Abstract
The cAMP-dependent protein kinase (PKA) is known to play a critical role in both transcription-independent short-term or intermediate-term memory and transcription-dependent long-term memory (LTM). Although distinct phases of LTM already have been demonstrated in some systems, it is not known whether these phases require distinct temporal patterns of learning-induced PKA activation. This question was addressed in a robust form of associative LTM that emerges within a matter of hours after single-trial food-reward classical conditioning in the pond snail Lymnaea stagnalis. After establishing the molecular and functional identity of the PKA catalytic subunit in the Lymnaea nervous system, we used a combination of PKA activity measurement and inhibition techniques to investigate its role in LTM in intact animals. PKA activity in ganglia involved in single-trial learning showed a short latency but prolonged increase after classical conditioning. However, while increased PKA activity immediately after training (0–10 min) was essential for an early phase of LTM (6 h), the late phase of LTM (24 h) required a prolonged increase in PKA activity. These observations indicate mechanistically different roles for PKA in recent and more remote phases of LTM, which may underpin different cellular and molecular mechanisms required for these phases.
In both invertebrates and vertebrates there is ample evidence that the cAMP-dependent protein kinase (PKA) plays a key role in the formation of transcription-dependent long-term memory (LTM) and long-lasting synaptic plasticity (Schacher et al. 1988; Abel et al. 1997; Müller 2000; Davis 2005). Within a dynamic network of molecular signaling cascades activated by learning, highly conserved PKA-mediated mechanisms are critical for triggering transcriptional processes that ultimately lead to protein synthesis required for LTM and long-lasting synaptic plasticity (Selcher et al. 2002; Roberts and Glanzman 2003; Barco et al. 2006; Schwärzel et al. 2007). In various species it has been shown that training procedures that induce long-lasting changes also induce a prolonged activation of PKA, which is a critical step for the induction of molecular processes underlying LTM (Müller and Carew 1998; Chain et al. 1999; Müller 2000; Locatelli and Romano 2005). Although recently it has been suggested that LTM itself has different phases (Dudai 2004), it is not known whether these phases require distinct temporal domains of PKA activation.
Similar to the effect of multi-trial classical conditioning in other studies, transcription-dependent LTM is present at 24 h after single-trial food-reward classical conditioning in the pond snail Lymnaea stagnalis (Kemenes et al. 2001). However, there is also a much earlier phase of transcription-dependent LTM in Lymnaea, which emerges several hours after single-trial training (Fulton et al. 2005). Activation of MAPK (mitogen-activated protein kinase) and NOS (nitric oxide synthase) as well as increased phosphorylation of CREB (cAMP-response element binding protein), well-known molecular components necessary for LTM formation in other systems (Silva et al. 1998; Müller 2000; Sharma and Carew 2004), also occur early after this type of associative learning (Kemenes et al. 2002; Ribeiro et al. 2003, 2005). A major advantage of the use of this single-trial paradigm is that, unlike multi-trial protocols, it allows the investigation of amnestic effects of sharply timed manipulations of key molecular pathways (Kemenes et al. 2002; Ribeiro et al. 2005). Therefore, Lymnaea offers the ideal opportunity to analyze the role of PKA in the formation of both early and late phases of LTM.
Since in Lymnaea PKA has not been characterized in detail, we first establish its molecular identity and biochemical modus operandi in its nervous system. On the basis of this information, we address the temporal characteristics of learning-induced PKA activation and its potential impact on memory formation by both measuring and inhibiting PKA activity at different time points after single-trial classical reward conditioning. This paradigm, which is based on a single pairing of a neutral chemical conditioned stimulus (amyl acetate, the CS) with a salient food unconditioned stimulus (sucrose, the US) has been well established in both behavioral and cellular/molecular studies of learning in Lymnaea (Alexander Jr. et al. 1984; I. Kemenes et al. 2002, 2006; Ribeiro et al. 2003; Fulton et al. 2005; G. Kemenes et al. 2006). After conditioning, snails show a much stronger feeding response to amyl acetate alone than they did before training or compared with appropriate control treatments, such as explicitly unpaired application of the CS and US or handling (Alexander Jr. et al. 1984; G. Kemenes et al. 2006). Here we demonstrate that different phases of LTM induced by a single associative learning trial require increased PKA activity in distinct time windows after training. This finding points to a more complex function of learning-induced PKA activity in LTM formation than previously thought, which may also apply to other systems and preparations.
Results
PKA in Lymnaea nerve tissue
Recent work in Lymnaea showed that after memory retrieval, the phosphorylation level of a specific substrate molecule of PKA increases and the injection of KT5720, a potent inhibitor of PKA in other systems, prevents memory reconsolidation (G. Kemenes et al. 2006), providing the first experimental evidence of PKA catalytic subunit (PKA C) activity in this species. However, no previous work has directly demonstrated PKA C mRNA or proteins in the Lymnaea central nervous system (CNS), an increasingly important experimental model for studies of the cellular and molecular mechanisms of classical and operant conditioning (Benjamin et al. 2000; Kemenes 2008). In these experiments we therefore addressed this issue by cloning Lymnaea PKA C, as well as by using biochemical purification methods and mass spectrometry, PKA-specific substrate phosphorylation and inhibition assays, Western blotting, and immuno/in situ histochemistry.
Cloning of PKA C yielded a highly homologous and conserved kinase enzyme sequence (NCBI GenBank accession number DQ084243). The data revealed functionally pivotal residues (Johnson et al. 2001) to be fully conserved with an overall protein identity to the human α PKA C isoform of 83%. Phylogenetic analysis of PKA C messages from representative species showed that both the DNA and protein sequences diverge in accordance with what can be predicted by evolutionary theory, sorting the Lymnaea message alongside Aplysia into the molluscan branch (data not shown).
