Abstract
Sleep deprivation alters mood and anxiety in man. In rats, 24 h of treadmill-induced total sleep deprivation or sleep fragmentation increased exploratory behavior in an open field test of anxiety compared to cage or exercise controls. Plasma corticosterone (CORT) levels of sleep disturbed and exercise control rats were elevated compared to cage controls, suggesting that the increased exploration observed in the sleep disturbed rats was not due to a hypothalamic–pituitary–adrenal (HPA) stress response.
Keywords: Sleep deprivation, Sleep fragmentation, Anxiety, Corticosterone, Rat
It has been well documented that loss of sleep in man results in changes in emotional behavior, personality and psychopathology [28]. For example, sleep deprivation has been shown to relieve symptoms of depression [30], trigger manic episodes [3,8], and cause temporary states of paranoid schizophrenic-like behavior [27]. In addition, both sleep deprivation and sleep fragmentation have been shown to adversely affect subjective measures of mood such as vigor and fatigue [4,5,21]. There is also a strong relationship between sleep loss and anxiety in that sleep loss is a complaint in up to 70% of people suffering from generalized anxiety disorder and nocturnal panic attacks occur in up to 45% of panic episodes [28]. In humans, experimental sleep deprivation is reported to contribute to, or exacerbate anxiety [14,20].
While conducting a previous study of the effect of sleep fragmentation on attentional set shifting, we observed that 24 h of experimental sleep fragmentation altered rats’ behavior in an open field and their response to handling by the investigators [12]. Hence, the present study was designed to quantify the effect of 24 h of sleep disruption on exploratory behavior in an open field test that has been used to assess neophobia and anxiety in rats [7]. Although many previous studies have examined the effects of total sleep deprivation in animals [6,17], few animal studies have used an experimental sleep fragmentation paradigm to model sleep fragmentation and none, to our knowledge, have looked at the effects of sleep fragmentation on anxiety. While the effects of sleep deprivation on measures of anxiety and mood in man have been more often studied [reviewed in [14]], sleep fragmentation is clinically relevant, as humans are more likely to experience fragmented sleep than sleep deprivation [24]. Plasma corticosterone (CORT) levels were measured to determine if a hypothalamic–pituitary–adrenal (HPA) stress response could mediate the behavioral changes observed after the treadmill-induced fragmentation or deprivation of sleep.
Adult (250–300 g) male Fischer–Norway (Harlan Laboratories, Ltd) rats were housed under constant temperature (23 °C) and 12:12 light dark cycle (lights-on at 0700 h) with food and water available ad libitum. All animals were treated in accordance with the American Association for Accreditation of Laboratory Animal Care’s policy on care and use of laboratory animals. All procedures were approved by the institutional animal care and use committee (IACUC) of the Boston VA Healthcare System.
To manipulate sleep, the rats lived in a treadmill cage (l × w × h = 50.8 cm × 16.51 cm × 30.48 cm) in which the floor is a horizontal belt automatically programmed to move slowly at a rate of 0.02 m/s as previously described [26]. To induce sleep fragmentation, the treadmill ran at this slow speed for 30 s, followed by no treadmill movement for 90 s. To induce total sleep deprivation, the treadmill ran for 4 s followed by no treadmill movement for 12 s. These schedules ran continuously for 24 h starting at 0700 h. In order to habituate the rats to the treadmill movement, the treadmills were turned on (5 min treadmill on followed by 5 min off) for one hour on each of the 2 days prior to the experiment. There were two control groups, an untreated cage control group, and an exercise control group. To control for the non-specific effects of locomotor activity, the exercise control group obtained an equivalent amount of treadmill movement/exercise, but with a treadmill schedule of 10 min on/30 min off, allowing for longer periods of undisturbed sleep. The use of treadmills to disturb the sleep of rats in this laboratory has been characterized previously [12,13,26].
Plasma CORT levels were measured in a group of rats (cage control n = 7, exercise control n = 6, sleep fragmentation n = 6, sleep deprivation n = 6) to determine if the treadmill procedures altered this endocrinological measure of stress, and, to determine if the sleep loss induced increase in open field exploration correlated with corticosterone levels. Rats were rapidly decapitated immediately after 24 h of sleep fragmentation, sleep deprivation, or exercise control (or at the equivalent time of day for the untreated cage control groups, i.e. 0700 h). Trunk blood was collected from each rat (6 ml per rat) into polyethylene tubes on ice containing 600 μl (Na2-EDTA) at 20 μg/μl. Blood samples were centrifuged at 20 °C for 7 min at 1000× gravity. The plasma fraction was isolated, aliquotted, and frozen at −80 °C. Plasma corticosterone was shipped on dry ice to the Cornell University Animal Health Diagnostic Laboratory for quantification of plasma corticosterone levels.
