Abstract
Endoscopic ultrasound (EUS) has been adopted into numerous interventional techniques and strategies that promise to improve diagnosis and management of gastrointestinal (GI) cancers. EUS-guided fine-needle aspiration (EUS-FNA) is recommended as a procedure of choice for tissue diagnosis of pancreatic cancer. Potential benefits of EUS-FNA in diagnosis of pancreatic cancer include the ability to detect small, discrete lesions compared with conventional imaging and the ability to provide staging information by examination of blood vessels surrounding the pancreas. EUS-FNA currently is being evaluated in strategies for improving diagnosis in pancreatic cancer through analysis of molecular markers, including strategies for distinguishing malignant pancreatic cysts. EUS-guided fineneedle injection currently is being investigated in a broad range of settings in GI cancers, including use in intratumoral injection in pancreas and esophageal cancers, ethanol lavage for nonmalignant pancreatic cystic tumors, and brachytherapy in nonresectable pancreatic cancer. Other applications of EUS currently being evaluated include EUS-guided biliary access in patients with unsuccessful endoscopic retrograde cholangiopancreatography and EUS-guided anastamoses in the GI tract. EUS-guided interventions have enormous potential to advance diagnosis and treatment of GI cancers.
“Do not go where the path may lead; go instead where there is no path and leave a trail”
- Ralph Waldo Emerson
In 2002, the American Joint Committee on Cancer referred to endoscopic ultrasound- guided fine needle aspiration (EUSFNA) as “the procedure of choice” for the tissue diagnosis of pancreatic cancer, since it provides information for clinical staging.1 Since then, interest in applying EUS-FNA (Figure 1) and other EUS techniques has only increased, with EUS-guided interventions forming the centerpiece of numerous novel strategies for improving diagnosis and management in gastrointestinal (GI) cancers. Indeed, EUS-FNA has bridged the divide between conventional sonographic imaging and interventional techniques, and EUS-guided interventions promise to have an important influence in at least three areas in GI oncology: molecular marker analysis, intratumoral injection, and EUS-guided biliary access and anastomosis. This paper reviews some of the emerging concepts in application of EUS-FNA and other interventional EUS techniques in GI oncology.
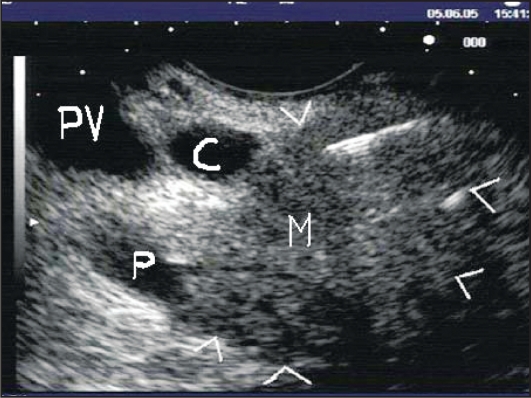
Figure 1.
Linear EUS image demonstrating transduodenal EUS-FNA of a pancreatic head mass using a 22-gauge needle. Note the needle tip within the mass (M) that is outlined by arrowheads. C: common bile duct. P: pancreatic duct. PV: portal vein.
