Abstract
The purpose of this study was to investigate the short-term effects of rosuvastatin (RSV), a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, on transient, focal cerebral ischemia in C57BL/6J ob/ob mice with insulin resistance (IR). Male ob/ob, lean, or wild-type (WT) mice were treated with RSV (10 mg/kg per day, i.p.) or vehicle for 3 days. Ischemia was induced by 60 mins of middle cerebral artery occlusion (MCAO) and cortical blood flow (CBF) was monitored by laser-Doppler flowmetry. Infarct volumes were measured 24 h after reperfusion. IR mice exhibited a higher infarct volume compared with Lean or WT mice, and RSV reduced infarct volume only in obese mice (40%±3% versus 32%±3%, P < 0.05). Blood cholesterol and insulin levels were elevated in ob/ob mice but were unaffected by RSV. The CBF reductions during MCAO were similar in all groups and were not affected by RSV. Although RSV did not increase cortical endothelial NO synthase (eNOS) levels in the ob/ob mice, it attenuated the increased cortical expression of intracellular adhesion molecule-1 (ICAM-1) after MCAO from ob/ob mice. Thus, RSV protects against stroke in IR mice by a mechanism independent of effects on the lipid profile, CBF, or eNOS but dependent on suppression of post-MCAO ICAM-1 expression.
Keywords: middle cerebral artery occlusion, cerebral circulation, endothelial nitric oxide synthase, ICAM-1, statins, strokes
Introduction
Insulin resistance (IR), defined as a defect in the ability of insulin to stimulate glucose uptake, has become a major health problem throughout the world and is associated with an increased risk for stroke, Alzheimer’s disease, and perhaps other neurological diseases (Arenillas et al, 2007; Bravata et al, 2005). Insulin resistance can exist in the absence of diabetes, or it can be a prolonged, initiating event preceding the development of type II diabetes. Whichever is the case, IR can be present for many years before detection and treatment. However, the potential consequences of IR to the neurovascular unit, comprising cerebral blood vessels and neural tissues, during this period are poorly understood. We have shown previously, that cerebrovascular responsiveness is impaired in both genetic (Zucker obese rat) (Erdös et al, 2004, 2006) and nutritional (fructose-fed rat) (Erdös et al, 2002) models of IR. Cerebrovascular dysfunction to many, but not all, dilator stimuli occurred at both the endothelium and smooth muscle levels, and the mechanism of impaired responsiveness involved actions of augmented reactive oxygen species (ROS) through the nicotinamide adenine dinucleotide phosphate oxidase system (Erdös et al, 2006). Although it is probable that impaired cerebrovascular reactivity or other features of IR would lead to enhanced brain damage after experimental strokes, this possibility has never been tested. Previous studies with rodent models of diabetes have shown cerebrovascular dysfunction and/or altered patterns of neuronal damage after experimental strokes (Ergul et al, 2007; Rizk et al, 2006; Toung et al, 2000; Vannucci et al, 2001), but the physical status of the diabetic animals, including body weight and blood glucose levels, usually is more compromised than in IR rodents.
Several studies have indicated that statins, which are inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, the rate-limiting enzyme in cholesterol synthesis, could prove beneficial to the neurovascular unit in IR (Çakmak et al, 2007; Hess et al, 2000). These beneficial effects of statins might involve their cholesterol decreasing effects, or may include other pleiotrophic effects, which apparently do not involve effects on blood or tissue levels of cholesterol (Akdim et al, 2007; Cimino et al, 2007). For example, prolonged treatment with statins in healthy rats and mice has been shown to reduce infarct volume after middle cerebral artery occlusion (MCAO) by maintaining cerebrovascular function because of augmented vascular levels and activation of endothelial NO synthase (eNOS) (Asahi et al, 2005; Endres et al, 1998; Laufs et al, 2000, 2002). More recently, we have shown that statin treatment is beneficial to the cerebral circulation in Zucker obese rats not only after 4 weeks of treatment with rosuvastatin (RSV), but also after a shorter treatment regimen (Erdös et al, 2006). With this shorter period of RSV treatment, elevated blood cholesterol levels seen in Zucker obese rats were not affected, but cerebral vascular ROS values and cerebral vascular responsiveness were restored to normal. Whether similar short-term statin treatment could affect the severity of experimental strokes in IR animals has never been tested. In terms of human health care, short-term statin treatment could be beneficial when given before surgery in compromised patients. From a mechanistic perspective, this approach may be able to separate the cholesterol decreasing properties of statins from those involving other mechanisms, which protect the brain.
The purpose of this study was to examine the short-term effects of RSV on the severity of neurological injury of experimental strokes in IR (C57BL/6J, ob/ob) mice. We tested whether infarct volume was enhanced in ob/ob mice and whether a 3-day treatment with RSV can protect the brain from ischemia-reperfusion injury in these animals. In addition, we examined possible mechanisms of neuroprotection by RSV in ob/ob mice.
Materials and methods
Animals
Adult male 8-week-old IR mice (C57BL/6J, ob/ob) and lean (Lean) littermates were purchased from Harlan (Indianapolis, IN, USA). Wild-type (WT) male, control mice (C57BL/6J) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The ob/ob mice lack functional leptin and become obese and IR. The animals were housed in an air-conditioned environment with constant temperature and a standard light/dark schedule. All procedures were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals. The procedures were approved by the Animal Care and Use Committee of Wake Forest University Health Sciences.