The open field test was designed and used in accordance with the procedures described by Devine and co-workers [7]. Briefly, a separate group of rats (cage control n = 7, exercise control n = 8, sleep fragmentation n = 7, sleep deprivation n = 6) were placed in a start box (20 cm × 30 cm) attached to an open field (90 cm × 90 cm), separated by a guillotine door. After 60 s in the start box, the door was opened remotely and rats were allowed to explore the open field for 5 min while being recorded by a video camera. Video tapes of the rats’ behavior were manually scored by a researcher blinded to the experimental manipulations. Latency to enter the open field, latency to enter the inner zone (54 cm × 54 cm), time spent in the open field, and time spent in the inner zone were scored. Failure to enter the inner zone or outer zone resulted in a latency score of 300 (5 min) for each measure. An entry into the open field or inner zone was scored when the rat placed all four paws in the respective area. Time spent in the open field began when the rat placed all four paws in the open field and ended when the rat had completely returned (all four paws) to the start box. Time spent in the inner zone began when the rat placed all four paws in the inner zone and ended when the rat completely left the region (i.e. all four paws out).
1. Open field test of anxiety
Fig. 1 illustrates the rodents’ behaviors in the open field task. As shown in Fig. 1A, the disruption of sleep in rats resulted in a significant change in the percent time spent in the open field (F(3,24) = 8.599, p < 0.001) so that when compared to exercise control rats (n = 8), rats spent significantly more time in the open space in both the 24 h total sleep deprivation (n = 6, Fisher’s, p = 0.002) and 24 h sleep fragmentation (n = 7, p < 0.001) groups. The same relationship was seen when cage controls (n = 7) were compared to sleep deprivation (p = 0.017) and sleep fragmentation (p = 0.002). In measures of thigmotactic behavior, the percent of time rats spent in either the outer zone or inner zone while in the open field was not significantly different among groups (F(3,24) = 0.216, p = 0.885, data not shown). As shown in Fig. 1B, the number of entries individual rats made into the open field significantly varied across groups (F(3,24) = 3.231, p = 0.040). After 24 h of total sleep deprivation, rats made significantly more entries into the open field than both cage control (p = 0.050) and exercise control rats (p = 0.030). A similar trend was noted when sleep interrupted animals are compared to cage controls (p = 0.049) and exercise controls (p = 0.029). The latency for rats to enter the open field also trended in the predicted direction (see Fig. 1C), though it failed to reach significance (F(3,24) = 2.962, p = 0.052). The lack of significance was likely due to a failure of the data to meet the assumption of homogeneity of variance (Levene = 7.304, p = 0.001). Groups were significantly different when analyzed with a statistic robust to violations of homogeneity of variance (Welch = 4.023, p = 0.040). While the mean (±S.E.M.) latency for rodents to enter the inner zone of the open field was shorter for sleep fragmented (56.29 ± 17.67) and sleep deprived (49.67 ± 17.27) rats as compared to cage control (111.94 ± 42.31) and exercise control (130.75 ± 41.27), this difference was not significant (F(3,24) = 1.56, p = .224).
Fig. 1.