EUS-FNA IN PANCREATIC CANCER
Traditionally, computed tomography (CT) scan-guided biopsy has been used to diagnose potentially unresectable pancreatic masses seen on imaging studies. CT scanguided biopsy, which has an accuracy of about 85%, can be successful in diagnosing pancreatic cancer in up to 95% of cases.2,3 In centers with available equipment and expertise, EUS-FNA also achieves accuracy rates of about 85%.4 Although sedation is required during its performance, the complication rate of EUS-FNA remains low at about 2%.5 In a recent randomized crossover trial6 comparing EUS-FNA with CT- or transabdominal ultrasound (US)-guided FNA among 84 patients suspected to have pancreatic cancer based on imaging tests, the sensitivity of EUS-FNA vs. CT/US-FNA was 84% vs. 62% (P = .074, chi square). CT/US-FNA had a higher false-negative rate compared with EUS-FNA. Interestingly, there were 6 patients with small, discrete lesions (mean size 23 ± 4.9 mm) missed by CT scan but identified by EUS-FNA; on the other hand, no malignancies were missed among the EUS-FNA-negative group that crossed over to CT/US-FNA. The study was underpowered due to failure to meet target enrollment. Nonetheless, it is reasonable to conclude that although EUS-FNA and percutaneous biopsy have similar overall accuracy rates, EUS-FNA has the advantage of being able to detect small, discrete pancreatic tumors that can be missed on CT scan. This benefit of EUS-FNA appears to be present even when compared with newer generation multidetector CT scans with multiplanar reconstruction.5
The preoperative imaging criteria for resectability of pancreatic cancer include absence of involvement of the celiac axis, hepatic artery, and superior mesenteric artery and demonstration of patency of the portal and superior mesenteric veins. EUS examination of blood vessels surrounding the pancreas can have an important role in the local staging of pancreatic cancer by avoiding unnecessary surgeries in a number of patients.7 However, CT and/or positron emission tomography (PET) scans are obviously still needed to rule out distant metastatic disease. Therefore, EUS should be viewed as a complementary modality along with other imaging tests for the staging of pancreatic cancer. EUS is also a helpful supplementary examination to CT scans when there is a strong suspicion of malignancy in a patient with equivocal findings and if a histologic diagnosis is required.
MOLECULAR MARKERS
Predicting the presence of malignancy in a pancreatic lesion seen on imagery has remained difficult, despite technological advances in CT scans, PET scans, and diagnostic EUS. Focal chronic pancreatitis in the head of the pancreas can mimic pancreatic cancer and present with jaundice and weight loss. The same is true for autoimmune pancreatitis, which is increasingly recognized as the “other” differential diagnosis for a pancreatic mass lesion.
When clinical suspicion for cancer is reasonably high, conventional pathologic examination obtained by nonsurgical means (EUS-FNA or CT-guided) cannot be totally relied upon when the result shows no cancer cells. A false-negative result, which occurs in at least 20% of cases identified by EUS-FNA,8–10 can lead to delay in management when a malignant lesion is missed or, in cases of high suspicion, can misdirect a patient with benign disease to pancreatic surgery. This diagnostic problem becomes more complicated when one takes into account the number of patients undergoing EUS-FNA who get categorized under “atypical cells, inconclusive for malignancy” or “inadequate sampling for diagnosis,” frustrating interventional endosonographers in cases in which the pretest probability of malignancy is high. Thus, we need to identify better ways to analyze tissue specimens obtained by EUS-FNA by improving accuracy and minimizing falsenegative or inconclusive findings due to inadequate sampling.
Molecular markers have been used to diagnose pancreatic cancer at an early stage. Trümper and colleagues11 studied 358 consecutive, nonselected patients who underwent endoscopic retrograde cholangiopancreatography (ERCP) with pancreatic and bile juice aspiration. Using surgical resection or imaging combined with clinical course as standard management, patients were classified as positive if either pancreatic or bile juice contained mutated K-ras genes. The sensitivity and specificity of polymerase chain reaction (PCR)—restriction fragment length polymorphism K-ras analysis for the detection of pancreatic cancer were 38.1% and 90.5%, respectively. Of note, a significant proportion of patients in this study had to be excluded from final analysis because of lack of amplifiable DNA (n=65), not enough material recovered for a second round of PCR (n=10), and availability of only duodenal secretions for assessment (n=40). A major cause of the poor test performance was difficulty in obtaining sufficient amounts of pancreatic juice for analysis, especially in cases of periampullary stenosis. The limited accuracy of PCR in this setting has lead to further investigation of more sensitive methods to diagnose pancreatic cancer at the molecular level, as well as a search for more efficient ways to obtain adequate specimens.
