Abstract
Extinction, a form of learning that has the ability to reshape learned behavior based on new experiences, has been heavily studied utilizing fear learning paradigms. Mechanisms underlying extinction of positive-valence associations, such as drug self-administration and place preference, are poorly understood yet may have important relevance to addiction treatment. Data suggest a major role for the noradrenergic system in extinction of fear-based learning. Employing both pharmacological and genetic approaches, we investigated the role of the α2-adrenergic receptor (α2-AR) in extinction of cocaine-conditioned place preference (CPP) and glutamatergic transmission in the bed nucleus of the stria terminalis (BNST). We found that pre-extinction systemic treatment with the α2-AR antagonist yohimbine impaired cocaine CPP extinction in C57BL/6J mice, an effect that was not mimicked by the more selective α2-AR antagonist, atipamezole. Moreover, α2A-AR knockout mice exhibited similar cocaine CPP extinction and exacerbated extinction impairing effects of yohimbine. Using acute brain slices and electrophysiological approaches, we found that yohimbine produces a slowly evolving depression of glutamatergic transmission in the BNST that was not mimicked by atipamezole. Further, this action was extant in slices from α2A-AR knockout mice. Our data strongly suggest that extinction-modifying effects of yohimbine are unlikely to be due to actions at α2A-ARs.
Extinction is a form of learning that is thought to involve the formation of a new memory that suppresses behavioral responses to a learned stimulus (Bouton 2002; Gale et al. 2004; Myers and Davis 2007), although degradation of the original memory may also be involved (Mao et al. 2006). The modulation of extinction processes is increasingly recognized for its clinical potential to reshape maladaptive behavior. A major focus of research has been on the possibility of combining pharmaceutical agents with extinction-based behavioral therapy to enhance therapeutic outcome for anxiety disorders (e.g., phobia, post-traumatic stress disorder [PTSD]) (Ressler et al. 2004). Extinction therapies could also be of benefit in the treatment of addiction. Although there have been studies investigating extinction of cue-induce craving responses in humans (Childress et al. 1993; Carter and Tiffany 1999), there have been relatively few clinical or preclinical studies investigating pharmacological manipulations of extinction of human drug seeking and addiction-related behaviors in animal models (Self et al. 2004).
Norepinephrine (NE) plays a role in a variety of aspects of learning and memory (Ferry and McGaugh 2008). Further, NE has emerged as a key regulator of various aspects of addiction-related behaviors (Aghajanian 1978; Redmond Jr. and Krystal 1984; Weinshenker and Schroeder 2007; Schank et al. 2008). The source of central NE arises from two projections, the ventral noradrenergic bundle (VNAB) and the dorsal noradrenergic bundle (DNAB), that both heavily innervate brain regions that are strongly implicated in extinction: the prefrontal cortex and the amygdaloid complex encompassing the bed nucleus of the stria terminalis (BNST) (Moore and Bloom 1979; Aston-Jones et al. 1999). Additionally, the infralimbic cortex (a prefrontal cortex region) is also critical for fear conditioning (Quirk et al. 2000; Wellman et al. 2007) and has been implicated in extinction behaviors. NE exerts its actions at these and other regions by signaling through adrenergic receptors (ARs), of which there are nine distinct AR receptors that fall into α1-, α2-, and β-AR categories, which are all G-protein, seven-transmembrane receptors. The α2-ARs, the focus of our studies, are widely distributed in the central nervous system (Nicholas et al. 1993; Wang et al. 2001).
Previous work has demonstrated that manipulation of the NE system affects extinction of conditioned fear behaviors (Cain et al. 2004; Mueller et al. 2008). Cain et al. (2004) found that systemic administration of the α2-AR antagonist yohimbine, a compound with strong anxiety-promoting properties in humans (Holmberg and Gershon 1961; Redmond Jr. and Huang 1979; Murburg et al. 1991) and laboratory animals (Lang and Gershon 1963; Davis et al. 1979; Holmes et al. 2002), facilitated long-term extinction of conditioned fear in mice. While these studies suggest a contribution of α2-ARs (as assayed by yohimbine) to fear extinction, little is known about their role in the extinction of reward-related memories formed by exposure to drugs of abuse.
In the present study, we investigated the role of α2-ARs in extinction of cocaine-induced conditioned place preference (CPP; and for comparison, fear extinction), using a combination of pharmacological and genetic strategies. In contrast to the facilitating effects of yohimbine on fear extinction (Cain et al. 2004; Hefner et al. 2007), we observed an impairment of extinction learning of cocaine CPP after yohimbine administration. Moreover, we found that the impairment of cocaine CPP produced by yohimbine in C57BL/6J mice was not mimicked by the more specific α2-AR antagonist atipamezole in this strain and was actually exacerbated rather than attenuated in α2A-AR knockout mice. Additionally, we found yohimbine elicited a slowly evolving decrease in glutamatergic transmission in the extended amygdala, which is also not mimicked by atipamezole. Overall, our study provides converging lines of evidence suggesting that yohimbine has complex actions on extinction of reward behaviors that are likely independent of their effects on α2-ARs.
Results
Cocaine CPP and extinction
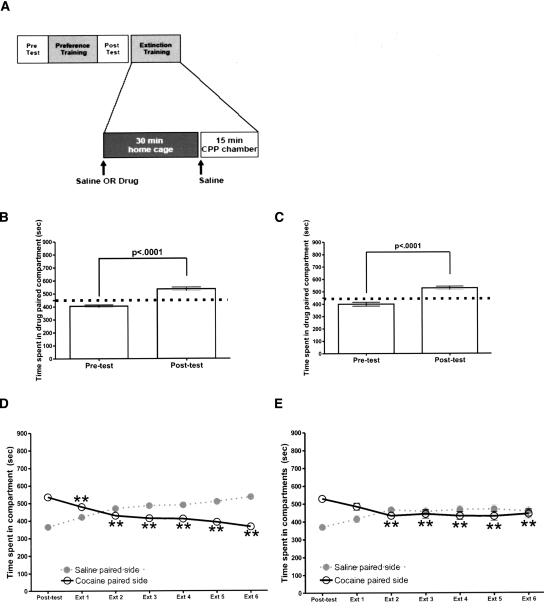
Two groups of C57BL/6J mice underwent CPP training (Fig. 1A) with cocaine (20 mg/kg) at two different times; there were no group differences in time spent on the drug-paired side of the two groups (546 ± 18 sec, n = 18; 520 ± 20 sec, n = 12; t-test P = ns), and thus the groups were collapsed. There was a significant increase in time spent on the CS+ side from the preconditioning and post-test, as expected (t = 7.77, df = 58, P < 0.0001, t-test) (Fig. 1B). We show the time spent on each side of the chamber for each of the extinction sessions (Fig. 1D), and we compared the post-test time value with each extinction value (previously cocaine-paired side [CS+]).