Animals were treated with either vehicle (physiological saline) or RSV (10 mg/kg, i.p.) 72, 48, and 24 h before the induction of ischemia. This dose of RSV was chosen based on effectiveness determined in previous studies in mice (Laufs et al, 2002; Li et al, 2005). The 3-day pre-MCAO treatment regimen was chosen because this period of treatment is too short to reduce blood cholesterol levels, but sufficient for RSV to reach appropriate levels in the body and to have effects on the cerebral vasculature (Erdös et al, 2006). Furthermore, studies in our laboratory using cultured cortical neurons have shown that three, daily treatments provide optimal protection against anoxia (Gaspar et al, 2008a, b). Studies have indicated that RSV is cleared from the body within 24 h of administration (McTaggart et al, 2001).
Middle Cerebral Artery Occlusion
Mice were allowed free access to food and water before surgery. Anesthesia was induced with 4% isoflurane and maintained at 1.7% in a 70:30 gas mixture of N2O and O2 under spontaneous respiration. Transient focal brain ischemia was induced using the filament method as previously described, with some modifications (Mayanagi et al, 2007). In brief, the common carotid artery and the external carotid artery were dissected from the surrounding connective tissues via a ventral neck midline incision. A 7-0 monofilament nylon suture with its tip rounded by heating and then coated with silicon was introduced into internal carotid artery from the external carotid artery stump and advanced around 9 mm distal to the carotid bifurcation to occlude the MCA. After awakening from anesthesia, mice were kept in the cage during 60 mins of MCAO. The animals were then reanesthetized to remove the suture and to ligate the stump of the external carotid artery. After reperfusion, mice were kept in a cage and allowed free access to food and water for 24 h after reperfusion. During surgery, the temperature of the right temporal muscle was monitored and maintained in a range between 36.5°C and 37.0°C by a heating pad and heating lamp. After termination of anesthesia, when spontaneous movement was observed, the mice were kept under a heating lamp for 60 mins. For measurement of brain infarct volume, the animals were anesthetized with 5% isoflurane in O2 and decapitated. The brain was removed quickly and sliced into seven sequential, 1 mm thick, coronal sections. Each slice was immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride (Sigma, St Louis, MO, USA) for 20 mins and then fixed in a 10% formaldehyde solution. The infarct area in each brain slice was determined using a standard image analysis system (Scion Image) and the indirect method (Swanson et al, 1990). Infarct volumes were calculated by the summation of the infarct area of the seven brain slices integrated by the thickness and were expressed as the percentage of contralateral hemispheric volume (percent infarction).
Collection of Cortical Blood Flow and Physiological Data
In similarly treated groups of animals, but in which infarct volume was not determined, femoral artery catheterization was performed for arterial blood pressure measurement and blood sampling. The right temporal muscle was removed from the skull and a laser-Doppler probe was placed onto the skull over the perfusion area of the middle cerebral artery. All animals were subjected to the same anesthesia and surgical protocols. Arterial blood pressure and cortical blood flow (CBF) were monitored continuously throughout the procedure. After the CBF measurements, blood samples were withdrawn from the femoral artery catheter for the measurement of glucose, total cholesterol, and insulin levels, as described previously (Erdös et al, 2006).
Western Blotting Analysis
In a parallel group of animals, 24 h after the 3-day treatment with RSV or vehicle, animals were killed by cervical dislocation under deep anesthesia with isoflurane. After decapitation, brains were removed and frozen with liquid nitrogen. Cortical samples were first homogenized in five volumes of phosphate-buffered saline compared with tissue weight and protease inhibitors were added as mentioned above. Next, sodium dodecyl sulfate buffer (1% sodium dodecyl sulfate, 10 mmol/L Tris, pH 7.6) was added in the amount of the homogenate and sonicated. Equal amounts of protein for each sample were separated by 4% to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto a polyvinylidine difluoride membrane and blocked for 1 h at room temperature in Tris-buffered saline containing 5% skimmed milk powder and 0.1% Tween 20. Blots were incubated overnight at 4°C with a polyclonal anti-eNOS antibody (1:5,000; BD Biosciences, San Jose, CA, USA). The membranes were then washed three times in Tris-buffered saline containing 0.05% Tween 20 and incubated for 1 h in the blocking buffer with anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (1:50,000; Jackson Immuno-Research, West Grove, PA, USA). The bound antibodies were visualized using enhanced chemiluminescence (SuperSignal West Pico; Pierce, Rockford, IL, USA) and recorded on X-ray film. The bands were scanned and the densities of the bands were quantitated.
