Abstract
Adult learning and memory functions are strongly dependent on neonatal experiences. We recently showed that neonatal odor-shock learning attenuates later life odor fear conditioning and amygdala activity. In the present work we investigated whether changes observed in adults can also be observed in other structures normally involved, namely olfactory cortical areas. For this, pups were trained daily from postnatal (PN) 8 to 12 in an odor-shock paradigm, and retrained at adulthood in the same task. 14C 2-DG autoradiographic brain mapping was used to measure training-related activation in amygdala cortical nucleus (CoA), anterior (aPCx), and posterior (pPCx) piriform cortex. In addition, field potentials induced in the three sites in response to paired-pulse stimulation of the olfactory bulb were recorded in order to assess short-term inhibition and facilitation in these structures. Attenuated adult fear learning was accompanied by a deficit in 2-DG activation in CoA and pPCx. Moreover, electrophysiological recordings revealed that, in these sites, the level of inhibition was lower than in control animals. These data indicate that early life odor-shock learning produces changes throughout structures of the adult learning circuit that are independent, at least in part, from those involved in infant learning. Moreover, these enduring effects were influenced by the contingency of the infant experience since paired odor-shock produced greater disruption of adult learning and its supporting neural pathway than unpaired presentations. These results suggest that some enduring effects of early life experience are potentiated by contingency and extend beyond brain areas involved in infant learning.
Neonatal experiences have profound effects on adult learning and memory, as well as the supporting neural structures. Most studies in this domain are related to the effect of early stress, mainly maternal separation/deprivation (Anisman et al. 1998; Fleming et al. 1999; Levine 2001, 2005; Pryce and Feldon 2003; Teicher et al. 2006), or alterations of level of maternal care (Zhang et al. 2006). Recently, we showed that some aspects of the enduring impact of early life adverse experience are dependent upon the learned contingencies of the experience. Specifically, infant paired odor-shock produces significantly greater attenuation of adult fear learning as assessed through freezing response, compared to infant unpaired presentations (Sevelinges et al. 2007). We also showed that this attenuated learning was associated with a decrease in amygdala 2-DG uptake whereas an increase was observed in the olfactory bulb (OB). This led us to suggest that, under the influence of learning, early experiences may be able to shape the neural circuits that would normally be used at adulthood. This question was further addressed in the present work by investigating more specifically the effects of neonatal odor-shock learning on other potential targets of the adult network, namely olfactory cortical areas including piriform cortex and cortical nucleus of the amygdala (CoA).
Recent studies on the ontogeny of fear learning (i.e., odor–0.5 mA shock conditioning) have highlighted the emergence of this amygdala-dependent learning at postnatal (PN) day 10. Before this age, odor-shock associations paradoxically induce an odor preference (Stehouwer and Campbell 1978; Haroutunian and Campbell 1979; Camp and Rudy 1988; Sullivan et al. 2000) despite pups feeling pain from shock (Collier and Bolles 1980; Barr 1995; Fitzgerald 2005). The neural network sustaining this paradoxical preference involves OB and anterior piriform cortex (aPCx) whereas the amygdala does not appear to participate in the circuit (Sullivan and Wilson 1995; Roth and Sullivan 2005; Moriceau et al. 2006). In contrast around PN 10, pups readily learn aversion to odors paired with shock and the network involved in this learned aversion includes posterior piriform cortex (pPCx) and amygdala (Sullivan and Wilson 1995; Sullivan et al. 2000; Roth and Sullivan 2005; Moriceau et al. 2006).
In adult rats, consistent with auditory fear conditioning (Davis 1997; LeDoux 2000; Fanselow and Gale 2003), odor fear learning was shown to involve the basolateral complex (BLcA) of the amygdala (Cousens and Otto 1998; Funk and Amir 2000; Schettino and Otto 2001; Rosenkranz and Grace 2002; Kilpatrick and Cahill 2003; Sevelinges et al. 2004; Walker et al. 2005). In addition, we recently showed that the CoA, which constitutes the primary amygdaloid target of olfactory information (Price 1973; McDonald 1998; Swanson and Petrovich 1998), as well as the pPCx both showed synaptic changes following olfactory fear conditioning (Sevelinges et al. 2004), suggesting their involvement in this learning. Therefore, the neural network sustaining odor-shock learning evolves from infancy to adulthood from a circuit including OB and aPCx supporting odor preference, toward a circuit including pPCx, BLcA, and CoA supporting odor aversion.
In our previous study (Sevelinges et al. 2007) we showed that early odor-shock learning induced a deficit in adult odor fear learning associated with opposite changes in the amygdala (decrease) and OB (increase) as revealed by 2-DG mapping. The main entrance for the olfactory information from the OB onto the amygdala is through the CoA, either directly or by way of the piriform cortex. Therefore, the aim of the present work was to assess whether early learning induced changes in these two intermediate areas. For this, pups were trained daily from PN8 to PN12 in an odor-shock conditioning paradigm, and retrained at adulthood in the same task. In adult rats, two complementary neurobiological techniques were used to assess the CoA, pPCx, and aPCx functioning. First, autoradiographic 14C 2-DG brain mapping was used to visualize the activity of these structures during learning acquisition. Second, because the 2-DG technique does not distinguish between excitatory and inhibitory neural activity, evoked field potentials (EFPs) were induced in these sites after learning, using paired-pulse electrical stimulation of the OB, in order to assess short-term inhibition and facilitation phenomenon at these different levels. In a subset of animals, we also recorded EFPs in the OB, which provides the main input to these cortical areas, using electrical stimulation of the lateral olfactory tract (LOT). This last experiment was made in order to further investigate whether the changes observed in the olfactory cortical areas were local or could be explained by modifications in the OB.
Results
Behavioral data
Infant odorant CS test
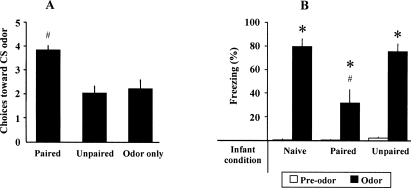
Pups’ learning was verified in 15 pups not trained at adulthood. PN13 pups trained in the Paired condition from PN8 to PN12 demonstrated a shock-induced odor preference, whereas control pups (Unpaired or Odor Only) did not exhibit such preference (Fig. 1A). ANOVA confirmed a main effect of condition (F(2,12) = 10.429, P < 0.01). Post-hoc Fisher tests showed that the Paired group was significantly different of the two control groups (P < 0.01) whereas Unpaired and Odor-Only groups were not different (P = 0.65).