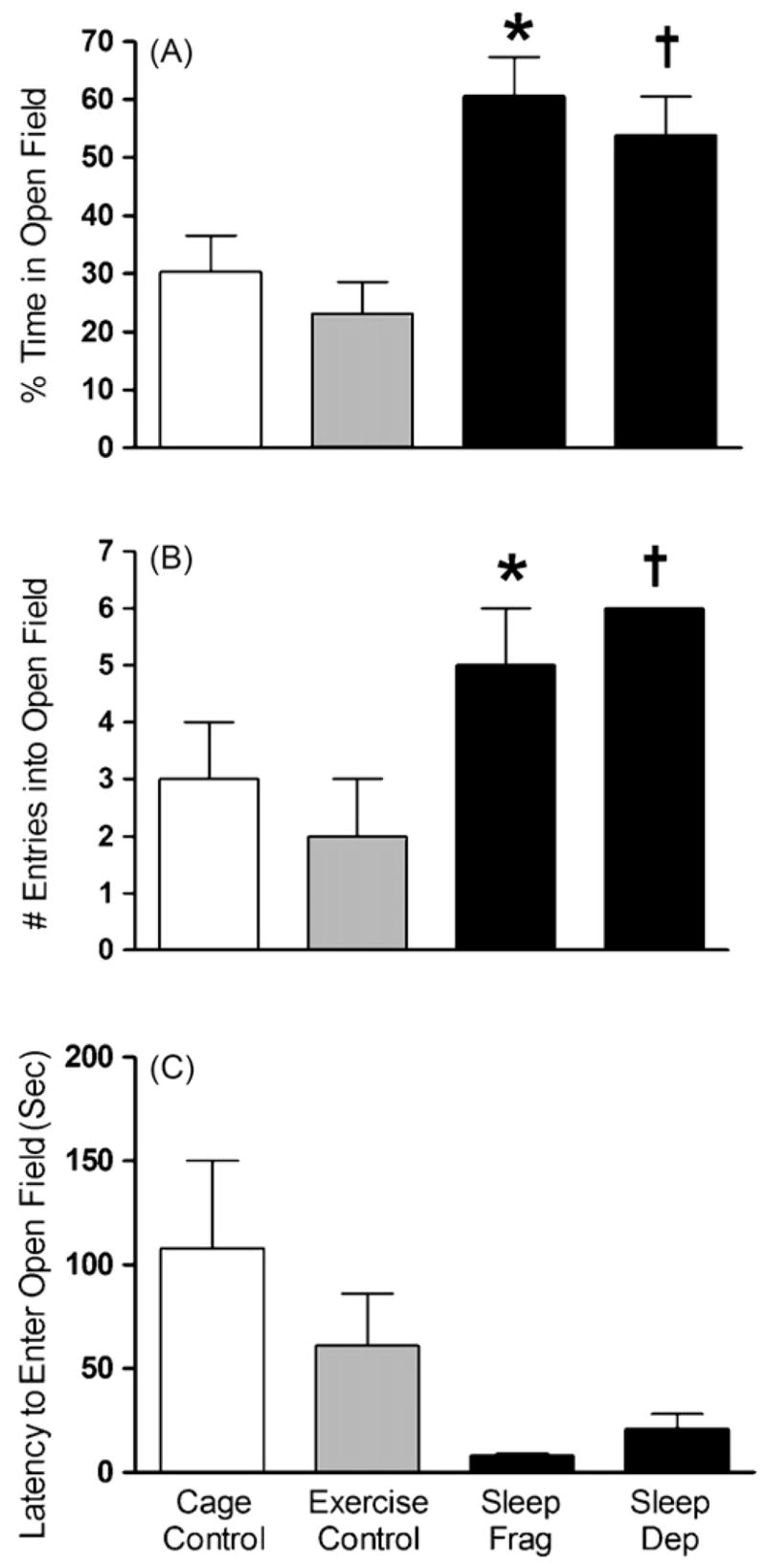
Rats that underwent 24 h of either total sleep deprivation (sleep dep, n = 6) or sleep fragmentation (sleep frag, n = 6), but not exercise (n = 6) or cage control groups (n = 7), showed decreases in anxiety-related behavior by: (A) spending more time in the open field; (B) entering the open field more often (note each sleep dep rat made six entries, therefore S.E.M. = 0); (C) and entering the open field more quickly. Figures represent mean ± S.E.M. (*) Indicates sleep fragmentation is significantly different from cage and exercise control (p < 0.05, Fisher) and † indicates sleep deprivation is significantly different from cage and exercise control (p < 0.05, Fisher).
2. Plasma corticosterone
Plasma CORT levels in rats undergoing 24 h of sleep fragmentation, sleep deprivation, and exercise control were highly variable and failed to meet the statistical assumption of homogeneity of variance (Levene = 6.556, p = .003). Therefore, the nonparametric Kruskal–Wallis test was utilized to analyze the CORT data. As can be seen in Fig. 2, an overall significant main effect of treatment was observed for all manipulations (χ2 = 12.826, p = 0.005) and a follow up with chi-square analysis indicated that compared to cage control rats (n = 7), plasma CORT was significantly elevated in the exercise control (n = 6, p = 0.002), sleep fragmentation (n = 6, p = 0.014), and total sleep deprivation (n = 6, p = 0.001) groups. There were no significant differences in plasma CORT between the exercise control, sleep fragmentation, or total sleep deprivation groups.