Figure 1.
C57BL/6J mice administered yohimbine 5 mg/kg (i.p.) during extinction sessions display impaired extinction. (A) Schematic of cocaine CPP extinction. Mice are trained with 20 mg/kg cocaine during CPP training. After mice show a preference for the cocaine-paired side (CS+), they are subjected to extinction training. (B,C) C57BL/6J mice acquire place preference for the cocaine-paired side (n = 30, saline group; n = 18, yohimbine group). (D,E) Mice were extinguished over 6 d. Shown is time spent on the cocaine-paired side and saline-paired side during post-test and extinction. Mice were given an injection of saline or yohimbine and placed in home cage; 30–35 min later, mice were given an injection of saline and placed in chambers. Error bars, ±SEM. **P < 0.01 comparison of time spent on white side of chamber in comparison to the CS+ post-test value (solid black line). Dotted line in panels B and C indicates half of test period (450 sec).
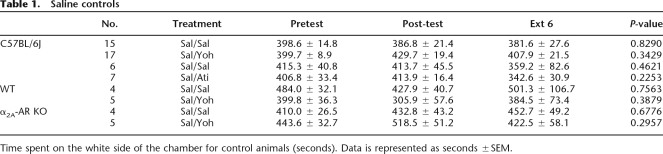
For extinction, a one-way RMANOVA of CS+ values was significant (F(6,209) = 27.08, P < 0.0001). Post hoc analysis shows that extinction sessions 1–6 are all significantly different from the post-test value (Fig. 1D). In regard to control groups, a one-way RMANOVA did not find a difference between preconditioning, post-test, and day 6 extinction for a group that received only saline during CPP training and pretreatment of saline during extinction (P = 0.8290) (Table 1).
Table 1.
Saline controls
Time spent on the white side of the chamber for control animals (seconds). Data is represented as seconds ±SEM.
Effect of yohimbine on cocaine CPP extinction
We next investigated the effects of yohimbine on cocaine CPP extinction. Significant cocaine CPP was produced in mice cocaine trained, as demonstrated by a significant increase in time spent in the cocaine-paired side during the preconditioning relative to the post-test (n = 18, t-test [t = 7.77, df = 17], P < 0.0001) (Fig. 1C).
For extinction, a one-way repeated-measures RMANOVA of CS+ values was significant (F(6,125) = 5.59, P < 0.0001). Post hoc analysis shows that extinction sessions 2–6 are significantly different from the post-test value (Fig. 1E). Yohimbine did not affect locomotor activity during extinction sessions (session 1: 2276 ± 115 cm traveled; session 6: 2508 ± 162 cm traveled).
Effect of yohimbine on fear extinction
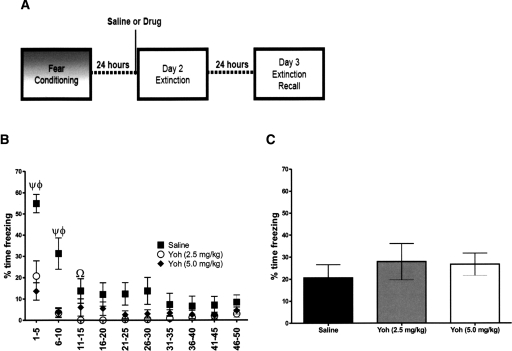
Because yohimbine appeared to impair, rather than facilitate, extinction of cocaine CPP, we reassessed the action of yohimbine on extinction of a fear-based memory. Two-way RMANOVA (treatment × trial block) found a significant effect of drug (F(2,249) = 7.93, P = 0.01), trial-block (F(9,249) = 28.12, P < 0.01), and a significant drug × trial-block interaction (F(18,249) = 6.50, P < 0.01) for freezing during day 2 extinction training (Fig. 2B). Post hoc analysis showed freezing was significantly lower in yohimbine- than saline-treated mice during the first two trial blocks (both P < 0.01), and freezing remained lower than saline for the 2.5 mg/kg yohimbine treatment group on trials 11–15 (P < 0.05). Freezing during day 3 extinction recall testing was no different between groups (Fig. 2C).
Figure 2.
Effects of yohimbine on extinction of fear in C57BL/6J mice. (A) Schematic of fear extinction time line. (B) Yohimbine was administered to mice 20 min prior to extinction session. Shown is the percentage of time freezing during the test during conditioned stimulus exposures (five exposures per bin). ψ is P < 0.01 saline vs. yoh (2.5 mg/kg). Φ is P < 0.01 saline vs. yoh (5.0 mg/kg). Ω is P < 0.05 saline vs. yoh 2.5 mg/kg. (C) Shown is the percentage of time freezing on day 3 of extinction. (n = 8–9 per group). Error bars, ±SEM.
Cocaine CPP extinction in α2A-AR knockout mice and wild-type littermates
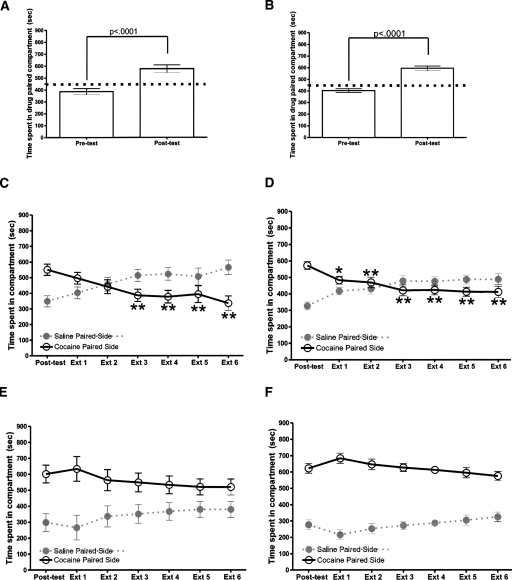
In order to investigate α2-AR subtype contribution of yohimbine’s effect on extinction of cocaine CPP, we tested α2A-AR knockout (KO) mice. Both KO and wild-type (WT) littermates acquired place preference after cocaine CPP training (KO: t = 12.05, df = 21, P < 0.0001; WT: t = 5.84, df = 21, P < 0.0001, t-test) (Fig. 3A,D).
