
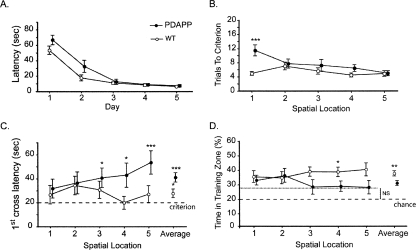
Experiment 2: Accumulating interference in spatial memory in 6-mo-old PDAPP mice. (A) Cue task. Escape latency as a function of days showing only a transitory deficit in young PDAPP mice. (B) Training to criterion in the serial spatial learning task. Number of trials to the strict training criterion of three trials averaging <20 sec reveals no deficit. (C) Latency to cross the correct platform location during probe tests conducted 10 min after reaching criterion on each spatial task reveals no deficit initially, but the PDAPP mice display an accumulating sensitivity to interference. (D) Proportion of time spent searching in the training platform “zone” during these successive probe tests conducted 10 min after reaching criterion. The dashed line represents the chance level (20%). Note equivalence of PDAPP and WT mice for the first two problems only. All data are plotted ±1 SEM.
Serial spatial learning task
In this task, the mice are trained until they reach the criterion of an average escape latency of 20 sec across three consecutive trials on each of a series of spatial locations. Although more modest than the training criterion used in Experiment 1, the demands upon memory retrieval are challenged in a different way by requiring the mice to learn a new spatial location, and then another one, and so on in serial fashion. Analyzing the data from this serial spatial learning task, the young PDAPP animals were slower to reach criterion on the first spatial task (t-test: t = −4.72, p < 0.001) but, from the second problem onward, did not differ from WT controls (F(1,32) = 1.78, p > 0.05) (Fig. 5B). Moreover, we observed a clear effect of Tasks (F(3.078,98.503) = 4.18, p < 0.01), Group (F(1,32) = 8.53, p < 0.01), and an interaction between them (F(3.078,98.503) = 3.74, p < 0.025). These data are similar to those of Chen et al. (2000), but, as noted above, successful learning of a series of novel spatial locations in the water maze to a modest criterion may nonetheless mask altered access to memory traces in a sensitive probe test.
Probe tests
To examine this possibility, probe tests were conducted 10 min after reaching criterion on each of the five successive spatial locations. A conventional quadrants analysis could not be used as the locations of the hidden platform for these five tasks were in 10 different places (counterbalanced between two series of five locations). Instead, we examined the latency to cross the correct platform location (first-crossing latencies) and how much the mice swam in a zone centered on the correct location (zone analysis).
For a PT taking place 10 min after the completion of training, a first-crossing latency of around or slightly greater than 20 sec is to be expected. The WT mice did not differ from this value for each of the five successive locations (Fig. 5C) (t-test: t < 1.89, p > 0.05). However, the PDAPP mice expressed a progressive increase in the time required to approach and cross this correct location. For the first two locations, they were successful in doing so at near criterion levels (t-test: t < 1.90, p > 0.05), just like the controls. However, from the third problem onward, it took significantly longer than 20 sec to successfully approach the platform location for the first time (t-test: t > 2.35, p < 0.05). Averaged across all five locations, PDAPP animals took significantly more time to reach the platform location than WT mice (41 vs. 27 sec; F(1,32) = 6.45, p < 0.025).
In a probe test, the zone analysis measures the extent to which, having reached the vicinity of the correct platform location, the animals continue to search there rather than elsewhere in the pool. The time spent in this target zone (18-cm radius) was analyzed relative to the total time spent in each of the five possible target locations (i.e., a chance level of 20%). The ANOVAR revealed an overall difference between PDAPP and WT mice (F(1,32) = 7.26, p < 0.025). Across successive locations, the WT mice expressed a relatively focused search in the correct platform zone (t-test: t > 3.64, p < 0.003 relative to chance), whereas PDAPP mice were successful in searching at above chance levels for the first two locations (t-test: t > 3.624, p < 0.01) but not thereafter (Fig. 5D) (t-test: t < 2.045, p > 0.05).
These gradually accumulating indications of memory interference were not secondary to changes in other parameters of the animals’ behavior in the water maze as the PDAPP and WT mice did not differ with respect to pathlength during these post-criterion probe tests, swimming speed, or thigmotaxic behavior (all ps > 0.05).
