Abstract
Eyelid conditioning has proven useful for analysis of learning and computation in the cerebellum. Two variants, delay and trace conditioning, differ only by the relative timing of the training stimuli. Despite the subtlety of this difference, trace eyelid conditioning is prevented by lesions of the cerebellum, hippocampus, or medial prefrontal cortex (mPFC), whereas delay eyelid conditioning is prevented by cerebellar lesions and is largely unaffected by forebrain lesions. Here we test whether these lesion results can be explained by two assertions: (1) Cerebellar learning requires temporal overlap between the mossy fiber inputs activated by the tone conditioned stimulus (CS) and the climbing fiber inputs activated by the reinforcing unconditioned stimulus (US), and therefore (2) trace conditioning requires activity that outlasts the presentation of the CS in a subset of mossy fibers separate from those activated directly by the CS. By use of electrical stimulation of mossy fibers as a CS, we show that cerebellar learning during trace eyelid conditioning requires an input that persists during the stimulus-free trace interval. By use of reversible inactivation experiments, we provide evidence that this input arises from the mPFC and arrives at the cerebellum via a previously unidentified site in the pontine nuclei. In light of previous PFC recordings in various species, we suggest that trace eyelid conditioning involves an interaction between the persistent activity of delay cells in mPFC-a putative mechanism of working memory-and motor learning in the cerebellum.
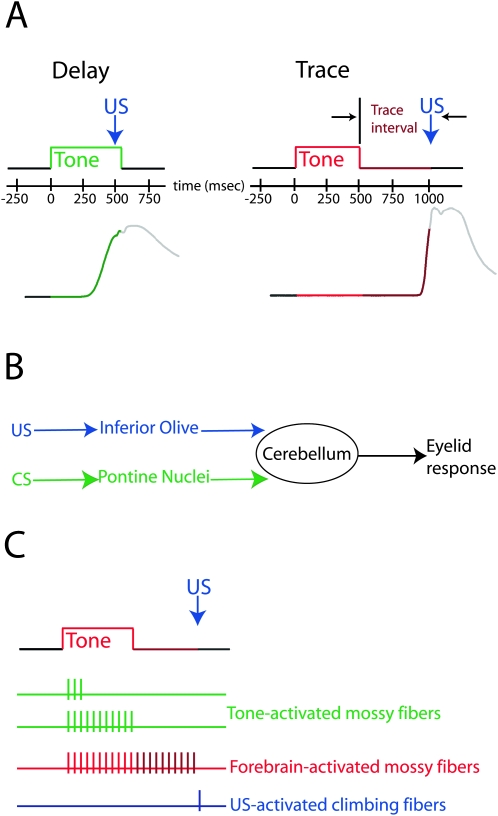
Eyelid conditioning is a form of associative learning that has proven useful for mechanistic studies of learning (Thompson 1986). All variants of eyelid conditioning involve pairing a conditioned stimulus (CS, typically a tone) with a reinforcing unconditioned stimulus (US, mild electrical stimulation near the eye) to promote learned eyelid closure in response to the CS (also known as a conditioned response). Delay eyelid conditioning, where the CS and US overlap in time (Fig. 1A , left), is largely unaffected by forebrain lesions (Solomon et al. 1986; Mauk and Thompson 1987; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Powell et al. 2001; McLaughlin et al. 2002) and engages the cerebellum relatively directly (but see Halverson and Freeman 2006). Presentation of the tone and the US are conveyed to the cerebellum via activation of mossy fibers and climbing fibers, respectively (Fig. 1B; Mauk et al. 1986; Steinmetz et al. 1987, 1989; Sears and Steinmetz 1991; Hesslow 1994; Hesslow et al. 1999). In addition, output via a cerebellar deep nucleus is required for the expression of conditioned responses (McCormick and Thompson 1984). This relatively direct mapping of stimuli onto inputs and of output onto behavior makes delay eyelid conditioning a powerful tool for the analysis of cerebellar learning and computation (Mauk and Donegan 1997; Medina and Mauk 2000; Medina et al. 2000, 2002; Hansel et al. 2001; Ohyama et al. 2003).
Figure 1.
The procedures, neural pathways, and putative signals involved in delay and trace eyelid conditioning. (A) Stimulus timing for delay (left) and trace (right) training trials. For delay conditioning, the US overlaps in time with the tone CS. In this and subsequent figures, green is used to indicate the presentation of the CS for delay conditioning. For trace conditioning, the US is presented after CS offset, and “trace interval” refers to the period between CS offset and US onset. For convenience, we used red and maroon regions to represent the CS and trace interval, respectively. Sample conditioned eyelid responses are shown below, for which an upward deflection indicates closure of the eyelid. (B) Schematic representation of the pathways engaged by delay conditioning. The CS and US, respectively, engage mossy fibers and climbing fibers relatively directly, and forebrain input is not required for normal learning. (C) The signals hypothesized to engage the cerebellum during trace conditioning. The activity of mossy fibers directly activated by the tone CS does not significantly outlast the stimulus. Thus, a forebrain structure is thought to provide an input that overlaps in time with the US and is necessary to produce cerebellar learning.
Trace eyelid conditioning, where the US is presented after tone offset (Fig. 1A, right), has attracted interest for its potential to reveal the nature of interactions between the forebrain and cerebellum as well as the learning mechanisms within these systems. This potential stems from the sensitivity of trace conditioning not only to lesions of cerebellum but also to lesions of hippocampus, medial prefrontal cortex (mPFC), or mediodorsal thalamic nucleus (Woodruff-Pak et al. 1985; Moyer Jr. et al. 1990; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Powell et al. 2001; McLaughlin et al. 2002; Powell and Churchwell 2002; Simon et al. 2005). Given the general inability of forebrain lesions to affect delay conditioning, these results have promoted the general interpretation that the forebrain and cerebellum interact to mediate trace conditioning (Weiss and Disterhoft 1996; Clark and Squire 1998; Clark et al. 2002).
Here we test the specific hypotheses that (Fig. 1C) (1) cerebellar learning requires that mossy fiber and climbing fiber inputs overlap in time (or nearly so) and (2) that cerebellar learning in trace conditioning occurs in response to a forebrain-driven mossy fiber input that outlasts the CS to overlap with the US rather than the inputs activated by the tone CS (Clark et al. 2002). The data provide direct support for both assertions and, together with recent anatomical studies (Buchanan et al. 1994; Weible et al. 2007), reveal a pathway between the mPFC and cerebellum that is necessary for the expression of trace eyelid responses. When combined with previous recordings from PFC in primates and rodents (Funahashi et al. 1989; Bodner et al. 1996; Fuster et al. 2000; Narayanan and Laubach 2006), these data support the hypothesis that trace eyelid conditioning is mediated by interactions between working memory-related persistent activity in mPFC and motor learning mechanisms in the cerebellum.