Real-Time Polymerase Chain Reaction
An additional subgroup of ob/ob mice were treated with either vehicle or RSV (10 mg/kg) for 3 days and exposed to 60 mins MCAO using the method just described. Twentyfour hours after reperfusion, animals were killed by cervical dislocation under deep anesthesia with isoflurane. After decapitation, brains were removed, divided into right (ipsilateral to MCAO) and left (nonischemic) cerebral hemispheres, and frozen with liquid nitrogen. Total cellular RNA was extracted from the cortical homogenates using an SVtotal RNA isolation system (Promega, Madison, WI, USA). Real-time reverse transcription-polymerase chain reaction was performed using an ABI Prism 7700 Sequence Detection System and Taqman probe and primer set for intracellular adhesion molecule-1 (ICAM-1) (Applied Biosystems, Foster City, CA, USA). Polymerase chain reaction products were detected by probes using reporter dye FAM (6-carboxy-fluorescein) at the 5′ end and using quencher dye TAMRA (6-carboxy-tetramethyl-rhodamine) at the 3′ end. Specificity of the polymerase chain reaction products was verified by agarose gel electrophoresis. The 2-ΔΔCt method was used to analyze the results. In brief, the Ct (threshold cycle) value of a gene was subtracted from the Ct value of the reference housekeeping gene (β-actin) to standardize the amounts of RNA template and efficiencies of reverse transcription. The resulting change in Ct values was then converted to a linear form using 2-ΔΔCt.
Statistical Analysis
All data are expressed as mean±s.e.m. One-way analysis of variance followed by Fisher’s least significant difference test was performed to assess statistical differences for infarct volumes and physiologic parameters except where noted in the text. A probability value < 0.05 was regarded as statistically significant.
Results
Physiological and Biochemical Characteristics
Resting values for blood glucose (Figure 1A), mean arterial blood pressure, arterial blood gases, and arterial blood pH (Table 1) were all within the normal range for all of the experimental groups, although arterial blood pressure and PaCO2 were modestly elevated in the ob/ob groups (Table 1). In contrast, blood insulin (Figure 1B) and cholesterol (Figure 1C) levels were substantially increased in ob/ob mice compared with WT or Lean littermates. Rosuvastatin treatment did not change any of the values reported in Figure 1 or Table 1.
Figure 1.
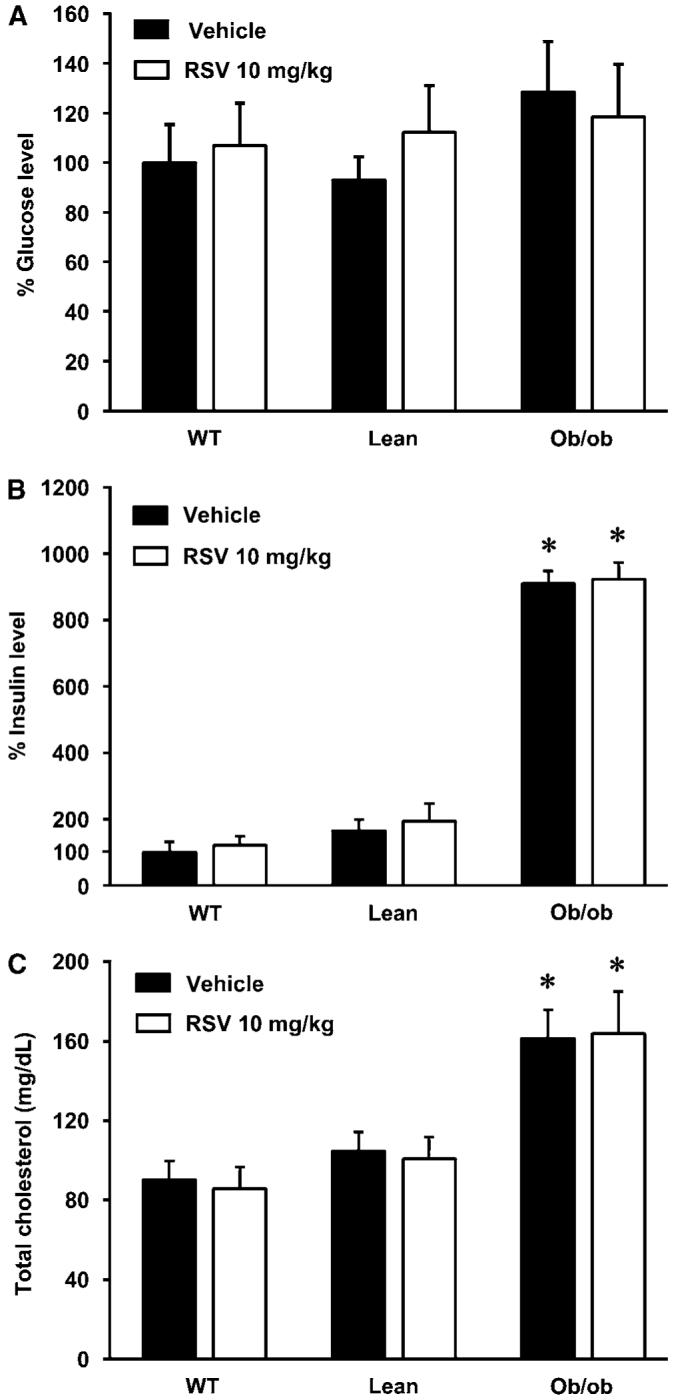
Effect of acute treatment with rosuvastatin (RSV) on glucose (A), insulin (B), and total cholesterol levels (C) in wild-type (WT), lean (Lean), and obese (ob/ob) mice (WT—vehicle: n = 5; WT—RSV n = 7; Lean—vehicle n = 7; Lean—RSV n = 5; ob/ob—vehicle n = 5; ob/ob—RSV n = 4). Ob/ob mice had significantly higher insulin and total cholesterol levels than Lean or WT mice. However RSV had no effect on these values in any animal group. The glucose and insulin levels were normalized to untreated WT mice values and expressed as 100%. Values are mean±s.e.m. *P < 0.05 ob/ob versus WT and Lean.