Immunocytochemistry
As in the previous study, the animals were killed, their brains were removed, and sections were cut on a cryostat. Analysis of the brains of these young animals revealed no amyloid plaque deposition in the PDAPP group (Fig. 4B). We also used 8E5 antibody against mutant APP to confirm group status (data not shown).
Discussion
The central finding of these two experiments is that PDAPP mice display faster forgetting and increased interference in spatial memory. When “overtrained” to an equivalent level of navigational performance, PDAPP mice could remember the platform location for a short period (10 min), but were unable to remember the location of the hidden platform 7 d later, whereas WT mice had no difficulty. This faster rate of forgetting is distinct from any deficit in memory encoding that PDAPP mice may also have. Furthermore, we obtained evidence that this faster forgetting may, in part, be due to a deficit in memory retrieval rather than merely a failure of consolidation. Specifically, brief retraining (of only four swim trials) was sufficient to return both PDAPP and WT 16-mo-old mice to equivalent good levels of spatial memory. Moreover, in 6-mo-old PDAPP mice tested at a time when elevated Aβ starts to occur but prior to overt β-amyloid plaque deposition (Johnson-Wood et al. 1997), memory probe tests conducted shortly after training on a series of spatial problems revealed gradually increasing interference.
Definitive evidence that an experimental intervention selectively affects dissociable memory processes (encoding, storage, consolidation, or retrieval) requires procedures that are applied in a restricted manner at appropriate points in training or retention. This is rarely possible in human studies, easiest in animal work using reversible treatments (e.g., drugs), but analytically difficult in mice harboring permanent genetic mutations. The PDAPP mouse shares with other human mutant APP transgenic mice that it is engineered to harbor a specific mutation throughout its life to model of certain aspects of progressive neurodegenerative disease (mutant β-amyloid plaque deposition). This obviates the possibility of a definitive test of whether human mutant APP overexpression is causally associated with a particular cognitive consequence, such as a failure of memory retrieval, because a definitive test would require the animal to be “normal” during training and the subsequent memory consolidation period, and only affected by the mutation at the time of retrieval. This limitation in what is feasible can, however, be offset by the use of analytically relevant behavioral manipulations of standard task procedures that enable partial dissociations between memory processes that might be compromised selectively.
Our first new finding is that 16-mo-old PDAPP mice show faster forgetting of spatial information learned in a water maze than WT mice. In making such a claim, it is insufficient merely to show poorer spatial memory after a retention delay (e.g., 7 d); it is also necessary to have established an equivalent level of good memory at a short delay that is within the domain of long-term memory (e.g., 10 min). This was achieved here, using the procedure of overtraining the animals individually to a stringent criterion, with the faster forgetting observed as a statistically significant interaction between groups and retention interval during the retention tests (Fig. 2). That is, using the standard water-maze measure of proportion of time spent searching in the training quadrant, the PDAPP mice were as good as the WT mice immediately after reaching criterion, but much poorer 7 d later. The “learning deficit” on the standard reference memory water-maze task is, nonetheless, likely to be due in part to an encoding/consolidation deficit. Further evidence for this came from the failure to observe a “carry-over” effect in PDAPP mice in the escape latency scores over the last 2 d of training, consistent with both a consolidation and a memory retrieval deficit. However, we did not give a retention test 24 h after training to criterion and thus do not know the rate of forgetting by the 16-mo-old PDAPP mice over this shorter time period.
Our second finding relates to the basis of this faster forgetting. Forgetting can occur for two broad reasons—trace decay (a deficit of consolidation), or interference from other learned material (a retrieval deficit). It is notoriously difficult to separate these contributions to amnesia in humans (McCarthy and Warrington 1990) and no less difficult in animal studies. However, the retraining data of Figure 3 are suggestive of retrieval failure. Following a suitably long interval that enabled WT mice also to reach chance performance in the series of retention tests (7 wk), all mice were given only four retraining trials followed by a probe test 10 min later. Spatially focused search performance recovered to equivalent above-chance levels that did not differ significantly in either group from those shown at the end of the more extended period of training to criterion earlier on. Demonstration of “savings” in retraining is a well-established way of revealing residual memory in groups whose performance in a memory test falls below the threshold of detection (de Hoz et al. 2004). It follows that it is unlikely that consolidation failure is the only reason for the forgetting seen here, although it may be a contributor in older transgenic animals that display deficits in immediate early gene expression (Dickey et al. 2004). Better evidence for this retrieval interpretation would be secured if performance levels could be modulated as a function of the presence and salience of retrieval cues, a matter that could be examined in future work. Unfortunately, the water maze is not the easiest task in which to do this because extramaze cues are multimodal and include such unchangeable factors as the geometry of the room. One way of doing this might be to use a “partial cueing” protocol (Nakazawa et al. 2002). Such an approach has revealed a specific role of NMDA receptors within area CA3 of the hippocampus during memory encoding that could later enable pattern completion at the point of recall. In a cue-controlled enclosure surrounding the water maze, we predict that PDAPP animals might also show poor retrieval of spatial memory with partial cues.