Figure 3.
Yohimbine (5 mg/kg) administered during extinction sessions impairs extinction of cocaine CPP in α2A-AR KO and WT littermates. Mice were administered either saline or yohimbine and placed in the home cage; 30–35 min later, mice were given an injection of saline and placed in chambers. (A) WT littermates obtain place preference. (n = 16). (B) Shown is time spent on each side of the chamber of WT cocaine trained and saline extinguished. (n = 8). (C) Shown is time spent on each side of chamber of WT cocaine trained and yohimbine extinguished. (n = 8). (D) KO mice obtain place preference. (n = 22). (E) Shown is time spent on each side of the chamber of KO mice cocaine trained and saline extinguished. (n = 12). (F) Shown is time spent on each side of chamber of KO mice cocaine trained and yohimbine extinguished. (n = 10). Error bars, ±SEM. *P < 0.05, **P < 0.01. Dotted line (panels A,D) indicates half of test period (450 sec).
For extinction within the WT saline pretreated mice, a one-way RMANOVA comparing CS+ time across sessions was significant (F(6,55) = 5.64, P < 0.001). Post hoc analysis shows that extinction sessions 3–6 are significantly different from the post-test value (Fig. 3B). For WT yohimbine pretreated mice, a one-way RMANOVA comparing CS+ time was not significant F(6,55) = 21.83, P = 0.07) (Fig. 3C). Locomotor activity was not affected in WT mice that received yohimbine during extinction (session 1: 1347 ± 129 cm traveled; session 6: 1839 ± 113 cm traveled).
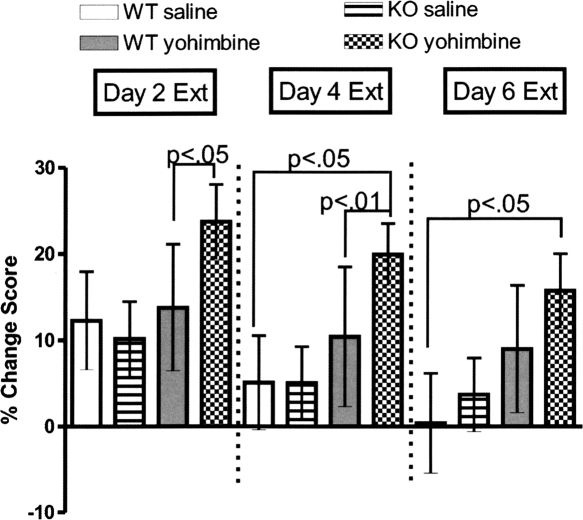
For extinction within the KO saline pretreated mice, a one-way RMANOVA comparing CS+ time across sessions was significant (F(6,83) = 8.10, P < 0.0001). Post hoc analysis shows extinctions sessions 1–6 were significantly different from the post-test value (Fig. 3E). For KO yohimbine pretreated mice, a one-way RMANOVA comparing CS+ time was significant (F(6,69) = 7.54, P < 0.05) (Fig. 3F). Post hoc analysis did not find any difference between the pretest and days 1–6 of extinction. Additionally, a three-way ANOVA (genotype × session × treatment) of the percentage for the three extinction sessions 2, 4, and 6 revealed an effect of treatment (F(1,76) = 4.97, P < 0.05) and session (F(2,113) = 9.38. P < 0.02). Post hoc analysis showed a difference between KO yohimbine pretreated and KO saline pretreated on extinction session 2, and between KO yohimbine pretreated and both WT saline pretreated (P < 0.05) and KO saline pretreated (P < 0.01) on extinction session 4 (Fig. 4). For a three-way RMANOVA for CS+ values for the WT and KO, both treatment groups revealed an effect of genotype (F(1,265) = 12.00, P < 0.001), a genotype × treatment interaction (F(1,265) = 125.26, P < 0.0001), an effect of session (F(6,265) = 79.75, P < 0.0001), treatment × session interaction (F(6,265) = 17.48, P < 0.0001), and a genotype × treatment × session interaction (F(6,265) = 8.10, P < 0.05). Post hoc analysis revealed WT saline pretreated mice differed from WT yohimbine treated mice on days 3 and 6 of extinction; KO saline pretreated mice were significantly different from KO yohimbine pretreated mice on days 1–6 of extinction. There were no differences found either between WT and KO saline pretreated mice or between WT and KO yohimbine pretreated mice. Regarding controls, a one-way RMANOVA did not find a difference between preconditioning, post-test, and day 6 extinction for both WT mice that received saline during cocaine CPP training and WT mice pretreated with either saline or yohimbine prior to extinction sessions (Table 1). Additionally, one-way RMANOVA found no difference between preconditioning, post-test, and day 6 of extinction for KO mice that received saline during cocaine CPP and KO mice pretreated with either saline or yohimbine prior to extinction sessions (Table 1).
Figure 4.
KO mice extinguished with yohimbine exhibit impaired extinction compared to saline extinguished KO mice. Percentage difference from preconditioning and extinction days for each genotype on 2, 4, and 6 for cocaine trained mice. Shown is the percentage of time on CS+ side during preconditioning minus percentage of time on CS+ during extinction. Bars, ±SEM. WT (n = 8), KO (n = 10–12).
