Abstract
Ovarian hormones influence memory formation by eliciting changes in neural activity. The effects of various concentrations of progesterone (P4) on synaptic transmission and plasticity associated with long-term potentiation (LTP) and long-term depression (LTD) were studied using in vitro hippocampal slices. Extracellular studies show that the highest concentration of P4 tested (10−6 M) decreased the baseline synaptic transmission and magnitude of LTP, but did not affect LTD. Intracellular studies suggest the P4 effect to be mediated, at least in part, by GABAA activity. These results establish a general effect of P4 on synaptic transmission, multiple forms of synaptic plasticity, and a possible mechanism of P4 action in hippocampus.
Progestagens, natural or synthetic steroid hormones that produce effects similar to progesterone (P4), are used in hormone therapy to counteract estrogen-induced proliferation of the endometrium (Rodgers and Falcone 2008). Aside from their local action, progestagens act at different body sites, including central nervous system (CNS) structures such as the prefrontal cortex, hippocampus, amygdala, and hypothalamus. In the CNS, ovarian hormones are involved in the control of several physiological functions lying outside the realm of immediate regulation of reproduction, including neuroplasticity and neuroprotection (Singh 2006). Although the issue of ovarian hormone function in CNS has engendered much debate, many studies using both in vitro and in vivo animal models support a protective role for estrogen (Foy et al. 1999; Foy 2001; Suzuki et al. 2006; Singh et al. 2008). In contrast to the extensive studies regarding the physiological role of estrogen in non-reproductive function, including neuroplasticity and neuroprotection, the effects of P4 on nonreproductive function have yet to be fully investigated.
There has been very little work, and most of that contradictory, on the acute effects of P4 on synaptic transmission and plasticity. One study reported that P4 (10−5 M) had no effect on long-term potentiation (LTP) recorded in vitro from hippocampal (CA1) slices in adult rats, but no non-drug control was graphed to compare the experimental (P4) condition, and the experimental subjects were a combined group of gonadally intact male and female rats (Ito et al. 1999). Another study reported that P4 (10−8 M) significantly enhanced synaptic transmission in CA1, but following seizure-inducing tetanus, P4 decreased both field potential and population spike responses and decreased the duration of after-discharges (Edwards et al. 2000). In a study using whole-cell patch clamp of pyramidal neurons from slices of prelimbic cortex, it was reported that P4 (100 μM) had no effect on the frequency of spontaneous and miniature EPSCs, but inhibited dopamine-induced increases in spontaneous EPSCs (Feng et al. 2004). P4 dose-response functions were not performed in any of these studies. In terms of interactions between estradiol and P4, estradiol and P4 both enhanced a glutamate-mediated increase in intracellular calcium in primary cultures of disassociated hippocampal neurons, estradiol more than P4, but P4 reduced the estradiol enhancement to the level of P4 alone enhancement (Nilsen and Brinton 2002). In summary, the data support the hypothesis that progestagens and hormone therapy modulate certain properties of neural plasticity and LTP.
In this study, we evaluated several concentrations of P4 on basal synaptic transmission and two forms of synaptic plasticity: LTP and LTD. The results from these experiments will help to determine effective P4 concentrations to be used in subsequent investigations on P4 in in vitro brain investigations and its regulation of synaptic plasticity and memory.
There were a total of 39 young adult (3 mo old) female ovariectomized Sprague-Dawley rats (Harlan) used in these experiments. All procedures were performed in accordance with protocols approved by the University of Southern California Institutional Animal Care and Use Committee. All efforts were made to reduce the number of rats used. Rats were housed in plastic cages under a 12-h light/dark cycle, at a constant temperature of 25°C ± 1°C, with food and water available ad libitum.
After deep isoflurane anesthesia, each rat was decapitated, and the brain was rapidly removed and immersed for 3 min in a cooled artificial cerebrospinal fluid (aCSF). The brain was then blocked, and 400-μm-thick coronal hippocampal slices with surrounding cortical tissue were cut using a vibratome (Series 1000). Sections were then transferred to a holding chamber, where they remained submerged in oxygenated aCSF (room temperature) that consisted of 124 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1.3 mM MgSO4, 26 mM NaHCO3, 2.4 mM CaCl2, and 10 mM glucose.