The third finding concerns the increased sensitivity to interference in a serial spatial learning task. This was observed in young PDAPP mice, prior to overt β-amyloid plaque deposition and arguably prior to the deficits in immediate early gene expression seen in older APP transgenic mice (Dickey et al. 2004). In certain respects, this finding is paradoxical because the faster forgetting of PDAPP mice observed in Experiment 1 might be expected to lead to decreased sensitivity to interference in the serial task. In practice, the opposite was observed—a finding that also points to retrieval failure being the basis of the faster forgetting in PDAPP mice. A difficulty, as already noted, is that we measured forgetting over 7 d in Experiment 1, but the interval between successive problems in Experiment 2 was only 1 d. Nonetheless, the gradual nature of the deficit in Experiment 2 is also suggestive: No deficit was seen for the first and second spatial locations, but it emerged as the animals were trained on successive spatial locations in the same water maze with the same spatial cues. An interference effect of this kind cannot be observed with “standard” water-maze training protocols (such as those of Experiment 1) because only one escape platform location is used. In contrast, by the time five separate spatial locations had been trained in a single context, the mice would have been in a position where their memory retrieval mechanism would need to successfully distinguish between five separate long-term memory traces and somehow identify which was the appropriate memory to recall that day—a process that would entail contextual pattern separation. This appears to be a difficulty for PDAPP mice (Savonenko et al. 2005), with episodic-like tasks (such as serial reversal) being particularly sensitive for revealing such a deficit (Morris 2001). The process of encoding highly similar but distinct episodes as separate memory traces is thought to require neural activity and plasticity in the dentate gyrus (DG). Given this, it is worth noting that certain lines of APP transgenic mice show decreased levels of calcium-binding proteins in the dentate gyrus (Palop et al. 2003). Interestingly, using mice lacking the NR1 subunit of the NMDA receptor specifically in DG granule cells, McHugh et al. (2007) observed normal contextual fear conditioning but an impairment in distinguishing two similar contexts. These mutant mice are also impaired in a delayed-matching-to-place (DMP) task in a water maze, but only after a certain amount of training (T.J. McHugh and M.W. Jones, pers. comm.). This suggests there is a developing sensitivity to memory interference. Although we did not attempt to block NMDA receptors pharmacologically, previous studies on PDAPP mice have revealed a decreased volume of DG and a shortening of branches of granule cells, apparent as early as 90 d of age (Redwine et al. 2003). These changes occurring prior to β-amyloid plaque deposition, like those seen in Tg2576 mice (Jacobsen et al. 2006), have been attributed to a selective vulnerability of projections that terminate in the molecular layer of DG. In this respect, one can speculate that the proactive memory interference effect observed in our study could, at least in part, be associated with the morphological changes observed in the dentate gyrus of PDAPP mice.
A fourth observation, though not one we investigated systematically here, is that these changes in memory retrieval are seen in young PDAPP animals prior to amyloid plaque deposition as well as in animals that have reached an age at which amyloid deposits have occurred. This is of interest given growing interest in the possibility that the accumulation of pathogenic Aβ-derived diffusible ligands (ADDLs or oligomers), rather than overt cell death and degeneration, is responsible for the learning and memory deficits seen in human mutant APP mice (Lambert et al. 1998; Walsh et al. 2002; Lesne et al. 2006, 2008; Klein et al. 2007; LaFerla et al. 2007).
In conclusion, it appears that the faster forgetting displayed by PDAPP mice is partly due to difficulties in accessing memory traces. These findings raise the possibility that the age-related “learning deficit” of these and perhaps other human mutant APP transgenic mice is due, in part, to impaired hippocampal-dependent memory retrieval. While speculative, this interpretation of the learning deficit in hAPP mice has parallels to retrieval deficits that have been observed in patients with AD, such as the faster forgetting on recall tasks (Christensen et al. 1998) and relatively gradual gradients of remote memory (Bright and Kopelman 2004). These data suggest that examining memory interference might also be helpful in the early diagnosis of AD and that further attention be paid to memory retrieval as well as to impairments of new memory encoding.