Figure 1.
Behavioral performances in infant and adult animals during CS retention test carried out the day after odor-shock conditioning. (A) Infant learning: mean number of choices toward CS odor at PN13. (B) Adult learning: mean percentage (±SEM) of adult freezing behavior before and during CS odor presentation. (*) Significant intragroup difference with the pre-odor period (P < 0.05). (#) Significant intergroup difference (P < 0.05).
Adult learning
At adulthood, animals of the different groups were trained in a paired odor-shock paradigm, using the same odor as in infancy. Freezing behavior measured 24 h later in response to odor cue alone in the different experimental groups (Infant Paired/Adult Paired, Infant Unpaired/Adult Paired, Infant Naive/Adult Paired) is illustrated in Figure 1B. ANOVA revealed a main effect for condition (F(2,17) = 9.39, P < 0.005), odor (F(1,17) = 155.65, P < 0.001), and their interaction (F(2,17) = 9.44, P < 0.005). Further comparisons showed that, although animals in the three groups presented a significant increase in freezing behavior when the odor was introduced (P < 0.05), animals in Infant Paired/Adult Paired group presented a significantly lower level of freezing than animals in the others two groups (post-hoc Fisher test, P < 0.005). Infant Unpaired/Adult Paired and Infant Naive/Adult Paired animals displayed similar levels of freezing. These data replicate those obtained previously (Sevelinges et al. 2007).
2-DG autoradiography data
Autoradiographs, which were derived from animals during odor-shock conditioning in adulthood, focused on the piriform cortex and CoA during acquisition. Twenty animals were used for this experiment and did not overlap with animals used for electrophysiology. The data are presented in Figure 2.
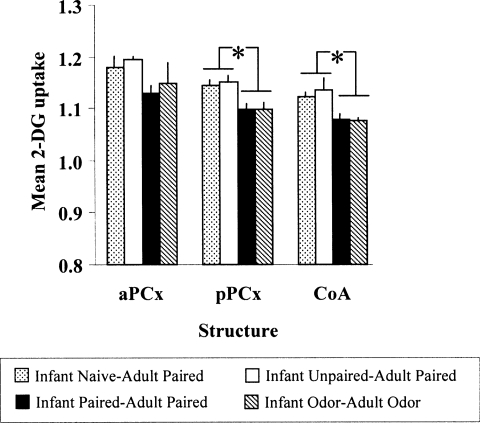
Figure 2.
Mean 2-DG uptake (±SEM) relative to corpus callosum during adult fear conditioning in the different experimental groups, in three structures: anterior piriform cortex (aPCx), posterior piriform cortex (pPCx), and cortical nucleus of the amygdala (CoA). (*) Significant intergroup differences (P < 0.05).
Piriform cortex
During conditioning, aPCx activity did not significantly differ between groups (F(3,16) = 1.149, P = 0.27). In contrast, in the pPCx ANOVA revealed a main effect of group condition (F(3,16) = 4.736, P < 0.05). Post-hoc Fisher tests revealed that the Infant Naive/Adult Paired and Infant Unpaired/Adult Paired animals exhibited higher levels of 2-DG uptake than animals of each of the Infant Paired/Adult Paired and Infant Odor/Adult Odor animals at the P < 0.05 level. Infant Paired/Adult Paired animals did not differ significantly from Infant Odor/Adult Odor animals.
Cortical amygdala nucleus
ANOVA revealed a main effect of group condition for the CoA (F(3,15) = 6.610, P < 0.005). Post-hoc Fisher tests revealed that the Infant Naive/Adult Paired and Infant Unpaired/Adult Paired animals exhibited higher levels of 2-DG uptake than animals of each of the Infant Paired/Adult Paired and Infant Odor/Adult Odor animals at the P < 0.05 level. Infant Paired/Adult Paired animals did not differ significantly from Infant Odor/Adult Odor animals.
In summary, whereas normal fear learning was associated with increased 2-DG uptake in pPCx and CoA, the attenuated freezing seen in Infant Paired/Adult Paired animals was associated with reduced 2-DG uptake in pPCx and in the CoA during conditioning.
Electrophysiological data
To better characterize the consequences of neonatal experience in the adult brain, field potentials were induced in aPCx, pPCx, and CoA in response to electrical stimulation of the OB, and in the OB in response to electrical stimulation of the LOT. Paired-pulse stimulation with different interpulse interval (IPI) durations was used. EFPs were collected in the absence of odor presentation.
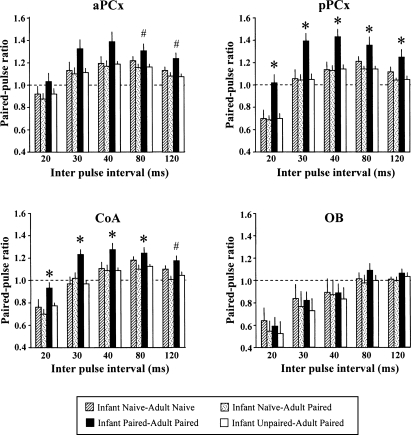
Figure 3 represents the paired-pulse ratios obtained in the different recording sites for the five IPI durations across the four experimental groups. A decrease in the size of the response to the second pulse suggests inhibition and is reflected in a score <1.
Figure 3.
Mean paired-pulse ratios (mean test signal amplitude/mean conditioning signal amplitude ± SEM) obtained in the different experimental groups, in four structures: anterior (aPCx) and posterior (pPCx) piriform cortex, cortical nucleus of the amygdala (CoA), and olfactory bulb (OB). Paired-pulse stimulations were delivered using different interpulse interval durations (20–120 msec). A ratio <1 characterizes paired-pulse inhibition, whereas a ratio >1 corresponds to paired-pulse facilitation. (*) Significant difference with all the other groups (P < 0.05). (#) Significant differences with all but Infant Naive/Adult Naive groups (P < 0.05).