After at least 1 h of equilibrium, hippocampal slices were transferred to a Haas-type interface recording chamber and perfused with aCSF at a rate of 1.5 to 2 mL/min at 35°C, with the surface of the slices exposed to warm, humidified 95% O2 to 5% CO2. For extracellular experiments, field excitatory post-synaptic potentials (fEPSPs) were recorded from stratum radiatum of CA1 using a glass pipette filled with aCSF (2–3 MΩ resistance) in response to orthodromic stimulation from a stimulating electrode (twisted nichrome wires, 50 μm), also positioned within the stratum radiatum. Pulses of 0.1 msec duration were delivered to the stimulating electrode every 30 sec. Evoked responses were amplified by an Axoclamp 2A DC amplifier, conditioned by a CyberAmp 320 (Axon Instruments), filtered at 6 kHz and digitized at 20 kHz. Data acquisition was controlled by Clampex 7.0 software (Axon Instruments). Input/output curves were generated using stimulus intensities from 20 to 150 μA in increments of 10 μA. During 30-sec intervals, baseline fEPSPs were evoked at 50% of the maximal fEPSP slope values recorded at 150 μA. After establishing a 15-min baseline period, P4 (Steraloids, Q2600-000) was introduced to the perfusion chamber by switching from control aCSF to P4-containing aCSF (10−9, 10−8, 10−7, and 10−6 M concentrations) for another 30 min (infusion period) before delivering high-frequency stimulation (HFS) or low-frequency stimulation (LFS). Control slices continued being perfused with vehicle aCSF, containing 0.01% ethanol. LTP was induced at baseline intensity using HFS consisting of two trains of 1 sec of 100-Hz stimulation separated by 20-sec intervals. Recording continued for 30 min after HFS. The LFS stimulation train for LTD induction consisted of 900 pulses delivered at 1 Hz for 15 min. HFS or LFS was delivered directly from baseline conditions in which slices were being perfused with either aCSF or P4.
For intracellular experiments, GABAA receptor-mediated currents were recorded in CA1 hippocampal neurons, via a discontinuous single electrode voltage clamp (dSEVC) mode of an Axoclamp 2A amplifier, with sharp intracellular electrodes filled with 2 M CsCl and 100 mM QX-314. After impaling a cell in current clamp mode, the amplifier was switched to discontinuous current clamp (DCC) to adjust optimal capacitance compensation and sampling rate. After switching to dSEVC mode, the gain was increased carefully with further sampling rate correction. This process was observed on a separate oscilloscope to avoid instability and overshooting. Usually it was possible to bring the sampling rate to 5–7 kHz. Currents were recorded with the low-pass filter set at 3 kHz and sampled at 10 kHz.
To estimate LTP and LTD values, fEPSP slopes are expressed as the percent of averaged fEPSP slope values recorded during the 10-min period before HFS or LFS, and compared with data following HFS or LFS induction protocols. Statistical significance between groups was evaluated by unpaired two-tailed t-tests and conducted on data recorded during the last 5 min of the baseline period and the last 5 min of the infusion period (continued aCSF or P4) to assess the effect of the drug on basal synaptic transmission. To estimate the magnitude of LTP and LTD, the data collected during the last 5 min of the infusion period were compared with data collected during the last 5 min of the HFS or LFS periods. Data are presented as mean ± SEM.
Extracellular experiments: P4 and baseline synaptic transmission
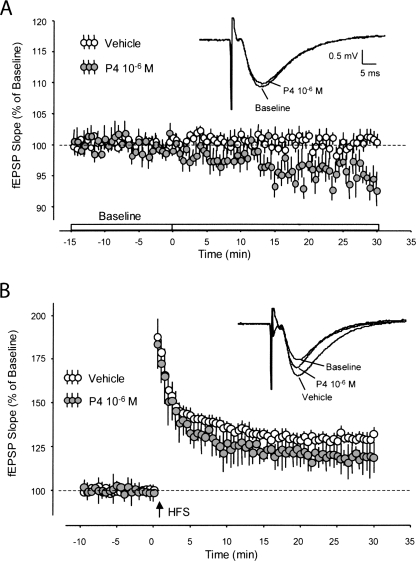
In the presence of the three lowest concentrations of P4 examined (10−9, 10−8, and 10−7 M), baseline fEPSPs evoked by Schaffer collateral-commissural stimulation were not found to be significantly different compared to slices perfused only with vehicle aCSF. Slices perfused with 10−9 M P4 were found to be 102% ± 2.7% above baseline values (P = 0.61, not significant [ns]); with 10−8 P4, 96% ± 1.7% below baseline values (P = 0.06, ns); and with 10−7 P4, 96% ± 1.7% below baseline values (P = 0.06, ns). However, at the highest concentration of P4 examined (10−6 M), baseline fEPSPs were found to be significantly attenuated compared to aCSF controls: 94% ± 1.7% (P < 0.01) (Fig. 1A).