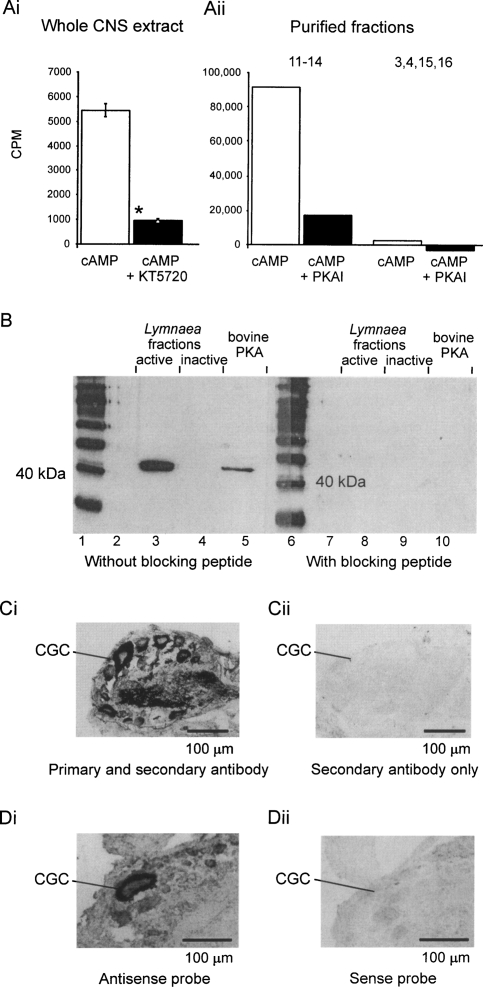
In biochemical assays using the PKA-specific phosphorylation substrate Kemptide, we demonstrated high cAMP-induced levels of substrate-phosphorylation activity in whole Lymnaea brain extracts (Fig. 1Ai) and fractions obtained by a PKA C purification procedure (Fig. 1Aii). This cAMP-induced phosphorylation activity was blocked by PKAI or KT5720, two potent inhibitors of PKA (Fig. 1Ai, Aii).
Figure 1.
A conserved functional PKA catalytic subunit (PKA C) is present in the Lymnaea CNS. (A) cAMP stimulates PKA C activity in Lymnaea nervous system extracts (Ai) and purified PKA fractions (Aii), which can be blocked by KT5720 or PKAI. The whole CNS data (mean ±SEM) come from experiments with single brains (n = 19). *, P < 0.005, paired t-test. The purified fraction data come from a single experiment in which 66 brains were used to obtain the purified fractions. Fractions 11–14 show PKA catalytic subunit activity that can be blocked by PKAI; fractions 3, 4, 15, and 16 are inactive. (B) Western blot results. An anti-human PKA αC antibody strongly labels a protein band in pooled active fractions 11–14 from the PKA purification procedure (lane 3). This band has the same molecular weight as the recombinant bovine PKA C (lane 5). There is no reaction in the sample containing pooled inactive fractions 3, 4, 15, and 16 (lane 4). The active Lymnaea fractions in lane 8 and bovine PKA in lane 10 do not react with the primary antibody preincubated with its specific blocking peptide. Lanes 1 and 6 are marker lanes, lanes 2 and 7 are blank. (C) Immunohistochemistry. The same PKA antibody that was used for Western blotting selectively labels individual neurons in the Lymnaea CNS. This figure shows ubiquitous PKA C expression in cell bodies, axons, and the neuropile in a section of the cerebral ganglia (Ci), with no staining in a section that was only treated with the secondary antibody (Cii). (CGC) Cerebral giant cell, an important identified modulatory neuron of the feeding network. (D) In situ hybridization. An antisense probe raised against LymPKA-C reacts with neurons in the cerebral ganglia (Di), with a particularly strong signal in the CGC, whereas the sense probe (control, Dii) does not react with LymPKA-C.
Using a mammalian anti α PKA C antibody, we demonstrated the presence of a single band in Western blots of Lymnaea brain homogenates (not shown) and eluates obtained by a PKA C specific protein purification procedure (Fig. 1B). Only fractions with PKA-specific substrate phosphorylation activity (Fig. 1A) showed immunoreactivity with the PKA C antibody (Fig. 1B, left). The protein in the single band had a molecular weight very similar to that of the recombinant bovine PKA C, which was run in parallel with the Lymnaea sample (Fig. 1B, left). Incubation of the Western blot with the PKA C antibody preabsorbed with its antigenic peptide failed to label recombinant bovine PKA C or Lymnaea fractions containing PKA C activity (Fig. 1B, right). Neither the bovine nor the molluscan PKA C was detected with the secondary antibody alone (not shown).
We subsequently isolated a band of a similar molecular weight to the recombinant bovine PKA C from the purified Lymnaea PKA C fractions by means of a silver-stained SDS-PAGE gel. Mass spectrometry (MS) and coupled MS/MS revealed this band to be a PKA-like protein. The fragments obtained by MALDI-TOF could be matched to both Aplysia and Lymnaea PKA-like protein sequences by means of a Mascot database search. Three fragments were further analyzed by MS/MS, of which two spectra could be matched to the Aplysia and Lymnaea sequences.
Immunohistochemical experiments using the same anti-PKA C antibody that was used in the Western blot experiments as well as in situ hybridization using digoxigenin (DIG)-labeled oligonucleotides demonstrated a ubiquitous, correlating neuronal expression of a PKA C signal in the Lymnaea CNS (Fig. 1Ci, Di). The signal was absent in control sections treated with the secondary antibody alone (Fig. 1Cii), treated with primary antibodies preincubated with the antigen (not shown), or by using a sense-probe in the in situ hybridization (Fig. 1Dii).
These experiments provided the first comprehensive characterization of the LymPKA C mRNA and protein in the nervous system and confirmed their high structural and functional homology with PKA C in other systems. This now presents a firm basis for the functional analysis of PKA in memory formation after single-trial conditioning.
Single-trial reward conditioning induces fast and prolonged PKA activation in Lymnaea
Previous work in honeybees and Aplysia showed that multi-trial training leading to LTM or long-lasting neuronal plasticity induces prolonged PKA activation (Müller and Carew 1998; Müller 2000), and we wanted to examine whether this was also the case after single-trial conditioning in Lymnaea, which is also known to lead to LTM (Alexander Jr. et al. 1984; Fulton et al. 2005).
To identify time windows of PKA activation induced by a single pairing of the CS with the US, we measured PKA activity in the cerebral ganglia at different times after training (Fig. 2A). The cerebral ganglia were targeted in these experiments because they show selective CREB phosphorylation (Ribeiro et al. 2003) and are important sites for neuronal plasticity (Straub et al. 2004) after single-trial learning.