Table 1.
Physiological parameters of IR (ob/ob), lean, and wild-type (WT) mice treated with rosuvastatin (RSV) or vehicle
| MABP | pH | PaCO2 (mm Hg) | PaO2 (mm Hg) | |
|---|---|---|---|---|
| WT—vehicle | 82.4±3.7 (5) | 7.383±0.011 (4) | 35.0±0.8 (4) | 141.3±5.6 (4) |
| WT—RSV (10 mg/kg) | 86.1±3.8 (5) | 7.334±0.019 (6) | 35.0±1.1 (6) | 136.2±2.8 (6) |
| ob/ob—vehicle | 93.4±2.3*# (5) | 7.350±0.009 (4) | 42.0±2.7*# (4) | 126.3±5.3 (4) |
| ob/ob—RSV (10 mg/kg) | 91.5±3.3 (5) | 7.376±0.015 (5) | 40.6±2.7** (5) | 133.2±7.1 (5) |
| Lean—vehicle | 85.7±3.5 (5) | 7.328±0.023 (5) | 37.2±3.4 (5) | 137.0±5.0 (5) |
| Lean—RSV (10 mg/kg) | 82.3±4.0 (6) | 7.335±0.019 (6) | 37.0±2.2 (6) | 139.3±6.9 (6) |
MABP, mean arterial blood pressure.
Values are mean±s.e.m. Sample sizes are in parentheses.
P < 0.05, compared with WT-vehicle
P < 0.05, compared with WT-RSV
P < 0.05, compared with Lean-RSV (MABP) or WT-RSV (PaCO2).
Infarct Volumes
In vehicle-treated animals, the infarct volumes were similar in the WT and Lean groups whereas the obese mice showed significantly larger infarcts. Rosuvastatin treatment did not reduce infarct volume in WT or Lean mice. However, a significant reduction of infarct volume was found in ob/ob mice treated with RSV (Figure 2). The character of the infarcted tissue varied among the different groups. The percentage of strokes that showed significant hemorrhages was greater in the ob/ob mice (10/11) compared with the WT (4/14) or Lean (2/11) groups (P < 0.05; Fisher’s exact test). Despite reducing infarct volume in the ob/ob mice, RSV treatment did not significantly affect the presence of hemorrhages in the infarcted tissue (9/13; P > 0.05; Fisher’s exact test).
Figure 2.

Effect of acute treatment with rosuvastatin (RSV) on infarction volume in mice (WT—vehicle: n = 14; WT—10 mg/kg RSV n = 8; Lean—vehicle n = 11; Lean—10 mg/kg RSV n = 11; ob/ob—vehicle n = 11; ob/ob—10 mg/kg RSV n = 13). Infarction volume in ob/ob mice was larger than that in WT and Lean mice. Three-day treatment with 10 mg/kg reduced the infarct volume in ob/ob mice. Values are mean±s.e.m. *P < 0.05 versus WT or Lean, **P < 0.05 versus ob/ob—vehicle.
Cortical Blood Flow Measurements
Cortical blood flow decreased during MCAO but recovered afterward and showed the same basic patterns in all three groups (Figure 3A (WT); Figure 3B (Lean); Figure 3C (ob/ob)). However, the reduction of CBF during MCAO tended to be slightly less severe in ob/ob mice compared with WT or Lean littermates (WT, 13±3% of baseline; Lean, 17±2% of baseline; ob/ob, 22±3% of baseline).
Figure 3.

Effects of rosuvastatin (RSV) on cortical blood flow (rCBF) during ischemia and reperfusion (Panel A represents WT—vehicle (n = 5) and WT—RSV (n = 6); Panel B represents Lean—vehicle (n = 5) and Lean—RSV (n = 6); Panel C represents ob/ob—vehicle (n = 6) and ob/ob—RSV (n = 6)). The reduction in CBF during middle cerebral artery occlusion (MCAO) in ob/ob mice was slightly smaller than that in WT or Lean mice. Treatment with RSV had no significant effect on CBF changes in any animal group. Values are mean±s.e.m. *P < 0.05 ob/ob versus WT or Lean.
Endothelial NO Synthase Determinations
The baseline eNOS protein levels tended to be higher in the cerebral hemisphere of ob/ob mice compared with lean mice, but the difference was not statistically significant (Figure 4). However, RSV treatment produced a significant 28% increase in Lean animals whereas it tended to decrease eNOS levels in obese mice.
Figure 4.

Effect of acute treatment with rosuvastatin (RSV; 10 mg/kg) on endothelial NO synthase (eNOS) protein expression (n = 6 in each experimental group). RSV induced eNOS overexpression in Lean but not in ob/ob mice. Values are mean±s.e.m. *P < 0.05 Lean—RSV treated versus Lean—vehicle treated.