Piriform cortex
In the aPCx, ANOVA revealed a main effect of group condition (F(3,43) = 0.039) and IPI (F(4,172) < 0.001) but not their interaction. One-way ANOVAs were then carried out for each IPI duration, followed when authorized by post-hoc comparisons. For IPI of 30 and 40 msec, despite a trend to intergroups difference, ANOVA did not reach the significance level (P = 0.052 and 0.051, respectively), thus precluding further post-hoc comparisons. For IPI of 80 and 120 msec, paired-pulse ratios were significantly higher in Infant Paired/Adult Paired animals than in the Infant Naive/Adult Paired and Infant Unpaired/Adult Paired groups, whereas they did not differ significantly from Infant Naive/Adult Naive group.
In the pPCx, ANOVA revealed a main effect of condition (F(3,43) = 7.49, P < 0.001), IPI (F(4,172) = 164.23, P < 0.001), and their interaction (F(12,172) = 2.34, P < 0.01). Further post-hoc comparisons showed that, for all IPI, paired-pulse ratios were significantly higher in Infant Paired/Adult Paired animals than in the other three groups (P < 0.05). Specifically, while paired-pulse inhibition was observed with the 20-msec interval (Infant Naive/Adult Naive, Infant Naive/Adult Paired, and Infant Unpaired/Adult Paired animals), it was absent in Infant Paired/Adult Paired animals. Moreover, for the 30–120-msec intervals, facilitation of the second pulse amplitude was observed in Infant Paired/Adult Paired animals compared to the other three groups.
Cortical amygdala nucleus
ANOVA revealed a main effect of group condition (F(3,43) = 5.42, P < 0.01), IPI (F(4,172) = 115.08, P < 0.001), and their interaction (F(12,172) = 1.98, P < 0.05). Further post-hoc comparisons showed that, for IPI of 20–80 msec, paired-pulse ratios were significantly higher in Infant Paired/Adult Paired animals than in the three other groups. For IPI of 120 msec, paired-pulse ratios were significantly higher in Infant Paired/Adult Paired animals than in Infant Naive/Adult Paired and Infant Unpaired/Adult Paired groups, whereas they did not differ significantly from the Infant Naive/Adult Naive group despite a strong tendency (P = 0.053). As for the pPCx, compared to the other groups, Infant Paired/Adult Paired animals did not show paired-pulse inhibition for the 20-msec interval and exhibited paired-pulse facilitation for the 30–120-msec intervals.
Olfactory bulb
ANOVA revealed no significant effect of group condition on the paired-pulse ratios in the OB (F(3,22) = 0.21, P = 0.89). Paired-pulse ratios were similar in the four groups and were not significantly modified by early experience.
In summary, electrophysiological data highlighted long-term effects of early odor-shock learning, as observed in Infant Paired/Adult Paired group. In these animals, compared to the other groups, paired-pulse inhibition normally observed for 20-msec interval in pPCx and CoA was abolished and paired-pulse facilitation was observed for 30–120-msec interval. Paired-pulse ratios in aPCx were only slightly affected in Infant Paired/Adult Paired group. Finally, no significant changes in paired-pulse ratios were detected in OB.
Discussion
The present study replicates our previous work suggesting that adult fear learning is altered by early life experience but also extends this work by expanding the assessment of the adult olfactory cortical areas of the learning circuit. Taken together, the present data show that Infant Paired/Adult Paired animals, which exhibited an attenuated adult odor fear, presented a deficit in 2-DG activation in pPCx and CoA compared to control animals. Moreover, electrophysiological assessment carried out in the absence of the learned odor, revealed that in these sites paired-pulse inhibition was lower than in control animals. In contrast 2-DG uptake in aPCx was not affected and paired-pulse inhibition was only slightly modified. The most salient implications of early life experience effects on adult cognition are the following: (1) The effects are widespread and include sensory cortical areas in addition to amygdala and (2) areas important for learning in adulthood are modified by early life experience, despite their lack of plasticity in early life learning.
Attenuated adult fear conditioning after early life experience
Our behavioral results confirm the data reported in our previous study indicating that early life experience with paired (but not unpaired) odor-shock conditioning attenuates adult fear learning (Sevelinges et al. 2007). While early life experiences (handling, maternal deprivation, level of maternal care) have previously been shown to alter later life context and cue fear learning (Kosten et al. 2005, 2006; Diorio and Meaney 2007), our results suggest the contingency of early life experience plays an important role in determining outcome (present results, Sevelinges et al. 2007). These attenuating effects of infant stress on adult fear learning are in sharp contrast to stress effects in adulthood, which facilitate learning (Cordero et al. 2003; Roozendaal et al. 2006).
Early learning alters the functioning of olfactory structures involved in adult odor fear learning
Infant Naive/Adult Paired and Infant Unpaired/Adult Paired animals, which showed normal odor fear conditioning, exhibited increased 2-DG uptake in the CoA and pPCx but not the aPCx, compared to Infant Odor/Adult Odor animals. These results complement previous data showing that odor fear conditioning was associated with synaptic plasticity within these two structures, as assessed during the CS odor retention test (Sevelinges et al. 2004), and further document the differential role of anterior and posterior parts of the piriform cortex in olfactory learning. Indeed there is now increasing evidence that, whereas the aPCx is involved in sensory processes and simple forms of short-term memory like habituation (Wilson 1998a, b, 2000; Kadohisa and Wilson 2006) or perceptual learning (Wilson 2003), the pPCx is involved in more cognitive mnesic processes including the learning and recall of associations between odorants and information from other sensory modalities (Litaudon et al. 1997, 2003; Chabaud et al. 1999, 2000; Haberly 2001; Mouly et al. 2001; Mouly and Gervais 2002; Sevelinges et al. 2004; Jones et al. 2007).
In contrast to Infant Naive/Adult Paired animals, acquisition-induced 2-DG uptake was attenuated in pPCx and CoA of Infant Paired/Adult Paired animals and appeared similar to nonlearning groups such as the Infant Odor/Adult Odor animals. It should be noted that the lack of a significant difference in 2-DG uptake between Infant Paired/Adult Paired animals and nonlearning groups does not indicate similar processing since 2-DG requires prolonged and robust differences in neural activity, thus precluding the detection of subtle differences.