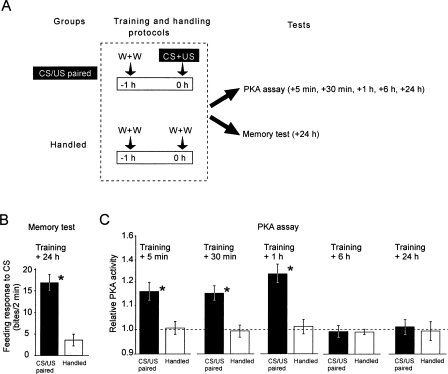
Figure 2.
Single-trial reward conditioning leading to LTM results in prolonged elevation of PKA activity in the Lymnaea cerebral ganglia. (A) The experimental protocol. Single-trial reward conditioning or handling (0 h time point) is followed by PKA assays at different time points or a memory test at 24 h post-training. (W) Water; (CS) conditioned stimulus; (US) unconditioned stimulus. (B) The feeding response to the CS tested 24 h after CS/US paired conditioning (n = 16) is significantly higher than in naive animals handled in parallel (n = 15) (*, unpaired t-test, df = 29, t = 4.88, P < 0.001). (C) Comparisons of relative PKA activity in the cerebral ganglia at different time points after training measured in samples from the same groups of animals as in B. All individual values were normalized to the mean level of activity measured in a naïve control group (n = 10) taken as 1 (broken line). At 5 min, 30 min, and 1 h post-training the PKA activity in the CS/US paired groups (n = 14, 15, 15, respectively) is significantly higher than in the corresponding handled groups (n = 15 each) (*, unpaired t-tests, df = 27, 28, 28, t = 3.42, 4.76, 4.90, P < 0.002, 0.001, 0.001, respectively). At 6 h and 24 h post-training, no significant differences in PKA activity between CS/US paired (n = 13 and 14, respectively) and handled groups (n = 15 and 14, respectively) are detectable.
Cerebral ganglia from animals conditioned at the same time as a group of animals saved for memory tests (Fig. 2B) showed significantly increased PKA activity at 5 min, 30 min, and 1 h post-training (Fig. 2C), while 6 h and 24 h after conditioning PKA activity in the CS/US paired group was back to baseline level (broken line in Fig. 2C). That the post-training increase was due to a close temporal association between the CS and US rather than nonassociative factors was verified in independent experiments using a CS/US paired and a handled group each, together with a variety of standard control groups (CS alone and US alone, or CS/US explicitly unpaired). In each of these experiments, only the CS/US paired group showed a significant increase in the behavioral response at 24 h as well as prolonged PKA activity after training (Fig. 3).
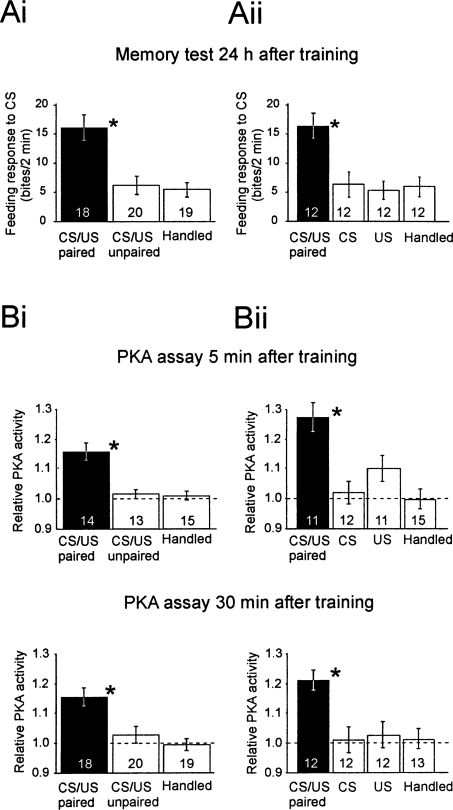
Figure 3.
PKA activation and LTM after single-trial food-reward classical conditioning and nonassociative control procedures. In two separate experiments, groups of animals were subjected to training or control procedures as indicated. After each procedure, the groups were randomly subdivided into three groups to test the feeding response to the CS 24 h later or to determine PKA activity in brain samples (the paired cerebral ganglia) at 5 min or 30 min post-training. The data show the means ±SEM for the feeding response to the CS and relative PKA activity. The number of independent samples is indicated for each group. (Ai,Aii) In both experiments, the memory tests at 24 h show robust learning in the CS/US paired group compared with each of the different control groups (*; one-way ANOVAs, F(2,56) = 7.4, P < 0.001 and F(2,47) = 8.2, P < 0.001; Tukey’s HSD tests, P < 0.002 to 0.006). (Bi,Bii) At both 5 min and 30 min, the PKA activity in the cerebral ganglia of animals that received CS/US paired conditioning are significantly above control levels, (*; one-way ANOVAs, F(2,42) = 12.1, P < 0.001 and F(2,41) = 8.4, P < 0.001; Tukey’s HSD tests P < 0.002 to 0.005).
LTM at 6 h only requires a short burst of PKA activity, whereas LTM at 24 h requires more prolonged activity after a single training trial
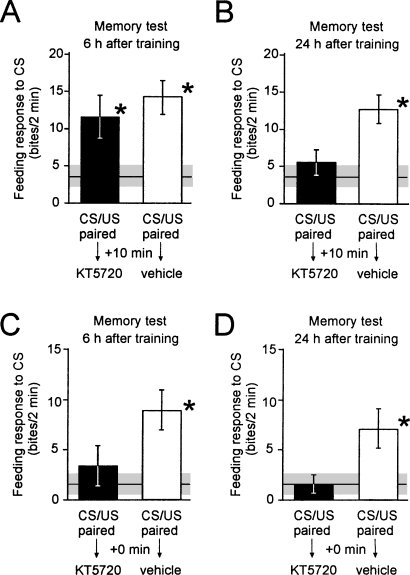
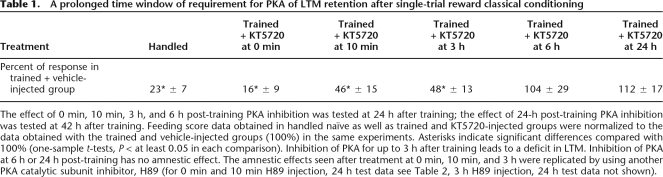
To investigate the role of the measured prolonged PKA activation in memory formation, we injected the PKA inhibitor KT5720 into intact animals to reduce the duration of conditioning-induced PKA activation. As shown in Figure 4, PKA inhibition by KT5720 injection at 10 min after training had no significant effect on the feeding response to the CS at 6 h post-training (Fig. 4A), but significantly reduced the feeding response to the CS presented 24 h after training (Fig. 4B). Injections of KT5720 up to 3 h but not 6 h after training impairs 24-h memory (Table 1), indicating a long-lasting but transient PKA requirement for the 24-h memory phase.