Microdissection-based genotyping is a technique available in a few centers that allows detailed DNA analysis of specimens obtained by EUS-FNA. Khalid et al12 retrospectively studied 11 patients with inconclusive or negative cytology and found that the mean fractional mutation rate (defined as the number of mutated markers divided by the total number of microsatellite markers plus 1—to account for K-ras-2 point mutation, which they also studied) was significantly different between benign and malignant groups (P < .0001). In another study, Nodit et al13 analyzed specimens obtained by EUS-FNA (22- or 25-gauge needle) from 25 patients with pancreatic endocrine tumors. Using a cutoff value of 0.2 or greater for fractional allele loss, there was a significant association with disease progression, providing important prognostic information. We hope to see larger studies that shed further light on the utility and cost-effectiveness of this promising diagnostic tool.
Pancreatic cysts are a common incidental finding now that CT scans and magnetic resonance imaging of the abdomen are routinely ordered in clinical practice. It is often difficult to predict whether a pancreatic cyst is malignant, premalignant, or benign by relying on imaging tests alone. In fact, even a negative cyst fluid cytologic analysis cannot rule out a malignant or premalignant pancreatic cyst. Khalid and colleagues14 analyzed pancreatic cyst fluid obtained by EUS-FNA and compared DNA quantification, K-ras point mutation, and broad panel tumor suppressor linked microsatellite marker allelic loss analysis with pathology. After studying 36 pancreatic cysts (11 malignant, 15 premalignant, and 10 benign), malignant cysts could be differentiated from premalignant cysts based on quality or amount of amplifiable DNA (P = .009), number of mutations (P = .002), and sequence of mutations acquired (P < .001). An initial K-ras mutation followed by allelic loss was most predictive of a malignant cyst (sensitivity 91%, specificity 93%). It is quite possible that clinical practice in the not too distant future will include sending out pancreatic cyst fluid not just for routine cytology and tumor markers, but for DNA analysis, as well.
Investigators have gone beyond investigating the operating characteristics of molecular markers and are looking into whether they can predict response to chemotherapy agents. Ashida et al15 used a gene scoring system based on the mRNA level extracted from tissue samples obtained by EUS-FNA from 11 patients with pancreatic cancer. They measured specific chemoresistant genes (factors DCD and FFM1 and gemcitabine-sensitive gene factor DCK) to predict non-response to gemcitabine. A score of +1 or more predicted gemcitabine efficacy with an accuracy of 91%. Although this method can help select chemotherapy agents in pancreatic cancer, we need further studies to identify which groups of patients should be tested and to determine how use of such a method will affect management.
EUS-GUIDED INTRATUMORAL INJECTION
The ability to target accessible organs through transesophageal, transgastric, or transduodenal approaches has enabled intratumoral injection of chemotherapy agents by EUS-guided fine needle injection (FNI) to be developed as an adjunctive treatment to systemic chemotherapy. Chang et al16 injected TNFerade™ into pancreatic tumors by EUS-guided FNI (n = 17) or percutaneous injection (n = 20). TNFerade is a replication-deficient adenovector containing the human tumor necrosis factoralpha gene that is regulated by the radiation- inducible promoter Egr-1. Weekly intratumoral injections (4 × 109 – 4 × 1011 particle units in 2 mL) of TNFerade were given for 5 weeks in combination with continuous infusion 5-fluorouracil (5-FU, 200 mg/m2/d × 5 days/wk) and radiation (50.4 Gy). Some signs of benefit in local control of disease and progression-free survival were seen during a short-term follow-up of 3 months.
The same group of investigators expanded their use of intratumoral injection of TNFerade into locally advanced esophageal cancer.17 In a multicenter, doseescalating trial, patients with resectable stage II or III disease on CT and EUS received TNFerade injections in 1-log increments via esophagogastroduodenoscopy (n = 18) or EUS (n = 6) once weekly every 5 weeks along with neoadjuvant chemoradiation with 5-FU plus cisplatin on days 1 and 29. In the top three dose cohorts, pathologic complete response was seen in 6 (40%) of 15 patients with resected tumors, and 10 (59%) of 17 remain disease free at follow-up of 13 to 25 months. The treatment was generally well tolerated; however, one case of pulmonary embolism was reported.
