Abstract
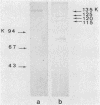
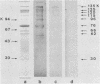
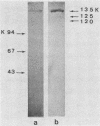
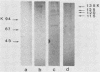
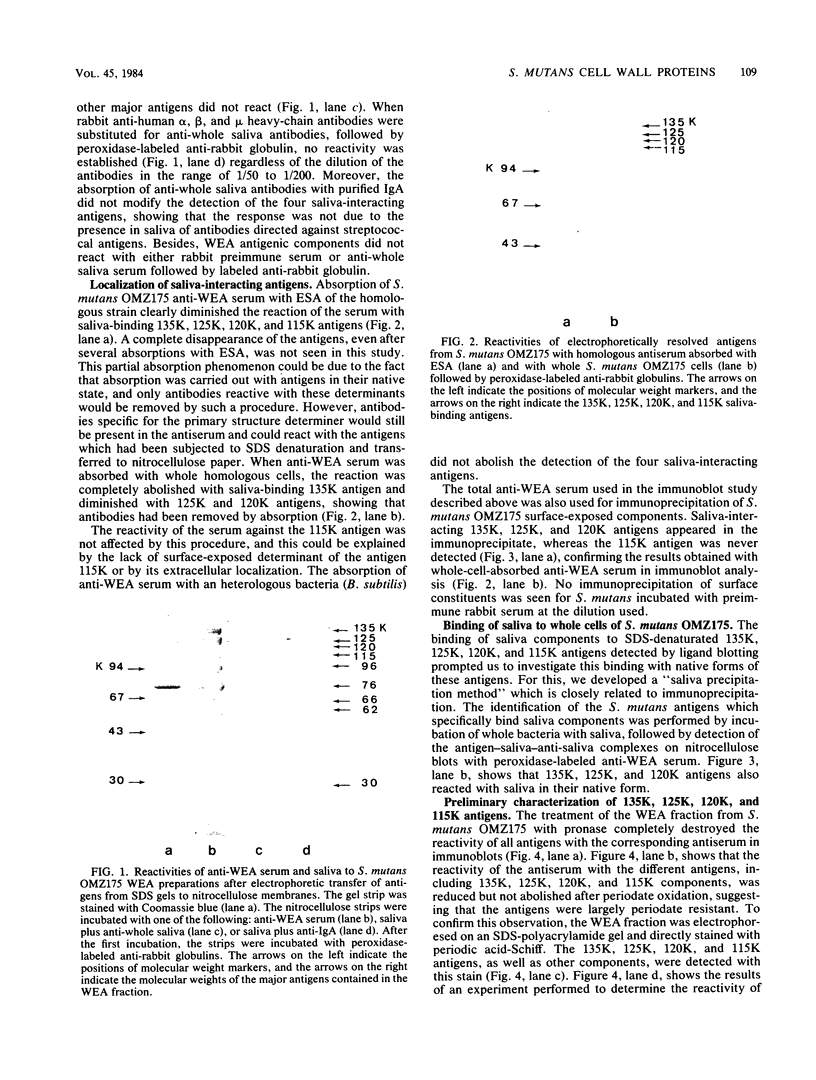
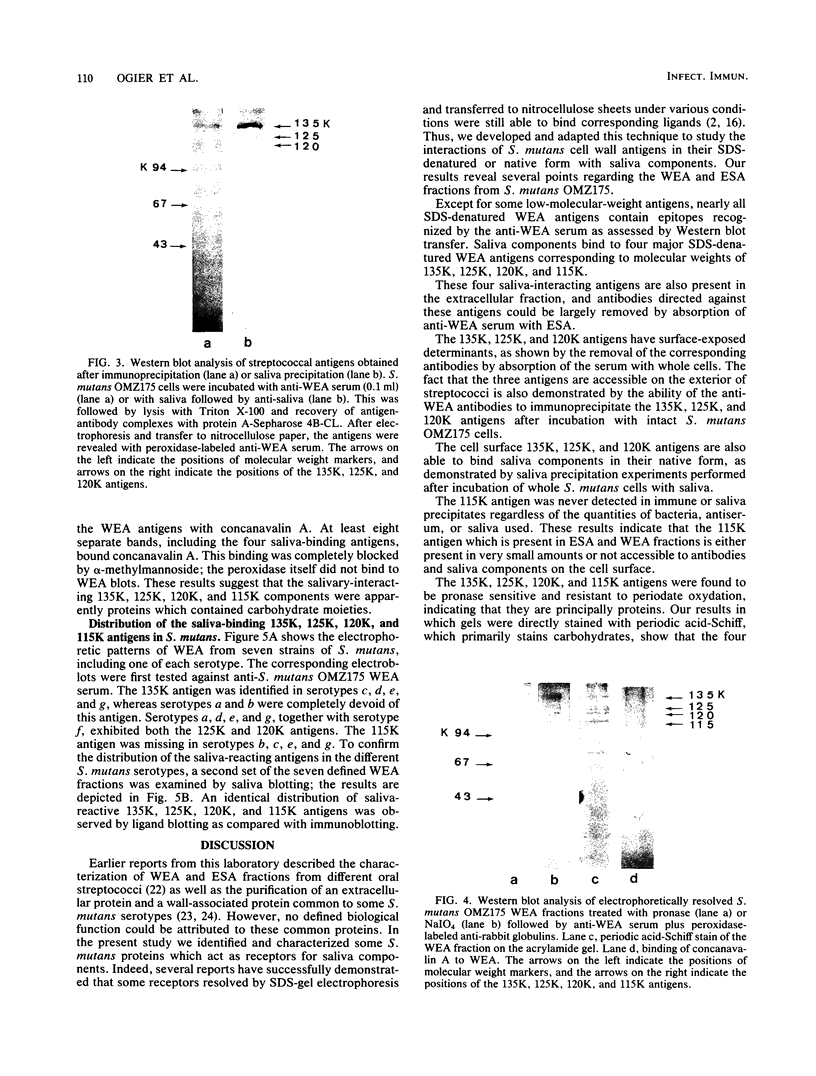
The ability of surface protein antigens of Streptococcus mutans to interact with salivary components was examined by Western blot and immunoprecipitation methods. Immunoblotting of S. mutans OMZ175 wall-associated antigens revealed 10 major antigens, designated according to their estimated molecular weights. Four of them, with molecular weights of 135,000, 125,000, 120,000, and 115,000 in their denaturated form, bound salivary components. This property was further investigated by immunoprecipitation experiments: the reactivity with saliva was confirmed for antigens with molecular weights of 135,000, 125,000, and 120,000 in their native form, and their locations on the bacterial cell surface were established. These three antigens were characterized as glycoproteins; they directly bound concanavalin A, and pronase abolished their antigenicity, which was partly retained after treatment with NaIO4. Because of their distribution in several other stains of S. mutans, it will be of interest to study their possible implication in the mechanism of attachment of streptococcal strains to saliva-coated tooth surfaces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clegg J. C. Glycoprotein detection in nitrocellulose transfers of electrophoretically separated protein mixtures using concanavalin A and peroxidase: application to arenavirus and flavivirus proteins. Anal Biochem. 1982 Dec;127(2):389–394. doi: 10.1016/0003-2697(82)90192-0. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Schneider W. J., Goldstein J. L., Brown M. S. Visualization of lipoprotein receptors by ligand blotting. J Biol Chem. 1983 Apr 10;258(7):4606–4611. [PubMed] [Google Scholar]
- Ericson T., Rundegren J. Characterization of a salivary agglutinin reacting with a serotype c strain of Streptococcus mutans. Eur J Biochem. 1983 Jun 15;133(2):255–261. doi: 10.1111/j.1432-1033.1983.tb07456.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Guggenheim B. Dextran-induced aggregation in a mutant of Streptococcus sobrinus 6715-13. Infect Immun. 1983 Jul;41(1):264–274. doi: 10.1128/iai.41.1.264-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Purification and immunochemical characterization of type e polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):68–76. doi: 10.1128/iai.14.1.68-76.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Adhesion of dextran to Streptococcus mutans. J Gen Microbiol. 1974 Apr;81(2):485–489. doi: 10.1099/00221287-81-2-485. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnusson I., Ericson T. Effect of salivary agglutinins of reactions between hydroxyapatite and a serotype c strain of Streptococcus mutans. Caries Res. 1976;10(4):273–286. doi: 10.1159/000260208. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M. Multiple forms of dextran-binding proteins from Streptococcus mutans. Adv Exp Med Biol. 1978;107:749–759. doi: 10.1007/978-1-4684-3369-2_84. [DOI] [PubMed] [Google Scholar]