Figure 4.
Effects of inhibition of PKA in different post-training time windows on 6 h and 24 h memory after single-trial classical conditioning. Groups of animals were injected with the PKA inhibitor KT5720 or its vehicle 10 min (A,B) or immediately (0 min, C,D) after single-trial reward conditioning. Feeding response to the CS was tested either 6 h (A,C) or 24 h (B,D) after training. Naive groups were handled in parallel to provide baseline values for CS-induced feeding. (A) Inhibition of PKA at 10 min post-training does not induce amnesia at 6 h post-training. The feeding response does not differ between KT5720 (n = 17) and vehicle (n = 17) injected groups (ANOVA, F(2,52) = 5.449, P < 0.007; Tukey’s HSD test, P = 0.402). Both are significantly (*) higher than the baseline feeding response to the CS in the handled group (n = 18) (Tukey’s HSD tests P < 0.005 and 0.05, respectively). (B,C,D) Inhibition of PKA at 10 min post-training induces amnesia at 24 h post-training (B) and PKA inhibition immediately after training induces amnesia at both 6 h (C) and 24 h (D) post-training. The feeding responses of the vehicle-injected groups (n = 18, 20, and 23) are significantly (*) higher than those of the handled groups (n = 18, 22, and 22) and the KT5720-injected groups (n = 17, 17, and 17) in the same experiment. Statistics: (B) ANOVA, F(2,53) = 5.581, P < 0.006; Tukey’s HSD tests, P < 0.02; (C) ANOVA, F(2,58) = 6.583, P < 0.003; Tukey’s HSD tests, P < 0.01, 0.05; (D) ANOVA, F(2,61) = 6.067, P < 0.004; Tukey’s HSD tests, P < 0.05.
Table 1.
A prolonged time window of requirement for PKA of LTM retention after single-trial reward classical conditioning
The effect of 0 min, 10 min, 3 h, and 6 h post-training PKA inhibition was tested at 24 h after training; the effect of 24-h post-training PKA inhibition was tested at 42 h after training. Feeding score data obtained in handled naïve as well as trained and KT5720-injected groups were normalized to the data obtained with the trained and vehicle-injected groups (100%) in the same experiments. Asterisks indicate significant differences compared with 100% (one-sample t-tests, P < at least 0.05 in each comparison). Inhibition of PKA for up to 3 h after training leads to a deficit in LTM. Inhibition of PKA at 6 h or 24 h post-training has no amnestic effect. The amnestic effects seen after treatment at 0 min, 10 min, and 3 h were replicated by using another PKA catalytic subunit inhibitor, H89 (for 0 min and 10 min H89 injection, 24 h test data see Table 2, 3 h H89 injection, 24 h test data not shown).
These experiments suggested that the 6-h memory was either insensitive to PKA inhibition or that the early phase of learning-induced PKA activity (in the range of minutes after training, Fig. 2C) is sufficient to trigger the molecular events required for 6-h memory. Thus, we examined whether PKA inhibition starting immediately after training had an effect on memory retention 6 h later. These experiments showed that unlike injection at 10 min, injection of the PKA inhibitor immediately after acquisition had a significant deleterious effect on the 6-h memory (Fig. 4C,D).
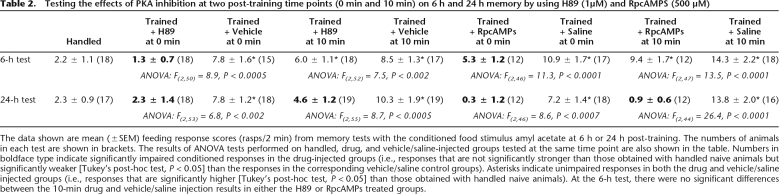
Although KT5720 has been used in several previous studies to analyze the effect of inhibiting the catalytic activity of PKA on behavioral or synaptic plasticity in both vertebrates and invertebrates (Bernabeu et al. 1997; Müller and Hildebrandt 2002; Hu et al. 2003; Scheiner et al. 2003; Hou et al. 2004; Khabour et al. 2004; G. Kemenes et al. 2006), in vitro kinase inhibition assays raised concerns about its selectivity (Davies et al. 2000). Therefore, to confirm the observations made by using this inhibitor we also used RpcAMPs, a selective inhibitor of the cAMP-triggered dissociation of PKA regulatory and catalytic subunits and H89, which is similar in its mode of action to KT5720 (i.e., inhibits PKA catalytic subunit activity) but has a lower IC50 value (half-maximal inhibitory concentration) for PKA and a different profile of nonspecific effects compared with KT5720 (Davies et al. 2000). The results obtained with these two inhibitors (Table 2) were very similar to those obtained with KT5720, confirming that inhibition of PKA at both 0 min and 10 min post-training was specifically responsible for the amnestic effects seen at both 6 h and 24 h post-training.
Table 2.
Testing the effects of PKA inhibition at two post-training time points (0 min and 10 min) on 6 h and 24 h memory by using H89 (1μM) and RpcAMPS (500 μM)
The data shown are mean (±SEM) feeding response scores (rasps/2 min) from memory tests with the conditioned food stimulus amyl acetate at 6 h or 24 h post-training. The numbers of animals in each test are shown in brackets. The results of ANOVA tests performed on handled, drug, and vehicle/saline-injected groups tested at the same time point are also shown in the table. Numbers in boldface type indicate significantly impaired conditioned responses in the drug-injected groups (i.e., responses that are not significantly stronger than those obtained with handled naive animals but significantly weaker [Tukey’s post-hoc test, P < 0.05] than the responses in the corresponding vehicle/saline control groups). Asterisks indicate unimpaired responses in both the drug and vehicle/saline injected groups (i.e., responses that are significantly higher [Tukey’s post-hoc test, P < 0.05] than those obtained with handled naive animals). At the 6-h test, there were no significant differences between the 10-min drug and vehicle/saline injection results in either the H89 or RpcAMPs treated groups.