Another application of EUS-guided FNI may be in ethanol lavage of nonmalignant cystic tumors of the pancreas. This approach has been proposed as a less invasive option for cyst resolution and epithelial ablation, akin to methods that have been employed for cysts of the liver, kidney, and thyroid. In a study by Gan et al,18 25 patients with asymptomatic pancreatic cysts underwent ethanol lavage of increasing concentrations. Of the 23 patients completing 6 to 12 months of followup, 8 (35%) had complete resolution of cysts on imaging. No procedure-related symptoms were noted. Five patients underwent surgical resection, providing histologic evidence of epithelial ablation. This experience was extended to a multicenter, randomized double-blind study19 in 39 patients, with preliminary results indicating similar success; one case of post-procedure pancreatitis was observed in a patient who underwent ethanol lavage.
Brachytherapy has been performed under EUS guidance, as well. Jin et al20 implanted radioactive iodine (125I) seeds in 25 patients with unresectable pancreatic cancer and 3 with metastatic disease who received no chemotherapy. A significant drop in pain score was observed 1 month after implantation. Seed translocation to the liver occurred in 1 patient, but no other major complications were observed. It would be of interest to compare the efficacy and tolerability of this approach with outcomes using conventional external beam radiation.
Table 1 shows other prospects for use of EUS-guided FNI as indicated by representative animal studies, case reports, case series, and a randomized trial.21–27 Exciting as these prospects appear, further validation of these techniques is required before they can be recommended for mainstream GI oncology practice.
Table 1.
Other techniques using EUS-guided FNI
|
EUS-GUIDED BILIARY ACCESS AND ANASTOMOSES
Endoscopic retrograde cholangiopancreotagraphy (ERCP) with stenting is the procedure of choice for malignant causes of obstructive jaundice. In up to 10% of cases, biliary drainage cannot be achieved via ERCP, usually in association with difficult anatomy or variable operator skill. These patients are often sent for percutaneous transhepatic cholangiography; however, this method carries a complication rate of up to 30%, including risk of infection and fistula formation.28 EUS allows the placement of a guidewire and stent by transduodenal or transgastric puncture of a dilated biliary system without the need for an external drain. Initial reports in this field included choledochoduodenostomy with EUS in a patient with failed ERCP with a mass at the head of the pancreas, in whom deployment of a plastic transduodenal stent into the obstructed biliary system was performed under EUS guidance.29 This technique later evolved into a transpapillary method that involves the antegrade insertion of a guidewire beyond the level of the obstruction and passage through the major papilla, thus allowing a “rendezvous procedure” wherein ERCP with biliary stent placement can be performed.
Kahaleh et al30 recently described 3- years experience with 23 patients (mean age, 61.5 years) who presented after failed ERCP attempts. By puncturing the biliary system with an EUS needle, the investigators were able to perform cholangiography in all patients and to provide resolution of jaundice in 21. The technique was not successful in 2 patients, one of whom had an impacted common bile duct stone and required a percutaneous transhepatic drain with subsequent internalization of the drain. During follow-up of at least 30 days, the authors observed one case of bile leak that developed 48 hours after the procedure (requiring percutaneous drainage for ascites) and two cases of selflimited pneumoperitoneum.
Perez-Miranda and colleagues31 used a similar technique to access the common bile duct under EUS guidance when ERCP attempts had failed; biliary obstruction was due to cancer in the majority of the 41 patients in the study. A 79% success rate in placing stents for drainage (transgastric or transduodenal) was achieved. Complications occurred in 7 (18%) of 38 patients who had needle puncture, including 3 serious adverse events, mostly occurring in the early phase of the study period. The potential for this procedure to expand options in patients with high-grade biliary obstruction and improve management in this setting is obvious. However, EUSguided biliary duct drainage is a technically challenging procedure with a steep learning curve and, at this time, should be reserved for use in high-volume centers.