Results
Cerebellar learning requires that mossy fiber and climbing fiber input nearly overlap in time
Recordings show that the activity of auditory-driven mossy fibers does not outlast the presentation of the tone—the majority of those recorded respond phasically to tone onset and a smaller percentage show sustained responses that persist until tone offset (Boyd and Aitkin 1976; Aitkin and Boyd 1978; Freeman Jr. and Muckler 2003). Thus, during trace eyelid conditioning, mossy fiber activity directly driven by the tone does not overlap in time with the climbing fiber activity elicited by the US. To evaluate whether the cerebellum requires activity in mossy fibers other than those activated directly by the tone, we asked whether cerebellar learning fails when mossy fiber and climbing fiber activity do not overlap in time.
Identifying the temporal patterns of input necessary to engage cerebellar learning requires selective and precise temporal control over the activation of mossy fiber and climbing fiber inputs. Therefore, we used direct electrical stimulation of mossy fibers (see Fig. 3B) as a replacement for the tone (therefore precluding contributions from the forebrain) and periorbital stimulation as the US since its activation of climbing fibers is relatively straightforward (Mauk et al. 1986; Sears and Steinmetz 1991; Hesslow 1994).
Figure 3.
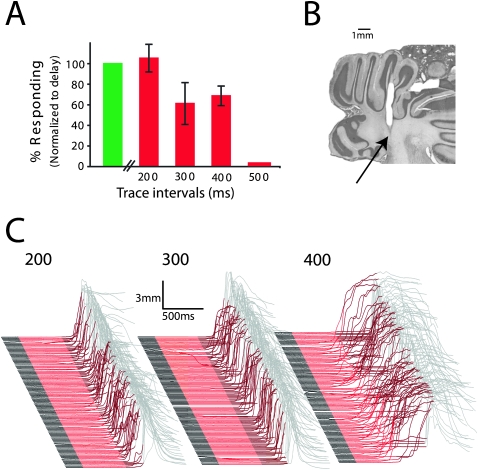
The ability of subjects to learn trace conditioning with a mossy fiber stimulation CS decreases as the trace interval increases. (A) Average response rates, normalized to delay conditioning trials, from the session after criterion was reached (n = 6). (B) Coronal section through the cerebellum shows a representative stimulation site in the middle cerebellar peduncle for the studies in this figure and in Figure 2. All stimulation sites were in the middle cerebellar peduncle. (C) Representative traces from the session after criterion was reached for trace intervals of 200, 300, and 400 msec.
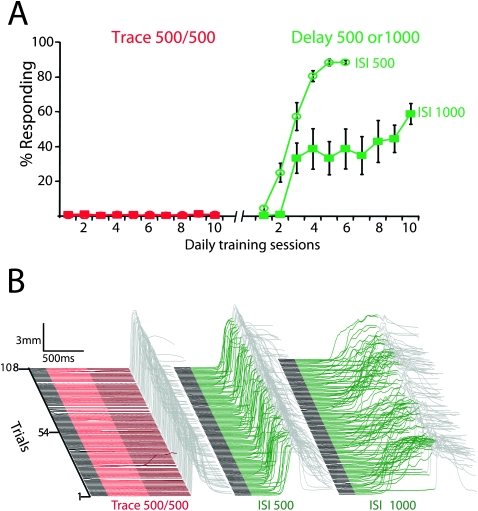
Trace eyelid conditioning using mossy fiber stimulation as the CS failed to promote learning, even though the same stimulation subsequently supported robust delay conditioning (Fig. 2). Nine rabbits were trained for 10 d using standard trace conditioning procedures (500 msec of mossy fiber stimulation and a 500-msec interval between stimulation offset and US). All subjects failed to learn (Fig. 2A,B, left) (F (9,72) = 0.78; P = 0.63; main effect of trace conditioning session on response rate). The same subjects were then tested for their ability to learn eyelid responses with delay conditioning using the same stimulation parameters. For five subjects the interstimulus interval (ISI; the interval between CS and US onset) was 500 msec to match the stimulation duration used in trace conditioning. For the remaining four subjects, the CS was 1000 msec to match the ISI used in trace conditioning. All animals in both delay ISI 500 (Fig. 2A,B, middle) (F (5,20) = 21.93; P < 0.0001; main effect of delay ISI 500 session on response rate) and delay ISI 1000 (Fig. 2A,B, right) (F (9,27) = 3.43; P = 0.006; main effect of delay 1000 session on response rate) conditions showed robust learning.
Figure 2.
The timing of inputs driven directly by trace conditioning stimuli in the absence of the forebrain is beyond the capabilities of cerebellar learning. (A) Direct electrical stimulation of mossy fibers as the CS failed to support trace conditioning with a 500-msec trace interval (red circles and squares; n = 9) but subsequently supported delay conditioning at 500 msec (green circles; n = 5) and 1000 msec (green squares; n = 4) ISIs. (B) Response traces of the last conditioning session of each protocol from representative animals. In this and subsequent figures, an upward deflection of the trace in the colored region represents a learned response and in the gray region represents a reflexive blink to the US. Trials in this and subsequent figures are stacked from first on the bottom to last on the top.
Trace eyelid conditioning in rabbits is prevented by forebrain lesions when the trace interval (interval between CS offset and US onset) is 500 msec but not 300 msec (Moyer Jr. et al. 1990). To test whether this reflects the temporal limitations of cerebellar learning, we subsequently examined the ability of the cerebellum to learn at various trace intervals. We trained six additional rabbits using a 500-msec mossy fiber stimulation train as the CS and a trace interval of 200, 300, or 400 msec until a criterion level of responding (see Materials and Methods). If this criterion was achieved, the trace interval was increased by 100 msec and training continued. If not, the trace interval was decreased until learning was possible. This procedure permitted us to vary the trace interval several times in the same subject. We found that the capacity for learning decreased as the trace interval increased (Fig. 3A) (F (4,18) = 11.11; P = 0.0001; main effect of trace interval on response rate). A 200-msec interval (Fig. 3C, left) supported robust learning in all animals, whereas learning was variable with trace intervals of 300 msec (Fig. 3C, middle) and 400 msec (Fig. 3C, right) and always failed with a 500-msec trace interval.
These data indicate that the cerebellum can learn when the climbing fiber input arrives no more than 200–400 msec after the offset of a mossy fiber input. This supports the hypothesis that the ability of forebrain lesions to impair trace conditioning with trace intervals longer than 300 msec (Moyer Jr. et al. 1990), and their inability to prevent delay conditioning (Solomon et al. 1986; Mauk and Thompson 1987; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Powell et al. 2001; McLaughlin et al. 2002) relates to the temporal requirements of cerebellar learning. Combined with observations that the activity of auditory-driven mossy fibers does not outlast the presentation of a stimulus (Boyd and Aitkin 1976; Aitkin and Boyd 1978; Freeman Jr. and Muckler 2003), the data also support the assertion that during trace conditioning with relatively long trace intervals, the cerebellum requires mossy fiber activity during the trace interval that is distinct from activity driven directly by the tone. If this assertion is true and if the two sets of mossy fibers are anatomically segregated, it should be possible to abolish trace and not delay responding by inactivating the relevant mossy fiber inputs. We test this possibility in the next section.
