Abstract
Purpose
Strabismus in human infants is linked strongly to nasotemporal asymmetries of smooth pursuit, but many features of this co-morbidity are unknown. The purpose of this study was to determine how the duration of early-onset strabismus (or timeliness of repair) affects the severity of pursuit asymmetries in a primate model.
Methods
Binocular image decorrelation was imposed on infant macaques by fitting them with prism goggles on day 1 of life. The goggles were removed after 3 weeks (n=2), 12 weeks (n=2) or 24 weeks (n=3), emulating surgical repair of strabismus in humans at 3, 12, and 24 months of age, respectively. Two control monkeys wore plano lenses. Several months after the goggles were removed, horizontal smooth pursuit was recorded using binocular search coils and a nasal-bias index (NBI) was calculated.
Results
Each animal in the 12- and 24-week groups developed a constant, alternating esotropic strabismus and a nasotemporal asymmetry of pursuit when viewing with either eye. Spatial vision was normal (no amblyopia). The 3-week duration monkeys were indistinguishable from control animals; they had normal eye alignment and symmetric pursuit. In the 12- and 24-week monkeys, the longer the duration of binocular decorrelation, the greater the pursuit asymmetry: for 15 deg/s target motion, the NBI in the 12-week and 24-week animals was 16 X and 22 X greater respectively, than that in the 3 week animals (ANOVA, p = 0.03).
Conclusions
Binocular decorrelation in primates during an early period of fusion development causes permanent smooth pursuit asymmetries when the duration exceeds the equivalent of 3 months in human. These findings support the conclusion that early correction of infantile strabismus promotes normal development of cerebral gaze pathways.
Keywords: visual development, ocular motor system, fusion, esotropia, visual cortex, brain repair
INTRODUCTION
Infantile (congenital) esotropia is a convergent misalignment of the visual axes with onset in the first six months of life (Costenbader, 1961, Taylor, 1972, von Noorden, 1988a). It represents over 90% of all strabismus occurring in infancy, and ~ 40% of all pediatric strabismus (Graham, Lorenz). In addition to subnormal fusion and stereopsis, children and adults with infantile esotropia exhibit a defect of conjugate gaze, evident as asymmetric smooth pursuit (or optokinetic nystagmus) (Tychsen et al., 1985, Tychsen and Lisberger, 1986a, Kommerell, 1987, Demer and von Noorden, 1988). The asymmetry is evident as a bias favoring nasalward target motion when viewing monocularly with either eye.
The appropriate age for surgical repair of infantile esotropia is controversial (Ing et al., 1966, Jampolsky, 1977, Parks, 1977). In North America, the average age of repair ranges from 10 – 18 months (von Noorden, 1996, Tychsen, 1999). Despite surgical repair at this age, deficits of binocular fusion persist permanently, including defective stereopsis, defective fusional vergence, and asymmetric pursuit (Tychsen et al., 1985, Tychsen and Lisberger, 1986a, Wright, 1996). Surgery before age 4–6 months (“early repair”) has been advocated because of an enhanced probability of restoring stereopsis (Ing et al., 1966, Taylor, 1972, Ing, 1981, Wright et al., 1994, Birch et al., 1995, Ing, 1995b, Birch et al., 2000a). However, little detailed information is known regarding improvement in motor functions.
Behavioural studies have shown that the postnatal development of binocular sensory and motor functions in normal infant monkeys closely parallel that of normal infant humans, but on a compressed time scale (i.e. one week of monkey development is equivalent to one month of human) (Atkinson, 1979, Atkinson and Braddick, 1981, Boothe et al., 1985, O'Dell and Boothe, 1997). Infant monkeys with strabismus display the same constellation of perceptual and ocular motor abnormalities found in strabismic humans, including defective stereopsis, abnormal vergence, and gaze asymmetries (Kiorpes et al., 1996, Tychsen and Boothe, 1996, Tychsen et al., 1996, Tychsen et al., 2000, Tychsen and Scott, 2003). Thus, strabismic monkeys are an appropriate animal model for study of the human disorder.
We reported preliminary results describing early vs. delayed repair of esotropia in infant monkeys, using a model of optically-induced strabismus (Wong et al., 2003). The “repair” (i.e. removal of prism goggles) was deliberately timed to mimic shorter (less than or equal to 3 months) vs. longer (12 to 24 months) durations of unrepaired esotropia in human infants. Early repair (shorter duration) was able to restore fusional vergence and stereopsis, whereas delayed repair (longer duration) caused permanent deficits. The purpose of the current study was to determine how the timing of repair influences the severity of pursuit asymmetries.
METHODS
Animals and Goggle-rearing Groups
Nine normal infant rhesus monkeys (Macaca mulatta, 7 male, 2 female) were used. They were born at the Yerkes Regional Primate Research Center in Atlanta, Georgia, and fitted with goggles on the first day of life. The fitting procedure, similar to the method described by Crawford (Crawford et al., 1996), was not stressful to the monkeys and did not require anaesthesia. Padded head straps held the goggle helmet firmly in place, preventing the infant from removing the apparatus. The lens holders unscrewed to allow thin plastic Fresnel prisms (Fresnel Prism & Lens, Eden Prairie, MN) to be inserted and secured in place before each eye. The animals were observed during bottle feedings and periodically in the primate nursery to ensure that the goggles remained clear and in proper position. Normal play and interactions with other infant monkeys were not affected noticeably by the goggles. Once daily, the goggle helmet was removed for cleaning and, if necessary, adjustment. During these brief periods the animal was placed in a dark enclosure to prevent normal binocular experience.
The goggles created a combined horizontal and vertical optical strabismus to prevent fusion. As listed in Table 1, the experimental animals viewed through an 11.4° base-in prism in the right eye and an 11.4° base-down prism in the left eye. The 2 control animals (WE and AY) wore the same goggle apparatus but with plano lenses in place of prisms. The experimental animals wore the prism goggles for durations of 3 weeks, 12 weeks, or 24 weeks, corresponding to durations of unrepaired strabismus in human of 3, 12, and 24 months respectively. Once the defined period of goggle-rearing ended, the monkeys were transported to Washington University in St. Louis, Missouri where they were trained to perform visual fixation tasks without goggles using fruit juice as a positive feedback reward (Foeller and Tychsen, 2002).
TABLE 1.