Materials and Methods
Animals
Hemizygous PDAPP and wild-type (WT) littermate mice were used. They were of common parentage and derived from a hybrid background of Swiss Webster, DBA, and C57Bl/6J strains. The animals were group housed and had free access to food and water. They were maintained on a light/dark cycle of 14:10 h with lights on at 08.00 h. In Experiment 1, the 22 male mice were 13–16 mo (PDAPP, n = 7; WT, n = 15). In Experiment 2, there were 34 mice aged from 6 to 8 mo (PDAPP, n = 14; WT, n = 20); both genders were used in this study, but data have been pooled as no differences in performance were observed. All mice were trained and tested “blind” with respect to group identity.
Apparatus
Behavioral testing was conducted using an open-field water maze (Morris 1981, 1984). The water maze consisted of a large circular tank (2.0 m diameter) filled with water (depth 0.5 m; temperature 25°C ± 1°C) made opaque with the addition of 400 mL of liquid latex. An escape platform (20 cm diameter in Experiment 1, and 13 cm in Experiment 2) had its top surface submerged 1.5 cm below the water surface. The mice were placed into the water facing the sidewalls, being transported there by hand from a transport cage in the same room. Prominent extramaze cues were placed around the testing room to enable the animals to learn the platform’s location. The animals’ swimming behavior was monitored by a video tracking system. A camera was fixed directly above the pool in a position where its entire surface area could be viewed. An appropriate level of diffuse lighting was created (4 × 500 W halogen floor-mounted floodlight angled toward the corners of the ceiling) to allow the mice to be detected against the white water of the pool by an image analyzer (HVS, model VP112) or video frame grabbing software.
During the cued training, an object (20 cm high) was used to mark the platform (20-cm diameter) (which was randomly placed in different locations across trials), and the pool was surrounded by white curtains to occlude extramaze cues. During place navigation, the curtains were drawn together at one point, and the hidden platform was unmarked by any local cues.
Immunocytochemistry
After the completion of behavioral training, all animals were sacrificed and the brain was removed to measure the extent of β-amyloid plaque burden in the hippocampus.
One hemisphere of the brain was fixed in formalin and sliced in 30-mm coronal sections using a cryostat. Every fifth section throughout the extent of the brain was stored in antifreeze solution (30% glycerol/30% ethylene glycol in 40 mM NaPO4). These sections were incubated with 3D6 or 8E5-biotinylated antibody overnight at 4°C and then reacted with the horseradish peroxidase-avidin biotin complex (Vector Laboratories) and developed using 3,3′-diaminobenzidine (DAB) as the chromagen. Diffuse amyloid plaque disposition was quantified automatically using Leica Q-Win image analysis (for 3D6 treated sections). Video images of the brain were captured, the hippocampal area was outlined, and a threshold optical density was obtained that discriminated staining from background. We made no attempt to quantify “plaque burden” in this study (this has been reported many times before in other studies), seeking only to confirm the presence and absence of plaques at the two ages tested. Group status was confirmed by 8E5 APP antibody. However, in Experiment 2, we intended to use the other hemisphere collected to measure β-amyloid levels with ELISA, but these samples were unfortunately “mislaid” by the courier on transit from Scotland to California.
Experiment 1
Experimental protocol
Training was divided into three conceptually distinct phases—training to criterion, forgetting, and memory reactivation.
Training to criterion
Initial training consisted of cued navigation to a visible platform (3 d) followed by spatial reference memory training (5 d). All animals received four trials/day with a maximum trial duration of 90 sec (+30 sec on the platform at the end of each trial) and an intertrial interval of 10 min. Ten minutes after the last trial on day 5, a probe test (PT1) was conducted in which the platform was removed and the animals were placed in the pool to swim for 60 sec. To investigate forgetting independently of learning, it is necessary to have all animals at an equivalent high level of memory performance. Therefore, the critical last part of this training phase involved training to criterion (eight trials/day; mean of eight consecutive trials <20 sec, or a maximum of 40 trials). Reaching criterion was followed, after 10 min, by probe test 2 (PT2).