Effect of atipamezole on fear extinction
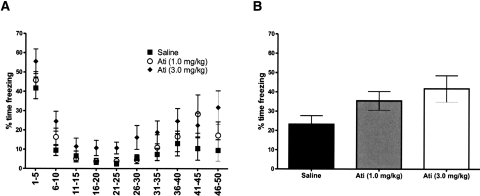
The actions of yohimbine in α2A-AR KO mice raise the possibility that its actions are independent of the α2-AR function. To further test this idea, we next investigated the effects of the specific α2-AR antagonist atipamezole on extinction. Two-way RMANOVA (treatment × time) found a significant effect of drug (F(2,429) = 3.28, P < 0.05) and trial-block (F(9,429) = 26.51, P < 0.01) but no drug × trial-block interaction for freezing during day 2 extinction training (Fig. 5A). Freezing during day 3 extinction recall testing was no different between groups, although there was a nonsignificant trend (F(2,43) = 3.14, P < 0.0539) due to higher freezing in atipamezole-treated mice (Fig. 5B).
Figure 5.
Effect of atipamezole (s.c.) on fear extinction in C57BL/6J mice. (A) Atipamezole (ati) was administered to mice 20 min prior to extinction session. Shown is the percentage of time freezing during the test during conditioned stimulus exposures (five exposures per bin). (B) Shown is the percentage of time freezing on the third test day of extinction. Error bars, ±SEM. (n = 13–14 per group).
Effect of atipamezole on cocaine CPP extinction
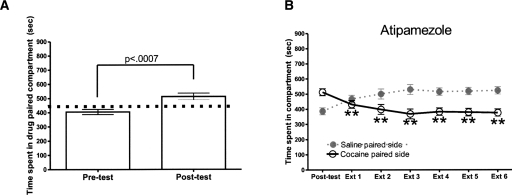
Mice in this experiment demonstrated significant cocaine CPP (t = 4.66, df = 11, P < 0.0007, t-test) (Fig. 6A). For extinction, a one-way RMANOVA of CS+ values was significant (F(6,83) = 10.96, P < 0.0001). Post hoc analysis shows that extinction sessions 1–6 are all significantly different from the post-test value (Fig. 6B).
Figure 6.
C57BL/6J mice administered atipamezole extinguish place preference. (A) Mice acquire place preference for the cocaine-paired side. (B) Mice were given atipamezole (n = 12) and placed in home cage; 30–35 min later, mice were given an injection of saline and placed in chambers. Error bars, ±SEM. **P < 0.01 comparison of time spent on white side of chamber in comparison to the CS+ post-test value (solid black line).
Atipamezole did not affect locomotor activity during extinction training (session 1: 2944 ± 149 cm traveled; session 6: 2957 ± 193 cm traveled). For mice pretreated with atipamezole before extinction sessions, a two-way RMANOVA was significant for treatment (F(5,359) = 9.95, P < 0.0001).
Next we analyzed differences in the percentage of time spent on the CS+ side during preconditioning and extinction days 2, 4, and 6 for saline, yohimbine, and atipamezole pretreated groups. Two-way ANOVAs for the extinction days 2, 4, and 6 between treatment groups were not significant, but there was a trend toward an effect of session (F(2,179) = 2.01, P = 0.08). Additionally, a two-way RMANOVA of CS+ time values between the C57BL/6J saline, yohimbine, and atipamezole pretreated groups revealed a significant effect of extinction sessions (F(6,419) = 30.25, P < 0.0001); for post hoc analysis shows on day 6 of extinction, there is a significant difference between saline pretreated mice and yohimbine pretreated mice (P < 0.05). As for controls, a one-way RMANOVA did not find a difference between preconditioning, post-test, and day 6 extinction for mice that received saline during cocaine CPP training and either saline or atipamezole during extinction (Table 1); thus, the drug (yohimbine or atipamezole) did not have an effect on its own.
Yohimbine depresses glutamatergic transmission in the BNST in an α2-AR-independent manner
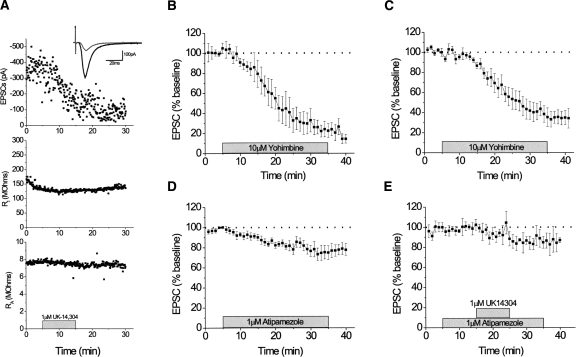
Our behavioral data suggested that actions of yohimbine on cocaine CPP extinction are independent of α2-AR function. Central NE derives from two primary sources, the VNAB and the DNAB. Lesion of the VNAB impairs extinction learning (Cole and Robbins 1987). The VNAB projections terminate in the lateral hypothalamus and the extended amygdala, including the BNST (Aston-Jones et al. 1999). Thus, we examined the effects of yohimbine and atipamezole on glutamatergic transmission in the dorsolateral (dl) BNST. We previously reported that the α2-AR agonists clonidine and UK14,304 elicit an acute depression of glutamatergic transmission in this region (UK14,304, 41.7 ± 7.7% of baseline, P < 0.05) (Egli et al. 2005). We have replicated these data and show a representative experiment for comparison (Fig. 7A). Paradoxically, we have shown here that a 30-min 10-μM application of yohimbine in the dlBNST unexpectedly caused a similar decrease (31.37% ± 6.90% of baseline; t = 11.10, df = 5, P < 0.001) in glutamatergic transmission (Fig. 7B). Further, while the effect of UK14,304 was absent in slices prepared from α2A-AR KO mice (Egli et al. 2005), yohimbine still caused a decrease (42.6 ± 7.5% of baseline; t = 5.44, df = 5, P < 0.003) in glutamatergic transmission in the dlBNST of these mice (Fig. 7C). We then compared the actions of yohimbine with that of the more selective α2-AR antagonist atipamezole. A 30min 1-μM application of atipamezole did not reproduce yohimbine’s effect (P < 0.001) on glutamatergic transmission (atipamezole: 75.6% ± 4.9% of baseline, n = 10; t = 5.01, df = 9) (Fig. 7D). This concentration of atipamezole was sufficient to antagonize effects of UK14,304 (85.64% ± 6.82% of baseline; t = 2.59, df = 2, P > 0.12) on glutamatergic transmission in the dlBNST (Fig. 7E). These experiments suggest yohimbine modulates glutamatergic transmission in the dlBNST independent of α2-ARs.