Characteristics of the 9 macaque monkeys used in the experiments.
| Animal / Sex / Age Testing | Rearing Conditions (RE prism; LE prism) | Eye Alignment | Latent Nystagmus | Pursuit/OKN Asymmetry | DVD | Visual Acuity SSVEP (cpd) | Refractive Error (SE) |
|---|---|---|---|---|---|---|---|
| CONTROL | |||||||
| WE / M / 1.5 yr | 3 weeks plano lens | Orthophoric | No | No | No | RE: 22.85 | +1.00 |
| (0°; 0°) | LE: 20.50 | +1.00 | |||||
| AY / M / 2 yr | 3 months plano lens | Orthophoric | No | No | No | RE: 18.09 | +1.75 |
| (0°; 0°) | LE: 16.17 | +1.75 | |||||
| 3 WK DURATION | |||||||
| TE / M / 1.5 yr | 3 weeks prism | Orthophoric | No | No | No | RE: 19.85 | +2.50 |
| (11.4° BI; 11.4° BD) | LE: 21.40 | +2.00 | |||||
| SY / M / 1.7 yr | 3 weeks prism | Orthophoric | No | No | No | RE: 17.95 | +1.75 |
| (11.4° BI; 11.4° BD) | LE: 22.80 | +1.50 | |||||
| 12 WK DURATION | |||||||
| YO / M / 2 yr | 3 months prism | ET: 16° | Yes | Yes | Yes | RE: 21.10 | +2.25 |
| (11.4° BI; 11.4° BD) | RHT: 5° | LE: 19.06 | +2.37 | ||||
| GO / M / 1.5 yr | 3 months prism | ET: 8° | Yes | Yes | Yes | RE: 8.25 | +0.75 |
| (11.4° BI; 11.4° BD) | LHT: 4° | LE: 10.64 | +1.25 | ||||
| 24 WK DURATION | |||||||
| HA / F / 2 yr | 6 months prism | ET: 15° | Yes | Yes | Yes | RE: 19.23 | +1.50 |
| (11.4° BI; 11.4° BD) | RHT: 4° | LE: 18.65 | +1.50 | ||||
| QN / F / 2 yr | 6 months prism | ET: 12° | Yes | Yes | Yes | RE: 23.28 | +2.00 |
| (11.4° BI; 11.4° BD) | LHT: 4° | LE: 24.01 | +2.25 | ||||
| EY / M / 1.5 yr | 6 months prism | ET: 12° | Yes | Yes | Yes | RE: 8.91 | −1.50 |
| (11.4° BI; 11.4° BD) | LE: 7.66 | −2.00 | |||||
BI = base-in Fresnel prism; BD = base-down Fresnel prism; RE = right eye; LE = left eye; ET = esotropia; HT = hypertropia; OKN = optokinetic nystagmus; DVD = dissociated vertical deviation; SSVEP = spatial sweep VEP; cpd = cycles per degree. SE = spherical equivalent; spherical +50% any astigmatic error.
Monocular visual acuity was measured using spatial sweep visually-evoked potentials (SSVEP) (Norcia and Tyler, 1985) without correction for refractive error. Attention to the grating stimulus display (viewing distance 1 meter) for testing of visual acuity was ensured by rewarding the animals (a bolus of juice) for fixating the center of the display. Cycloplegic refractions revealed a refractive error ≤ +3.00 spherical equivalent in each of the experimental and control animals. In the months before coil implantation, eye alignment was assessed using 35 mm photographs and video recordings (Hirschberg method) of each monkey ((Quick and Boothe, 1992). All procedures were performed in compliance with the ARVO resolution on the use of animals in research and were approved by the Washington University Animal Care and Use Committee.
Eye Movement Recording
Detailed descriptions of the surgical and recording methods have been published in previous reports; an abbreviated description is provided here (Foeller and Tychsen, 2002). Using deep general inhalation anaesthesia (supplemented by local infiltration and topical anaesthesia), scleral search coils were implanted in both eyes and a custom-built, polycarbonate head-restraint-device was attached to the skull.
Eye movements were recorded using standard magnetic search coil techniques (Robinson, 1963, Fuchs and Robinson, 1966). The monkey sat in a primate chair in the middle of field coils. The head restraint was locked to preclude head movement and the room was lit with dim background illumination. Eye position was calibrated at the start of each recording session by using a calibration coil and by having the animal maintain eye position within a 2° window of target position. The target was a laser spot subtending ~ 0.05° projected onto the back of a translucent screen located 50 cm in front of the animal. The calibration sequence was repeated separately for each eye.
Recordings of eye alignment were performed under conditions of binocular and alternate, monocular viewing. Monocular viewing was achieved by use of liquid-crystal shutter goggles which cycled from transparent to opaque (or the reverse) in 80 microseconds (0.08 msec) (Scott et al., 1999). Voltages proportional to horizontal and vertical eye position were digitized at 500 Hz. Eye velocity signals were obtained by passing the eye position signals through a Finite Impulse Response filter (DC to 90 Hz) and differentiated. Angular resolution of the system was about 0.05°. Experiments were controlled and the data were acquired and analyzed with the aid of a computer and interactive signal processing software (Spike2 for Macintosh, Cambridge Electronic Design, United Kingdom; and Igor Graphics, Wave Metrics, Lake Oswego, Oregon).
Visual Stimuli, Trial Design and Data Analysis
Horizontal smooth pursuit was recorded under conditions of monocular viewing using a modification of the “step-ramp” paradigm of Rashbass (Rashbass, 1961, Tychsen and Lisberger, 1986b). At the beginning of each trial, the animal fixated on the stationary spot at straight-ahead gaze. When the animal’s eye remained within a 2° window continuously for an interval of 2 to 5 seconds, the stationary spot disappeared and a second spot appeared, moving rightward or leftward at 15°/s or 30°/s. The moving spot started either from the point of fixation (zero eccentricity) or from one of four other initial positions along the horizontal meridian (5° and 10° to the right or to the left of zero eccentricity). The “step-ramp” approach allowed us to present target motion at a precise location on the retina as determined by the relative positions of the stationary and moving spots. When viewing with the right eye, leftward (negative) target velocities represented nasalward motion, and rightward (positive) target velocities temporalward motion in the visual field (for clarity, all plots in the Figures of the Results are shown as viewing with the right eye; the inverse was true when viewing with the left eye). To receive the juice reward, the monkey had to track the stimulus within the 2° window for a duration > 750 milliseconds. The onset, direction (left, right), and speed of the target were controlled by the computer program, which selected combinations of initial target position and direction in a pseudorandom fashion to preclude prediction.
Individual “step-ramp” trials were judged acceptable for analysis if the trial contained at least 50 milliseconds of smooth eye velocity after the onset of pursuit. If an accepted trial contained a saccade, the saccade was removed and replaced with a straight line using a cursor-controlled interactive computer program. In cases of the linear interpolation not fitting smoothly into the velocity trace, the trial was excluded from analysis. An average of >1000 trials were analyzed for each animal.