To better characterize mechanisms responsible for attenuated fear learning in Infant Paired/Adult Paired animals, we performed EFP recordings in response to paired-pulse stimulation of the OB to assess the time course of short-term inhibition and facilitation processes in the different sites. In Infant Naive/Adult Naive animals, paired-pulse stimulation of the OB induced inhibition or facilitation depending on the duration of the interpulse interval. In accordance with data reported in a previous study (Mouly and Di Scala 2006), inhibition was observed in the three recording sites for short interpulse intervals (20 msec), with a lower degree of inhibition in aPCx than in the pPCx and CoA. At longer interpulse intervals (40–120 msec), paired-pulse facilitation was observed in the three structures which confirm previous data (Haberly 1973; Schwob et al. 1984; Patneau and Stripling 1992; Best and Wilson 2004; McNamara et al. 2004). This approach first revealed that normal adult odor fear learning did not alter paired-pulse inhibition or facilitation in the three recorded areas since paired-pulse ratios were similar in Infant Naive/Adult Paired and Infant Naive/Adult Naive animals. In contrast, Infant Paired/Adult Paired animals presented alterations in the level of inhibition in the pPCx and CoA and to a lesser extent in the aPCx. Specifically, paired-pulse inhibition normally observed for short interpulse intervals was strongly attenuated in pPCx and CoA, and switched to paired-pulse facilitation for longer stimulation intervals in Infant Paired/Adult Paired animals. Therefore, the pPCx and CoA which are not involved in infant odor-shock learning presented alterations in adult animals, whereas aPCx, a structure involved in infant odor-shock learning, only showed minor modifications.
Paired-pulse inhibition mainly reflects the inhibitory feedback exerted by local GABAergic interneurons onto the main glutamatergic projection neurons (for the piriform cortex: Tseng and Haberly [1988] and Princivalle et al. [2000]; for the amygdala: Sah et al. [2003] and Muller et al. [2006]). Paired-pulse facilitation is usually considered to reflect an increase in transmitter release due to residual Ca2+ accumulation in the presynaptic terminal (for reviews, see Fortune and Rose 2001; Zucker and Regehr 2002). In Infant Paired/Adult Paired animals both phenomena seem to have been altered. Indeed paired-pulse inhibition was strongly attenuated in pPCx and CoA, and paired-pulse facilitation was increased for longer stimulation intervals. At first sight, these data could seem contradictory with the decrease in 2-DG uptake observed in the same sites in this experimental group. Indeed, attenuation in inhibition would logically predict an increase in 2-DG uptake. However, one must keep in mind that variations in 2-DG uptake can be interpreted as changes in either excitatory or inhibitory neuron activity. Interestingly, pairing of an odor with stroking in rat pups was shown to induce an odor preference which correlated with an increase in 2-DG uptake in the OB (Sullivan and Leon 1986) and a parallel increase in inhibition of mitral/tufted cells response to the learned odor (Wilson et al. 1987). In the present study, the decrease in 2-DG uptake observed in pPCx and CoA could therefore reflect a decrease in inhibitory neuron activity, although further experiments are needed to properly address this question. In addition, this explanation only stands for short IPIs and cannot explain the increase in paired-pulse facilitation at longer intervals.
Are the changes observed in cortical areas a consequence of changes in the OB?
In our previous study (Sevelinges et al. 2007) we reported that early experience resulted in an increased 2-DG uptake in the OB during adult odor fear learning. These data suggest the OB is sending altered odor input to the olfactory cortical areas and may contribute to their altered response to adult conditioning. Although further experiments are needed to address this question, two arguments seem to discard this hypothesis. First, in Paired–Paired animals, 2-DG assessment showed that the activity of aPCx, which is the main target of the OB output neurons, is not modified by early experience, despite a strong increase in OB 2-DG uptake. Secondly, in the present study, paired-pulse inhibition measured in the OB was not modified in Infant Paired/Adult Paired animals whereas it was altered in pPCx and CoA. These results suggest a dissociation between modifications observed in the OB and olfactory structures on which it projects.
Are the changes observed in cortical areas responsible for changes in amygdala?
In our previous study (Sevelinges et al. 2007) we reported that early experience resulted in a decrease in 2-DG uptake in the basolateral amygdala during adult odor fear learning. In the present study, as indicated by 2-DG autoradiography and electrophysiology, the CoA appears altered by early life paired odor-shock conditioning. The CoA is an amygdala nucleus considered part of the olfactory cortex and receives input directly from the OB but also from the aPCx and pPCx (Kajiwara et al. 2007), although the link between the pPCx and CoA is much greater (Price 1973; McDonald 1998; Swanson and Petrovich 1998). In contrast the basolateral amygdala does not receive projections from the OB and receives relatively weak projections from the piriform cortex (Krettek and Price 1978; Ottersen 1982; Luskin and Price 1983). However, it receives fairly dense projections from the corticomedial amygdala including the CoA (Savander et al. 1996; Sah et al. 2003). The reliance of the basolateral amygdala on CoA input may indicate altered input rather than local changes and could, at least in part, underlie the altered functioning of this important site for fear learning plasticity.
Functional interpretation
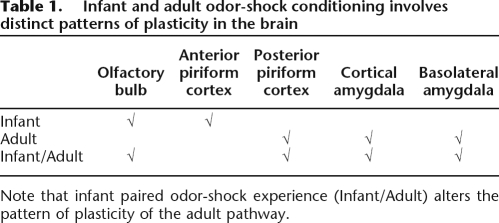
Taken together these data suggest that early life paired odor-shock conditioning has enduring effects on a wide network of structures, from the first relay of olfactory information (OB, Sevelinges et al. [2007]) to the olfactory cortical areas (pPCx, CoA, present study) and the basolateral amygdala (Sevelinges et al. 2007). Thus, each structure along the olfactory pathway likely alters the signal as it travels to the amygdala, which may result in the observed attenuated learning. Interestingly, the sites which presented an altered functioning in adult animals were different, at least in part, from those involved in odor memory formation in infant learning (see Table 1; Sullivan and Wilson 1995; Roth and Sullivan 2005; Moriceau et al. 2006). The present work complements earlier data on compromised adult learning and emotionality derived from other early life experience paradigms, such as painful shock or separation from the mother (Levine 1957, 1962; Denenberg and Bell 1960; Bell and Denenberg 1962; Lindholm 1962; Denenberg 1963; Henderson 1965; Coplan et al. 1996; Caldji et al. 1998, 2000; Pryce and Feldon 2003; Seckl and Meaney 2004; Card et al. 2005; Kosten et al. 2005, 2006). Here, we present data suggesting that mechanisms associated with the enduring effects of early life experience are greatly dependent upon the contingent presentation of odor and shock (i.e., not found in unpaired infant experience groups), and the resulting effects are not limited to brain areas, showing learning-induced plasticity in infancy (see Table 1).