Figure 7.
EPSCs in the dlBNST: (A) 1 μM UK14,304 (n = 1); (B) 10 μM yohimbine (n = 10); (C) 10 μM yohimbine in α2A-AR KO mouse (n = 6); (D) 1 μM atipamezole (n = 10); (E) 1 μM UK14,304 in the presence of 1 μM atipamezole (n = 3). Error bars, ±SEM.
Discussion
Here we investigated the effects of a widely used anxiogenic compound, yohimbine, on extinction of cocaine CPP. We found that yohimbine impaired extinction of cocaine CPP. Additionally, we found that mice lacking the α2A-AR extinguished cocaine CPP just as WT littermates and were hypersensitive, rather than insensitive, to the extinction impairing effects of yohimbine. We reassessed previously reported facilitatory effects on extinction of fear by yohimbine administration (Cain et al. 2004) and found that yohimbine impaired fear recall without having unambiguous effects on within- or between-session fear extinction in the paradigm we employed. The specific α2-AR antagonist, atipamezole, did not mimic yohimbine in terms of either extinction of fear conditioning or cocaine CPP. Further, we found yohimbine produced a significant depression of glutamatergic transmission in the dlBNST in brain slices prepared from both WT and α2A-AR KO mice that was not mimicked by atipamezole.
Yohimbine impairs extinction of cocaine CPP
It has been reported that NE and α2-ARs play a role in both positive and negative valence-learned behaviors (Ventura et al. 2007). However, most studies exploring extinction behavior have utilized negative valence-learned behaviors. By use of fear-based learning, yohimbine has been shown to facilitate long-term extinction of fear in mice (Cain et al. 2004), although we did not observe this under current conditions, possibly due to it being precluded by the strong extinction produced by our extensive massed training protocol. To investigate the effect of yohimbine on extinction of a positive valence-learned behavior, we asked whether yohimbine could facilitate extinction of cocaine CPP, a widely used paradigm (Tzschentke 2007). It has been reported that yohimbine causes an anxiety response when administered to humans (Murburg et al. 1991), as well as enhancing morphine place preference (Zarrindast et al. 2002). Further, it has been shown that stress enhances acquisition of morphine-induced CPP (Haile et al. 2001). Thus, it may be expected that yohimbine acting as a stressor would prolong preference, i.e., impair extinction, for the CS+. We found yohimbine, indeed, impaired extinction of cocaine CPP.
To provide more insight into the mechanistic basis of yohimbine’s effects on extinction of cocaine CPP after showing cocaine CPP extinction was inconsistent with the effect of yohimbine on fear extinction, we utilized mice with targeted deletion of the α2A-AR gene. The α2A-ARs make up 90% of the centrally located α2-ARs (Bucheler et al. 2002). By using the α2A-AR KO mice, we asked whether yohimbine was acting through the α2A-ARs or other receptors. Interestingly, we found not only that loss of α2A-AR failed to prevent the extinction impairing effects of cocaine CPP but that mice lacking the α2A-AR actually showed significantly exaggerated impairment of extinction by yohimbine. A noteworthy point was that despite being on a C57BL/6J background, WT littermates of the KO mice exhibited a stronger yohimbine impairment profile than we saw in C57BL/6J mice. C57BL/6J mice and WT littermates could be subtly different because of (1) maternal effects from the heterozygote breeders as well as (2) handling issues of ordered mice (C57BL/6J from Jackson), while the WT littermates were bred in house. The difference is interesting and would be beneficial to examine in the future.
Next, we tested the effects of atipamezole, a more specific α2-AR antagonist, which does not have subtype selectivity (Newman-Tancredi et al. 1998). In contrast to yohimbine, atipamezole failed to alter cocaine CPP extinction and showed a trend toward impairing long-term fear extinction. Taken together, these data suggest (1) that yohimbine regulates cocaine CPP through an α2A-AR-independent mechanism and (2) that the α2A-AR is not required for extinction learning but plays a permissive role in modulation of extinction. It should be noted that only a single dose of atipamezole was used for these experiments. However, these data are consistent with previous suggestions that anxiety behaviors emitted after yohimbine administration are independent of the α2-ARs in mice (Cole and Robbins 1987) and rats (Redfern and Williams 1995). However, the role of the α2-ARs was largely unexplored in extinction behaviors.
Yohimbine produces depression of glutamatergic transmission in the dlBNST through an off-target action
To address the possible mechanistic basis of the behavioral effects of yohimbine and atipamezole, we utilized whole cell patch clamp techniques and recorded from dlBNST neurons. The BNST receives a dense noradrenergic input from the VNAB (Aston-Jones et al. 1999). When the VNAB is lesioned, there is an impairment of negative valence-learned extinction (Cole and Robbins 1987), suggesting regions innervated are essential to extinction of learned behaviors. We found atipamezole was able to reverse the depression caused by UK14,304, an α2-AR agonist, whereas yohimbine did not reverse the depression. Furthermore, we found that in α2A-AR KO mouse slices, the depression caused by yohimbine persists. One interpretation is that yohimbine is acting through a non-α2-AR mechanism in this brain region that may contribute to impairment of extinction in cocaine CPP.
Implications
A number of behavioral studies in rats and mice have used yohimbine for a variety of assays as a prototypical α2-AR antagonist. It has been recently used in the study of reinstatement of positive valence behaviors (Shepard et al. 2004; Ledgerwood et al. 2005; Ghitza et al. 2006, 2007). Our data suggest that yohimbine may impair extinction of the learned behavior; thus, the mice may still have a preference for the CS+ (side or lever) when tested. In cases where mice must reach criteria for extinction and then receive yohimbine to reinstate drug seeking, the reinstatement is likely not due to impaired extinction. Further, we are aware of only one behavioral study in rats that utilized atipamezole and made a comparison to yohimbine treated rats (Powell et al. 2005). Thus, for various behaviors, it is unknown whether yohimbine’s actions in reinstating positive valence behaviors are mediated by α2-ARs or off target sites.