Delay and trace conditioning engage cerebellar learning via separate sets of mossy fibers
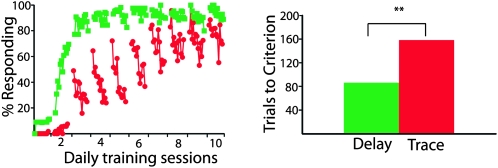
One way to test this prediction is to train subjects on delay conditioning in the first phase and then trace conditioning in the second, while examining the effects of inactivating a specific set of mossy fibers after each training phase. However, any selective abolition of trace but not delay conditioned responses could be attributed to a number of differences during the separate test sessions (differences in drug concentration or diffusion, time-dependent effects, etc.) rather than successful inactivation of trace conditioning relevant mossy fibers. A more powerful way to control for such differences is to probe the effects of inactivation on delay and trace eyelid conditioning within the same animal and test session. We therefore developed a novel dual delay/trace conditioning procedure in which delay and trace conditioning trials were intermixed within the same training session. The CSs were two different tones (1 kHz and 9.5 kHz), with one used as the delay CS and the other as the trace CS (counterbalanced across subjects). We found that animals readily learned both delay and trace conditioned responses using this procedure, with delay responses learned in significantly fewer trials than trace responses (Fig. 4) (n = 11; t (10) = 3.69; P = 0.004; delay versus trace trials to criterion).
Figure 4.
Learning during dual delay/trace conditioning. (Left) The acquisition of delay responses (green squares) was faster than the acquisition of trace responses (red circles) during dual delay/trace training. Data points represent the mean response rate during nine-trial blocks of training (n = 11). There were 12 blocks per training session. Note that both delay and trace responses decrease during each session. We also observe this phenomenon in animals trained with only trace or only delay conditioning. This effect increases with the ISI and will be presented in a forthcoming paper along with a more detailed description and mechanistic implications. (Right) The faster acquisition of delay conditioning can also be seen in the trials needed to reach a criterion level of responding (see Materials and Methods). **P < 0.01.
To ensure that learning resulting from this procedure is comparable to that in which animals are trained with only trace or only delay conditioning, we examined the effects of inactivating the caudal mPFC and anterior interpositus nucleus (AIN) on learned responses. If learning in the dual training paradigm is comparable to learning established by training only one condition at a time, then inactivating the caudal mPFC should impair only trace conditioning (Mauk and Thompson 1987; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Powell et al. 2001), while inactivating the AIN should abolish delay and trace conditioning (McCormick and Thompson 1984; Woodruff-Pak et al. 1985).
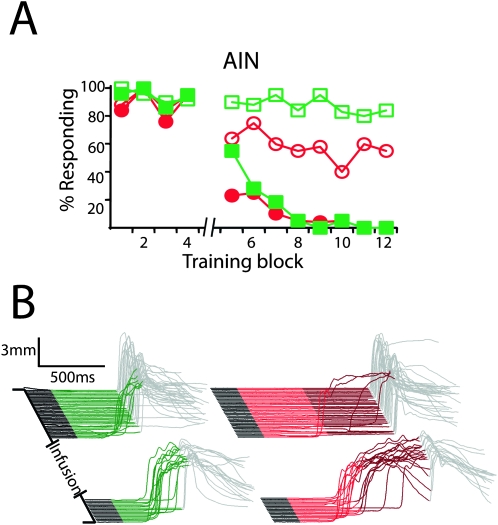
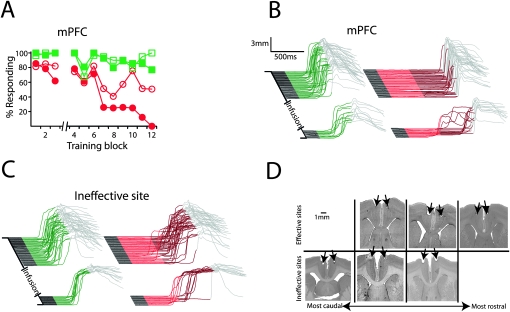
We trained animals using the dual delay/trace procedure and then tested the effects of reversibly inactivating the caudal mPFC or the AIN with the GABAA agonist muscimol on the expression of learned responses. Consistent with previous observations, we found that infusing muscimol into the AIN abolished delay (Fig. 5, filled symbols) (F (11,44) = 11.12; P < 0.0001; block by session type [muscimol vs. last day of training] interaction) and trace conditioning (n = 5; F (11,44) = 16.13; P < 0.0001; block by session type [muscimol vs. last day of training] interaction). Also consistent with previous observations, muscimol infusions abolished trace (F (11,55) = 5.03; P < 0.0001; block by session type [muscimol vs. last day of training] interaction) but not delay conditioning (n = 6; F (11,55) = 1.09; P = 0.39; block by session type [muscimol vs. last day of training] interaction) in subjects with cannula placements in or near the anterior cingulate or prelimbic areas of caudal mPFC (Fig. 6A , filled symbols, B, D, top row). Artificial cerebrospinal fluid (ACSF) infusions had no effect on trace responses in these subjects (Fig. 6A, open symbols) (F (11,55) = 1.15; P = 0.34; block by session type [ACSF vs. last day of training] interaction). Furthermore, muscimol infusions failed to abolish either delay (n = 7; F (11,66) = 0.85; P = 0.59; block by session type [muscimol vs. last day of training] interaction) or trace conditioning (F (11,66) = 0.69; P = 0.75; block by session type [muscimol vs. last day of training] interaction) in subjects with cannula placements dorsal or caudal to effective cannula placements (Fig. 6C,D, bottom row).
Figure 5.
The AIN is necessary for the expression of delay and trace conditioning. (A) Inactivating the AIN with muscimol after the fourth block of dual delay/trace conditioning (break in abscissa) abolished delay (filled green squares) and trace responses (filled red circles), while infusing ACSF had no effect (open squares indicate delay; open circles, trace; n = 5). (B) Representative traces taken from a muscimol infusion session. Delay and trace responses in this and subsequent figures have been separated for clarity but were intermixed during testing.
Figure 6.
The mPFC is necessary for the expression of trace, but not delay, conditioning. (A) Infusing muscimol bilaterally into the mPFC after the third block of dual delay/trace conditioning (break in abscissa) abolished trace (filled red circles) but not delay responses (filled green squares), while infusing ACSF had no affect (open squares indicate delay; open circles, trace; n = 6). (B) Representative traces taken from an effective muscimol infusion session (same subject as shown in D, top left). (C) Representative traces taken from an ineffective muscimol infusion session (same subject as in D, bottom right). Note that in this example, neither trace nor delay responses were affected by the infusion. (D) Histological verification of cannula placements revealed that all cannula placements were in the vicinity of the caudal mPFC. Closer examination revealed that effective infusion sites tended to be more rostral and ventral than ineffective sites, suggesting that the necessary site(s) for trace eyelid conditioning is in the anterior cingulate and/or prelimbic cortices.