Table 1.
Infant and adult odor-shock conditioning involves distinct patterns of plasticity in the brain
Note that infant paired odor-shock experience (Infant/Adult) alters the pattern of plasticity of the adult pathway.
Materials and Methods
Subjects and husbandry
A total of 67 animals were used in this experiment. Male Long Evans rats were born in the respective institutions animal care facilities from dams housed in polypropylene cages (34 × 29 × 17 cm) lined with abundant pine shavings, ad libitum food and water, and kept in a temperature (23°C) and light (7–19 h) controlled room. Mothers were either purchased pregnant (France) or bred (USA) in the facilities. The day of parturition was considered PN0 and culling of litters to 12 pups occurred on PN0–1. To prevent litter effects on statistical analysis, no more than one male from a litter was used in an experimental training/testing condition. Pups were weaned at PN21–23 and adult fear conditioning occurred when rats were 4–6 mo old. Institutional approval was received for all procedures, which followed the NIH (USA) and European guidelines (France). An overlap in personnel training/testing of both infant and adult rats in both France and the USA ensured consistency of conditioning and testing of infant and adult animals between laboratories.
Infant odor-shock conditioning
PN8 pups were assigned to one of the following experimental groups: (1) Paired odor-shock, (2) Unpaired odor-shock, (3) Odor Only, and (4) Naive. Pups were trained daily for five consecutive days in order to induce a strong learning. In paired condition, this paradigm causes pups to learn an odor preference provided conditioning is begun during the sensitive period (Sevelinges et al. 2007). The Paired group received 11 presentations of a 30-sec conditioned stimulus (CS; McCormick Pure Peppermint; 2 L/min 1:10 peppermint vapor to air) and a 1-sec unconditioned stimulus (US; hind limb shock; 0.5 mA). Peppermint odor was delivered via an olfactometer controlled by a Chrontrol with an intertrial interval of 4 min. The Unpaired group was shocked 1.5–2 min following the CS odor presentation and the Odor-Only group received only the odor CS. Naive pups did not receive odor or shock and were always from a litter without any conditioned pups. Pups were placed in individual 600-mL clear plastic cylinders, immediately after being removed from the nest, given 10 min to recover from experimenter handling, and were returned immediately to the nest following the 45-min training.
Infant odorant CS test
To ensure that odor-shock conditioning from PN8 to PN12 leads to an odor preference in our conditions 15 extra pups were trained in one of these three conditions: (1) Paired, (2) Unpaired, or (3) Odor Only. The day after conditioning, pups were given a Y-maze test. This test required pups to choose between two arms of a Plexiglas Y maze (start box: 8.5 × 10 × 8 cm; choice arms: 8.5 × 24 × 8 cm): one containing the peppermint odor CS (20 μL of peppermint odor placed on a Kim Wipe), and the other containing the familiar odor of pine shavings (20 mL of clean shaving in a Petri dish). A pup was placed in the start box (habituation chamber) during the 5 sec before the door to each alley was opened. Each pup was given 60 sec to choose an arm. A response was considered a choice when a pup's entire body was past the entrance to the alley. Pups received five trials with 30 sec between trials, and the floor was wiped clean between each trial (Sullivan and Wilson 1991). Testing was done blind to the training condition. These pups were not trained at adulthood in order to avoid possible interferences of this unreinforced CS exposure on the strength of the previous CS-US learning.
Adult odor-shock conditioning
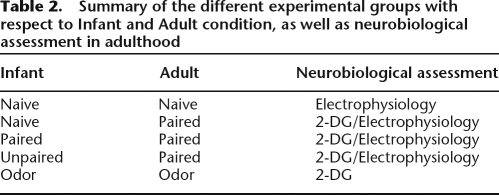
Conditioning took place in a Plexiglas transparent cylinder (diameter = 21 cm, height = 21.5 cm) with a lateral door (Vigouroux and Royet 1981), housed in a sound attenuating enclosure. The floor of the cage consisted of 17 stainless steel bars, 0.5 cm in diameter, spaced 1 cm apart. The floor was connected to a Coulbourn shock generator, which delivered 0.4-mA scrambled shock. The ceiling of the cage allowed the branching of three Tygon tubing connected to an olfactometer located outside the apparatus. Deodorized air constantly flowed through the cage. At appropriate times, the odor was introduced in the air stream for 30 sec. The conditioning cage was placed above a cubic Plexiglas chamber (30 × 30 × 15 cm) on which an exhaust fan was mounted allowing a continuous evacuation of the odorant stream from the conditioning cage. Conditioning took place in a single session. Rats were introduced in the conditioning cage and given 2 min of free exploration. At the third minute, peppermint was introduced in the cage for 30 sec, the last 2 sec of which overlapped with the delivery of 0.4-mA foot-shock (US). The animals received six pairings of odor and shock with an intertrial interval of 4 min. Five different experimental groups were used based on the infant and adult training conditions: Infant Naive/Adult Naive, Infant Paired/Adult Paired, Infant Unpaired/Adult Paired, Infant Naive/Adult Paired, Infant Odor/Adult Odor (Table 2). Animals were divided in two distinct experiments. A first set of rats (n = 20) served for 2-DG autoradiography experiment. The second set (n = 47) served for cue testing and electrophysiological studies.
Table 2.
Summary of the different experimental groups with respect to Infant and Adult condition, as well as neurobiological assessment in adulthood
Autoradiography analysis of the olfactory cortical areas
The first set of adult rats (Infant Paired/Adult Paired, n = 5; Infant Unpaired/Adult Paired, n = 4; Infant Naive/Adult Paired, n = 6; Infant Odor/Adult Odor, n = 5) was injected with 14C 2-deoxyglucose (2-DG; 40 μCi sc/rat) 5 min before adult odor-shock conditioning. Brains were removed immediately after conditioning, frozen in 2-methylbutane (−45°C) and stored at −70°C. At −20°C brains were sectioned (20 μm) in a cryostat with alternating sections mounted and exposed for 5 d with standards (14C methylmethacrylate standard 10 × 0.02 mCi; American Radiolabeled Chemicals, Inc.) (DiRocco and Hall 1981; Coopersmith and Leon 1986; Nudo and Masterton 1986; Sullivan and Wilson 1995). Analysis was done by an experimenter blind to the experiment conditions using the NIH computer-based digital image system that allows pseudocolor imaging and quantitative optical densitometry of autoradiographs.