Overall, our data suggest that it is likely that extinction of negative and positive valence-learned behavior recruits different circuitries. Additionally, with a high overlap of afflictions, such as PTSD and addiction, a drug may extinguish one behavior and exacerbate the other. In addition to PTSD, age may play a significant role in extinction. It has been shown that there are high levels of NE in the BNST of young animals compared with old animals (Jorm and Stamford 1993), as well as a retardation of extinction in animals that receive drugs when young (Rodd-Henricks et al. 2002). Lastly, it is important for future studies that utilize yohimbine to also use a more specific α2-AR antagonist to help elucidate the mechanism behind the behaviors measured after yohimbine administration.
Materials and Methods
Subjects
Experiments on C57BL/6J mice were conducted using males obtained from The Jackson Laboratory (Bar Harbor, ME) aged 8–12 wk. Male α2A-AR KO mice were generated as previously described (Altman et al. 1999) and backcrossed onto a C57BL/6J genetic background for a minimum of eight generations. KO and WT littermate controls were bred from heterozygous parents to minimize any potential genotype-related maternal abnormalities (Millstein and Holmes 2007). Mice were housed on a 12-h light/ dark cycle in groups of two to five with ad libitum access to food and water. Testing commenced at least 1 wk after acclimation to the facilities. All procedures were approved by the Vanderbilt University and NIAAA Animal Care and Use Committees and were in accordance with the Animal Welfare Act and the guidelines outlined in “Using Animals in Intramural Research.” The number of mice used is reported in the figure legends.
Drug treatment
Cocaine (20 mg/kg) was administered (i.p.) in a saline vehicle in a volume of 10 mL/kg body weight based on previous experiments in adult mice (Schramm-Sapyta et al. 2004). For cocaine CPP and fear extinction, yohimbine was administered (i.p. and s.c.) in saline vehicle in a volume of 10 mL/kg body weight 30–35 min prior to extinction testing at a dose of 2.5 mg/kg or 5 mg/kg, based upon extinction facilitating doses in C57Bl6/j mice (Cain et al. 2004). For cocaine CPP and fear extinction, atipamezole was administered s.c. in saline vehicle in a volume of 10 mL/kg body weight 30–35 min prior to extinction testing at a dose of 3 mg/kg, based upon studies in rats and mice (Seppala et al. 1994; Newman-Tancredi et al. 1998; Millan et al. 2000; Powell et al. 2005; Risbrough and Geyer 2005).
Cocaine CPP and extinction
The apparatus was as previously described (Schramm-Sapyta et al. 2004). Med Associates open field chambers were fitted with acrylic inserts that created two distinct environments. One environment had a black smooth floor/black ceiling; the other, a white sanded floor/white ceiling (cleaned with 30% EtOH), which creates a moderately biased chamber with a preference for the black smooth floor/black ceiling side of the chamber. Mice were first acclimated to handling and i.p. injections for 5 d before testing. There was then a preconditioning session in which each a mouse was placed in the black compartment and allowed to explore the two compartments for 15 min (900 sec). Any mouse spending >67% of the preconditioning session in any one compartment was excluded from the study. Mice in the saline group received saline (i.p.) on both sides of the chamber. Mice in the cocaine group received CS− conditioning sessions occurring on experimental days 1, 3, and 5 and CS+ conditioning sessions occurring on experimental days 2, 4, and 6. For CS+ sessions, mice received an i.p. injection of 20 mg/kg cocaine and were exposed to the white compartment alone for 15 min. For CS− sessions, mice received an i.p. injection of saline and were exposed to the black compartment alone for 15 min.
Place preference was tested on day 7 (post-test). Mice received an i.p. injection of saline, were placed in the black compartment, and were for allowed to freely explore both compartments for 15 min. Extinction of the CPP was then tested on days 8–13. Extinction sessions were the same as the preference session, with the exception that mice received injections of saline (i.p.), 5 mg/kg yohimbine (i.p.), or 3 mg/kg atipamezole (s.c.) 30–35 min prior to session and then (to mimic preference testing) i.p. saline again immediately before each session. All drugs and saline were administered in a volume of 10 mL/kg body weight. Doses of yohimbine hydrochloride (Tocris) were based on fear extinction affiliating doses in mice (Cain et al. 2004; Hefner et al. 2007). Dose of atipamezole hydrochloride (Pfizer) was based on previous behavioral studies in mice (Seppala et al. 1994; Newman-Tancredi et al. 1998; Millan et al. 2000; Powell et al. 2005; Risbrough and Geyer 2005; Boyce-Rustay et al. 2008). Two groups of C57BL/6J mice cocaine trained and saline pretreated during extinction were run with the yohimbine and atipamezole pretreated animals as controls. There was no difference, and the groups were collapsed (Fig. 1B). Additional control groups, mice that received saline during cocaine CPP training and were pretreated with yohimbine or atipamezole, were run along with mice that were cocaine trained and pretreated with drug (yohimbine or atipamezole).
The main dependent measure of behavior during preference and extinction sessions was time spent in each compartment during each session as recorded by Med Associates Activity Monitor software. As for preference, it was inferred from a significantly greater amount of time spent in the cocaine-paired side relative to the saline-paired side. Extinction was indicated by (1) a significantly greater amount of time spent in the saline-paired side relative to the cocaine-paired side, and (2) the absence of a percentage of difference in time spent on the cocaine-paired side during extinction days relative to during preconditioning. Additionally, locomotor activity (centimeters traveled) was recorded to determine whether drug administration altered activity.
Pavlovian fear conditioning and extinction
Pavlovian fear conditioning and extinction was assessed based on methods previously described (Kim and Fanselow 1992; Izquierdo et al. 2006). Mice were placed in a 27 × 27 × 11-cm chamber with transparent walls and a metal rod floor. To provide a distinctive olfactory environment, the chamber was cleaned between subjects with a 79.5% water/19.5% ethanol/1% vanilla extract solution. After a 180-sec acclimation period, the mouse received three pairings (60- to 120-sec interval after each pairing) between an auditory tone (30 sec, 80 dB, white noise) and footshock (2-sec, 0.6-mA scrambled footshock), in which the shock was presented during the last 2 sec of the tone. The presentation of stimuli was controlled by the Freeze Monitor system (Med Associates Inc.).