Fig. 2.
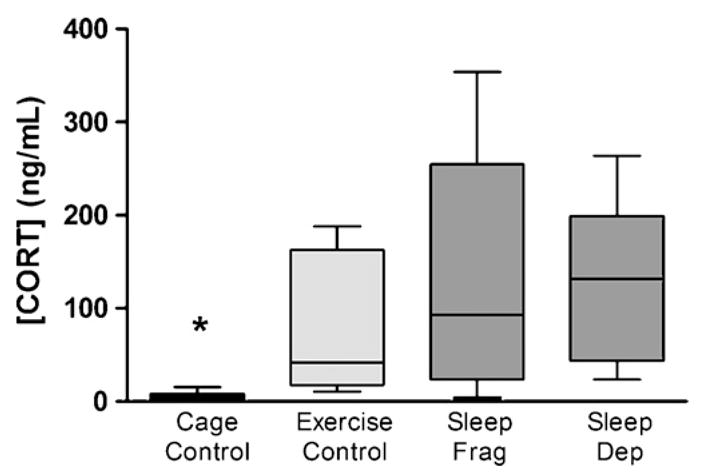
Rats that underwent 24 h of total sleep deprivation (sleep dep, n = 6), sleep fragmentation (sleep frag, n = 7), or exercise (n = 8) showed an increase in plasma corticosterone levels as compared to the cage control group (n = 7). Box represents 25th to 75th percentile of data with the median indicated by the line across the box. (*) Indicates the cage control is significantly different from the three other groups (p < 0.05, chi-square).
Here we show that after 24 h of either sleep fragmentation or total sleep deprivation rats were less fearful in a modified open field, exhibiting a behavioral pattern associated with reduced anxiety [7]. An exercise control group that received an equivalent amount of time walking on the treadmill responded similarly to cage control rats in the open field test, but showed a significant CORT elevation similar to the sleep deprivation and sleep fragmentation groups. Thus, the changes in open field behavior produced by sleep deprivation and sleep fragmentation were not due to non-specific effects such as treadmill exercise or an HPA stress response (i.e. plasma CORT levels).
We were interested in determining if our sleep manipulations resulted in increased CORT levels since an HPA stress reaction in itself could potentially explain changes in exploratory activity observed in the open field. We found that compared to the rats in the cage control group, the rats in the exercise control, sleep deprived, and sleep fragmented groups had significantly elevated CORT levels. For several reasons, we do not interpret these elevations as an indication of an HPA stress response in reaction to our sleep manipulations. Firstly, in rats, CORT is released in a pulsatile manner and the circulating levels follow a specific circadian rhythmicity. The circadian nadir for CORT is at the ‘lights-on’ (inactive) phase and higher plasma levels are found in ‘lights-off’ (active) phase. At the nadir, CORT levels are virtually undetectable, while during the active/lights-off phase, these levels climb to approximately 60 ng/ml [2]. Importantly, this normal level of CORT during rats’ active period is similar to the elevated CORT level in our exercise control group (76 ng/ml). Secondly, the CORT elevations in the sleep loss groups observed here (~130 ng/ml) are not as high as those produced experimentally from an acute stressor (~400 ng/ml) [23]. It is possible the loss of normal circadian rhythm in the sleep loss and the exercise control groups caused a dysregulation in the normal diurnal variation in CORT. Thirdly, like the sleep loss groups, the exercise control group also had a significant increase in plasma CORT, but the open field behavior of the exercise control group was similar to the cage control group. This further indicates that a mechanism independent of CORT is likely responsible for the behavioral changes in the open field test. Finally, using an activity wheel instead of a treadmill to produce sleep fragmentation and deprivation does not result in CORT elevations but does produce behavioral impairments (unpublished observation). Combined, the above arguments suggest that the effects of sleep disturbances on open field behavior are independent of the effects on the plasma CORT.
Another important consideration in our study is that the apparent reduction in anxiety observed in the rats after sleep deprivation and sleep fragmentation can potentially be explained by the finding that rodents can experience locomotor “hyperactivity” following short periods of REM sleep deprivation [1]. REM sleep deprivation in rats can cause an increase in locomotor activity [29] and has even been suggested as a model of mania [8]. Thus, our finding of increased number of entries into and time spent in the open field observed following sleep deprivation and sleep fragmentation, might be a result of hyperactivity, and not directly due to anxiolytic effects of the sleep perturbations. However, sleep deprived rodents have been shown to freeze in tests of conditioned fear [19]. In light of the aforementioned findings of an increase in manic or hyperactivity behaviors after sleep deprivation, it remains uncertain if our findings demonstrate a true anxiolytic effect from sleep loss or an increase in locomotor hyperactivity.