ICAM-1 mRNA Levels
The expression of ICAM-1 markedly increased at 24 h after reperfusion in the MCAO hemisphere compared with the nonischemic, contralateral hemisphere in vehicle-treated ob/ob mice (Figure 5). However, RSV treatment reduced ICAM-1 expression in the MCAO cerebral hemisphere without affecting levels on the nonischemic side.
Figure 5.

Effects of rosuvastatin (RSV) on intracellular adhesion molecule-1 (ICAM-1) mRNA expression in middle cerebral artery occlusion (MCAO) and contralateral hemisphere of ob/ob mice 24 h after reperfusion (n = 8 in each experimental group). ICAM-mRNA was elevated in ischemic brain and this increase was significantly suppressed with RSV treatment. Values are mean±s.e.m. #P < 0.05 MCAO versus nonischemic, *P < 0.05 vehicle versus RSV treated.
Discussion
The major, new findings of our study are as follows: (1) The infarction volumes in ob/ob mice after transient MCAO were significantly larger than those in WT or Lean mice. Furthermore, brains of ob/ob mice also showed greater levels of hemorrhage in the infarcted tissue than those of other animals; (2) Short-term treatment with RSV (10 mg/kg per day for 3 days), which did not affect blood cholesterol levels, protected the brains of ob/ob mice against MCAO but did not reduce infarct volume in WT or Lean mice; (3) Cortical blood flow during and after MCAO showed the same basic patterns in all three groups; (4) Although RSV treatment increased eNOS levels in Lean animals, it did not affect eNOS levels in ob/ob mice; (5) The elevated expression of ICAM-1 after MCAO was reduced by RSV treatment in ob/ob mice. We suggest that one of the beneficial effects of short-term RSV treatment is the suppression of adhesion molecule expression after MCAO in ob/ob mice, rather than on CBF responses during MCAO or reperfusion, brain levels of eNOS, or blood cholesterol status.
The impact of IR on the degree of brain injury after experimental strokes without associated diabetes has not been previously described. Our novel finding is that MCAO leads to both quantitative and qualitative differences between IR and non-IR mice. First, infarct area in was enhanced in the IR mice. This finding is consistent with our previous study in heart showing that infarct area was augmented after coronary artery occlusion and reperfusion in Zucker obese rats (Katakam et al, 2007). Enhanced tissue and organ damage after ischemic events may be a general feature of IR and diabetes (Katakam et al, 2007; Rizk et al, 2006). Second, the incidence of pronounced hemorrhages was increased in the infarcted tissue of IR mice compared with non-IR mice. Ergul et al (2007) found that the incidence of brain hemorrhages after experimental strokes increased in a type II model of diabetes in rats, although the size of the infarcted area was reduced in the brains from diabetic animals. The reason for the greater susceptibility to vascular injury, expressed as hemorrhagic transformation, after MCAO in the ob/ob mice is unclear, but apparently is not due to the size of the infarct because the incidence of significant hemorrhages was still elevated after reduction in infarct volume with RSV treatment. Studies in our laboratory as well as other laboratories have showed that elements of the metabolic syndrome lead to extensive vascular dysfunction in the brain, including decreased vascular responsiveness to dilator stimuli and disruption of the blood-brain barrier (Erdös et al, 2002, 2004, 2006; Phillips et al, 2005). It is possible, however, that features such as altered morphology and vascular wall integrity of cerebral blood vessels could explain the greater propensity for hemorrhages in IR brains after MCAO.
Considerable evidence indicates that long-term treatment with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors is protective against strokes in people and lessens severity of experimental strokes in animals (Berger et al, 2008; Çakmak et al, 2007; Mazighi et al, 2007; Sironi et al, 2003). Although the cholesterol-decreasing abilities of statins are undoubtedly beneficial, recent studies have provided evidence of important cholesterol-independent or pleiotropic effect of statins on the cerebral vasculature and parenchyma (Akdim et al, 2007; Berger et al, 2008; Cimino et al, 2007). In our experiments, RSV treatment did not change arterial blood gases and pH, arterial blood pressure, and arterial blood levels of glucose, insulin, or cholesterol in any of the groups studied. The beneficial, pleiotropic effects of RSV could involve increased levels of eNOS and/or decreased inflammatory responses of cerebral blood vessels. In a previous study, we found that cerebral vascular dysfunction was associated with enhanced ROS generation in Zucker obese rats. In these IR rats, short-term treatment with RSV normalized both ROS levels and cerebrovascular dilator responses (Erdös et al, 2006). It is unlikely that neuroprotective effects of RSV are because of differences in CBF responses, as these were similar among groups studied during MCAO and reperfusion and were not affected by statin treatment. However, our method for measuring CBF is not a quantitative method and all that can be concluded is that perfusion decreased in all groups and returned toward normal during reperfusion. Nonetheless, we are unaware of studies which have shown that statin treatment affects absolute CBF in mice or that basal CBF values are different in lean or obese mice.