Twenty-four hours later, the initial expression of fear to the CS and extinction learning was tested following administration of either yohimbine (2.5 or 5.0 mg/kg) or, in a separate experiment, atipamezole (1.0 or 3.0 mg/kg). Mice were placed in a novel context (black/white-checkered walls and a solid-Plexiglas, opaque floor, cleaned with a 1% acetic acid/99% water solution) housed in a novel room. Following an initial 180-sec acclimation period, the mouse received 50 × 30-sec presentations of the CS (5-sec no-stimulus interval). Twenty-four hours later, extinction recall was tested using the same procedure as the previous day except that there were three CS presentations only. Freezing (no visible movement except that required for respiration) was manually scored every 5 sec and converted to a percentage ([number of freezing observations/total number of observations] × 100). Freezing during extinction trials were averaged into 10 × 5-trial blocks for analysis. Testing was conducted in a manner counterbalanced for strain and drug.
Electrophysiological recordings in BNST slices
Brain slices from the dlBNST were prepared as previously described (McElligott and Winder 2007). Briefly, mice were retrieved from the colony and allowed to rest in sound attenuating boxes for a minimum of 1 h, after which they were anesthetized (isoflurane) and decapitated in a separate room. Three-hundred-micrometer coronal slices were cut on a VT1000S vibratome (Leica Microsystems) in a 1°C–4°C, oxygenated (95% O2, 5% CO2), high-sucrose/low Na+ artificial cerebral spinal fluid (ACSF; in mM: 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, 26 NaHCO3).
Following slicing, hemisected slices were allowed to rest submerged in a holding chamber filled with oxygenated and heated (28°C) ACSF for at least 30 min. After this incubation time, an individual slice was moved to the recording chamber where it was submerged in oxygenated and heated (28°C) ACSF with added picrotoxin (25 μM included for the entirety of all experiments) to isolate currents evoked by glutamate receptor activation at a rate of 2 mL/min. A bipolar Nichrome (A-M Systems) stimulating electrode was placed dorsally to the recording electrode within the dlBNST. Patch electrodes (3–6 MΩ) were pulled on a Flaming/Brown microelectrode puller (Sutter Instruments) and filled with either cesium (Cs)-gluconate intracellular solution (in mM: Cs-gluconate 135, NaCl 5, MgCl2 2, HEPES 10, EGTA 0.6, Na2ATP 4, Na2GTP 0.4). In all whole-cell experiments, cells were clamped at −70 mV throughout and excitatory post-synaptic currents (EPSCs) were recorded using Clampex 9.2 (Molecular Devices). Series resistance was monitored throughout each experiment, and a change greater than 20% resulted in the exclusion of the experiment from the data set. EPSCs were evoked at a frequency of 0.167 Hz, and 100–400 pA EPSCs were recorded. Consistent with the field experiments, drugs were bath applied at their final concentrations.
A 5- to 10-min baseline was acquired prior to drug application, and all points were normalized to minutes 8–10 within each. Baseline is an average of 2 min before dug application, and the experimental value is 24–25 min following drug application. Points are 1-min averages on plotted time course.
Statistical analysis
Student’s t-test, ANOVA, two-factor, and three-factor RM ANOVAs were used. The Levene’s test for homogeneity was used to test for homogeneity of variances. If the criterion of homogeneity of variances was met, Newman Keuls or Dunnett’s multiple comparison post hoc test was used; if not, the Games-Howell post hoc test was used. All data points were reported as the mean ± SEM, and significance (determined by paired and unpaired Student’s t-test) is reported in the text and figure legends. Differences were defined were considered significant if P < 0.05.
Acknowledgments
We thank Dr. Chris Olsen for a critical reading of the manuscript and William Nobis for writing a helpful script to gather data. Research was supported by NIDA (D.G.W.), NIDA supplement (A.D. and D.G.W.), and the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (A.H., J.B., and M.N.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1079308.
References
- Aghajanian G.K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Altman J.D., Trendelenburg A.U., MacMillan L., Bernstein D., Limbird L., Starke K., Kobilka B.K., Hein L. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol. Pharmacol. 1999;56:154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Delfs J.M., Druhan J., Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann. N. Y. Acad. Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Bouton M.E. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay J.M., Palachick B., Hefner K., Chen Y.C., Karlsson R.M., Millstein R.A., Harvey-White J., Holmes A. Desipramine potentiation of the acute depressant effects of ethanol: Modulation by α2-adrenoreceptors and stress. Neuropharm. 2008 doi: 10.1016/j.neuropharm.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheler M.M., Hadamek K., Hein L. Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Cain C.K., Blouin A.M., Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn. Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B.L., Tiffany S.T. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Childress A.R., Hole A.V., Ehrman R.N., Robbins S.J., McLellan A.T., O'Brien C.P. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res. Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Cole B.J., Robbins T.W. Dissociable effects of lesions to the dorsal or ventral noradrenergic bundle on the acquisition, performance, and extinction of aversive conditioning. Behav. Neurosci. 1987;101:476–488. doi: 10.1037//0735-7044.101.4.476. [DOI] [PubMed] [Google Scholar]
- Davis M., Redmond D.E., Baraban J.M. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology. 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- Egli R.E., Kash T.L., Choo K., Savchenko V., Matthews R.T., Blakely R.D., Winder D.G. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Ferry B., McGaugh J.L. Involvement of basolateral amygdala α2-adrenoceptors in modulating consolidation of inhibitory avoidance memory. Learn. Mem. 2008;15:238–243. doi: 10.1101/lm.760908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale G.D., Anagnostaras S.G., Godsil B.P., Mitchell S., Nozawa T., Sage J.R., Wiltgen B., Fanselow M.S. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J. Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza U.E., Gray S.M., Epstein D.H., Rice K.C., Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: A role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza U.E., Nair S.G., Golden S.A., Gray S.M., Uejima J.L., Bossert J.M., Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J. Neurosci. 2007;27:11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile C.N., GrandPre T., Kosten T.A. Chronic unpredictable stress, but not chronic predictable stress, enhances the sensitivity to the behavioral effects of cocaine in rats. Psychopharmacology. 2001;154:213–220. doi: 10.1007/s002130000650. [DOI] [PubMed] [Google Scholar]