Due to the availability of echoendoscopes with larger-diameter accessory channels allowing the passage of specialized instruments, there has been interest in evaluating the utility of EUS in creating various forms of anastomoses in the GI tract. Fritscher-Ravens and colleagues32 reported use of a through-the-scope device for endoscopic suturing and tissue approximation under EUS control in an animal model. Using a 19-gauge EUS needle, transgastric access under EUS guidance allowed real- time visualization of the suturing device and the surrounding tissue. Natural orifice transluminal endoscopic surgery is a procedure that avoids abdominal wall incision and can potentially offer a clinical advantage over conventional surgery, and EUS may play a supportive role in this exciting, new field.
DISCUSSION
EUS has matured beyond its conventional role in locoregional staging of GI cancers. We look forward to further refinement of molecular markers that can improve our confidence in ruling out suspicious pancreatic lesions, phase III clinical trials of intratumoral injection in GI cancers, and more experience with EUS-guided biliary access for patients with obstructive jaundice who fail ERCP. Indeed, it would appear that the close collaboration between oncologists and endosonographers will be a most rewarding scientific journey.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.American Joint Committee on Cancer . Cancer Staging Handbook. 6th ed. New York: Springer-Verlag; 2002. Exocrine pancreas; pp. 179–188. [Google Scholar]
- 2.Brandt KR, Charboneau JW, Stephens DH, et al. CT- and US-guided biopsy of the pancreas. Radiology. 1993;187:99–104. doi: 10.1148/radiology.187.1.8451443. [DOI] [PubMed] [Google Scholar]
- 3.DelMaschio A, Vanzulli A, Sironi S, et al. Pancreatic cancer versus chronic pancreatitis: diagnosis with CA 19–9 assessment, US, CT, and CT-guided fine-needle biopsy. Radiology. 1991;178:95–99. doi: 10.1148/radiology.178.1.1984331. [DOI] [PubMed] [Google Scholar]
- 4.Harewood GC, Wiersema MJ. Endosonographyguided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 5.Rafique A, Freeman S, Carroll N.A clinical algorithm for the assessment of pancreatic lesions: utilization of 16- and 64-section multidetector CT and endoscopic ultrasound Clin Radiol 621142–1153.2007. Epub Sep 25, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–975. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Erickson RA, Garza AA. Impact of endoscopic ultrasound on the management and outcome of pancreatic carcinoma. Am J Gastroenterol. 2000;95:2248–2254. doi: 10.1111/j.1572-0241.2000.02310.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–393. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 9.Bhutani MS, Hawes RH, Baron PL, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854–858. doi: 10.1055/s-2007-1004321. [DOI] [PubMed] [Google Scholar]
- 10.Fritscher-Ravens A, Brand L, Knofel WT, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–2775. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 11.Trümper L, Menges M, Daus H, et al. Low sensitivity of the k-ras polymerase chain reaction for diagnosing pancreatic cancer from pancreatic juice and bile: a multicenter prospective trial. J Clin Oncol. 2002;20:4331–4337. doi: 10.1200/JCO.2002.06.068. [DOI] [PubMed] [Google Scholar]
- 12.Khalid A, Nodit L, Zahid M, et al. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–2500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 13.Nodit L, McGrath KM, Zahid M, et al. Endoscopic ultrasound-guided fine needle aspirate microsatellite loss analysis and pancreatic endocrine tumor outcome Clin Gastroenterol Hepatol 41474–1478.2006. Epub Sep 25, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Khalid A, McGrath KM, Zahid M, et al. The role of pancreatic cyst fluid molecular analysis in predicting cyst pathology. Clin Gastroenterol Hepatol. 2005;3:967–973. doi: 10.1016/s1542-3565(05)00409-x. [DOI] [PubMed] [Google Scholar]