- McGhee J. R., Michalek S. M. Immunobiology of dental caries: microbial aspects and local immunity. Annu Rev Microbiol. 1981;35:595–638. doi: 10.1146/annurev.mi.35.100181.003115. [DOI] [PubMed] [Google Scholar]
- Oblas B., Boyd N. D., Singer R. H. Analysis of receptor-ligand interactions using nitrocellulose gel transfer: application to Torpedo acetylcholine receptor and alpha-bungarotoxin. Anal Biochem. 1983 Apr 1;130(1):1–8. doi: 10.1016/0003-2697(83)90641-3. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Zanders E. D., Bergmeier L. A., Lehner T. Affinity purification and characterization of protease-susceptible antigen I of Streptococcus mutans. Infect Immun. 1980 Sep;29(3):999–1006. doi: 10.1128/iai.29.3.999-1006.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R. R., Peach S. L., Colman G., Cohen B. Antibody responses to antigens of Streptococcus mutans in monkeys (Macaca fascicularis) immunized against dental caries. J Gen Microbiol. 1983 Mar;129(3):865–875. doi: 10.1099/00221287-129-3-865. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Frank R. M. Common antigens of streptococcal and non-streptococcal oral bacteria: immunochemical studies of extracellular and cell-wall-associated antigens from Streptococcus sanguis, Streptococcus mutans, Lactobacillus salivarius, and Actinomyces viscosus. Infect Immun. 1981 Jan;31(1):52–60. doi: 10.1128/iai.31.1.52-60.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Sommer P., Frank R. Common antigens of streptococcal and non-streptococcal oral bacteria: isolation and biochemical characterization of the extracellular protein antigen. J Gen Microbiol. 1982 Sep;128(9):2113–2120. doi: 10.1099/00221287-128-9-2113. [DOI] [PubMed] [Google Scholar]
- Schöller M., Klein J. P., Sommer P., Frank R. Common antigens of streptococcal and nonstreptococcal oral bacteria: characterization of wall-associated protein and comparison with extracellular protein antigen. Infect Immun. 1983 Jun;40(3):1186–1191. doi: 10.1128/iai.40.3.1186-1191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suginaka H., Ebisu S., Kotani S. Mechanism of glucan-induced agglutination in Streptococcus mutans. I. Binding of radioactive glucan to whole cells of S. mutans OMZ-176. Microbiol Immunol. 1978;22(12):745–754. doi: 10.1111/j.1348-0421.1978.tb00428.x. [DOI] [PubMed] [Google Scholar]
- Swanson J. Surface-exposed protein antigens of the gonococcal outer membrane. Infect Immun. 1981 Dec;34(3):804–816. doi: 10.1128/iai.34.3.804-816.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]