- Hefner K., Cameron H.A., Karlsson R.M., Holmes A. Short-term and long-term effects of postnatal exposure to an adult male in C57BL/6J mice. Behav. Brain Res. 2007;182:344–348. doi: 10.1016/j.bbr.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Holmberg G., Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacology. 1961;2:93–106. doi: 10.1007/BF00592678. [DOI] [PubMed] [Google Scholar]
- Holmes A., Yang R.J., Crawley J.N. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J. Mol. Neurosci. 2002;18:151–165. doi: 10.1385/JMN:18:1-2:151. [DOI] [PubMed] [Google Scholar]
- Izquierdo A., Wellman C.L., Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm C.M., Stamford J.A. Early age-dependent changes in noradrenaline efflux in the bed nucleus of stria terminalis: Voltammetric data in rat brain slices. Neurobiol. Aging. 1993;14:499–501. doi: 10.1016/0197-4580(93)90108-n. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Fanselow M.S. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lang W.J., Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch. Int. Pharmacodyn. Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- Ledgerwood L., Richardson R., Cranney J. D-cycloserine facilitates extinction of learned fear: Effects on reacquisition and generalized extinction. Biol. Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Mao S.C., Hsiao Y.H., Gean P.W. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J. Neurosci. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott Z.A., Winder D.G. α1-Adrenergic receptor-induced heterosynaptic long-term depression in the bed nucleus of the stria terminalis is disrupted in mouse models of affective disorders. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J., Lejeune F., Gobert A., Brocco M., Auclair A., Bosc C., Rivet J.M., Lacoste J.M., Cordi A., Dekeyne A. S18616, a highly potent spiroimidazoline agonist at α2-adrenoceptors: II. Influence on monoaminergic transmission, motor function, and anxiety in comparison with dexmedetomidine and clonidine. J. Pharmacol. Exp. Ther. 2000;295:1206–1222. [PubMed] [Google Scholar]
- Millstein R.A., Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Moore R.Y., Bloom F.E. Central catecholamine neuron systems: Anatomy and physiology of the norepinephrine and epinephrine systems. Annu. Rev. Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Mueller D., Porter J.T., Quirk G.J. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murburg M.M., Villacres E.C., Ko G.N., Veith R.C. Effects of yohimbine on human sympathetic nervous system function. J. Clin. Endocrinol. Metab. 1991;73:861–865. doi: 10.1210/jcem-73-4-861. [DOI] [PubMed] [Google Scholar]
- Myers K.M., Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A., Nicolas J.P., Audinot V., Gavaudan S., Verriele L., Touzard M., Chaput C., Richard N., Millan M.J. Actions of α2 adrenoceptor ligands at α2A and 5-HT1A receptors: The antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for α2A adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Nicholas A.P., Pieribone V., Hokfelt T. Distributions of mRNAs for α2 adrenergic receptor subtypes in rat brain: An in situ hybridization study. J. Comp. Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Powell S.B., Palomo J., Carasso B.S., Bakshi V.P., Geyer M.A. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not α2-adrenoceptors. Psychopharmacology. 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Russo G.K., Barron J.L., Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern W.S., Williams A. A re-evaluation of the role of α2-adrenoceptors in the anxiogenic effects of yohimbine, using the selective antagonist delequamine in the rat. Br. J. Pharmacol. 1995;116:2081–2089. doi: 10.1111/j.1476-5381.1995.tb16415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond D.E., Huang Y.H. Current concepts. II. New evidence for a locus coeruleus-norepinephrine connection with anxiety. Life Sci. 1979;25:2149–2162. doi: 10.1016/0024-3205(79)90087-0. [DOI] [PubMed] [Google Scholar]
- Redmond D.E., Krystal J.H. Multiple mechanisms of withdrawal from opioid drugs. Annu. Rev. Neurosci. 1984;7:443–478. doi: 10.1146/annurev.ne.07.030184.002303. [DOI] [PubMed] [Google Scholar]
- Ressler K.J., Rothbaum B.O., Tannenbaum L., Anderson P., Graap K., Zimand E., Hodges L., Davis M. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch. Gen. Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Risbrough V.B., Geyer M.A. Anxiogenic treatments do not increase fear-potentiated startle in mice. Biol. Psychiatry. 2005;57:33–43. doi: 10.1016/j.biopsych.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks Z.A., Bell R.L., Kuc K.A., Murphy J.M., McBride W.J., Lumeng L., Li T.K. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol. Clin. Exp. Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Schank J.R., Liles L.C., Weinshenker D. Norepinephrine signaling through β-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol. Psychiatry. 2008;63:1007–1012. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta N.L., Pratt A.R., Winder D.G. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology. 2004;173:41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Self D.W., Choi K.H., Simmons D., Walker J.R., Smagula C.S. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learn. Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala T., Idanpaan-Heikkila J.J., Stromberg C., Mattila M.J. Ethanol antagonism by atipamezole on motor performance in mice. Life Sci. 1994;55:245–251. doi: 10.1016/0024-3205(94)00886-8. [DOI] [PubMed] [Google Scholar]
- Shepard J.D., Bossert J.M., Liu S.Y., Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol. Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Tzschentke T.M. Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Ventura R., Morrone C., Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cen X., Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur. J. Pharmacol. 2001;432:153–161. doi: 10.1016/s0014-2999(01)01487-x. [DOI] [PubMed] [Google Scholar]
- Weinshenker D., Schroeder J.P. There and back again: A tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Wellman C.L., Izquierdo A., Garrett J.E., Martin K.P., Carroll J., Millstein R., Lesch K.P., Murphy D.L., Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast M.R., Bahreini T., Adl M. Effect of imipramine on the expression and acquisition of morphine-induced conditioned place preference in mice. Pharmacol. Biochem. Behav. 2002;73:941–949. doi: 10.1016/s0091-3057(02)00951-6. [DOI] [PubMed] [Google Scholar]