- 15.Ashida R, Nakata B, Mizuno N, et al. Prediction of the chemotherapeutic efficacy for advanced pancreatic cancer by focused DNA array analysis using endoscopic ultrasound-guided fine needle aspiration. Gastrointest Endosc. 2007;65:AB101. [Google Scholar]
- 16.Chang KC, Senzer N, Chung T, et al. A novel gene transfer therapy against pancreatic cancer (TNFerade) delivered by endoscopic ultrasound (EUS) and percutaneous guided fine needle injection (FNI) Gastrointest Endosc. 2004;59:AB–92. [Google Scholar]
- 17.Chang KJ, Senzer N, Swisher S, et al. Multicenter clinical trial using endoscopy and endoscopic ultrasound (EUS) guided fine needle injection (FNI) of anti-tumor agent (TNFerade™) in patients with locally advanced esophageal cancer. Gastrointest Endosc. 2006;63:AB84. [Google Scholar]
- 18.Gan SI, Thompson CC, Lauwers GY, et al. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746–752. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 19.Dewitt JM, Mcgreevy K, Schmidt CM, et al. Ethanol pancreatic injection of cysts (EPIC): preliminary results of a prospective multicenter randomized double blinded study. Gastrointest Endosc. 2007;65:AB106. [Google Scholar]
- 20.Jin Z, Li Z, Du Y, et al. Endoscopic ultrasonography-guided interstitial implantation of iodine 125 seeds combined with chemotherapy in the treatment of unresectable abdominal carcinoma: a prospective study. Gastrointest Endosc. 2007;65:AB105. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 21.Gress FG, Barawi M, Kim D, et al. Preoperative localization of a neuroendocrine tumor of the pancreas with EUS-guided fine needle tattooing. Gastrointest Endosc. 2002;55:594–597. doi: 10.1067/mge.2002.122580. [DOI] [PubMed] [Google Scholar]
- 22.Ayub K. Endoscopic ultrasound-guided superior hypogastric plexus neurolysis: a new technique for the management of pelvic pain. Gastrointest Endosc. 2002;56:S143. [Google Scholar]
- 23.Robbins DH, Block M, Lewin D, et al. Control of traumatic chylothorax with EUS-guided thoracic duct injection sclerotherapy. Gastrointest Endosc. 2004;59:AB213. [Google Scholar]
- 24.Parasher VK, Hernandez LV, Leveen RF, et al. Lymph sampling and lymphangiography via EUS-guided transesophageal thoracic duct puncture in a swine model. Gastrointest Endosc. 2004;59:564–567. doi: 10.1016/s0016-5107(03)02880-3. [DOI] [PubMed] [Google Scholar]
- 25.Barclay RL, Perez-Miranda M, Giovannini M. EUS-guided treatment of solid hepatic metastasis. Gastrointest Endosc. 2002;55:266–270. doi: 10.1067/mge.2002.120784. [DOI] [PubMed] [Google Scholar]
- 26.Gunter E, Lingenfelser T, Eitelbach F, et al. EUS-guided ethanol injection for treatment of a GI stromal tumor. Gastrointest Endosc. 2003;57:113–115. doi: 10.1067/mge.2003.39. [DOI] [PubMed] [Google Scholar]
- 27.Magno P, Giday SA, Gabrielson KL, et al. EUSguided implantation of radio-opaque marker into mediastinal and retroperitoneal lymph nodes. Gastrointest Endosc. 2007;65:AB105. doi: 10.1016/j.gie.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 28.Lameris JS, Stoker J, Nijs HG, et al. Malignant biliary obstruction: percutaneous use of selfexpandable stents. Radiology. 1991;179:703–707. doi: 10.1148/radiology.179.3.2027978. [DOI] [PubMed] [Google Scholar]
- 29.Giovannini H, Moutardier B, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomoses: a new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 30.Kahaleh M, Hernandez AJ, Tokar J, et al. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52–59. doi: 10.1016/j.gie.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Miranda M, Saracibar E, Mata L, et al. EUS-guided pancreatic and biliary ductal drainage (EUS-PBD): a first line strategy after unsuccessful ERCP drainage. Gastrointest Endosc. 2007;65:AB106. [Google Scholar]
- 32.Fritscher-Ravens A, Mosse CA, Mills TN, et al. A through-the-scope device for suturing and tissue approximation under EUS control. Gastrointest Endosc. 2002;56:737–742. doi: 10.1067/mge.2002.129084. [DOI] [PubMed] [Google Scholar]