Olfactory cortical areas examined were the aPCx and pPCx, as well as the CoA. Brain areas were identified by counterstaining sections with cresyl violet and a template constructed to overlay on the autoradiographs to determine the areas for analysis (Paxinos and Watson 1986). The 2-DG uptake was expressed relative to 2-DG uptake in the corpus callosum (which did not vary between conditioning group) to control for differences in section thickness and exposure (Sullivan et al. 2000; Moriceau and Sullivan 2004; Moriceau et al. 2006).
Cue testing in adulthood
Assessment of CS-conditioned fear in the second set of adult rats was performed 24 h after the conditioning session, in a testing cage different from the conditioning cage in order to avoid the influence of fear conditioning to the training context (Holland and Bouton 1999). Rats were introduced in the testing cage and conditioned fear to the odor was assessed in an 8-min session. During the first two minutes of testing, no odorant was present and the rats were free to explore their environment. The CS odor was then presented during the first 30 sec of each of the following 6 min. Animals’ behavior was continuously monitored with a camera connected to a video-recorder for off-line analysis. Off-line, animals’ behavior recorded during the test sessions was rated using an ethological keyboard connected to customized Matlab software. Freezing, which was characterized by a crouching posture and an absence of any visible movement except that due to breathing (Blanchard and Blanchard 1969), was quantified as total percent time during baseline prior to the odor presentations (pre-odor period) and during the odor presentations (odor period).
Electrophysiological assessment of the olfactory cortical areas
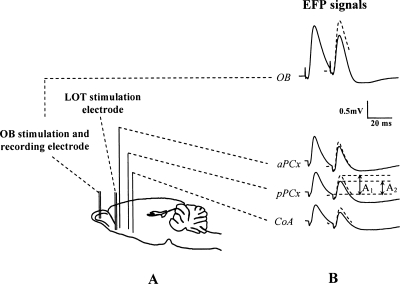
Two days after the cue-testing session, animals from different experimental groups (Infant Naive/Adult Naive, n = 11; Infant Paired/Adult Paired, n = 13; Infant Unpaired/Adult Paired, n = 12; Infant Naive/Adult Paired, n = 11) were anesthetized with Equithesin (mixture of chloral hydrate and sodium pentobarbital; 3 mL/kg, i.p.). The level of anesthesia was held constant with regular injections of Equithesin throughout the experiment. A bipolar stimulating electrode was lowered in the left OB (A/P −6 mm relative to the nasofrontal suture, L/M 1.3 mm relative to Bregma) at the level of the mitral cell layer. Three monopolar recording electrodes were implanted ipsilaterally in the aPCx (A/P +2.2 mm, L/M 4 mm relative to Bregma), the pPCx (A/P –1.8 mm, L/M 5.5 mm to Bregma), and the CoA (A/P –2.3 mm, L/M 3.3 mm relative to Bregma) (Fig. 4A). Accurate positioning of recording electrodes depth was achieved using the field potential profile evoked in each structure in response to electrical stimulation of the bipolar OB electrodes (Fig. 4B; Mouly and Di Scala 2006). In aPCx, pPCx, and CoA, recording electrode tips were positioned in the deep cortical layers (layers II–III) where the field potential signal presented large stable amplitude, which corresponded to the approximate depths of −7 mm, −8 mm, and −9 mm, respectively. In a subgroup of animals (n = 26), a second bipolar stimulating electrode was lowered in the left LOT in order to induce antidromic EFP in the OB collected via the stimulation electrode implanted at this level.
Figure 4.
Schematic representation of the implanted electrodes and recorded evoked field potential (EFPs) signals. (A) A bipolar stimulation electrode was inserted in the mitral cell layer of the olfactory bulb (OB) and in the lateral olfactory tract (LOT) for a subgroup of animals. Three monopolar recording electrodes were, respectively, implanted in the anterior piriform cortex (aPCx), posterior piriform cortex (pPCx), and cortical nucleus of the amygdala (CoA). Moreover, the bipolar electrode implanted in the OB also served as a recording electrode during stimulation of the LOT. (B) An example of EFPs induced in the four recording sites in response to paired-pulse stimulation of the OB (aPCx, pPCx, CoA) or the LOT (OB) using a 20-msec interpulse interval. Paired-pulse ratio was defined as the ratio of mean test signal amplitude (A2, second pulse) over mean conditioning signal (A1, first pulse). A decrease in the size of the response to the second pulse suggests inhibition and is reflected in a ratio <1.
Electrical stimulation was delivered in the OB or LOT through a Master-8 stimulator (AMPI). The electrical stimulus was a single monophasic square pulse, 0.1-msec duration, 0.1-Hz frequency. Stimulation test intensity (300–500 μA) was set to induce a response amplitude of ∼70% of maximum in the different recording sites. EFPs were amplified (Grass Model 12, Astro-Med, Inc.), filtered (1–300 Hz), and digitized (sampling frequency: 5 kHz) using a data acquisition system (Wavebook 512, Lotech, Inc.) for storage on computer hard disk.
Paired-pulse stimulation of the OB or LOT was used to assess the time course of short-term inhibition and facilitation in the different recording sites. In these tests, the effect of the conditioning (first) pulse was assessed by measuring changes in the response to the test (second) pulse. A ratio Test/Conditioning <1 is observed when the response to the test pulse is smaller than the response to the conditioning pulse and characterizes paired-pulse inhibition. Inversely, a ratio >1 is obtained when the response to the test pulse is greater than the response to the first pulse and corresponds to paired-pulse facilitation. Paired pulses were delivered at interpulse intervals of 20, 30, 40, 80, and 120 msec. Twelve responses were recorded for averaging at each interpulse interval.
Off-line, individual EFPs were averaged (n = 12 sweeps) and analyzed using the data acquisition software Dasylab (Iotech, Inc.). In each recording site, peak amplitudes of both the conditioning and test pulses were measured. Test signal amplitude was expressed as a ratio of conditioning signal amplitude. The obtained ratios were compared between the different experimental groups.