Irrespective of the treatment and time tested, nonassociative parameters, such as the US-induced feeding response, remained unaffected (data not shown).
Taken together, the above experiments showed that 6-h memory retention after single-trial conditioning is only dependent on the earliest period of training-induced PKA activity (up to ∼10 min), whereas 24-h memory retention requires a much more prolonged PKA-dependent consolidation process.
Discussion
Here we have provided the first evidence for the presence of a conserved and functional catalytic subunit of PKA in Lymnaea CNS tissue. We successfully purified a measured PKA C-like activity and performed mass spectrometry on the highly active fraction. The obtained spectra successfully identified predicted amino acid sequences from our cloned Lymnaea PKA C message. Further evidence from immuno- as well as in situ histochemistry revealed the ubiquitous presence of PKA C mRNA as well as protein in the Lymnaea nervous system. The molecular and functional identification of Lymnaea PKA C formed the foundation for further analysis in which we elucidated the role played by this identified PKA C in the associative reward learning and memory paradigm of Lymnaea.
Using a combination of specific PKA activity measurement and inhibition methods, we have provided evidence for the role of two temporally distinct patterns of PKA activity in the formation of two different phases of LTM (6 h and 24 h) induced by a single training trial.
Memory tested 6 h after training requires only a brief burst of PKA activity (just subsequent to training), whereas the prolonged learning-induced PKA activity is pivotal to memory tested 24 h after training. Since memory tested at both 6 h and 24 h depends on post-acquisition transcription and translation (Kemenes et al. 2001; Fulton et al. 2005), these findings demonstrate that distinct time windows of PKA activity are critical to triggering at least two different phases of LTM to emerge after the same single training trial. This finding also appears to indicate the existence of a so-far-unidentified transcriptional activation mechanism that can be activated even by a brief burst of PKA activity and is sufficient for the early phase of LTM at 6 h but not for the late phase of LTM at 24 h.
In other systems in which the role of PKA in memory formation was investigated, distinct temporal patterns of training-induced PKA activity leading to different memory phases also have been identified (Müller and Carew 1998; Chain et al. 1999; Müller 2000; Locatelli and Romano 2005). However, these different patterns were induced by differing numbers of training trials. A single trial (or its in vitro equivalent) resulted in a brief activation of PKA and short-term (transcription and translation independent) memory, whereas more trials led to a more prolonged PKA activity and intermediate-term (translation- but not transcription-dependent) or long-term (transcription- and translation-dependent) memory.
An interesting parallel can be drawn between our findings in intact animals and previous findings in an Aplysia sensory and a motoneuronal cell culture system, where mechanisms of more transient and more persistent forms of long-term facilitation were investigated (Casadio et al. 1999). Both of these forms of long-term facilitation depend on transcription, translation, and PKA, but unlike persistent facilitation (lasting for at least 72 h), the more transient form (lasting for up to 24 h) is not associated with synaptic growth, but rather seems to be based on an increase in the incidence of active synaptic zones. It is possible that in intact Lymnaea single-trial classical conditioning similarly induces a more persistent and a more transient form of LTM, which might differ in their underlying cellular mechanisms.
In Aplysia, a late phase of PKA activation is required for the activation of transcription necessary for long-term facilitation (Chain et al. 1999). In Lymnaea, however, the same single conditioning trial leads to the emergence of two phases of transcription-dependent memory with distinct temporal requirements for PKA, one early and one late. It is possible that the early phase of PKA activity in Lymnaea is involved in the activation of different genes compared with the later phase, which may have a similar role in LTM as the persistent PKA activity described in the Aplysia long-term facilitation studies (Chain et al. 1999).
Interestingly, recent work using electrophysiological techniques already has revealed differences in the cellular mechanisms of more recent (<16 h post-training) and more remote (>24 h post-training) LTM after single-trial classical conditioning in Lymnaea. A learning-induced persistent depolarization of a key modulatory neuron (cerebral giant cell [CGC]) of the Lymnaea feeding system occurred only >24 h after single-trial classical conditioning, when it was shown to encode information that enables the expression of long-term associative memory (I. Kemenes et al. 2006). It is tempting to speculate that prolonged PKA activity after learning is necessary for this delayed and persistent neuronal plasticity, whereas a shorter burst of PKA activity is sufficient to support the so-far-unidentified cellular mechanisms that underlie behavioral memory in more recent time windows (e.g., 6 h). Although both phases of memory are dependent on the transcription and translation of new proteins (Kemenes et al. 2001; Fulton et al. 2005), the identity of these proteins remains to be established. The learning-induced delayed and persistent depolarization of the CGC recently has been linked to a concomitant up-regulation of the persistent sodium current INa(P) of this neuron (Nikitin et al. 2008), which also shows a delayed and prolonged increase after intracellular injection of the CGC with cAMP (Nikitin et al. 2006). These observations suggest that one of the proteins whose expression increases at >24 h after learning in a PKA-dependent manner might be the neuronal sodium channel carrying INa(P). However, this attractive hypothesis requires further verification by molecular biological tools.
Materials and Methods
Experimental animals and chemicals
A laboratory-bred stock of L. stagnalis was maintained before the learning experiments as described previously (Kemenes et al. 2002; Ribeiro et al. 2003, 2005; Fulton et al. 2005). All chemicals were from Sigma unless stated otherwise.
In vitro PKA activation and inhibition assays
The techniques used were modified from Kemp and Pearson (1991) and Fiala et al. (1999). Individual brains were homogenized in 50 mM Tris pH 7.7, 1 mM EDTA, 1 mM EGTA, and 10 mM BME and incubated at room temperature for 10 min (in a final concentration of 50 mM Tris pH 7.7, 1 mM EDTA, 1 mM EGTA, 10 mM BME, 10 mM MgCl, 1 μCi 32P [Amersham], 100 μM Kemptide [a specific substrate for PKA-mediated phosphorylation], and 50 μM ATP). PKA activity and the effect of KT5720 on PKA activity were determined by addition of 5 μM 8-bromo-cAMP and 5 μM 8-bromo-cAMP together with 10 μM KT5720, respectively. The samples were handled on ice. After 10 min, the reaction was stopped by spotting the samples onto P81 paper (Whatman), which was immediately washed with 5% (w/v) phosphoric acid. The radioactivity of the samples was measured in a scintillation counter. Baseline count values were determined from the samples containing no cAMP or KT5720 in the incubation mixture.