Forgetting
From this baseline of effective memory, there were a series of two further probe tests. No additional training intervened between each test. After the second probe test above (PT2), the animals were returned to their home cage for 7 d before PT3, and then again for 7 wk, before PT4.
Memory reactivation
Five days after PT4, the animals were given a limited set of only four trials of retraining to the previous platform location. After a further interval of 10 min, a final probe test was given (PT5) to monitor the outcome of this retraining.
Experiment 2
Experimental protocol
Training was divided into three distinct phases—cuetask, training to criterion task, and a probe test.
Cuetask
The initial training consisted of cued navigation to a visibly cued platform as in Experiment 1, excepting that it continued for 5 d.
Training to criterion
The animals were then subjected to serial spatial learning, a series of five spatial reference memory tasks trained to a criterion of performance as described by Chen et al. (2000). Each task constituted a separate spatial problem, with all five problems taking place in the same water maze in the same experimental room. The platform location was varied from placement on an inner “virtual” ring (1-m diameter) or an outer ring (1.5-m diameter). In this way, the location differed between problems but remained the same within each day of training and each problem until criterion was reached. The animals had a maximum of 32 trials to acquire a task, but if it reached the criterion of an average escape latency of <20 sec on three consecutive trials, the training was stopped. On the following day, the training for the next spatial problem was begun. There was a maximum of eight trials per day, with an intertrial interval of 10 min. If the animal did not reach the PF within 90 sec, it was transported from the pool using a small paint roller, and allowed a 30-sec rest on this before being placed under a heat lamp to avoid hypothermia.
Probe tests
In order to assess the strength of memory for each platform location, a probe test was conducted 10 min after reaching criterion on each of the five problems.
Acknowledgments
We are grateful to Patrick Spooner for computing assistance. This work was supported by the Fyssen Foundation to S.D., the Cunningham Trust, an MRC Programme Grant, and a Royal Society/Wolfson Merit Award to R.G.M.M.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.990208.
References
- Billings L.M., Green K.N., McGaugh J.L., LaFerla F.M. Learning decreases Aβ*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J. Neurosci. 2007;27:751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright P., Kopelman M.D. Remote memory in Alzheimer’s disease. In: Morris R.G., Becker J.T., editors. Cognitive neuropsychology of Alzheimer’s disease. Oxford, London, UK: 2004. pp. 141–144. [Google Scholar]
- Chapman P.F., White G.L., Jones M.W., Cooper-Blacketer D., Marshall V.J., Irizarry M., Younkin L., Good M.A., Bliss T.V., Hyman B.T., et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chen G., Chen K.S., Knox J., Inglis J., Bernard A., Martin S.J., Justice A., McConlogue L., Games D., Freedman S.B., et al. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Christensen H., Kopelman M.D., Stanhope N., Lorentz N., Owen P. Rates of forgetting in Alzheimer’s disease. Neuropsychologia. 1998;36:547–557. doi: 10.1016/s0028-3932(97)00116-4. [DOI] [PubMed] [Google Scholar]
- de Hoz L., Martin S.J., Morris R.G. Forgetting, reminding, and remembering: The retrieval of lost spatial memory. PLoS Biol. 2004;2:e225. doi: 10.1371/journal.pbio.0020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C.A., Gordon M.N., Mason J.E., Wilson N.J., Diamond D.M., Guzowski J.F., Morgan D. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J. Neurochem. 2004;88:434–442. doi: 10.1111/j.1471-4159.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Dodart J.-C., Mathis C., Bales K.R., Paul S.M., Ungerer A. Early regional cerebral glucose hypometabolism in transgenic mice overexpressing the V717F B-amyloid precursor protein. Neurosci. Lett. 1999;277:49–52. doi: 10.1016/s0304-3940(99)00847-2. [DOI] [PubMed] [Google Scholar]
- Dodart J.-C., Mathis C., Bales K.R., Paul S.M., Ungerer A. Behavioral deficits in APP V717F transgenic mice deficient for the apolioprotein E gene. Neuroreport. 2000;11:603–607. doi: 10.1097/00001756-200002280-00034. [DOI] [PubMed] [Google Scholar]