Figure 1.
fEPSP baseline synaptic transmission and LTP with progesterone. (A) fEPSP baseline slope values (vehicle) were measured for 15 min prior to the infusion of P4 (10−6 M). P4 began to elicit a significant decrease in fEPSP during the next 30 min. fEPSP traces reflect five sweeps that were averaged immediately prior to the addition of P4, and the last five sweeps averaged at the end of the experiment, in the presence of P4. (B) fEPSP slope values (vehicle and P4) were measured prior to and following high-frequency stimulation (HFS) designed to induce long-term potentiation (LTP). Following HFS, slices perfused with P4 showed a significant decrease in fEPSP slope values compared to vehicle, resulting in decreased LTP. fEPSP traces reflect five sweeps that were normalized immediately prior to HFS, and the last five sweeps normalized at the end of the experiment from two different recording sessions (aCSF vehicle vs. P4).
Extracellular experiments: P4 and long-term potentiation
Consistent with the results above concerning dose-response experiments, when long-term potentiation was assessed following high-frequency stimulation following perfusion of P4, fEPSP slope values were significantly decreased in the P4-treated slices at the highest concentrations that were tested (10−7 and 10−6 M). At 10−7 M P4 following HFS, fEPSP slope values were 111% ± 3.4% (vehicle baseline values = 130% ± 3.6%), P < 0.007; at 10−6 M P4 following HFS, fEPSP slope values were 119% ± 3.4% (P < 0.05) compared to vehicle baseline values (130%). Hippocampal slices treated with P4 at the highest concentrations examined exhibited a pronounced, persisting, and significant decrease in LTP as measured by population fEPSP slope recordings (Fig. 1B).
Extracellular experiments: P4 and long-term depression
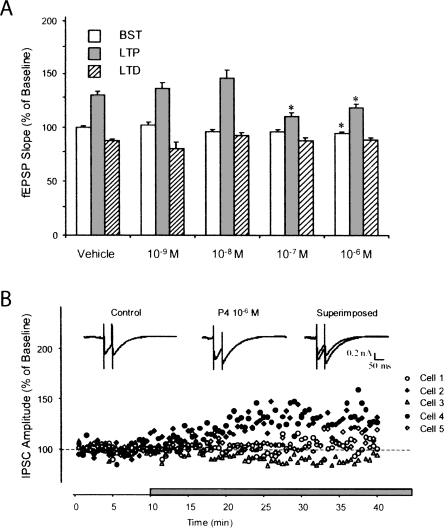
To assess long-term depression in hippocampal slices perfused with P4 at the four different concentrations used in the above experiments, there were no significant changes in LTD magnitude between any of the P4 groups studied compared to vehicle baseline control values. In contrast to its effect on LTP, the same concentrations of P4 did not affect LTD (Fig. 2A).
Figure 2.
Progesterone concentration, LTP, LTD, and IPSC recordings. (A) Vehicle and P4 at different concentrations (10−9, 10−8, 10−7, and 10−6 M) were compared with fEPSP values during the baseline synaptic transmission (BST), LTP, and LTD periods. At the highest concentration tested, P4 attenuated BST and LTP, but had no effect on LTD. (B) Using intracellular recordings of inhibitory post-synaptic currents (IPSCs), P4 at 10−6 M increased GABAA receptor-mediated current in two of five recorded cells. Representative current traces recorded before and during P4 application at 10−6 M.