Statistical analysis
For all the experiments, comparisons were made using an analysis of variance (ANOVA), followed by paired Student’s t-test for within group comparisons and post-hoc Fisher tests for between group comparisons. For all the statistical comparisons performed, the significance level was set at 0.05.
Acknowledgments
This work was supported by NICHD HD33402, NSF IOB-0544406, OCAST, OU Presidential International Travel Funds to R.M.S.; a Eurodoc grant to Y.S.; and financial support of the Agence Nationale de la Recherche (ANR), The French National Research Agency under the Programme National de Recherche en Alimentation et nutrition humaine project ANR-05-PNRA-1.E7 AROMALIM to A.M.M. We thank Charlis Raineki, Stephanie Moriceau, Parker Holman, and Kyle Muzny for assistance with this project.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.998508.
References
- Anisman H., Zaharia M.D., Meaney M.J., Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Barr G.A. Ontogeny of nociception and antinociception. NIDA Res. Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Bell R.W., Denenberg V.H. The interrelationships of shock and critical periods in infancy as they affect adult learning and activity. Anim. Behav. 1962;11:21–27. [Google Scholar]
- Best A.R., Wilson D.A. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J. Neurosci. 2004;24:652–660. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard R.J., Blanchard D.C. Crouching as an index of fear. J. Comp. Physiol. Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P.M., Meaney M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C., Diorio J., Meaney M.J. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Camp L.L., Rudy J.W. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev. Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Card J.P., Levitt P., Gluhovsky M., Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J. Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabaud P., Ravel N., Wilson D.A., Gervais R. Functional coupling in rat central olfactory pathways: A coherence analysis. Neurosci. Lett. 1999;276:17–20. doi: 10.1016/s0304-3940(99)00773-9. [DOI] [PubMed] [Google Scholar]
- Chabaud P., Ravel N., Wilson D.A., Mouly A.M., Vigouroux M., Farget V., Gervais R. Exposure to behaviourally relevant odour reveals differential characteristics in rat central olfactory pathways as studied through oscillatory activities. Chem. Senses. 2000;25:561–573. doi: 10.1093/chemse/25.5.561. [DOI] [PubMed] [Google Scholar]
- Collier A.C., Bolles R.C. The ontogenesis of defensive reactions to shock in preweanling rats. Dev. Psychobiol. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Coopersmith R., Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Res. 1986;371:400–403. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- Coplan J.D., Andrews M.W., Rosenblum L.A., Owens M.J., Friedman S., Gorman J.M., Nemeroff C.B. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proc. Natl. Acad. Sci. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero M.I., Venero C., Kruyt N.D., Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm. Behav. 2003;44:338–345. doi: 10.1016/s0018-506x(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Cousens G., Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behav. Neurosci. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: The role of the amygdala. J. Neuropsychiatry Clin. Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Denenberg V.H. Early experience and emotional development. Sci. Am. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Denenberg V.H., Bell R.W. Critical periods for the effects of infantile experience on adult learning. Science. 1960;131:227–228. doi: 10.1126/science.131.3395.227. [DOI] [PubMed] [Google Scholar]
- Diorio J., Meaney M.J. Maternal programming of defensive responses through sustained effects on gene expression. J. Psychiatry Neurosci. 2007;32:275–284. [PMC free article] [PubMed] [Google Scholar]
- DiRocco R.J., Hall W.G. Metabolic neural mapping in neonatal rats. J. Neurosci. Res. 1981;6:13–19. doi: 10.1002/jnr.490060103. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Gale G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Fleming A.S., O'Day D.H., Kraemer G.W. Neurobiology of mother-infant interactions: Experience and central nervous system plasticity across development and generations. Neurosci. Biobehav. Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Fortune E.S., Rose G.J. Short-term synaptic plasticity as a temporal filter. Trends Neurosci. 2001;24:381–385. doi: 10.1016/s0166-2236(00)01835-x. [DOI] [PubMed] [Google Scholar]
- Funk D., Amir S. Enhanced Fos expression within the primary olfactory and limbic pathways induced by an aversive conditioned odor stimulus. Neuroscience. 2000;98:403–406. doi: 10.1016/s0306-4522(00)00217-7. [DOI] [PubMed] [Google Scholar]
- Haberly L.B. Summed potentials evoked in opossum prepyriform cortex. J. Neurophysiol. 1973;36:775–788. doi: 10.1152/jn.1973.36.4.775. [DOI] [PubMed] [Google Scholar]
- Haberly L.B. Parallel-distributed processing in olfactory cortex: New insights from morphological and physiological analysis of neuronal circuitry. Chem. Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haroutunian V., Campbell B.A. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Henderson N.D. Acquisition and retention of conditioned fear during different stages in the development of mice. J. Comp. Physiol. Psychol. 1965;59:439–442. doi: 10.1037/h0022039. [DOI] [PubMed] [Google Scholar]
- Holland P.C., Bouton M.E. Hippocampus and context in classical conditioning. Curr. Opin. Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Jones S.V., Stanek-Rattiner L., Davis M., Ressler K.J. Differential regional expression of brain-derived neurotrophic factor following olfactory fear learning. Learn. Mem. 2007;14:816–820. doi: 10.1101/lm.781507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadohisa M., Wilson D.A. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc. Natl. Acad. Sci. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R., Tominaga T., Takashima I. Olfactory information converges in the amygdaloid cortex via the piriform and entorhinal cortices: Observations in the guinea pig isolated whole-brain preparation. Eur. J. Neurosci. 2007;25:3648–3658. doi: 10.1111/j.1460-9568.2007.05610.x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L., Cahill L. Modulation of memory consolidation for olfactory learning by reversible inactivation of the basolateral amygdala. Behav. Neurosci. 2003;117:184–188. doi: 10.1037//0735-7044.117.1.184. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Miserendino M.J., Bombace J.C., Lee H.J., Kim J.J. Sex-selective effects of neonatal isolation on fear conditioning and foot shock sensitivity. Behav. Brain Res. 2005;157:235–244. doi: 10.1016/j.bbr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Lee H.J., Kim J.J. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Krettek J.E., Price J.L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J. Comp. Neurol. 1978;178:255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary–adrenal axis in the rat. Physiol. Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Lindholm B.W. Critical periods and the effects of early shock on later emotional behavior in the white rat. J. Comp. Physiol. Psychol. 1962;55:597–599. [PubMed] [Google Scholar]