To assess pursuit performance, two parameters were calculated: (1) Pursuit gain, which is defined as the ratio of eye velocity to target velocity during steady-state pursuit (i.e. 200 milliseconds after pursuit onset). Nasalward pursuit gains for the right eye (i.e. target motion to the left) and the left eye (i.e. target motion to the right) are reported as the pooled means of both eyes, as are temporalward gains; (2) A nasal bias index (NBI), which is defined as (Sn−St) / (Sn+St), where Sn is nasalward and St is temporalward mean eye speed. A nasalward bias yields a positive NBI, a temporalward bias a negative NBI, and a symmetric response zero NBI.
Pursuit gains were analyzed separately by a 4 (between) × 2 (within) repeated measures ANOVA. The between-subjects factor was the duration of binocular image decorrelation (controls, 3, 12 and 24 weeks) and the within-subjects factor was target speed (15°/s and 30°/s). The specific effects of each factor were analyzed further using post-hoc Sidak tests, with significance defined as p < 0.05.
RESULTS
Visual Acuity and Eye Alignment
Table 1 summarizes the ocular findings for the 9 animals at the time of testing, 1.5 to 2 years after prism goggle rearing. The control and 3-week prism-duration monkeys had normal eye alignment. The 12- and 24-week duration monkeys all had constant esotropia and vertical deviations, with the largest magnitudes recorded in the 24-week animals. None of the strabismic monkeys had strabismic amblyopia (defined as an inter-ocular acuity difference ≥ 0.25 octave) (Birch and Hale, 1988). Grating visual acuity thresholds, as measured by SSVEP (Table 1), varied idiosyncratically from animal-to-animal, but inter-ocular acuity differences were comparable in experimental and control monkeys.
Pursuit Eye Position And Eye Velocity Asymmetries
Figure 1 illustrates the step-ramp paradigm that was used to elicit smooth pursuit, showing eye and target positions and velocities. For these trials, the eye started from the position of fixation (zero eccentricity). The target stepped to ± 5 deg eccentricity, moving at a ramp speed of 15°/s in either a nasalward or temporalward direction. The control monkey WE (Figure 1A) responded by initiating smooth eye acceleration ~ 80 ms after onset of target motion, with eye velocity approaching target velocity ~ 200 ms later. Eye velocity was equivalent (i.e. within ± 2 deg/s symmetric) for both the nasalward and the temporalward trial. In contrast, the 24-week, esotropic monkey QN (Figure 1B) showed a striking naso-temporal pursuit asymmetry. After latencies of ~ 100 ms, QN initiated pursuit, but the eye velocity response to nasalward target motion was substantially stronger than that to temporalward motion. The greater fixation (baseline) and pursuit eye velocity fluctuations of QN, as well as the serial “catch up” saccades for temporalward target motion, were typical of the esotropic animals.
Figure 1.
Individual trials of step-ramp smooth pursuit in (A) control monkey WE and (B) 24-week duration monkey QN, viewing with the right eye. The target stepped from 0 deg to 5 deg eccentricity, and maintained ramp motion at 15 deg/s, nasalward or temporalward. (A) In the control monkey, pursuit was robust in both nasalward and temporalward directions. (B) In the esotropic monkey, pursuit was asymmetric: strong nasalward but weak temporalward (with “catch up” compensatory saccades). Saccadic deflections excised from the velocity tracings using interpolation software in both monkeys (see Methods). Dashed lines = target position and speed, solid lines = eye position and speed. Downward = leftward/nasalward with respect to right eye. Upward = rightward/temporalward.
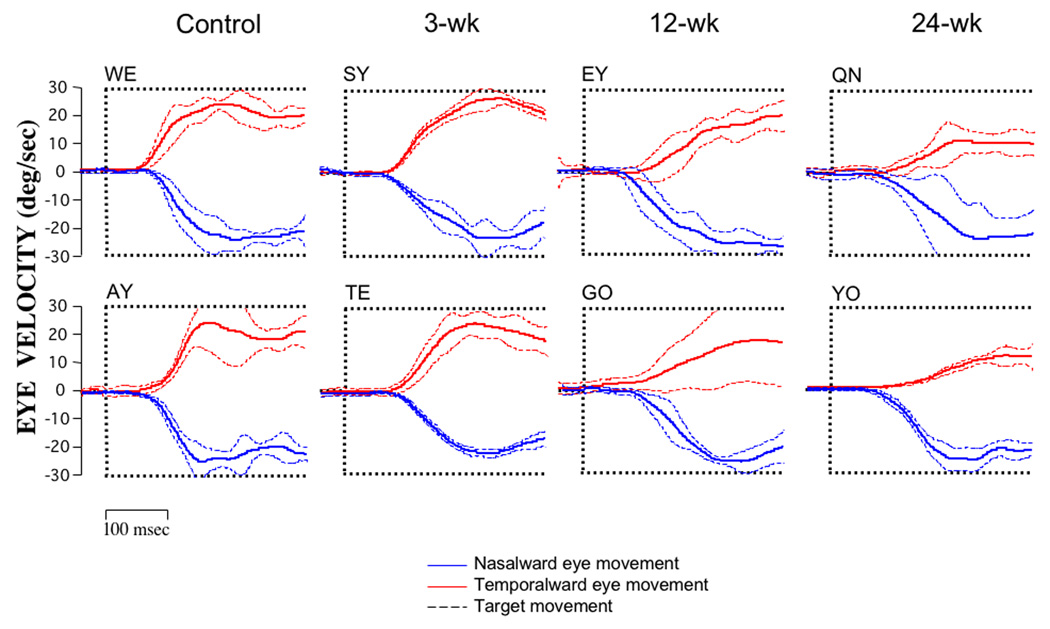
The four columns of Figure 2 show mean eye velocity profiles (± SD) in response to 30 deg/s target motion for two animals from each duration-group (n = 50 trials for each profile plotted). Although considerable inter-individual variability is apparent, a clear trend is evident: nasalward pursuit velocities exceeded temporalward velocities in each of the 12- and 24-week monkeys, with the weakest temporalward pursuit in the 24-week animals.
Figure 2.
Average pursuit eye velocity (± SD) for nasalward vs. temporalward 30 deg/s target motion in a total of 8 monkeys, 2 from each duration-group, viewing with the right eye. Nasalward (downward deflection) pursuit was robust in all monkeys. Temporalward (upward deflection) pursuit was weaker in the 12- and 24-week duration monkeys. Solid lines = mean eye velocity profiles; dashed lines = standard deviation. Generated from an average 50 trials in each monkey.