Many studies have shown that enhanced cerebral vascular levels of eNOS because of statin administration are directly correlated with development of protection against experimental strokes, probably by increasing dilator responses (Endres et al, 1998; Laufs et al, 2000, 2002). However, despite slightly elevated cortical eNOS levels, the ob/ob mice had augmented infarct volumes after MCAO compared with Lean control mice. Furthermore, in spite of a tendency for cortical eNOS levels to fall with RSV treatment in ob/ob mice, cortical infarct volume actually decreased. Similarly, we showed that cerebral vascular eNOS levels are higher in Zucker obese rats compared with non-IR Lean rats, and that short-term administration of RSV did not increase eNOS expression (Erdös et al, 2006). In contrast, the cortical eNOS levels increased with RSV administration in non-IR mice and infarct volume was not decreased. However, the increases of eNOS protein levels in studies showing protective effect of statin treatment are twofold or more in magnitude, suggesting that higher baseline eNOS values in the ob/ob mice and the RSV-induced increases in eNOS in Lean mice in our study were not enough to produce neuroprotective effects (Endres et al, 1998; Laufs et al, 2000, 2002). Kilic et al (2005) also has shown that beneficial effects of delayed administration of statins against experimental strokes in mice may be independent of eNOS effects. However, we cannot exclude a potential role for enhanced cerebral vascular eNOS in ob/ob and Lean mice after more prolonged statin administration.
Numerous studies have shown that inflammatory responses after ischemia-reperfusion injury are involved in the pathogenesis of ischemia-related neuronal damage (Feuerstein et al, 1997; Kim, 1996; Kilic et al, 2005; Kochanek and Hallenbeck, 1992). A series of intracellular events triggered during and after MCAO, including increased calcium release and ROS production, activate second messenger systems such as extracellular signal-regulated kinase (ERK) and promote the expression of proinflammatory proteins and adhesion molecules on the endothelial cell membrane. We and others have previously shown that ICAM-1 expression is correlated with disruption of the blood-brain barrier (Lenzsér et al, 2007) and enhanced infarct volume in rodents (Wang et al, 1994; Zhang et al, 1995), and that suppression of expression or actions of adhesion proteins lessens the severity of injury after MCAO without affecting CBF during ischemia and reperfusion (Vemuganti et al, 2004; Kanemoto et al, 2002). Increased expression of adhesion molecules such as ICAM-1 on endothelial cells interact with complementary surface receptors on neutrophils and allow these blood-borne cells to bind to the endothelium, cross the blood-brain barrier, enter the parenchyma, and initiate inflammatory responses (Schroeter et al, 1994). In ob/ob mice, ICAM-1 expression was enhanced after 24 h of reperfusion after MCAO, but reduced after short-term RSV treatment. These findings suggest that the ICAM-1-restricting actions of RSV could be one factor leading to attenuated damage after cardiovascular insults in obesity or IR. Our results are supported by other investigators who have shown that statins inhibit ICAM-1 expression after ischemia/reperfusion in brain in the neonatal rat (Miao et al, 2005; Carloni et al, 2006). Furthermore, Gueler et al (2002) has shown that a 3-day treatment with statins blunts enhanced ICAM-1 expression after ischemic injury to the kidney. The signaling link may involve activation of ERK 1/2 because Chung et al (2002) have shown a functional link between suppression of ERK 1/2 activation and inhibition of ICAM-1 expression in endothelial cells. Although the similarity of the effects on infarction volumes and on ICAM-1 mRNA expression strongly suggests that inhibition of ICAM-1 mRNA expression by RSV leads to the limitation of infarct volumes in ob/ob mice, we cannot rule out the involvement of other factors contributing to neuroprotection. For example, Kilic et al (2005) has shown that postischemic delivery of RSV reduces brain infarct volume in mice and that this protection is associated with reduced inducible NOS expression, suppression of ERK 1/2 activation, and decreased levels of activated caspase-3 in mice. Thus, reduction in ICAM-1 expression is one of several potentially beneficial effects of RSV against ischemic injury.
In contrast to our results with obese mice, short-term RSV treatment did not reduce brain infarct volume after MCAO in WT or Lean mice. Although the reason for this difference is unclear, we believe that the predominant effect of short-term RSV was to reverse the underlying cerebral vascular inflammation associated with IR and that longer treatment periods are necessary for the additional beneficial effects, which were observed previously in healthy rodents. Vascular inflammation is a common feature of IR (Cimino et al, 2007; Mason et al, 2004), and the elevated ROS levels in the cerebral arteries in our previous study on Zucker obese rats (Erdös et al, 2006) appear to be secondary to IR-induced inflammation. Similarly, the elevated eNOS levels seen in the obese mice, which were reduced by RSV, probably occur in response to cerebral vascular inflammation. Our findings in IR mice appear to be consistent with protective effects described previously in healthy rats when statins are given immediately after initiation of MCAO (Berger et al, 2008; Sironi et al, 2003). In this situation, rapid beneficial effects of statins may alleviate or prevent the cerebral vascular inflammation, which occurs after ischemia/reperfusion. Additional protective effects of statins, associated with factors such as increased eNOS levels, usually take several weeks of treatment to be effective against MCAO (Asahi et al, 2005).
Insulin resistance especially in obese individuals leads to enhanced incidence of stroke as well as increased morbidity and mortality after stroke. The underlying basis for the greater vulnerability in people with the metabolic syndrome and in experimental animals with IR, obesity, and/or diabetes is not fully understood. Nonetheless, our findings provide evidence that statin therapies can provide a rapid and sustained protection of the neurovascular unit in patients experiencing IR.