Protein purification
The following protocol was modified from Zoller et al. (1979) and Altfelder and Müller (1991). Sixty-six Lymnaea CNS were homogenized in 2 mL of buffer (20 mM Na-phosphate buffer pH ∼6.6, 1 mM EDTA, 1 mM EGTA, 10 mM BME), centrifuged for 15 min at 13,000 RPM at 4°C, and the supernatant was retained. The pellet was resuspended in buffer and centrifuged as before, and the pooled supernatant was made up to 40 mL with buffer. The supernatant was then pumped over a DEAE-Sepharose-column (1 mL resin) at 4°C, washed with buffer for ∼30 min, and run in tandem with a CM-Sepharose-column (200 μL resin) for ∼90 min using 0.5 mM cAMP in buffer. The columns were washed in buffer, and the CM column was eluted using 0.5 M NaCl in buffer. Drop-sized fractions (∼50 μL) were collected and tested for PKA activity.
Western blotting
Whole brains were homogenized in 125 mM TRIS pH 6.8 and diluted to 125 mM TRIS pH 6.8, 2% SDS, 10% glycerol, 10% BME, 0.01% BFB. Protein content was measured with the Pierce BCA-kit. SDS gel electrophoresis was then carried out according to Sambrook et al. (1989) using a semi-dry blotting procedure. Antibody concentrations were as follows: primary PKA αcat (sc-903) 1:2000 and blocking peptide 1:1000 (both Santa Cruz); secondary peroxidase labeled anti-rabbit Ig-G 1:2000 (Vector). The blots were then developed with LumiGLO and peroxide (Cell Signaling).
Immunohistochemistry
Immunohistochemistry using the PKA antibody at a dilution of 1:500 was carried out according to Ribeiro et al. (2003).
In situ hybridization
In situ hybridization was adapted from Kellett et al. (1996) and carried out on 10-μm thick frozen sections. Oligonucleotide probes were synthesized by MWG Biotech as follows: PKA C sense: ATGCTGTTTAGACGACTTCGACAGAATCAA AACCCTTGG and ATTCTCAGCAAAGGCTATAAGCAAGGC AGTAGACTGGTGGGC; PKA C antisense: TACGACAAATCTGCT GAAGCTGTCTTAGTTTTGGGAACC and TAAGAGTCGTTTCC GATATTCGTTCCGTCATCTGACCACCCG. The oligonucleotides were subsequently DIG-labeled using a DIG-tailing kit (Roche), and pictures were taken with Zeiss AxioVision 3.0 software on a Zeiss camera-microscope setup.
PKA C cloning
PKA C cloning was performed as follows. A 150-bp fragment was generated by conventional PCR (on a Perkin Elmer Cetus 9600 thermocycler using Helena taq-polymerase, 94°C at 2 min, then 35 cycles of 94°C for 15 sec, 50°C for 30 sec, and 72°C for 1 min, finalized by a single 15-min extension at 72°C) with primer 1: 5′-CCAGAYTTYGGKTTYGCKAA-3′ and primer 2: 5′-TA NCCNGCNGCCATYTCRTA-3′ (according to the method of Eisenhardt et al. [2001]).
5′ rapid amplification of cDNA ends (5′RACE) was carried out according to the Ambion RLM RACE kit with the following gene-specific primers (GSPs): outer: 5′-ATGAGGACACCT AAAGCCCACC-3′, inner: 5′-GGGTGCCGCATAGTGTCCAG GTT-3′ in a nested approach at 55°C annealing temperature, cycling conditions as above.
3′RACE was carried out according to the method of Zhang and Frohman (1997). The GSPs used for the nested approach were outer: GAACCTGGACACTATGCGG and inner: CTG GTGGGCTTTAGGTGTC. PCR was carried out at 55°C for 35 cycles, 2-min extension. Fragments were cloned into an Invitrogen TA vector and sequenced by MWG Biotech.
Mass spectrometry
Active fractions from the purification procedure were precipitated (chloroform-methanol precipitation), re-suspended in 1× SDS buffer, and silver stained according to the method of Shevchenko et al. (1996). Mass spectrometry was carried out by Shimadzu Scientific Instruments using an Axima CFRplus (MALDI-TOF and MALDI-TOF-TOF) setup and analyzed using the MASCOT database (Perkins et al. 1999).
Phylogeny
Sequence alignments were produced with EBI’s ClustalW (Thompson et al. 1994). The accession numbers are AF238979, AF367428, AJ413218, BC046697, D10770, D23667, DQ084243.1, M63311, M63312, NM_002730, NM_002732, NM_008854, NM_011100, NM_174584, NM_174585, NM_182948, X16969, X57986, X63420, X63421, and XM_393285.3. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 3.1 (Kumar et al. 2004).
Single-trial conditioning protocol
Appetitive (food-reward) chemical classical conditioning of intact Lymnaea was carried out using established methods based on single-trial training, control, and handling protocols described in detail in previous publications (Alexander Jr. et al. 1984; Kemenes et al. 2002; Ribeiro et al. 2003; Fulton et al. 2005). The CS was amyl acetate (0.004% final concentration), and the US was sucrose (0.67% final concentration).
In-vitro PKA activity assay after single-trial classical conditioning with intact animals
Animals were trained or subjected to control treatments (handling, CS alone, US alone, CS/US unpaired) individually using a staggered experimental regime to allow each animal to be sacrificed at a precise time point (5 min, 30 min, 1 h, 6 h, or 24 h) after single-trial training (CS/US paired) or control treatments. Cerebral ganglia were quickly dissected (<30 sec) on ice and immediately homogenized in 100-μL micropipette tubes (Blaubrand) each containing 20 μL of frozen homogenization buffer (50 mM Tris-HCl, pH 7.7, 10 mM 2-mercaptoethanol, 1 mM EDTA, and 1 mM EGTA). All samples were stored in liquid N2 before they were subjected to a PKA assay previously used to determine in vivo-induced PKA activity in honeybees and Aplysia (Hildebrandt and Müller 1995a, b; Müller and Carew 1998; Fiala et al. 1999; Müller 2000).