Pursuit Gain Asymmetries And Nasal Bias Index
Pursuit gains (eye velocity/target velocity) are shown for each duration-group in Figure 3. Large naso-temporal gain asymmetries were observed in both the 12- and 24-week monkeys (ANOVA, p = .04). Temporalward gains were 30% and 36% lower than nasalward gains for 15 deg/s motion; for 30 deg/s motion, temporalward gains were 20% and 32% lower, respectively. Nasalward gains in the 12- and 24-week monkeys were minimally higher than those in the control and 3-week animals. Nasal Bias Indices (Figure 4) were negligible or negative in the control and 3-week duration monkeys, but substantial and positive in both the 12- and 24-week animals. The NBI for 15 deg/s target motion was 16 X greater in the 12-week monkeys, and 22 X greater in the 24-week monkeys, than that in the 3-week animals. NBI differences were 3 X and 6 X, respectively, for 30 deg/s motion. Small differences (< 20%) in pursuit gains and NBI were apparent between individual animals within group and between viewing eyes within animal. For example, 12-week monkey GO had a 14% greater pursuit gain asymmetry than 12-week monkey EY. We did not find a consistent relationship between visual acuity (or the preferred eye, if any) and pursuit gain or NBI.
Figure 3.
Nasalward and temporalward pursuit gains for each duration group in response to target speeds of 15 and 30 deg/s. Nasalward vs. temporalward gains were comparable in control and 3-week monkeys, but nasalward gains exceeded temporalward gains in the 12- and 24-week animals. Gain = eye velocity/target velocity. Group means ± SD.
Figure 4.
Average nasal bias index (NBI) for each duration group in response to target speeds of 15°/s and 30°/s. NBI were negligible or negative in the control and 3-week monkeys, but positive and substantial in the 12- and 24-week monkeys.
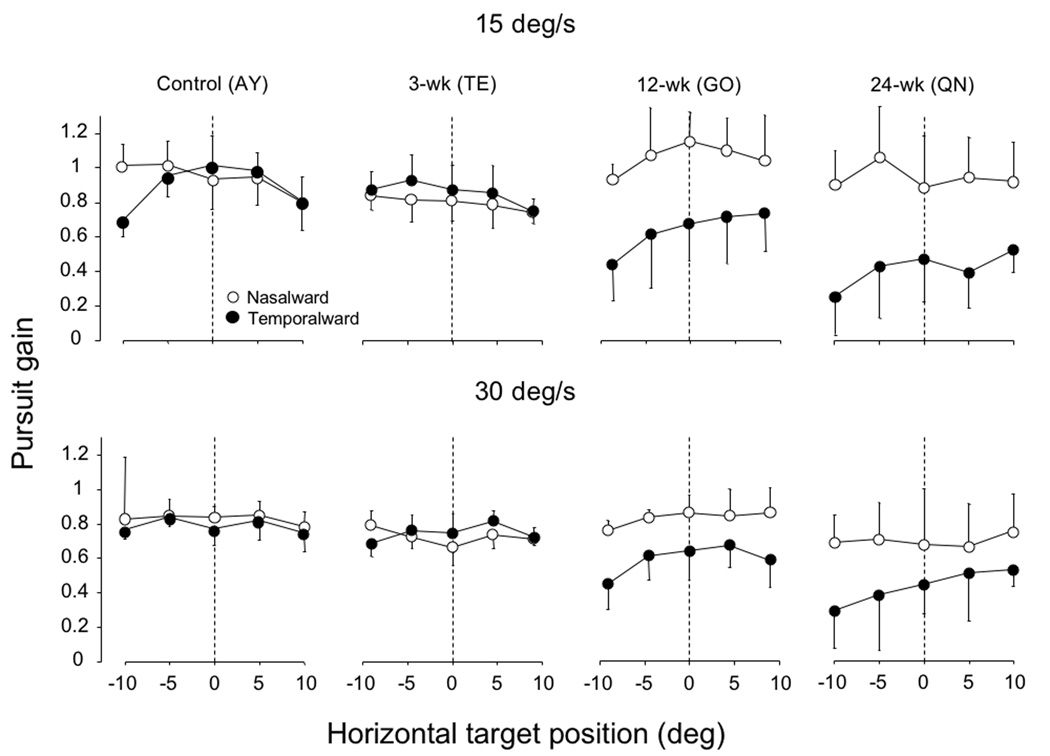
Pursuit asymmetries in humans with infantile esotropia (Tychsen and Lisberger, 1986a), and monkeys with esotropia induced by muscle sectioning in the first weeks of life (Kiorpes et al., 1996), have been shown to be: a) present for target starting positions at multiple eccentricities across the horizontal visual field, and b) slightly greater for eccentricities in the nasal hemi-field. To determine if our esotropic monkeys displayed similar findings, we measured gains in response to target starting positions in both the nasal (plotted as negative eccentricities) and temporal (positive eccentricities) hemi-fields (Figure 5). The asymmetries were apparent across both hemi-fields (for both 15 deg/s and 30 deg/s target speeds), but the greatest asymmetries tended to occur for target starting positions in the nasal hemi-field (negative eccentricities in the plots of Figure 5; p < .05 comparing −10 deg to +10 deg in 12-week monkey GO and 24-week monkey QN).
Figure 5.
Pursuit gains for target starting positions at multiple eccentricities across the horizontal visual field, nasalward vs. temporalward 15 and 30 deg/s motion. In the control and 3-week monkey, gains were symmetric. In the 12- and 24-week monkey, nasotemporal asymmetries were apparent across both hemi-fields, but the greatest asymmetries tended to occur for target starting positions in the nasal hemi-field. Nasal hemi field = negative eccentricities; temporal hemi field = positive eccentricities.
DISCUSSION
The major finding from this study is that binocular image decorrelation imposed early in life for a sufficient duration causes a permanent asymmetry of monocular smooth pursuit. The findings of the current report and previous reports from our laboratory (Wong et al., 2003, Tychsen et al., 2004a, Wong et al., 2005) also reinforce the utility of the macaque monkey as a model for exploring the critical period that dictates successful and unsuccessful correction of infantile strabismus in human. Each of the monkeys with the shortest duration of prism rearing (3 weeks) retained symmetric pursuit. The critical factor in development of normal, symmetric pursuit is therefore timely restoration of binocular image correlation for the development of fusion. In monkeys who had intermediate (12 weeks) and long (24 weeks) durations of prism rearing, a “dose-dependent” response was evident: the longer the duration of binocular image decorrelation, the more severe the directional deficit of monocular pursuit.Longer durations of binocular decorrelation were linked to both severe asymmetries of pursuit and larger angles of esotropia. A concordance between angle of esotropia and severity of pursuit asymmetry has been found in humans with infantile strabismus (Tychsen and Lisberger, 1986a), and monkeys with naturally-occuring esotropia (Tychsen et al., 2008). In general, larger angles of esotropia in monkey are associated with severe deficits of V1 binocular connections (Tychsen et al., 2004b, Tychsen, 2008), and metabolic suppression between ocular dominance columns of opposite ocularity (Tychsen and Burkhalter, 1997, Wong et al., 2005). These deficits within V1, early in the pursuit pathways of the visual cortex, may explain the severity of the pursuit asymmetry (see below Neural Mechanism for Pursuit Asymmetry).