Intracellular experiments: P4 and GABAA receptor-mediated currents
The attenuation of baseline synaptic transmission and decreased magnitude of LTP in hippocampal slices resulting from the application of the highest concentration of P4 examined (10−6 M) may be due to GABAA receptor activation (Mitchell et al. 2008), either by direct action of P4 or its metabolites. To test the effect of P4 on GABAA receptor-mediated currents of CA1 hippocampal pyramidal cells, we recorded from individual cells by using intracellular sharp electrodes in dSEVC mode in the presence of NMDA and non-NMDA glutamate receptor antagonists, 50 μM APV and 10 μM CNQX, respectively. Under these experimental conditions, the isolated currents were proven to be GABAA receptor-mediated since they were reversed at near 0 mV of holding potential and were completely blocked by the GABAA receptor antagonist picrotoxin (100 μM). In two of five cells that were recorded, P4 at 10−6 M increased the GABAA receptor-mediated current. Representative current tracesrecorded before and during P4 application are shown in Figure 2B.
The experiments reported here establish several fundamental characteristics of the effects of progesterone on synaptic transmission in the mammalian CNS. First, we demonstrate that P4 acts relatively quickly (∼30 min) to cause a decrease in baseline synaptic transmission. From the dose-response experiments reported in this study, the highest concentration of P4 tested (10−6 M) resulted in a decreased level of basal synaptic transmission that persisted for at least 45 min following initial P4 application.
Second, our results indicate that not only can P4 cause a decrease in synaptic transmission in the hippocampus, but also markedly decrease LTP in CA1 neurons from adult, ovariectomized rats. Furthermore, we found no evidence that P4 causes any change in synaptic activity following low-frequency stimulation used to induce long-term depression. In contrast to P4’s effect on LTP, the same concentrations of P4 did not affect LTD, suggesting that high-frequency stimulation could significantly increase inhibitory action of P4 on excitatory synaptic transmission in hippocampus.
Finally, our intracellular results suggest that the effects of P4 on hippocampal synaptic transmission may be mediated, at least in part, by activation of GABAA receptor-mediated activity. This is consistent with other studies that link GABAA with that of P4 (Herd et al. 2007).
In sum, we find that progesterone at concentrations greater than 10−7 M significantly decreases LTP, while having no effect on LTD. These findings suggest that P4 action is dependent on high-frequency stimulation and a compensatory recruitment of GABAA receptors, either by direct action of P4, or perhaps by its metabolites.
Acknowledgments
This work was supported by the National Institutes of Health Grant NIA P01 AG026572 (Roberta Diaz Brinton), Project 2 (R.F.T. and M.R.F.).
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1124708.
References
- Edwards H.E., Epps T., Carlen P.L., MacLusky N.J. Progestin receptors mediate progesterone suppression of epileptiform activity in tetanized hippocampal slices in vitro. Neuroscience. 2000;101:895–906. doi: 10.1016/s0306-4522(00)00439-5. [DOI] [PubMed] [Google Scholar]
- Feng X.Q., Dong Y., Fu Y.M., Zhu Y.H., Sun J.L., Wang Z., Sun F.Y., Zheng P. Progesterone inhibition of dopamine-induced increase in frequency of spontaneous excitatory postsynaptic currents in rat prelimbic cortical neurons. Neuropharmacology. 2004;46:211–222. doi: 10.1016/j.neuropharm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Foy M.R. 17β-Estradiol: Effect on CA1 hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 2001;76:239–252. doi: 10.1006/nlme.2001.4018. [DOI] [PubMed] [Google Scholar]
- Foy M.R., Xu J., Xie X., Brinton R.D., Thompson R.F., Berger T.W. 17β-Estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J. Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- Herd M.B., Belelli D., Lambert J.J. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol. Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Ito K., Skinkle K.L., Hicks T.P. Age-dependent, steroid-specific effects of oestrogen on long-term potentiation in rat hippocampal slices. J. Physiol. 1999;515:209–220. doi: 10.1111/j.1469-7793.1999.209ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E.A., Herd M.B., Gunn B.G., Lambert J.J., Belelli D. Neurosteroid modulation of GABAA receptors: Molecular determinants and significance in health and disease. Neurochem. Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Nilsen J., Brinton R.D. Impact of progestins on estrogen-induced neuroprotection: Synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Rodgers A.K., Falcone T. Treatment strategies for endometriosis. Expert Opin. Pharmacother. 2008;9:243–255. doi: 10.1517/14656566.9.2.243. [DOI] [PubMed] [Google Scholar]
- Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29:271–274. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- Singh M., Sumien N., Kyser C., Simpkins J.W. Estrogens and progesterone as neuroprotectants: What animal models teach us. Front. Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Brown C.M., Wise P.M. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]