Amnestic treatments and memory tests
To test the amnestic effect of inhibiting PKA after training, a batch of snails was conditioned with a single CS/US pairing and randomly divided into four groups. Snails in two of the four groups were injected with KT5720 (Alomone Laboratories), a potent PKA inhibitor, at 10 μM final concentration dissolved in DMSO (0.5% final concentration). Snails in the two remaining groups were injected with a mixture of saline and DMSO (vehicle, 0.5% final DMSO concentration) 10 min after conditioning. Snails from one of the KT5720 and vehicle-injected groups each were tested at 6 h while the remaining KT5720 and vehicle-injected snails were tested at 24 h post-training for their feeding responses to the CS.
The experiments were repeated with the use of two other PKA inhibitors, H89 (1 μM) and RpcAMPs (500 μM), to rule out possible nonspecific behavioral effects caused by KT5720 potentially inhibiting kinase enzymes other than PKA (Davies et al. 2000).
The above procedure was repeated in separate experiments with the only difference that the injection of drug or vehicle took place immediately after the single CS/US pairing (0 min post-training injection time point). In another separate experiment, vehicle or drug injection took place at 3 h, 6 h, or 24 h after the single CS/US pairing with memory tests conducted at 24 h or 42 h post-training.
In all the above experiments, handled snails were tested with the CS alongside the CS/US paired and vehicle- or drug-injected snails to obtain baseline CS feeding response levels.
After the CS tests, all snails were also tested with the sucrose US to assess their ability to produce the basic feeding motor pattern. All tests were carried out blind with the experimenter unaware of the treatment that each snail had undergone.
Acknowledgments
This work was supported by the Royal Society (G.K., U.M., and I.K., United Kingdom), the MRC (G.K., United Kingdom), the BBSRC (G.K., United Kingdom), and the Deutsche Forschungsgemeinschaft (U.M., Germany). M.M. was partly funded by a Graduate Teaching Assistantship from the School of Life Sciences, University of Sussex.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1088408.
References
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Alexander J., Audesirk T.E., Audesirk G.J. One-trial reward learning in the snail Lymnea stagnalis. J. Neurobiol. 1984;15:67–72. doi: 10.1002/neu.480150107. [DOI] [PubMed] [Google Scholar]
- Altfelder K., Müller U. Cyclic nucleotide-dependent protein kinases in the neural tissue of the honeybee Apis mellifera. Insect. Biochem. 1991;21:487–494. [Google Scholar]
- Barco A., Bailey C.H., Kandel E.R. Common molecular mechanisms in explicit and implicit memory. J. Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- Benjamin P.R., Staras K., Kemenes G. A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea. Learn. Mem. 2000;7:124–131. doi: 10.1101/lm.7.3.124. [DOI] [PubMed] [Google Scholar]
- Bernabeu R., Bevilaqua L., Ardenghi P., Bromberg E., Schmitz P., Bianchin M., Izquierdo I., Medina J.H. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio A., Martin K.C., Giustetto M., Zhu H., Chen M., Bartsch D., Bailey C.H., Kandel E.R. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Chain D.G., Casadio A., Schacher S., Hegde A.N., Valbrun M., Yamamoto N., Goldberg A.L., Bartsch D., Kandel E.R., Schwartz J.H. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. [DOI] [PubMed] [Google Scholar]
- Davies S.P., Reddy H., Caivano M., Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.L. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Annu. Rev. Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Eisenhardt D., Fiala A., Braun P., Rosenboom H., Kress H., Ebert P.R., Menzel R. Cloning of a catalytic subunit of cAMP-dependent protein kinase from the honeybee (Apis mellifera) and its localization in the brain. Insect Mol. Biol. 2001;10:173–181. doi: 10.1046/j.1365-2583.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Fiala A., Müller U., Menzel R. Reversible down-regulation of protein kinase A during olfactory learning using antisense technique impairs long-term memory formation in the honeybee, Apis mellifera. J. Neurosci. 1999;19:10125–10134. doi: 10.1523/JNEUROSCI.19-22-10125.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D., Kemenes I., Andrew R.J., Benjamin P.R. A single time-window for protein synthesis-dependent long-term memory formation after one-trial appetitive conditioning. Eur. J. Neurosci. 2005;21:1347–1358. doi: 10.1111/j.1460-9568.2005.03970.x. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H., Müller U. Octopamine mediates rapid stimulation of protein kinase A in the antennal lobe of honeybees. J. Neurobiol. 1995a;27:44–50. doi: 10.1002/neu.480270105. [DOI] [PubMed] [Google Scholar]
- Hildebrandt H., Müller U. PKA activity in the antennal lobe of honeybees is regulated by chemosensory stimulation in vivo. Brain Res. 1995b;679:281–288. doi: 10.1016/0006-8993(95)00246-m. [DOI] [PubMed] [Google Scholar]
- Hou J., Kuromi H., Fukasawa Y., Ueno K., Sakai T., Kidokoro Y. Repetitive exposures to nicotine induce a hyper-responsiveness via the cAMP/PKA/CREB signal pathway in Drosophila. J. Neurobiol. 2004;60:249–261. doi: 10.1002/neu.20021. [DOI] [PubMed] [Google Scholar]
- Hu J.Y., Meng X., Schacher S. Redistribution of syntaxin mRNA in neuronal cell bodies regulates protein expression and transport during synapse formation and long-term synaptic plasticity. J. Neurosci. 2003;23:1804–1815. doi: 10.1523/JNEUROSCI.23-05-01804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.A., Akamine P., Radzio-Andzelm E., Madhusudan M., Taylor S.S. Dynamics of cAMP-dependent protein kinase. Chem. Rev. 2001;101:2243–2270. doi: 10.1021/cr000226k. [DOI] [PubMed] [Google Scholar]