Latent Fixation Nystagmus
Monkeys in the 12-week and 24-week duration groups developed not only nasotemporal asymmetries of pursuit, but also latent fixation (nasalward slow phase) nystagmus, a co-morbidity typically found in the infantile strabismus syndrome (Tychsen and Boothe, 1996, Tychsen, 1999). The smooth pursuit deficits found in these monkeys, however, cannot be attributed to algebraic summation of the slow phase eye velocity of latent fixation nystagmus superimposed on a normal, symmetric pursuit response. The velocity of the latent nystagmus was too small (0.5 to 1.5 deg/s) to account for the pursuit deficits; it merely provided a non-zero baseline against which we measured deficits in eye velocity during the step-ramp paradigm (Tychsen and Lisberger, 1986a).
Visual Acuity
Fresnel prisms were used in the experimental monkeys and plano lenses in the normal controls. In addition to misalignment of the visual axes (i.e. image decorrelation), Fresnel prisms can cause image blur and mild chromatic aberration (Rubin, 1977). The Fresnel prisms in our monkeys did not impair the normal development of spatial vision (visual acuity) and did not cause amblyopia. Acuity measured using SSVEPs were comparable in the experimental and control groups.
Grating visual acuity varied idiosyncratically from animal to animal and was not related to individual refractive errors (none of the animals used in our study had high ametropia). Strabismic monkeys YO 12-week, HA 24-week and QN 24-week (Table 1) had visual acuities equal to or better than those of both the control monkeys (WE; AY) and the 3-week monkeys (TE; SY). Strabismic monkeys GO 12-week, and EY 24-week, had lower visual acuities in both eyes. We do not believe the lower acuities in these 2 monkeys were do to a deprivation effect caused by prism wear. The lower bilateral acuities are comparable to those we have reported in several, non-prism-reared normal adult monkeys used in previous studies (Wong et al., 2005, Narasimhan et al., 2007). Table 1 and the figures of the results show also that we found no consistent relationship between visual acuity and the severity of a pursuit asymmetry.
The retention of normal acuity reinforces the value of the animal model we employed; the majority of human infants with esotropia alternate fixation between the eyes and do not have significant, strabismic amblyopia (Tychsen, 1999). Our results indicate that binocular image decorrelation alone, for a duration exceeding 3 weeks in infant primates, is sufficient to cause maldevelopment of the pursuit pathways.
Prism-Induced Strabismus And Binocular Correlation
Removal of the prisms allowed the 3-week monkeys to recover normal alignment. Removal of the prisms reduced the total magnitude of decorrelation (angle of physical esotropia + prismatic angle), but did not restore alignment in the 12- and 24-week animals. Because the prism magnitude was identical in all animals, removal of the prisms mimicked a strabismus surgery--of identical dosage in all subjects—performed after shorter or longer durations of strabismus. Early removal/shorter duration was effective for recovery of fusion, delayed removal/longer duration was not.
The animal model replicates in important ways the response of infantile strabismus in children to surgical correction. Infants with identical magnitudes of esotropia—and identical dosages of eye muscle surgery—may respond in significantly different ways, depending on the timeliness of the repair (Ing et al., 1966, von Noorden, 1988b, Wright et al., 1994, Ing, 1995a, Birch et al., 2000b). Infants who have surgery within 2 to 3 months of onset of a constant misalignment have a substantially greater chance of development of robust stereopsis and fusional vergence (Birch et al., 2000b, Fawcett and Birch, 2000). Repair delayed to age 12 to 18 months is more commonly associated with abnormal stereopsis and permanent eye movement deficits (Tychsen et al., 1985, Demer and von Noorden, 1988, Birch et al., 1990, Birch et al., 1995, Wright, 1996, Westall et al., 1998). Our findings in the infant macaque are similar, taking into account the compressed 1:4 time scale of macaque visual development compared with human. When restoration of binocular image correlation was achieved by age 3 weeks (the equivalent of 3 months in human), the monkeys developed normal, symmetric pursuit.
Neural Mechanisms for Pursuit Asymmetry
The nasalward asymmetry of pursuit in strabismic primates represents persistence of a directional bias encoded within the immature ocular motor system. Normal human and non-human primates have naslward biases of pursuit and OKN in early infancy, before onset of binocular fusion (Atkinson and Braddick, 1981, Naegele and Held, 1982, Schor et al., 1983, Hainline et al., 1984); (Demer and von Noorden, 1988, Mohn, 1989, Jacobs et al., 1994, Tychsen et al., 2001). The biases resolve and adult-like symmetry is achieved if development of fusion proceeds normally. Maldevelopment of fusion causes permanent asymmetry (Schor and Levi, 1980, Tychsen et al., 1985, Tychsen and Lisberger, 1986a, Demer and von Noorden, 1988, Kiorpes et al., 1996).
Visual areas V1, V2, MT, and MST of the cerebral cortex are major components of the pursuit pathway (Tusa and Ungerleider, 1988). Each of these areas in normal primates contains directionally-selective, binocular neurons (Poggio and Fischer, 1977, Albright et al., 1984, Orban et al., 1986, DeAngelis et al., 1996). MST in each cerebral hemisphere encodes only ipsiversive pursuit (Figure 6A) (Dürsteler and Wurtz 1988; Newsome et al., 1988; Komatsu 1988; Yamasaki and Wurtz 1991). MST in turn projects downstream to the brainstem visuomotor nuclei that generate eye movements: the nucleus of the optic tract (NOT), medial vestibular nucleus, and interconnected abducens and ocular motor nuclei (Tusa and Ungerleider, 1988, Mustari et al., 1994). In primates, subcortical inputs to NOT may play a minor role (Hoffmann and Distler, 1992, Tychsen, 1992, Tusa et al., 2002). But the dominant pathway – as shown in Figure 6 – is cerebral, from MST to brainstem (Tusa and Ungerleider, 1988). The dominant role of the cortical pathway, and the minimal role of a subcortical pathway, is reinforced by studies of children. Neuroimaging of visual cortex, combined with eye movement recordings, have shown absence of visually-driven pursuit or OKN in cerebrally-blind infants (Tychsen, 1996, Werth, 2007).
Figure 6.