- Litaudon P., Mouly A.M., Sullivan R., Gervais R., Cattarelli M. Learning-induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur. J. Neurosci. 1997;9:1593–1602. doi: 10.1111/j.1460-9568.1997.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Litaudon P., Amat C., Bertrand B., Vigouroux M., Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur. J. Neurosci. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- Luskin M.B., Price J.L. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J. Comp. Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- McDonald A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McNamara A.M., Cleland T.A., Linster C. Characterization of the synaptic properties of olfactory bulb projections. Chem. Senses. 2004;29:225–233. doi: 10.1093/chemse/bjh027. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav. Neurosci. 2004;118:274–281. doi: 10.1037/0735-7044.118.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Dual circuitry for odor-shock conditioning during infancy: Corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly A.M., Di Scala G. Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat. Neuroscience. 2006;137:1131–1141. doi: 10.1016/j.neuroscience.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Mouly A.M., Gervais R. Polysynaptic potentiation at different levels of rat olfactory pathways following learning. Learn. Mem. 2002;9:66–75. doi: 10.1101/lm.45602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly A.M., Fort A., Ben-Boutayab N., Gervais R. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience. 2001;102:11–21. doi: 10.1016/s0306-4522(00)00476-0. [DOI] [PubMed] [Google Scholar]
- Muller J.F., Mascagni F., McDonald A.J. Pyramidal cells of the rat basolateral amygdala: Synaptology and innervation by parvalbumin-immunoreactive interneurons. J. Comp. Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo R.J., Masterton R.B. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J. Comp. Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- Ottersen O.P. Connections of the amygdala of the rat. IV: Corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. J. Comp. Neurol. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Patneau D.K., Stripling J.S. Functional correlates of selective long-term potentiation in the olfactory cortex and olfactory bulb. Brain Res. 1992;585:219–228. doi: 10.1016/0006-8993(92)91210-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Price J.L. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J. Comp. Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Princivalle A., Spreafico R., Bowery N., De Curtis M. Layer-specific immunocytochemical localization of GABABR1a and GABABR1b receptors in the rat piriform cortex. Eur. J. Neurosci. 2000;12:1516–1520. doi: 10.1046/j.1460-9568.2000.01060.x. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 2003;27:57–71. doi: 10.1016/s0149-7634(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Okuda S., de Quervain D.J., McGaugh J.L. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Rosenkranz J.A., Grace A.A. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Sullivan R.M. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S., De Lopez Armentia M., Power J. The amygdaloid complex: Anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Savander V., LeDoux J.E., Pitkanen A. Topographic projections from the periamygdaloid cortex to select subregions of the lateral nucleus of the amygdala in the rat. Neurosci. Lett. 1996;211:167–170. doi: 10.1016/0304-3940(96)12750-6. [DOI] [PubMed] [Google Scholar]
- Schettino L.F., Otto T. Patterns of Fos expression in the amygdala and ventral perirhinal cortex induced by training in an olfactory fear conditioning paradigm. Behav. Neurosci. 2001;115:1257–1272. doi: 10.1037//0735-7044.115.6.1257. [DOI] [PubMed] [Google Scholar]
- Schwob J.E., Haberly L.B., Price J.L. The development of physiological responses of the piriform cortex in rats to stimulation of the lateral olfactory tract. J. Comp. Neurol. 1984;223:223–237. doi: 10.1002/cne.902230206. [DOI] [PubMed] [Google Scholar]
- Seckl J.R., Meaney M.J. Glucocorticoid programming. Ann. N. Y. Acad. Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Gervais R., Messaoudi B., Granjon L., Mouly A.M. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learn. Mem. 2004;11:761–769. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y., Moriceau S., Holman P., Miner C., Muzny K., Gervais R., Mouly A.M., Sullivan R.M. Enduring effects of infant memories: Infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry. 2007;62:1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Stehouwer D.J., Campbell B.A. Habituation of the forelimb-withdrawal response in neonatal rats. J. Exp. Psychol. Anim. Behav. Process. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. Neural correlates of conditioned odor avoidance in infant rats. Behav. Neurosci. 1991;105:307–312. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. Dissociation of behavioral and neural correlates of early associative learning. Dev. Psychobiol. 1995;28:213–219. doi: 10.1002/dev.420280403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Landers M., Yeaman B., Wilson D.A. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L.W., Petrovich G.D. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Tomoda A., Andersen S.L. Neurobiological consequences of early stress and childhood maltreatment: Are results from human and animal studies comparable? Ann. N. Y. Acad. Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Tseng G.F., Haberly L.B. Characterization of synaptically mediated fast and slow inhibitory processes in piriform cortex in an in vitro slice preparation. J. Neurophysiol. 1988;59:1352–1376. doi: 10.1152/jn.1988.59.5.1352. [DOI] [PubMed] [Google Scholar]
- Vigouroux M., Royet J.P. An olfactometric cage suitable for short duration stimulations of unrestrained small animals. J. Neurosci. Methods. 1981;4:189–196. doi: 10.1016/0165-0270(81)90053-4. [DOI] [PubMed] [Google Scholar]
- Walker D.L., Paschall G.Y., Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn. Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.A. Habituation of odor responses in the rat anterior piriform cortex. J. Neurophysiol. 1998a;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson D.A. Synaptic correlates of odor habituation in the rat anterior piriform cortex. J. Neurophysiol. 1998b;80:998–1001. doi: 10.1152/jn.1998.80.2.998. [DOI] [PubMed] [Google Scholar]
- Wilson D.A. Odor specificity of habituation in the rat anterior piriform cortex. J. Neurophysiol. 2000;83:139–145. doi: 10.1152/jn.2000.83.1.139. [DOI] [PubMed] [Google Scholar]
- Wilson D.A. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J. Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson D.A., Sullivan R.M., Leon M. Single-unit analysis of postnatal olfactory learning: Modified olfactory bulb output response patterns to learned attractive odors. J. Neurosci. 1987;7:3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.Y., Bagot R., Parent C., Nesbitt C., Bredy T.W., Caldji C., Fish E., Anisman H., Szyf M., Meaney M.J. Maternal programming of defensive responses through sustained effects on gene expression. Biol. Psychol. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]