Our modified neophobic open field test is also an important concern in the interpretation of the present results. In general, open field tests of anxiety rely on the conflict between rats’ innate desire to explore/forage and their natural aversion to entering an open area. Several variations of the open field test exist [7,9,11]. The most commonly used open field test has only 2 zones (outer and inner parts of the open field), and manipulations that reduce anxiety produce an increase in the time spent in the inner zone relative to the outer zone. The present study used a modified open field with a small dark start box attached to a standard 2 zone open field, a design that has been previously shown to be sensitive to pharmacological manipulations of anxiety [7]. In this modified (3 zone) open field apparatus the rats “feel” most safe in the start box. As a consequence of this design, the number of entries into the larger attached 2 zone open field and the time spent in the 2 zone open field are the most sensitive measure in this apparatus (Fig. 1A). Herein, a comparison of the time spent in the inner zone versus the outer zone of the larger open field did not reveal a significant difference, a finding that we attribute to the presence of the start box.
In agreement with our findings, several published studies have reported that sleep disruption decreases anxiety-like behavior although studies vary on how they interpret the findings. For example, it has been shown that in humans, one night of total sleep deprivation decreases anxiety in depressed patients (although there was no change in anxiety in patients with panic disorder) [18]. REM sleep deprivation has also been shown to result in increased exploration in the elevated plus maze (which indicates a reduction in anxiety) in mice [16] and rats [11,25]. In addition, REM sleep deprivation has been shown to result in increased exploratory activity in a standard open field apparatus [[11,15]; but see [10]].
However, in contrast to these findings, some studies report that sleep deprivation increases anxiety. For example, in humans there is an increase in self-reported anxiety after 36 h of sleep deprivation [20]. In addition, it has been reported that 72 h of REM sleep deprivation in mice resulted in anxiogenic behaviors in the elevated plus maze and standard open field tests of anxiety [22]. The reported contradictions in the effect of sleep loss on anxiety may be due to differences in the duration of exposure to sleep disruption and the method used to produce sleep disruption.
Additional work is needed to fully characterize the emotional and cognitive state of the rat following sleep fragmentation and sleep deprivation, and also to better understand the role of the nervous system in these observations. Along with potential changes in anxiety state, there could be changes in the rats’ coping strategies and defensive responses [11]. In addition, because it remains uncertain if the present results reflect a true reduction in anxiety following sleep fragmentation and sleep deprivation, follow up studies should include additional neophobic tests of anxiety along with measures of locomotor activity, and activity independent tests such as fecal boli and postural measurements. In summary, here we extended previous findings by showing that 24 h of total sleep deprivation or 24 h of sleep fragmentation, which we have previously characterized with polysomnography measures [12,13,26] increases exploration in a modified open field test of anxiety, indicating that 24 h of sleep disruption alters emotional behavior in rats. The findings are compatible with several interpretations such as a reduction in anxiety, loss of fear, irritability, or possibly an increase in locomotor activity/restlessness.
Acknowledgments
We thank John Franco for care of the animals. This research was supported by: Department of Veterans Affairs Medical Research Service Award to R.E.S., NHLBI – P50 HL060292 (R.E.S. and R.W.M.), NHLBI – T32 HL07901 (J.L.T.).
Footnotes
Disclosure statement: This is not an industry supported study. The authors have indicated no financial conflicts of interest.