Diagram of circuit mediating pursuit in primates and the role of binocular connections. Shaded structures indicate less active visual and motor neurons caused by occlusion of one eye and/or inter-ocular suppression.Strabismic: Flowing top to bottom, starting from monocular visual field of viewing right eye. Retinal ganglion cell fibres from the nasal and temporal hemi-retinae decussate at the optic chiasm (chi), synapse at the lateral geniculate nucleus (LGN), and project to alternating, monocular right eye (RE) and left eye (LE) ocular dominance columns (ODC) in V1. In each V1, ODCs representing the nasal hemi-retinae (temporal visual hemi-field) occupy slightly more cortical territory than those representing the temporal hemi-retinae (nasal hemi-field), but each ODC contains neurons sensitive to nasalward vs. temporalward motion (neurons = half circles matching corresponding hemi-field, arrows = directional preference). Visual area neurons (including those beyond V1 in area MT) are sensitive to both nasalward and temporalward motion, but only those encoding nasalward motion are wired innately, through monocular connections, to gaze neurons in area MST. MST in each cerebral hemisphere encodes ipsi-versive gaze, which is nasalward gaze with respect to the contralateral eye. Normal: Access to MST for temporalward gaze requires binocular connections to homo-versive neurons within neighbouring ODCs that have opposite-ocularity (i.e. LE ODC neurons when viewing with the RE). Binocular horizontal connections = diagonal lines between RE and LE neurons. Call = efferent projections through splenium of corpus callosum. MST efferents project to ipsilateral brainstem nucleus of optic tract (NOT) and ipsi-versive motor nuclei. See text for references.
One mechanism for the pursuit asymmetry would be over-representation of nasally-directed neurons within visual areas V1 through MT in the immature/strabismic cortex. However, directional and binocular responses of neurons in V1, V2 and MT have been investigated in infant monkeys, and monkeys with early-onset strabismus, and no overrepresentation of neurons selective for nasalward motion has been found {Kiorpes, 1996 #5309; Hatta, 1998 #6300; Watanabe, 2005 #7603}.
But in comparison to normal animals, binocular (excitatory) responses in these strabismic monkeys are reduced and inter-ocular suppression is increased (Endo et al., 2000, Watanabe et al., 2005). These physiological abnormalities have neuroanatomic correlates. In V1 of strabismic monkeys, binocular connections are deficient (Tychsen and Burkhalter, 1995, Tychsen et al., 2004b), and interocular metabolic activity is suppressed (Tychsen and Burkhalter, 1997, Horton et al., 1999, Wong et al., 2005). Therefore, a more plausible explanation linking asymmetric pursuit to defective binocularity is that the pursuit pathway of the visual cortex in each cerebral hemisphere is wired innately for nasalward pursuit (Figure 6B). The innate wiring is monocular. To generate temporalward pursuit, signals must traverse binocular connections, unimpeded by interocular suppression. If normal binocularity fails to develop, the system remains predominantly monocular and asymmetric, incapable of driving robust, temporalward pursuit (Tychsen et al., 1985, Tychsen and Lisberger, 1986a, Kiorpes et al., 1996, Tychsen, 1999).
Acknowledgments
This work was supported by Grant EY10214 (LT) from the NIH, A Walt and Lilly Disney Award for Amblyopia Research from Research to Prevent Blindness (LT), Grant MOP 67104 (AW) and a New Investigator Award (AW) from the Canadian Institutes of Health Research.
ABBREVIATIONS
- ANOVA
Analysis of variance
- ARVO
Association for Research in Vision and Ophthalmology
- cm
Centimeter
- DC
Direct current
- Hz
Hertz
- msec
Millisecond
- MST
Medial superior temporal
- MT
Medial temporal
- NBI
Nasal bias index
- NOT
Nucleus of optic tract
- OKN
Optokinetic nystagmus
- SSVEP
Spatial sweep visually-evoked potential
- V1
Visual area 1 (striate cortex)
- V2
Visual area 2 (pre-striate cortex)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albright TD, Desimone R, et al. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophys. 1984;51:16–31. doi: 10.1152/jn.1984.51.1.16. [DOI] [PubMed] [Google Scholar]
- Atkinson J. Development of optokinetic nystagmus in the human infant and monkey infant: an analogue to development in kittens. In: Freeman RD, editor. Developmental neurobiology of vision. New York: Plenum; 1979. pp. 277–287. [Google Scholar]
- Atkinson J, Braddick O. Development of optokinetic nystagmus in the human infant and monkey infant. In: Fisher DF, et al., editors. Eye movements: cognition and visual perception. Hillsdale, NJ: Erlbaum; 1981. pp. 53–64. [Google Scholar]
- Birch EE, Fawcett S, et al. Co-development of VEP motion response and binocular vision in normal infants and infantile esotropes. Invest Ophthalmol Vis Sci. 2000a;41:1719–1723. [PubMed] [Google Scholar]
- Birch EE, Fawcett S, et al. Why does early surgical alignment improve stereopsis outcomes in infantile esotropia? J AAPOS. 2000b;4:10–14. doi: 10.1016/s1091-8531(00)90005-3. [DOI] [PubMed] [Google Scholar]
- Birch EE, Hale LA. Criteria for monocular acuity deficit in infancy and early childhood. Invest Ophthalmol Vis Sci. 1988;29:636–643. [PubMed] [Google Scholar]
- Birch EE, Stager DR, et al. Prospective assessment of acuity and stereopsis in amblyopic infantile esotropes following early surgery. Invest Ophthalmol Vis Sci. 1990;31:758–765. [PubMed] [Google Scholar]
- Birch EE, Stager DR, et al. Random dot stereoacuity following surgical correction of infantile esotropia. J Pediatr Ophthalmol Strabismus. 1995;32:231–235. doi: 10.3928/0191-3913-19950701-07. [DOI] [PubMed] [Google Scholar]
- Boothe RG, Dobson V, et al. Postnatal development of vision in human and nonhuman primates. Ann Rev Neurosci. 1985;8:495–546. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- Costenbader FD. Infantile esotropia. Trans Am Ophthalmol Soc. 1961;59:397–429. [PMC free article] [PubMed] [Google Scholar]
- Crawford ML, Harwerth RS, et al. Loss of stereopsis in monkeys following prismatic binocular dissociation during infancy. Behav Brain Res. 1996;79:207–218. doi: 10.1016/0166-4328(95)00247-2. [DOI] [PubMed] [Google Scholar]
- DeAngelis GC, Groh JM, et al. Society for Neuroscience. Washington, DC: 1996. Organization of disparity selectivity in macaque area MT; p. 146. [Google Scholar]