- Dodart J.C., Bales K.R., Gannon K.S., Greene S.J., DeMattos R.B., Mathis C., DeLong C.A., Wu S., Wu X., Holtzman D.M., et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Eriksen J.L., Janus C.G. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav. Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F., et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M.C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L., et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hsia A.Y., Masliah E., McConlogue L., Yu G.Q., Tatsuno G., Hu K., Kholodenko D., Malenka R.C., Nicoll R.A., Mucke L. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Irizarry M.C., Soriano F., McNamara M., Page K.J., Schenk D., Games D., Hyman B.T. Aβ deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J. Neurosci. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen J.S., Wu C.C., Redwine J.M., Comery T.A., Arias R., Bowlby M., Martone R., Morrison J.H., Pangalos M.N., Reinhart P.H., et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Wood K., Lee M., Motter R., Hu K., Gordon G., Barbour R., Khan K., Gordon M., Tan H., Games D., et al. Amyloid precursor protein processing and Aβ42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W.L., Krafft G.A., Finch C.E. Targeting small Aβ oligomers: The solution to an Alzheimer’s disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Klein W.L., Lacor P.N., De Felice F.G., Ferreira S.T. Molecules that disrupt memory circuits in Alzheimer’s disease: The attack on synapses by Aβ oligomers (ADDLs) In: Bontempi B., editor. Memories: Molecules and circuits. Springer-Verlag; Berlin, Germany: 2007. pp. 155–179. [Google Scholar]
- Kobayashi D.T., Chen K.S. Behavioral phenotypes of amyloid-based genetically modified mouse models of Alzheimer’s disease. Genes Brain Behav. 2005;4:173–196. doi: 10.1111/j.1601-183X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Lambert M.P., Barlow A.K., Chromy B.A., Edwards C., Freed R., Liosatos M., Morgan T.E., Rozovsky I., Trommer B., Viola K.L., et al. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J., Lynch G., Games D., Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- Lesne S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A., Gallagher M., Ashe K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lesne S., Kotilinek L., Ashe K.H. Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neuroscience. 2008;151:745–749. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Sisk A., Mallory M., Mucke L., Schenk D., Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F β-amyloid precursor protein and Alzheimer’s disease. J. Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy R.A., Warrington E.A. Cognitive neuropsychology. Academic Press; San Diego, CA: 1990. [Google Scholar]
- McHugh T.J., Jones M.W., Quinn J.J., Balthasar N., Coppari R., Elmquist J.K., Lowell B.B., Fanselow M.S., Wilson M.A., Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Spatial localisation does not depend on the presence of local cues. Learn. Motiv. 1981;12:239–260. [Google Scholar]
- Morris R.G.M. Developments of a watermaze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris R.G.M. Episodic-like memory in animals: Psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:1453–1465. doi: 10.1098/rstb.2001.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Quirk M.C., Chitwood R.A., Watanabe M., Yeckel M.F., Sun L.D., Kato A., Carr C.A., Johnston D., Wilson M.A., et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Jones B., Kekonius L., Chin J., Yu G.-Q., Raber J., Masliah E., Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate-gyrus is tightly linked to Alzheimer’s disease related cognitive deficits. Proc. Natl. Acad. Sci. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D.L., Tanzi R.E., Borchelt D.R., Sisodia S.S. Alzheimer’s disease: Genetic studies and transgenic models. Annu. Rev. Genet. 1998;32:461–493. doi: 10.1146/annurev.genet.32.1.461. [DOI] [PubMed] [Google Scholar]
- Redwine J.M., Kosofsky B., Jacobs R.E., Games D., Reilly J.F., Morrison J.H., Young W.G., Bloom F.E. Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: A magnetic resonance microscopy and stereologic analysis. Proc. Natl. Acad. Sci. 2003;100:1381–1386. doi: 10.1073/pnas.242746599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D.C., Fisher E.M., Brown S.D., Peters J., Hunter A.J., Martin J.E. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm. Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Savonenko A., Xu G.M., Melnikova T., Morton J.L., Gonzales V., Wong M.P., Price D.L., Tang F., Markowska A.L., Borchelt D.R. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: Relationships to β-amyloid deposition and neurotransmitter abnormalities. Neurobiol. Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Walsh D.M., Klyubin I., Fadeeva J.V., Cullen W.K., Anwyl R., Wolfe M.S., Rowan M.J., Selkoe D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Walsh D.M., Klyubin I., Shankar G.M., Townsend M., Fadeeva J.V., Betts V., Podlisny M.B., Cleary J.P., Ashe K.H., Rowan M.J., et al. The role of cell-derived oligomers of Aβ in Alzheimer’s disease and avenues for therapeutic intervention. Biochem. Soc. Trans. 2005;33:1087–1090. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]