References
- 1.Albert I, Cicala GA, Siegel J. The behavioral effects of REM sleep deprivation in rats. Psychophysiology. 1970;6(5):550–60. doi: 10.1111/j.1469-8986.1970.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL. Diurnal variation in the responsiveness of the hypothalamic–pituitary–adrenal axis of the male rat to noise stress. J Neuroendocrinol. 1970;18(7):526–33. doi: 10.1111/j.1365-2826.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res. 1996;65:121–5. doi: 10.1016/s0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet MH. Effect of sleep disruption on sleep, performance, and mood. Sleep. 1985;8:11–9. doi: 10.1093/sleep/8.1.11. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1992;71:1112–8. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 6.Everson CA. Functional consequences of sustained sleep deprivation in the rat. Behav Brain Res. 1995;69(1–2):43–54. doi: 10.1016/0166-4328(95)00009-i. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- 8.Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5:89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- 9.Jawahar MC, Brodnicki TC, Quirk F, Wilson YM, Murphy M. Behavioural analysis of congenic mouse strains confirms stress-responsive Loci on chromosomes 1 and 12. Behav Genet. 2008;38(4):407–16. doi: 10.1007/s10519-008-9206-3. [DOI] [PubMed] [Google Scholar]
- 10.Kovalzon VM, Tsibulsky VL. REM-sleep deprivation, stress and emotional behavior in rats. Behav Brain Res. 1984;14(3):235–45. doi: 10.1016/0166-4328(84)90191-8. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Gonzalez D, Obermeyer W, Fahy JL, Riboh M, Kalin NH, Benca RM. REM sleep deprivation induces changes in coping responses that are not reversed by amphetamine. Sleep. 2004;27(4):609–17. [PubMed] [Google Scholar]
- 12.McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, et al. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;1(30):52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 13.McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, et al. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;46(4):1462–73. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellman TA. Sleep and anxiety disorders. Behav Brain Res. 2006;29(4):1047–58. doi: 10.1016/j.psc.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Mogilnicka E, Boissard CG, Hunn C, Delini-Stula A. Suppressant effect of REM sleep deprivation on neophobia in normal rats and in rats with selective DSP-4 induced damage of locus coeruleus neurons. Pharmacol Biochem Behav. 1985;23(1):93–7. doi: 10.1016/0091-3057(85)90136-4. [DOI] [PubMed] [Google Scholar]
- 16.Pokk P, Vali M. Small platform stress increases exploratory activity of mice in staircase test. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25(7):1435–44. doi: 10.1016/s0278-5846(01)00195-6. [DOI] [PubMed] [Google Scholar]
- 17.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 18.Roy-Byrne PP, Uhde TW, Post RM. Effects of one night’s sleep deprivation on mood and behavior in panic disorder. Patients with panic disorder compared with depressed patients and normal controls. Arch Gen Psychiatry. 1986;43:895–9. doi: 10.1001/archpsyc.1986.01800090085011. [DOI] [PubMed] [Google Scholar]
- 19.Ruskin DN, Liu C, Dunn KE, Bazan NG, LaHoste GJ. Sleep deprivation impairs hippocampus-mediated contextual learning but not amygdala-mediated cued learning in rats. Eur J Neurosci. 2004;19(11):3121–4. doi: 10.1111/j.0953-816X.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 20.Sagaspe P, Sanchez-Ortuno M, Charles A, Taillard J, Valtat C, Bioulac B, et al. Effects of sleep deprivation on Color-Word, Emotional, and Specific Stroop interference and on self-reported anxiety. Brain Cogn. 2005;60(1):76–87. doi: 10.1016/j.bandc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Scott JP, McNaughton LR, Polman RC. Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav. 2006;87:396–408. doi: 10.1016/j.physbeh.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Silva RH, Kameda SR, Carvalho RC, Takatsu-Coleman AL, Niigaki ST, Abilio VC, et al. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology. 2004;176:115–22. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- 23.Simpkiss JL, Devine DP. Responses of the HPA axis after chronic variable stress: effects of novel and familiar stressors. Neuroendocrinol Lett. 2003;24:97–103. [PubMed] [Google Scholar]
- 24.Stepanski E, Lamphere J, Badia P, Zorick F, Roth T. Sleep fragmentation and daytime sleepiness. Sleep. 1984;7:18–26. doi: 10.1093/sleep/7.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Suchecki D, Tiba PA, Tufik S. Hormonal and behavioural responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14(7):549–54. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 26.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23(10):2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyler DB. Psychological changes during experimental sleep deprivation. Dis Nerv Syst. 1955;16:293–9. [PubMed] [Google Scholar]
- 28.Uhde TW. Anxiety disorders. In: Kryger MH, Roth T, Demet WC, editors. Sleep medicine. Philadelphia: W.B. Saunders Company; 2000. pp. 1123–39. [Google Scholar]
- 29.van Hulzen ZJ, Coenen AM. Paradoxical sleep deprivation and locomotor activity in rats. Physiol Behav. 1981;27(4):741–4. doi: 10.1016/0031-9384(81)90250-x. [DOI] [PubMed] [Google Scholar]
- 30.Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]