These data demonstrate that the effects of lesions of the forebrain and cerebellum on responses using the dual delay/trace procedure parallel those seen previously for animals trained using delay and trace conditioning procedures in separate experiments. Inactivation of the AIN abolished delay and trace responses, while inactivation of mPFC abolished only trace responses. Because effective mPFC infusion sites (Fig. 6D, top row) tended to be more ventral and rostral than ineffective ones (Fig. 6D), our data suggest that the anterior cingulate and/or prelimbic areas of caudal mPFC are important for trace eyelid conditioning. Moreover, consistent with the relatively direct connections between the caudal mPFC and cerebellum in rabbits (Weible et al. 2007), the data indicate that the mPFC is a candidate source of the forebrain signal that engages cerebellar learning during trace eyelid conditioning.
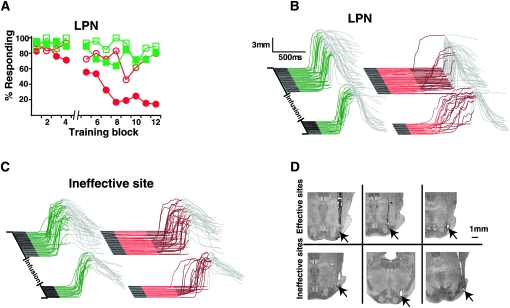
The above data demonstrate that the dual delay/trace conditioning procedure is an effective assay for identifying sites that are selectively important for trace eyelid conditioning. Therefore in another set of subjects, we used the procedure to identify mossy fibers important for trace conditioning. Muscimol infusions in eight well-trained animals with cannula placements in the lateral pontine nuclei (LPN), a source of mossy fibers that receives direct connections from areas of mPFC (Buchanan et al. 1994; Weible et al. 2007) close to our effective cannula placements (Fig. 6D, top), abolished trace (F (11,77) = 4.31; P < 0.0001; block by session type [muscimol vs. last day of training] interaction) but not delay responses (F (11,77) = 0.98; P = 0.47; block by session type [muscimol vs. last day of training] interaction) (Fig. 7A , filled symbols, B, D, top row). ACSF infusions in these animals had no effect on trace responses (n = 5; F (11,44) = 1.32; P = 0.25; block by session type [ACSF vs. last day of training] interaction) (Fig. 7A, open symbols). Furthermore, muscimol infusions failed to abolish either delay (F (11,66) = 1.32; P = 0.24; block by session type [muscimol vs. last day of training] interaction) or trace conditioning (n = 7; F (11,66) = 0.62; P = 0.80; block by session type [muscimol vs. last day of training] interaction) in subjects with cannula placements near but outside of the LPN (by as little as < 500 μm in some cases) (Fig. 7C,D, bottom row).
Figure 7.
Mossy fibers originating in the LPN are necessary for the expression of trace, but not delay, conditioning. (A) Infusing muscimol into the LPN after the fourth block of dual delay/trace conditioning (break in abscissa) abolished trace (filled red circles) but not delay responses (filled green squares), while infusing ACSF had no affect (open squares indicate delay; open circles, trace; n = 8 for muscimol and 5 for ACSF). (B) Representative traces taken from an effective muscimol infusion session (same subjects as shown in D, top middle). (C) Representative traces taken from an ineffective muscimol infusion session (same subjects as in D, bottom right). (D) Histological verification of cannula placements revealed that effective infusion sites were located in the LPN, while ineffective sites were located near (<1mm), but outside of, the LPN.
These data show that trace conditioning using a tone CS and a 500-msec trace interval requires activation of mossy fiber inputs that are distinct from those that are driven directly by auditory input and that are important for delay conditioning. The data also suggest that these mossy fibers originate in the LPN, as effective infusions sites were always located within the LPN. Furthermore, given that the caudal mPFC projects directly to the LPN (Buchanan et al. 1994; Weible et al. 2007), the data also indicate that the caudal mPFC provides the cerebellum with the mossy fiber activity necessary to engage learning during trace eyelid conditioning with a relatively long trace interval.
An examination of the response rates during muscimol infusions into the AIN, mPFC, and LPN (Figs. 5–7, respectively) reveals what appears to be a delayed effect. During each type of infusion, responses decrease over the first few post-infusion blocks. For the LPN and AIN infusions, this is an averaging effect-some subjects’ responses abolished quickly, while others’ abolished more slowly. Thus, the apparent delayed effect of LPN and AIN infusions are likely due to individual differences in cannula placement and/or drug diffusion. For the mPFC infusions, we believe that the actual effective infusion site may be a small distance from our cannulae. We have noticed a strong tendency for ventral and anterior infusion sites to produce more robust and rapid effects on trace conditioning. Defining the precise region of mPFC necessary for the expression of trace responses will require systematically placing cannulae over a wide range of mPFC. It is also possible that our mPFC infusions caused extinction, rather than abolition of responses. We are currently investigating these possibilities.
LPN involvement is specific to trace conditioning and is not related to the ISI
Trace conditioning differs from delay conditioning not only because it has a stimulus-free trace interval but also because it usually involves a longer ISI. Because of this, the effects of forebrain lesions on trace eyelid conditioning have sometimes been attributed to the difficulty of learning eyelid conditioning procedures with long ISIs rather than the presence of the trace interval (Beylin et al. 2001). Thus, it is not clear whether the necessity of the forebrain during trace eyelid conditioning is attributable to the presence of the trace interval or to the long ISI. In a final set of experiments, we used the dual delay/trace conditioning procedure along with reversible inactivation of the LPN to address this confound in a controlled setting with the advantages of a within-animal design.
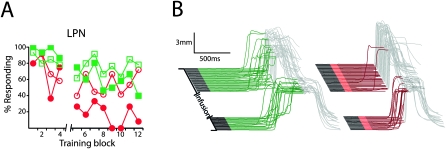
We trained three subjects using the dual delay/trace conditioning procedure in which the delay conditioning ISI was longer than the trace conditioning ISI. The trace conditioning tone was 100 msec and was followed by a 500-msec trace interval for a total ISI of 600 msec, while the delay conditioning ISI was 1000 msec. Post-training infusions of muscimol into the LPN abolished trace responses (F (11,22) = 3.58; P = 0.005; block by session type [muscimol vs. last day of training] interaction) but not delay responses (F (11,22) = 1.44; P = 0.22; block by session type ]muscimol vs. last day of training] interaction) (Fig. 8A, filled symbols, B). These data indicate that the LPN-and by inference the mPFC-is necessary for trace conditioning because of the presence of the trace interval and not because trace conditioning often has a longer ISI than delay conditioning (Beylin et al. 2001).
Figure 8.
The necessity of the LPN in trace conditioning is due to the trace interval and not a longer ISI. For these experiments, the trace conditioning tone was 100 msec, the trace interval was 500 msec, and the delay conditioning ISI was 1000 msec. (A) Infusing muscimol into the LPN after the fourth block of dual delay/trace conditioning (break in abscissa) abolished trace (filled red circles) but not delay responses (filled green squares), while infusing ACSF had no affect (open squares indicate delay; open circles, trace; n = 3). (B) Representative traces taken from an effective muscimol infusion session.