- Kellett E., Perry S.J., Santama N., Worster B.M., Benjamin P.R., Burke J.F. Myomodulin gene of Lymnaea: Structure, expression, and analysis of neuropeptides. J. Neurosci. 1996;16:4949–4957. doi: 10.1523/JNEUROSCI.16-16-04949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes G. Molecular mechanisms of associative learning 4: Lymnaea. In: Byrne J.H., editor; Sweatt J.D., editor. Learning and Memory: A Comprehensive Reference. Molecular Mechanisms of Memory. Elsevier; Oxford, UK: 2008. pp. 133–148. Vol. 4. [Google Scholar]
- Kemenes G., Ribeiro M.J., Kemenes I., Staras K., Jones N.G., Benjamin P.R., Müller U. Appetitive learning in Lynmaea: Towards an understanding at the cellular and molecular level. International Congress of Neuroethology Abstracts. 2001;6:364. [Google Scholar]
- Kemenes G., Kemenes I., Michel M., Papp A., Müller U. Phase-dependent molecular requirements for memory reconsolidation: Differential roles for protein synthesis and protein kinase A activity. J. Neurosci. 2006;26:6298–6302. doi: 10.1523/JNEUROSCI.0890-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes I., Kemenes G., Andrew R.J., Benjamin P.R., O'Shea M. Critical time-window for NO-cGMP-dependent long-term memory formation after one-trial appetitive conditioning. J. Neurosci. 2002;22:1414–1425. doi: 10.1523/JNEUROSCI.22-04-01414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemenes I., Straub V.A., Nikitin E.S., Staras K., O'Shea M., Kemenes G., Benjamin P.R. Role of delayed nonsynaptic neuronal plasticity in long-term associative memory. Curr. Biol. 2006;16:1269–1279. doi: 10.1016/j.cub.2006.05.049. [DOI] [PubMed] [Google Scholar]
- Kemp B.E., Pearson R.B. Design and use of peptide substrates for protein kinases. Methods Enzymol. 1991;200:121–134. doi: 10.1016/0076-6879(91)00134-i. [DOI] [PubMed] [Google Scholar]
- Khabour O., Levenson J., Lyons L.C., Kategaya L.S., Chin J., Byrne J.H., Eskin A. Coregulation of glutamate uptake and long-term sensitization in Aplysia. J. Neurosci. 2004;24:8829–8837. doi: 10.1523/JNEUROSCI.2167-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Locatelli F., Romano A. Differential activity profile of cAMP-dependent protein kinase isoforms during long-term memory consolidation in the crab Chasmagnathus. Neurobiol. Learn. Mem. 2005;83:232–242. doi: 10.1016/j.nlm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Müller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Müller U., Carew T.J. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- Müller U., Hildebrandt H. Nitric oxide/cGMP-mediated protein kinase A activation in the antennal lobes plays an important role in appetitive reflex habituation in the honeybee. J. Neurosci. 2002;22:8739–8747. doi: 10.1523/JNEUROSCI.22-19-08739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin E.S., Kiss T., Staras K., O'Shea M., Benjamin P.R., Kemenes G. Persistent sodium current is a target for cAMP-induced neuronal plasticity in a state-setting modulatory interneuron. J. Neurophysiol. 2006;95:453–463. doi: 10.1152/jn.00785.2005. [DOI] [PubMed] [Google Scholar]
- Nikitin E.S., Vavoulis D.V., Kemenes I., Marra V., Pirger Zs., Michel M., Feng J., O’Shea M., Benjamin P.R., Kemenes G. Persistent sodium current is a non-synaptic substrate for long-term associative memory. Curr. Biol. 2008;18 doi: 10.1016/j.cub.2008.07.030. (in press) [DOI] [PubMed] [Google Scholar]
- Perkins D.N., Pappin D.J., Creasy D.M., Cottrell J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro M.J., Serfozo Z., Papp A., Kemenes I., O'Shea M., Yin J.C., Benjamin P.R., Kemenes G. Cyclic AMP response element-binding (CREB)-like proteins in a molluscan brain: Cellular localization and learning-induced phosphorylation. Eur. J. Neurosci. 2003;18:1223–1234. doi: 10.1046/j.1460-9568.2003.02856.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro M.J., Schofield M.G., Kemenes I., O'Shea M., Kemenes G., Benjamin P.R. Activation of MAPK is necessary for long-term memory consolidation following food-reward conditioning. Learn. Mem. 2005;12:538–545. doi: 10.1101/lm.8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A.C., Glanzman D.L. Learning in Aplysia: Looking at synaptic plasticity from both sides. Trends Neurosci. 2003;26:662–670. doi: 10.1016/j.tins.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Schacher S., Castellucci V.F., Kandel E.R. cAMP evokes long-term facilitation in Aplysia sensory neurons that requires new protein synthesis. Science. 1988;240:1667–1669. doi: 10.1126/science.2454509. [DOI] [PubMed] [Google Scholar]
- Scheiner R., Müller U., Heimburger S., Erber J. Activity of protein kinase A and gustatory responsiveness in the honey bee (Apis mellifera L.) J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003;189:427–434. doi: 10.1007/s00359-003-0419-x. [DOI] [PubMed] [Google Scholar]
- Schwärzel M., Jaeckel A., Mueller U. Signaling at A-kinase anchoring proteins organizes anesthesia-sensitive memory in Drosophila. J. Neurosci. 2007;27:1229–1233. doi: 10.1523/JNEUROSCI.4622-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher J.C., Weeber E.J., Varga A.W., Sweatt J.D., Swank M. Protein kinase signal transduction cascades in mammalian associative conditioning. Neuroscientist. 2002;8:122–131. doi: 10.1177/107385840200800208. [DOI] [PubMed] [Google Scholar]
- Sharma S.K., Carew T.J. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: Facilitatory effects and inhibitory constraints. Learn. Mem. 2004;11:373–378. doi: 10.1101/lm.81104. [DOI] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Silva A.J., Kogan J.H., Frankland P.W., Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Straub V.A., Styles B.J., Ireland J.S., O'Shea M., Benjamin P.R. Central localization of plasticity involved in appetitive conditioning in Lymnaea. Learn. Mem. 2004;11:787–793. doi: 10.1101/lm.77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Frohman M.A. Using rapid amplification of cDNA ends (RACE) to obtain full-length cDNAs. Methods Mol. Biol. 1997;69:61–87. doi: 10.1385/0-89603-383-x:61. [DOI] [PubMed] [Google Scholar]
- Zoller M.J., Kerlavage A.R., Taylor S.S. Structural comparisons of cAMP-dependent protein kinases I and II from porcine skeletal muscle. J. Biol. Chem. 1979;254:2408–2412. [PubMed] [Google Scholar]