- Demer JL, von Noorden GK. Optokinetic asymmetry in esotropia. J Pedriatr Ophthalmol Strabismus. 1988;25:286–292. doi: 10.3928/0191-3913-19881101-08. [DOI] [PubMed] [Google Scholar]
- Endo M, Kaas JH, et al. Binocular cross-orientation suppression in the primary visual cortex (V1) of infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2000;41:4022–4031. [PubMed] [Google Scholar]
- Fawcett SL, Birch EE. Motion VEPs, stereopsis, and bifoveal fusion in children with strabismus. Invest Ophthalmol Vis Sci. 2000;41:411–416. [PubMed] [Google Scholar]
- Foeller P, Tychsen L. Eye movement training and recording in alert macaque monkeys: 1. Operant visual conditioning 2. Magnetic search coil and head restraint surgical implantation 3. Calibration and recording. Strabismus. 2002;10:5–22. doi: 10.1076/stra.10.1.5.8154. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Graham PA. Epidemiology of strabismus. Br J Ophthalmol. 1974;58:224–231. doi: 10.1136/bjo.58.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainline L, Lemerise E, et al. Orientational asymmetries in small-field optokinetic nystagmust in human infants. Behioural Brain Res. 1984;13:217–230. doi: 10.1016/0166-4328(84)90164-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann K-P, Distler C. IV Symposium of the Bielschowsky Society for the Study of Strabismus, vol. 1, p 46 Eye Clinic Heidelberg of the Ruprecht-Karls University, Germany. Aeolus Press; 1992. The optokinetic reflex in macaque monkeys with congenital strabismus. [Google Scholar]
- Horton JC, Hocking DR, et al. Metabolic mapping of suppression scotomas in striate cortex of macaques with experimental strabismus. J Neurosci. 1999;19:7111–7129. doi: 10.1523/JNEUROSCI.19-16-07111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ing M, Costenbader FD, et al. Early surgery for congenital esotropia. Am J Ophthalmol. 1966;61:1419–1427. doi: 10.1016/0002-9394(66)90480-6. [DOI] [PubMed] [Google Scholar]
- Ing MR. Early surgical alignment for congenital esotropia. Trans Am Ophthalmol Soc. 1981;79:625–663. [PMC free article] [PubMed] [Google Scholar]
- Ing MR. Outcome study of surgical alignment before six months of age for congenital esotropia. Ophthalmology. 1995a;102:2041–2045. doi: 10.1016/s0161-6420(95)30756-7. [DOI] [PubMed] [Google Scholar]
- Ing MR. Surgical alignment prior to six months of age for congenital esotropia. Trans Am Ophthalmol Soc. 1995b;93:135–146. doi: 10.1016/s0002-9394(14)70552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M, Harris C, et al. The Development of Eye Movements in Infancy. In: Lennerstrand G, editor. Update on Strabismus and Pediatric Ophthalmology Proceedings of the Joint ISA and AAPO&S Meeting; June 19 to 23, 1994; Vancouver, Canada. Boca Raton: CRC Press; 1994. pp. 140–143. [Google Scholar]
- Jampolsky A. When should one operate for congenital strabismus? In: Brockhurst RJ, et al., editors. Controversy in Ophthalmology. Philadelphia: W. B. Saunders Co.; 1977. pp. 416–422. [Google Scholar]
- Kiorpes L, Walton PJ, et al. Effects of artificial early-onset strabismus on pursuit eye movements and on neuronal responses in area MT of macaque monkeys. J Neurosci. 1996;16:6537–6553. doi: 10.1523/JNEUROSCI.16-20-06537.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommerell G. The pathophysiology of infantile strabismus. Giessen, Germany: European Strabismological Association; 1987. [Google Scholar]
- Lorenz B. Genetics of isolated and syndromic strabismus: facts and perspectives. Strabismus. 2002;10:147–156. doi: 10.1076/stra.10.2.147.8133. [DOI] [PubMed] [Google Scholar]
- Mohn G. The development of binocular and monocular optokinetic nystagmus in human infants. Invest Ophthalmol Vis Sci Suppl. 1989;30:49. [Google Scholar]
- Mustari MJ, Fuchs AF, et al. Anatomical connections of the primate pretectal nucleus of the optic tract. J Comp Neurol. 1994;349:111–128. doi: 10.1002/cne.903490108. [DOI] [PubMed] [Google Scholar]
- Naegele JR, Held R. The postnatal development of monocular optokinetic nystagmus in infants. Vision Res. 1982;22:341–346. doi: 10.1016/0042-6989(82)90149-3. [DOI] [PubMed] [Google Scholar]
- Narasimhan A, Tychsen L, et al. Horizontal rectus muscle anatomy in naturally and artificially strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48:2576–2588. doi: 10.1167/iovs.06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcia AM, Tyler CW. Spatial frequency sweep VEP: visual acuity during the first year of life. Vision Res. 1985;25:1399–1408. doi: 10.1016/0042-6989(85)90217-2. [DOI] [PubMed] [Google Scholar]
- O'Dell C, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vision Res. 1997;37:2675–2684. doi: 10.1016/s0042-6989(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Orban GA, Kennedy H, et al. Velocity sensitivity and direction selectivity of neurons in area V1 and V2 of the monkey: influence of eccentricity. J Neurophys. 1986;56:462–480. doi: 10.1152/jn.1986.56.2.462. [DOI] [PubMed] [Google Scholar]
- Parks MM. Operate early for congenital strabismus. In: Brockhurst RJ, et al., editors. Controversy in Ophthalmology. Philadelphia: W. B. Saunders Co.; 1977. pp. 423–430. [Google Scholar]
- Poggio GF, Fischer B. Binocular interaction and depth sensitivity in striate and prestriate cortex of behaving rhesus monkey. J Neurophysiol. 1977;40:1392–1405. doi: 10.1152/jn.1977.40.6.1392. [DOI] [PubMed] [Google Scholar]
- Quick MW, Boothe RG. A photographic technique for measuring horizontal and vertical eye alignment throughout the field of gaze. Invest Ophthalmol Vis Sci. 1992;33:234–246. [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Rubin ML. Optics for Clinicians. Gainesville, Florida: TRIAD Scientific Publishers; 1977. [Google Scholar]
- Schor CM, Levi DM. Disturbances of small-field horizontal and vertical optokinetic nystagmus in amblyopia. Invest Ophthalmol Vis Sci. 1980;19:668–683. [PubMed] [Google Scholar]
- Schor CM, Narayan V, et al. Postnatal development of optokinetic after nystagmus in human infants. Vision Res. 1983;23:1643–1647. doi: 10.1016/0042-6989(83)90178-5. [DOI] [PubMed] [Google Scholar]
- Scott C, Gusdorf G, et al. Automated cover test for eye misalignment in awake monkeys using spectacle-mounted liquid crystal shutters. Binocul Vis Strabismus Q. 1999;15:59–66. [PubMed] [Google Scholar]
- Taylor DM. Is congenital esotropia functionally curable? Trans Am Ophthalmol Soc. 1972;70:529–576. [PMC free article] [PubMed] [Google Scholar]