Discussion
We have tested two component assertions of the hypothesis that trace eyelid conditioning involves cerebellar learning in response to an input from the forebrain. First, we showed that the cerebellum cannot support learning when climbing fiber inputs arrive more than a few hundred milliseconds after the end of a mossy fiber input, as occurs for tone-activated mossy fibers during trace conditioning with trace intervals longer than 200–400 msec (Figs. 2, 3). Thus, our data indicate that under these conditions, mossy fiber inputs other than those activated by the auditory system must provide activity during the trace interval for learning to occur.
Second, we provided direct evidence that cerebellar learning during delay and trace conditioning using an auditory CS occurs to different mossy fiber inputs. We showed that cerebellar learning during trace eyelid conditioning occurs in response to activity in a set of mossy fibers that originate in the LPN and that are distinct from tone-CS activated mossy fibers (Fig. 7). Given that the mPFC projects to the LPN and that inactivating the caudal mPFC selectively abolishes trace conditioning (Fig. 6), we suggest that the mossy fibers necessary for trace conditioning convey input to the cerebellum from the mPFC. Together with previous observations (Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Simon et al. 2005), our data also indicate that this mPFC-driven input is necessary for both the acquisition and expression of trace conditioned responses.
Together, these observations provide a specific explanation for why trace conditioning is sensitive to lesions of both the cerebellum and forebrain structures when the trace interval is longer than ∼400 msec. Given that mossy fiber inputs driven directly by a tone CS terminate shortly after CS offset (Boyd and Aitkin 1976; Aitkin and Boyd 1978; Freeman Jr. and Muckler 2003), the pattern of mossy fiber and climbing fiber inputs driven directly by the CS and US during trace conditioning is not appropriately timed to engage cerebellar learning. Thus, a second mossy fiber input is required that arises from the LPN, is driven by input from the mPFC, and presumably provides activity that overlaps in time with the US. Indirect evidence suggests that this input is learned-neurons in the hippocampus (Moyer Jr. et al. 1996; McEchron and Disterhoft 1997; McEchron et al. 2001) and mPFC (Weible et al. 2003) show learning-related changes during trace conditioning, and the acquisition of trace conditioned responses requires more training trials than is required for delay conditioning (Fig. 4; Beylin et al. 2001). We therefore suggest that trace conditioning with a trace interval longer than ∼400 msec is mediated by a multistage process in which forebrain learning precedes, and is required for, subsequent cerebellar learning.
Implications for delay eyelid conditioning
It is interesting that inactivating the LPN did not affect the expression of delay conditioned responses, given that the LPN have previously been implicated as a locus of the auditory-driven mossy fibers that are necessary for delay eyelid conditioning to a tone (Steinmetz et al. 1987). Because the pontine nuclei are a large structure that span several millimeters in the anterior-posterior axis, our effective infusions may have been in an area of the LPN that lacks auditory-driven mossy fibers. This interpretation is supported by observations that short-latency auditory evoked activity is restricted to caudal aspects of the LPN and that lesions of the caudal, but not rostral, LPN affect delay conditioning to a tone (Steinmetz et al. 1987). Given that our effective infusions tended to be in rostral regions of the LPN (Fig. 7), mPFC-driven and auditory-driven mossy fibers may be segregated along the anterior-posterior axis of the LPN. A systematic mapping of single-unit, auditory, and mPFC-driven activity along the anterior-posterior axis of the LPN is needed to test this hypothesis.
The majority of auditory-driven mossy fibers that have been recorded do not show sustained activity in response to a tone (Boyd and Aitkin 1976; Aitkin and Boyd 1978; Freeman Jr. and Muckler 2003), suggesting the potential need for a mechanism to sustain mossy fiber activity during delay eyelid conditioning as well. Although the potential for selection bias with in vivo recordings requires that such conclusions remain tentative, the apparent majority of phasically responding auditory-driven mossy fibers would be unable to engage cerebellar learning mechanisms during delay conditioning because the offset of their activity would occur more than a few hundred milliseconds before a climbing fiber input. Thus, the cerebellum may require a mechanism that sustains mossy fiber activity to overlap with the US during delay conditioning. Our data indicate that this sustained input would not require the mPFC, as inactivating the mPFC did not abolish delay responses. There are a variety of alternatives. Sustained mossy fiber responses to tones may be driven by the forebrain-auditory system (e.g., the inferior colliculus or auditory thalamus) (Halverson and Freeman 2006; Freeman et al. 2007) or by feedback from the cerebellum or red nucleus (Cartford et al. 1997; Clark et al. 1997; Bao et al. 2000). Alternatively, it may simply be the case that the number of mossy fibers that naturally show sustained responses to a tone is sufficient to support cerebellar learning during delay conditioning.
Finally, we showed that the necessity of the forebrain is related specifically to the trace interval and is not the by-product of the long ISIs often used during trace conditioning. By use of combined delay/trace conditioning where the ISI for trace conditioning was shorter than the ISI for delay (600 msec for trace vs. 1000 msec for delay), we demonstrated that inactivating the LPN abolishes trace responses but not delay responses (Fig. 8). These data reinforce the interpretation that the forebrain activity relayed to the cerebellum via the LPN is required to span the stimulus-free trace interval and is not required because the ISI is long. This interpretation is also supported by the observation that subjects acquired delay conditioning with 1000-msec ISI, but not trace conditioning with 1000-msec ISI with mossy fiber stimulation as the CS (Fig. 2).
Forebrain-cerebellum interactions during trace eyelid conditioning
Our findings establish trace eyelid conditioning as a useful tool to study cerebellar computation during interactions with the forebrain. Delay conditioning has proven particularly useful in characterizing the input/output transformations of the cerebellum (Mauk and Donegan 1997; Medina and Mauk 2000; Medina et al. 2000, 2002; Ohyama et al. 2003), due to the relatively direct ways that it engages cerebellar inputs (Mauk et al. 1986; Steinmetz et al. 1987, 1989; Sears and Steinmetz 1991; Hesslow 1994; Hesslow et al. 1999) and output (McCormick and Thompson 1984) and to its insensitivity to forebrain lesions (Mauk and Thompson 1987; Moyer Jr. et al. 1990; Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Powell et al. 2001). Despite its utility in this regard, delay eyelid conditioning represents a somewhat atypical situation where cerebellar afferents are activated relatively directly by somatosensory and auditory stimuli. Large numbers of mossy and climbing fiber inputs to the cerebellum are, in contrast, driven by descending forebrain projections (Ito 1984; Schmahmann and Pandya 1995; 1997; Middleton and Strick 2000; Weible et al. 2007). Thus, the typical mode of cerebellar information processing in the mammalian brain likely involves interactions with the forebrain. Our results therefore highlight the potential for using trace conditioning to study cerebellar information processing in a more typical context involving interactions with the forebrain.
More generally, our findings highlight the potential importance of learned (or potentially sensory-driven) cortical inputs driving the mossy fiber system for cerebellar learning. Given the massive amount of cortio-pontine input, it may be that the patterns of mossy fiber input driven relatively directly by the outside world are normally too brief to engage cerebellar learning mechanisms. Forebrain learning may therefore serve to expand the repertoire of mossy fiber inputs available for cerebellar learning by internally generating mossy fiber inputs that overlap with climbing fiber input (Clark et al. 2002).