Acknowledgements
We thank Nancy Busija, MA, for editorial assistance.
This study was supported by NIH Grants HL-07731, HL-030260, and HL-065380, YF Wu Research and Education Fund, WFUSM Venture Fund, and KG Phillips Fund for the Prevention and Treatment of Heart Disease, WFUSM Interim Funding, and by the Hungarian Science Foundation (OTKA K 63401 and IN 69967).
References
- Akdim F, van Leuven SI, Kastelein JJ, Stroes ES. Pleiotropic effects of statins: stabilization of the vulnerable atherosclerotic plaque? Curr Pharm Des. 2007;13:1003–12. doi: 10.2174/138161207780487548. [DOI] [PubMed] [Google Scholar]
- Arenillas JF, Moro MA, Dávalos A. The metabolic syndrome and stroke: potential treatment approaches. Stroke. 2007;38:2196–203. doi: 10.1161/STROKEAHA.106.480004. [DOI] [PubMed] [Google Scholar]
- Asahi M, Huang Z, Thomas S, Yoshimura S, Sumii T, Mori T, Qiu J, Amin-Hanjani S, Huang PL, Liao JK, Lo EH, Moskowitz MA. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:722–9. doi: 10.1038/sj.jcbfm.9600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C, Xia F, Mauer MH, Schwab S. Neuroprotection by pravastatin in acute ischemic stroke in rats. Brain Res Rev. 2008;58:48–56. doi: 10.1016/j.brainresrev.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Wells CK, Kernan WN, Concato J, Brass LM, Gulanski BI. Association between impaired insulin sensitivity and stroke. Neuroepidemiology. 2005;25:69–74. doi: 10.1159/000086286. [DOI] [PubMed] [Google Scholar]
- Çakmak A, Yemişçi M, Köksoy C, Yazgan U, Dinçer D, Dalkara T. Statin pre-treatment protects brain against focal cerebral ischemia in diabetic mice. J Surg Res. 2007;138:254–8. doi: 10.1016/j.jss.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Carloni S, Mazzoni E, Cimino M, De Simoni MG, Perego C, Scopa C, Balduini W. Simvastatin reduces caspase-3 activation and inflammatory markers induced by hypoxia-ischemia in the newborn rat. Neurobiol Dis. 2006;21:119–26. doi: 10.1016/j.nbd.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Chung HK, Lee IK, Kang H, Suh JM, Kim H, Park KC, Kim DW, Kim YK, Ro HK, Shong M. Statin inhibits interferon-gamma-induced expression of intercellular adhesion molecule-1 (ICAM-1) in vascular endothelial and smooth muscle cells. Exp Mol Med. 2002;34:451–61. doi: 10.1038/emm.2002.63. [DOI] [PubMed] [Google Scholar]
- Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist. 2007;13:208–13. doi: 10.1177/1073858406297121. [DOI] [PubMed] [Google Scholar]
- Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1998;95:8880–5. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdös B, Miller AW, Busija DW. Impaired endothelium-mediated relaxation in isolated cerebral arteries from insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2002;282:H2060–5. doi: 10.1152/ajpheart.01124.2001. [DOI] [PubMed] [Google Scholar]
- Erdös B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes. 2004;53:1352–9. doi: 10.2337/diabetes.53.5.1352. [DOI] [PubMed] [Google Scholar]
- Erdös B, Tulbert C, Snipes JA, Busija DW. Rosuvastatin reduces NAD(P)H-oxidase-dependent superoxide anion production and improves vascular function in cerebral arteries of Zucker obese rats. Am J Physiol. 2006;290:H1264–70. doi: 10.1152/ajpheart.00804.2005. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann NY Acad Sci. 1997;825:179–93. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- Gaspar T, Katakam P, Snipes JA, Kis B, Domoki F, Bari F, Busija DW. Delayed neuronal preconditioning by NS1619 is independent of calcium activated potassium channels. J Neurochem. 2008a;105:1115–28. doi: 10.1111/j.1471-4159.2007.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar T, Snipes JA, Busija AR, Kis B, Domoki F, Bari F, Busija DW. ROS-independent preconditioning in neurons via activation of mitoKATP channels by BMS-191095. J Cereb Blood Flow Metab. 2008b;28:1090–103. doi: 10.1038/sj.jcbfm.9600611. [DOI] [PubMed] [Google Scholar]
- Gueler F, Rong S, Park JK, Fiebeler A, Menne J, Elger M, Mueller DN, Hampich F, Dechend R, Kunter U, Luft FC, Haller H. Postischemic acute renal failure is reduced by short-term statin treatment in a rat model. J Am Soc Nephrol. 2002;13:2288–98. doi: 10.1097/01.asn.0000026609.45827.3d. [DOI] [PubMed] [Google Scholar]
- Hess DC, Demchuk AM, Brass LM, Yatsu FM. HMG-CoA reductase inhibitors (statins): a promising approach to stroke prevention. Neurology. 2000;54:790–6. doi: 10.1212/wnl.54.4.790. [DOI] [PubMed] [Google Scholar]
- Kanemoto Y, Nakase H, Akita N, Sakaki T. Effects of anti-intercellular adhesion molecule-1 antibody on reperfusion injury induced by late reperfusion in the rat middle cerebral artery occlusion model. Neurosurgery. 2002;51:1034–41. doi: 10.1097/00006123-200210000-00033. [DOI] [PubMed] [Google Scholar]
- Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW. Myocardial preconditioning against ischemia-reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am J Physiol. 2007;292:R920–6. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- Kilic U, Bassetti CL, Kilic E, Xing H, Wang Z, Hermann DM. Post-ischemic delivery of the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor rosuvastatin protects against focal cerebral ischemia in mice via inhibition of extracellular-regulated kinase-1/2. Neuroscience. 2005;134:901–6. doi: 10.1016/j.neuroscience.2005.04.063. [DOI] [PubMed] [Google Scholar]
- Kim JS. Cytokines and adhesion molecules in stroke and related diseases. J Neurol Sci. 1996;137:69–78. doi: 10.1016/0022-510x(95)00338-3. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Hallenbeck JM. Polymorphonuclear leukocytes and monocytes/macrophages in the pathogenesis of cerebral ischemia and stroke. Stroke. 1992;23:1367–79. doi: 10.1161/01.str.23.9.1367. [DOI] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Dirnagl U, Bohm M, Nickenig G, Endres M. Rosuvastatin, a new HMG-CoA reductase inhibitor, upregulates endothelial nitric oxide synthase and protects from ischemic stroke in mice. Brain Res. 2002;942:23–30. doi: 10.1016/s0006-8993(02)02649-5. [DOI] [PubMed] [Google Scholar]
- Laufs U, Gertz K, Huang P, Nickenig G, Bohm M, Dirnagl U, Endres M. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31:2442–9. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- Lenzsér G, Kis B, Snipes JA, Gáspár T, Sándor P, Komjáti K, Szabó C, Busija DW. Contribution of poly (ADP-ribose) polymerase to postischemic blood-brain barrier damage in rats. J Cereb Blood Flow Metab. 2007;27:1318–26. doi: 10.1038/sj.jcbfm.9600437. [DOI] [PubMed] [Google Scholar]
- Li W, Asagami T, Matsushita H, Lee KH, Tsao PS. Rosuvastatin attenuates monocyte-endothelial cell interactions and vascular free radical production in hypercholesterolemic mice. J Pharmacol Exp Ther. 2005;313:557–62. doi: 10.1124/jpet.104.080002. [DOI] [PubMed] [Google Scholar]
- Mason RP, Walter MF, Jacob RF. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109:II34–41. doi: 10.1161/01.CIR.0000129503.62747.03. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Gaspar T, Katakam PV, Kis B, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:348–55. doi: 10.1038/sj.jcbfm.9600345. [DOI] [PubMed] [Google Scholar]
- Mazighi M, Lavallée PC, Labreuche J, Amarenco P. Statin therapy and stroke prevention: what was known, what is new and what is next? Curr Opin Lipidol. 2007;18:622–5. doi: 10.1097/MOL.0b013e3282f19ede. [DOI] [PubMed] [Google Scholar]
- McTaggart F, Buckett L, Davidson R, Holdgate G, McCormick A, Schneck D, Smith G, Warwick M. Preclinical and clinical pharmacology of Rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol. 2001;87:28B–32B. doi: 10.1016/s0002-9149(01)01454-0. [DOI] [PubMed] [Google Scholar]
- Miao H, Jiang L, Huang L. Effects of simvastatin on the expression of intercellular adhesion molecule-1 mRNA in neonatal brain with hypoxic-ischemic damage. J Nanosci Nanotechnol. 2005;5:1261–5. doi: 10.1166/jnn.2005.224. [DOI] [PubMed] [Google Scholar]
- Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol. 2005;288:R522–R530. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- Rizk NN, Rafols JA, Dunbar JC. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096:204–12. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Sironi L, Cimino M, Guerrini U, Calvio AM, Lodetti B, Asdente M, Balduini W, Paoletti R, Tremoli E. Treatment with statins after induction of focal ischemia in rats reduces the extent of brain damage. Arterioscler Thromb Vasc Biol. 2003;23:322–7. doi: 10.1161/01.atv.0000044458.23905.3b. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke. 2000;31:2701–6. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Willing LB, Goto S, Alkayed NJ, Brucklacher RM, Wood TL, Towfighi J, Hurn PD, Simpson IA. Experimental stroke in the female diabetic, db/db, mouse. J Cereb Blood Flow Metab. 2001;21:52–60. doi: 10.1097/00004647-200101000-00007. [DOI] [PubMed] [Google Scholar]
- Vemuganti R, Dempsey RJ, Bowen KK. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35:179–84. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- Wang X, Siren AL, Liu Y, Yue TL, Barone FC, Feuerstein GZ. Upregulation of intercellular adhesion molecule 1 (ICAM-1) on brain microvascular endothelial cells in rat ischemic cortex. Brain Res Mol Brain Res. 1994;26:61–68. doi: 10.1016/0169-328x(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Zhang R, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, Tang WX, Tsang W, Anderson DC, Manning AM. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682:182–8. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]