- Tusa RJ, Mustari MJ, et al. Animal models for visual deprivation-induced strabismus and nystagmus. Ann NY Acad Sci. 2002;956:346–360. doi: 10.1111/j.1749-6632.2002.tb02833.x. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Ungerleider LG. Fiber pathway of cortical areas mediating smooth pursuit eye movements in monkeys. Ann Neurol. 1988;23:174. doi: 10.1002/ana.410230211. [DOI] [PubMed] [Google Scholar]
- Tychsen L. Binocular Vision. In: Hart WM, editor. Adler's Physiology of the Eye: Clinical Applications. St. Louis: CV Mosby; 1992. pp. 773–853. [Google Scholar]
- Tychsen L. Absence of subcortical pathway optokinetic eye movements in an infant with cortical blindness. Strabismus. 1996;4:11–14. doi: 10.3109/09273979609087732. [DOI] [PubMed] [Google Scholar]
- Tychsen L. Infantile esotropia: Current neurophysiologic concepts. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management. Philadephia: WB Saunders; 1999. pp. 117–138. [Google Scholar]
- Tychsen L. Causing and curing infantile eostropia in primates: the role of de-correlated binocular input. Tr Am Ophth Soc. 2008;105:1–30. [PMC free article] [PubMed] [Google Scholar]
- Tychsen L, Boothe RG. Latent fixation nystagmus and nasotemporal asymmetries of motion visually-evoked potentials in naturally-strabismic primate. J Pediatr Ophthalmol Strabismus. 1996;33:148–152. doi: 10.3928/0191-3913-19960501-05. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Burkhalter A. Neuroanatomic abnormalities of primary visual cortex in macaque monkeys with infantile esotropia: preliminary results. J Pediatr Ophthalmol Strabismus. 1995;32:323–328. doi: 10.3928/0191-3913-19950901-13. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Burkhalter A. Nasotemporal asymmetries in V1: ocular dominance columns of infant, adult, and strabismic macaque monkeys. J Comp Neurol. 1997;388:32–46. doi: 10.1002/(sici)1096-9861(19971110)388:1<32::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Hurtig RR, et al. Pursuit is impaired but the vestibulo-ocular reflex is normal in infantile strabismus. Arch Ophthalmol. 1985;103:536–539. doi: 10.1001/archopht.1985.01050040078022. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Leibole M, et al. Comparison of latent nystagmus and nasotemporal asymmetries of optokinetic nystagmus in adult humans and macaque monkeys who have infantile strabismus. Strabismus. 1996;4:171–177. doi: 10.3109/09273979609057145. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Lisberger SG. Maldevelopment of visual motion processing in humans who had strabismus with onset in infancy. J Neurosci. 1986a;6:2495–2508. doi: 10.1523/JNEUROSCI.06-09-02495.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tychsen L, Lisberger SG. Visual motion processing for the initiation of smooth-pursuit eye movements in humans. J Neurophysiol. 1986b;56:953–968. doi: 10.1152/jn.1986.56.4.953. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Richards M, et al. Spectrum of infantile esotropia in primates: behavior, brains and orbits. J. AAPOS. 2008 doi: 10.1016/j.jaapos.2007.11.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tychsen L, Scott C. Maldevelopment of convergence eye movements in macaque monkeys with small and large-angle infantile esotropia. Invest Ophthalmol Vis Sci. 2003;44:3358–3368. doi: 10.1167/iovs.02-0698. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Scott C, et al. Defective accommodative vergence in monkeys who have unrepaired infantile esotropia. Society for Neuroscience. 2001:404–423. [Google Scholar]
- Tychsen L, Scott C, et al. Early versus delayed repair of infantile strabismus in macaque monkeys: III. Effects on short-latency fusional vergence. Invest Ophthalmol Vis Sci. 2004a;45:2543. [Google Scholar]
- Tychsen L, Wong AMF, et al. Paucity of horizontal connections for binocular vision in V1 of naturally-strabismic macaques: cytochrome-oxidase compartment specificity. J Comp Neurol. 2004b;474:261–275. doi: 10.1002/cne.20113. [DOI] [PubMed] [Google Scholar]
- Tychsen L, Yildirim C, et al. Macaque monkey as an ocular motor and neuroanatomic model of human infantile strabismus. In: Lennerstrand G, Ygge J, editors. Advances in Strabismus Research: Basic and Clinical Aspects. vol. 78. London, U.K: Wenner-Gren International Series, Portland Press Ltd.; 2000. pp. 103–119. [Google Scholar]
- von Noorden GK. A reassessment of infantile esotropia. XLIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1988a;105:1–10. doi: 10.1016/0002-9394(88)90113-4. [DOI] [PubMed] [Google Scholar]
- von Noorden GK. Current concepts of infantile esotropia. XLIV Edward Jackson Memorial Lecture. Amer J Ophthalmol. 1988b;105:1–10. doi: 10.1016/0002-9394(88)90113-4. [DOI] [PubMed] [Google Scholar]
- von Noorden GK. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. St. Louis: CV Mosby; 1996. [Google Scholar]
- Watanabe I, Bi H, et al. Directional bias of neurons in V1 and V2 of strabismic monkeys: temporal-to-nasal asymmetry? Invest Ophthalmol Vis Sci. 2005;46:3899–3905. doi: 10.1167/iovs.05-0563. [DOI] [PubMed] [Google Scholar]
- Werth R. Residual visual functions after loss of both cerebral hemispheres in infancy. Invest Ophthalmol Vis Sci. 2007;48:3098–3106. doi: 10.1167/iovs.06-1141. [DOI] [PubMed] [Google Scholar]
- Westall CA, Eizenman M, et al. Cortical binocularity and monocular optokinetic asymmetry in early-onset esotropia. Invest Ophthalmol Vis Sci. 1998;39:1352–1360. [PubMed] [Google Scholar]
- Wong AMF, Burkhalter A, et al. Suppression of metabolic activity caused by infantile strabismus and strabismic amblyopia in striate visual cortex of macaque monkeys. J AAPOS. 2005;9:37–47. doi: 10.1016/j.jaapos.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Wong AMF, Foeller P, et al. Early versus delayed repair of infantile stabismus in macaque monkeys: I. Ocular motor effects. J AAPOS. 2003;7:200–209. doi: 10.1016/s1091-8531(03)00014-4. [DOI] [PubMed] [Google Scholar]
- Wright KW. Clinical optokinetic nystagmus asymmetry in treated esotropes. J Pediatr Ophthalmol & Strabismus. 1996;33:153–155. doi: 10.3928/0191-3913-19960501-06. [DOI] [PubMed] [Google Scholar]
- Wright KW, Edelman PM, et al. High-grade stereo acuity after early surgery for congenital esotropia. Arch Ophthalmol. 1994;112:913–919. doi: 10.1001/archopht.1994.01090190061022. [DOI] [PubMed] [Google Scholar]