Why does cerebellar learning fail when mossy fiber and climbing fiber activity do not overlap in time? Long-term depression (LTD) at the granule cell-Purkinje cell synapse and an increase in the efficacy of the mossy fiber to deep cerebellar nucleus cell synapse are two types of plasticity presumed to underlie cerebellar learning (Mauk and Donegan 1997; Ohyama et al. 2006; Pugh and Raman 2006). Thus, the inability to induce either could explain why nonoverlapping mossy fiber activity and climbing fiber activity fail to support cerebellar learning. Several lines of evidence indicate that cerebellar LTD requires that climbing fiber and granule cell activity are separated in time by no more than ∼100 msec (Wang et al. 2000). Thus, assuming that granule cell activity does not long persist after the offset of the mossy fiber input that drives it, cerebellar LTD would not be induced when climbing fiber input arrives shortly after the offset of mossy fiber activity. Similarly, plasticity at the mossy fiber to deep nucleus synapse is induced by pairing mossy fiber activity with a release from Purkinje cell inhibition (Pugh and Raman 2006). Thus, this plasticity would not be induced if nonoverlapping mossy fiber and climbing fiber activity fails to produce an overlapping release from Purkinje cell inhibition and mossy fiber activity.
Finally, the straightforward way in which the forebrain and cerebellum interact suggests that trace eyelid conditioning may be useful for studying information processing in cortical circuits. Our infusion results, combined with previous lesion studies (Kronforst-Collins and Disterhoft 1998; Weible et al. 2000; Powell et al. 2001; Simon et al. 2005) suggest the mPFC as a strong candidate for the forebrain site that provides the cerebellum with the necessary mossy fiber activity during the trace interval. This hypothesis is strengthened by observations that mPFC projects directly to the LPN (Weible et al. 2007), which we have shown is necessary for trace, but not delay, conditioning. Furthermore, it is supported indirectly by the extensive observation in non-human primates and rodents of so-called delay cells in PFC, which have been implicated in working memory (Funahashi et al. 1989; Bodner et al. 1996; Fuster et al. 2000; Narayanan and Laubach 2006). These cells have been studied in the context of behavioral protocols in which a brief cue is paired with a reinforcing stimulus that is presented well after the offset of the cue. These cells fire persistently during the stimulus-free period between the offset of the cue and the reinforcing stimulus, which is the equivalent of the trace interval in trace conditioning. They have the appropriate firing properties, therefore, to be the source of a signal that overlaps with the US-activated climbing fiber input to engage cerebellar learning in trace eyelid conditioning.
On the basis of these considerations, we propose the hypothesis that trace eyelid conditioning is mediated by interactions between working memory mechanisms in mPFC and cerebellar learning. Specifically, we propose that during trace conditioning with trace intervals longer than ∼400 msec delay, cells of mPFC provide a mossy fiber input to the cerebellum via the LPN that overlaps in time with the US. From the cerebellum's point of view, this overlap, in affect, turns trace conditioning into delay conditioning with a relatively long ISI.
Future studies could test this forebrain-cerebellum hypothesis rather directly, given that the level of acetylcholine and certain monoamines in the PFC affect working memory and modulate its neural correlates (Williams and Goldman-Rakic 1995; Chudasama et al. 2004; Vijayraghavan et al. 2007; Wang et al. 2007). For example, relatively small doses of D1 dopamine receptor antagonists improve working memory and potentiate the task-related activity of PFC neurons (Williams and Goldman-Rakic 1995; Vijayraghavan et al. 2007). The working memory hypothesis predicts that trace eyelid conditioning should be similarly affected by these compounds. Another prediction is that recording from neurons in mPFC or LPN should reveal cells that fire persistently during the trace interval. This activity should gradually emerge early in training before overt, behavioral learning occurs and continue for as long as eyelid responses persist. Confirming this prediction would make trace eyelid conditioning a useful model system to study how delay cell activity in the PFC is learned.
Finally, the concrete framework provided by our hypothesis could help clarify the elusive role of the hippocampus in trace eyelid conditioning. Given the direct connections between the hippocampus and mPFC (Swanson 1981; Ferino et al. 1987; Jay and Witter 1991) and the time-dependent effects of hippocampal lesions on trace conditioning (Kim et al. 1995; Takehara et al. 2003), neural correlates of trace conditioning in the hippocampus (McEchron and Disterhoft 1997; McEchron et al. 2001) may play a crucial role in the establishment, but not the maintenance, of sustained activity in the PFC (Gilmartin and McEchron 2005; Hasselmo and Stern 2006).
Materials and Methods
Subjects
We obtained data from 57 male New Zealand albino rabbits (Oryctolagus cuniculus) (Myrtle's Rabbitry, TN). The animals weighed between 2.5 and 3 kg, were individually housed, and had free access to food and water. Treatment of animals and surgical procedures were in accordance with National Institutes of Health Guidelines and an institutionally approved animal welfare protocol.
Surgery
All animals were prepared with a head bolt cemented to the skull and a 26-gauge stainless steel guide cannula (Plastics One) or a tungsten stimulating electrode (A-M Systems; tip exposed to obtain impedance of ∼100–200 kΩ). Before surgery, animals were anesthetized with 5 mg/kg acepromazine, and their skulls were placed in a stereotaxic restrainer. Anesthesia was maintained with isofluorene (2% mixed in oxygen), and sterile procedures were used throughout the surgery. For all surgeries, lambda was positioned 1.5 mm below bregma. In five subjects, a guide cannula was placed at the stereotaxic coordinates corresponding to the AIN of the cerebellum ipsilateral to the training eye (5 mm lateral, 13.3 mm ventral, and 1 mm anterior from lambda). In 18 subjects, a guide cannula was placed at the coordinates corresponding to the LPN contralateral to the training eye (2.5–2.7 mm lateral, 19–21.5 mm ventral, and 9–10.5 mm posterior from bregma). Three of these subjects were chosen randomly to be included in the behavioral experiments of Figure 4. In 13 subjects, bilateral guide cannulae were placed at the coordinates corresponding to the caudal mPFC (1 mm lateral [2 mm distance between cannula], 2–4 mm ventral from the surface of the brain, and 2.5–3 mm anterior from bregma). Two of these subjects were chosen randomly to be included in the behavioral experiments of Figure 4. In 15 subjects, a stimulating electrode was placed in the coordinates corresponding to the middle cerebellar peduncle ipsilateral to the training eye (5.5 mm lateral, 16 mm ventral, and 3 mm anterior from lambda). Six subjects were prepared with only a head bolt and were used for behavioral experiments. The implant and head bolt were secured in place with dental acrylic. Finally, two stainless steel stimulating electrodes were implanted in the periorbital muscles of the eye. Training began at least 1 wk after surgery.
Conditioning
Subjects were trained in custom-designed chambers equipped with a speaker that was connected to an audio source module (Coulborn Instruments, model V85-05) used to generate tones and isolated pulse stimulators (A-M systems, model 2100) to deliver trains of electrical pulses through the periorbital electrodes. We also used isolated pulse stimulators to time the delivery of constant current pulses by stimulus isolators (World Precision Instruments, model A360) connected to the electrodes implanted in the middle cerebellar peduncle. As in previous studies, (Medina et al. 2000), an infrared emitter/detector was attached directly to the head stage of each subject to record movements of the left external eyelid by detecting changes in the amount of reflected light.
All daily conditioning procedures consisted of 12 blocks of nine trials (one CS-alone trial and eight paired trials/block). Trials were separated by a random intertrial interval in the range of 25 to 35 sec. The CS was either a 1-kHz, 85-dB tone; a 9.5-kHz, 85-db tone; or cathodal stimulation (100 Hz, 40-μsec pulse width, 100 μA) of the middle cerebellar peduncle. The US was a 50-msec train of constant current pulses (100-Hz, 1-msec pulse width, 2–3 mA) delivered through the periorbital electrodes. During all paired trace conditioning trials, the CS was presented for either 500 or 100 msec, and the US followed the offset of the CS by 100–500 msec, depending on the experiment. During paired delay conditioning trials, the CS was presented for either 550 or 1050 msec and coterminated with the US. For dual delay/trace conditioning procedures, a delay trial followed either every one or two trace trials. Stimulus presentation was controlled by custom-designed software.
Infusions
After subjects were well trained using the dual delay/trace conditioning paradigm (10–15 sessions), we infused 1 mM of the GABAA agonist muscimol (Tocris), or ACSF through a 33-gauge internal cannula that extended 1.2 mm beyond the guide cannula during test sessions. Muscimol was dissolved in ACSF consisting of (in mM): NaCl 119, KCl 2.5, NaH2PO4 1.2, MgCl2 2, CaCl2 2, NaHCO3 26, D-glucose 10, and HEPES 20 (pH 7.35∼7.4). The internal cannula was coupled to a 50-μL Hamilton syringe that was mounted on an automated injector system (Bioanalytical Systems; model MD-1001) and driven by an electronic pump (model MD-1020). Infusions into cannula aimed at the LPN and AIN began after the fourth block and continued at a rate of 0.1 μL/min until training resumed 20 min later. Infusions into cannula aimed at the mPFC began after the third block and continued at a rate of 0.1 μL/min until training resumed 20 min later. Infusions into a given brain region were conducted within subjects. The order of infusion type (muscimol vs. ACSF) was counterbalanced across subjects and at least one day of retraining was given between infusion sessions to ensure there were no long-term effects.
Data analysis
We analyzed digitized sweeps of eyelid movements made 200 msec before and 2300 msec after CS onset with custom software. A conditioned response was defined as an eyelid movement of at least 0.3 mm within the ISI. Trials in which eyelid movements greater than 0.3 mm were made within 200 msec before CS onset were excluded from analysis. ANOVA was used to test for within- and between-subject differences. The significance level for all tests was 0.05. To determine the effects of muscimol or ACSF infusions on responding, we compared infusion data with those taken from the last day of training. We defined response rates as affected if there was a significant block by training session (muscimol, ACSF, or last day of training) interaction. We made this comparison because we observed a significant and reliable decrease in responding that occurred naturally within sessions during both delay and trace conditioning (a phenomenon we will describe in more detail in a forthcoming paper), rendering a pre- versus post-infusion comparison inappropriate. We used the Bonferroni method to correct for type I errors associated with multiple comparisons.
Because the experiments of Figures 3 and 4 were designed for different purposes, we defined two different criteria for learning. In the experiments of Figure 3, where the objective was to detect the first signs of learning regardless of its robustness, the criterion for learning was defined as the session in which subjects made three responses in each of two consecutive blocks. In our hands, this criterion predicted well that a subject would reach asymptotic responding the day after criterion was reached (unpublished observations) and thus allowed us to vary the trace interval multiple times in the same subject. Three subjects were given the 200-msec trace interval first, one subject the 300-msec interval first, and two subjects the 400-msec interval first. When a subject reached criterion for a given trace interval, they were given one more session with that interval before switching to a session with a new interval. The response rate during the day after criterion was reached was used in analysis. Because not all subjects received the same trace procedures or order of training, we used a between-subjects ANOVA to analyze these data. In the experiments of Figure 4, where the objective was to detect asymptotic learning, the criterion for learning was defined as five responses made in each of two consecutive blocks. We used this criterion rather than the classic criterion of eight responses in any nine consecutive trials to accommodate the lower asymptote of trace conditioning relative to delay conditioning.
Histology
Infusion and electrode sites were marked by passing 200 μA, anodal current for 15 sec through a wire (cut to the length of the internal cannula and threaded through the guide cannula) or stimulating electrode, respectively. Animals were killed with an overdose of sodium pentobarbital and were perfused transcardially with 10% formalin. Brains were imbedded in gelatin and sectioned at 80 μm using a microtome. Sections were mounted and stained with cresyl violet.
Acknowledgments
We thank J. Knierim, D. Johnston, and J. Siegel for their helpful comments on an earlier version of this manuscript. This work was supported by NIMH grants MH74006 and MH46904 to M.D.M.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.1178309.
References
- Aitkin L.M., Boyd J. Acoustic input to the lateral pontine nuclei. Hear. Res. 1978;1:67–77. doi: 10.1016/0378-5955(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Bao S., Chen L., Thompson R.F. Learning- and cerebellum-dependent neuronal activity in the lateral pontine nucleus. Behav. Neurosci. 2000;114:254–261. doi: 10.1037//0735-7044.114.2.254. [DOI] [PubMed] [Google Scholar]
- Beylin A.V., Gandhi C.C., Wood G.E., Talk A.C., Matzel L.D., Shors T.J. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiol. Learn. Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bodner M., Kroger J., Fuster J.M. Auditory memory cells in dorsolateral prefrontal cortex. Neuroreport. 1996;7:1905–1908. doi: 10.1097/00001756-199608120-00006. [DOI] [PubMed] [Google Scholar]
- Boyd J., Aitkin L.M. Responses of single units in the pontine nuclei of the cat to acoustic stimulation. Neurosci. Lett. 1976;3:259–263. doi: 10.1016/0304-3940(76)90052-5. [DOI] [PubMed] [Google Scholar]
- Buchanan S.L., Thompson R.H., Maxwell B.L., Powell D.A. Efferent connections of the medial prefrontal cortex in the rabbit. Exp. Brain Res. 1994;100:469–483. doi: 10.1007/BF02738406. [DOI] [PubMed] [Google Scholar]
- Cartford M.C., Gohl E.B., Singson M., Lavond D.G. The effects of reversible inactivation of the red nucleus on learning-related and auditory-evoked unit activity in the pontine nuclei of classically conditioned rabbits. Learn. Mem. 1997;3:519–531. doi: 10.1101/lm.3.6.519. [DOI] [PubMed] [Google Scholar]
- Chudasama Y., Dalley J.W, Nathwani F., Bouger P., Robbins T.W. Cholinergic modulation of visual attention and working memory: Dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn. Mem. 2004;11:78–86. doi: 10.1101/lm.70904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.E., Squire L.R. Classical conditioning and brain systems: The role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Clark R.E., Gohl E.B., Lavond D.G. The learning-related activity that develops in the pontine nuclei during classical eye-blink conditioning is dependent on the interpositus nucleus. Learn. Mem. 1997;3:532–544. doi: 10.1101/lm.3.6.532. [DOI] [PubMed] [Google Scholar]
- Clark R.E., Manns J.R., Squire L.R. Classical conditioning, awareness, and brain systems. Trends Cogn. Sci. 2002;6:524–531. doi: 10.1016/s1364-6613(02)02041-7. [DOI] [PubMed] [Google Scholar]
- Ferino F., Thierry A.M., Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp. Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Freeman J.H., Jr, Muckler A.S. Developmental changes in eyeblink conditioning and neuronal activity in the pontine nuclei. Learn. Mem. 2003;10:337–345. doi: 10.1101/lm.63703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J.H., Halverson H.E., Hubbard E.M. Inferior colliculus lesions impair eyeblink conditioning in rats. Learn. Mem. 2007;14:842–846. doi: 10.1101/lm.716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S., Bruce C.J., Goldman-Rakic P.S. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster J.M., Bodner M., Kroger J.K. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Gilmartin M.R., McEchron M.D. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav. Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Halverson H.E., Freeman J.H. Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behav. Neurosci. 2006;120:880–887. doi: 10.1037/0735-7044.120.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C., Linden D.J., D'Angelo E. Beyond parallel fiber LTD: The diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Hasselmo M.E., Stern C.E. Mechanisms underlying working memory for novel information. Trends Cogn. Sci. 2006;10:487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J. Physiol. 1994;476:229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G., Svensson P., Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Ito M. Raven Press; New York: 1984. The cerebellum and neural control. [Google Scholar]
- Jay T.M., Witter M.P. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Clark R.E., Thompson R.F. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav. Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins M.A., Disterhoft J.F. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol. Learn. Mem. 1998;69:147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Mauk M.D., Donegan N.H. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn. Mem. 1997;4:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- Mauk M.D., Thompson R.F. Retention of classically conditioned eyelid responses following acute decerebration. Brain Res. 1987;403:89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Mauk M.D., Steinmetz J.E., Thompson R.F. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc. Natl. Acad. Sci. 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D.A., Thompson R.F. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- McEchron M.D., Disterhoft J.F. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J. Neurophysiol. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- McEchron M.D., Weible A.P., Disterhoft J.F. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. J. Neurophysiol. 2001;86:1839–1857. doi: 10.1152/jn.2001.86.4.1839. [DOI] [PubMed] [Google Scholar]
- McLaughlin J., Skaggs H., Churchwell J., Powell D.A. Medial prefrontal cortex and pavlovian conditioning: Trace versus delay conditioning. Behav. Neurosci. 2002;116:37–47. [PubMed] [Google Scholar]
- Medina J.F., Mauk M.D. Computer simulation of cerebellar information processing. Nat. Neurosci. 2000;3(Suppl):1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- Medina J.F., Garcia K.S., Nores W.L., Taylor N.M., Mauk M.D. Timing mechanisms in the cerebellum: Testing predictions of a large-scale computer simulation. J. Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J.F., Nores W.L., Mauk M.D. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- Middleton F.A., Strick P.L. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res. Brain Res. Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Moyer J.R., Jr, Deyo R.A., Disterhoft J.F. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav. Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Moyer J.R., Jr, Thompson L.T., Disterhoft J.F. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J. Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan N.S., Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T., Nores W.L., Murphy M., Mauk M.D. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Ohyama T., Nores W.L., Medina J.F., Riusech F.A., Mauk M.D. Learning-induced plasticity in deep cerebellar nucleus. J. Neurosci. 2006;26:12656–12663. doi: 10.1523/JNEUROSCI.4023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D.A., Churchwell J. Mediodorsal thalamic lesions impair trace eyeblink conditioning in the rabbit. Learn. Mem. 2002;9:10–17. doi: 10.1101/lm.45302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D.A., Skaggs H., Churchwell J., McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus) Behav. Neurosci. 2001;115:1029–1038. doi: 10.1037//0735-7044.115.5.1029. [DOI] [PubMed] [Google Scholar]
- Pugh J.R., Raman I.M. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Prefrontal cortex projections to the basilar pons in rhesus monkey: Implications for the cerebellar contribution to higher function. Neurosci. Lett. 1995;199:175–178. doi: 10.1016/0304-3940(95)12056-a. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Pandya D.N. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J. Neurosci. 1997;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears L.L., Steinmetz J.E. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Res. 1991;545:114–122. doi: 10.1016/0006-8993(91)91276-7. [DOI] [PubMed] [Google Scholar]
- Simon B., Knuckley B., Churchwell J., Powell D.A. Post-training lesions of the medial prefrontal cortex interfere with subsequent performance of trace eyeblink conditioning. J. Neurosci. 2005;25:10740–10746. doi: 10.1523/JNEUROSCI.3003-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon P.R., Vander Schaaf E.R., Thompson R.F., Weisz D.J. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav. Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Steinmetz J.E., Logan C.G., Rosen D.J., Thompson J.K., Lavond D.G., Thompson R.F. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proc. Natl. Acad. Sci. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz J.E., Lavond D.G., Thompson R.F. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Swanson L.W. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Takehara K., Kawahara S., Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J. Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.F. The neurobiology of learning and memory. Science. 1986;233:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S., Wang M., Birnbaum S.G., Williams G.V., Arnsten A.F. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Denk W., Hausser M. Coincidence detection in single dendritic spines mediated by calcium release. Nat. Neurosci. 2000;3:1266–1273. doi: 10.1038/81792. [DOI] [PubMed] [Google Scholar]
- Wang M., Ramos B.P., Paspalas C.D., Shu Y., Simen A., Duque A., Vijayraghavan S., Brennan A., Dudley A., Nou E., et al. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Weible A.P., McEchron M.D., Disterhoft J.F. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav. Neurosci. 2000;114:1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weible A.P., Weiss C., Disterhoft J.F. Activity profiles of single neurons in caudal anterior cingulate cortex during trace eyeblink conditioning in the rabbit. J. Neurophysiol. 2003;90:599–612. doi: 10.1152/jn.01097.2002. [DOI] [PubMed] [Google Scholar]
- Weible A.P., Weiss C., Disterhoft J.F. Connections of the caudal anterior cingulate cortex in rabbit: Neural circuitry participating in the acquisition of trace eyeblink conditioning. Neuroscience. 2007;145:288–302. doi: 10.1016/j.neuroscience.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Weiss C., Disterhoft J.F. Eyeblink conditioning, motor control, and the analysis of limbic-cerebellar interactions. Behav. Brain Sci. 1996;19:479–504. [Google Scholar]
- Williams G.V., Goldman-Rakic P.S. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak D.S., Lavond D.G., Thompson R.F. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]