Abstract
Despite significant advances in identifying the risk factors and elucidating atherosclerotic pathology, atherosclerosis remains the leading cause of morbidity and mortality in industrialized society. These risk factors independently or synergistically lead to chronic vascular inflammation, which is an essential requirement for the progression of atherosclerosis in patients. However, the mechanisms underlying the pathogenic link between the risk factors and atherosclerotic inflammation remain poorly defined. Significant progress has been made in two major areas, which are determination of the roles of the receptors for pathogen-associated molecular patterns (PAMPs) in initiation of vascular inflammation and atherosclerosis, and characterization of the roles of regulatory T cells in suppression of vascular inflammation and atherosclerosis. In this review, we focus on three related issues: (1) examining the recent progress in endothelial cell pathology, inflammation and their roles in atherosclerosis; (2) analyzing the roles of the receptors for pathogen-associated molecular patterns (PAMPs) in initiation of vascular inflammation and atherosclerosis; and (3) analyzing the advances in our understanding of suppression of vascular inflammation and atherosclerosis by regulatory T cells. Continuous improvement of our understanding of the risk factors involved in initiation and promotion of artherogenesis, will lead to the development of novel therapeutics for ischemic stroke and cardiovascular diseases.
Keywords: endothelial cells, inflammation, receptors for PAMPs, regulatory T cells and atherosclerosis
1. INTRODUCTION
Atherosclerosis is characterized by focal arterial lesions containing cholesterol, fibrosis, intense immunological activity, inflammatory cell infiltrates and cell death [1]. Several risk factors have been identified for atherogenic process including hyperlipidemia, high density lipoprotein (HDL), cigarette smoking, diabetes, hypertension, obesity[2] and excessive quiescence[3]. We and others confirmed that hyperhomocysteinemia (HHcy) acts as an independent risk factor in accelerating atherosclerosis, etc [4–8]. In addition, atherosclerosis is positively correlated with the endotoxin load in patients’ plasma[9]. Endotoxinemia at levels as low as 50 pg/ml, that results from bacterial infection, constitutes a strong risk factor for the development of atherosclerosis[10]. These risk factors independently or synergistically lead to chronic vascular inflammation, which is an essential requirement for the progression of atherosclerosis in patients[11]. Despite significant advances in elucidating atherosclerotic pathology, atherosclerosis remains the leading cause of morbidity and mortality in industrialized society. Therefore, continuous improvement of our understanding on the atherogenesis initiated and promoted by risk factors will lead to the future development of novel therapeutics for ischemic stroke and cardiovascular diseases.
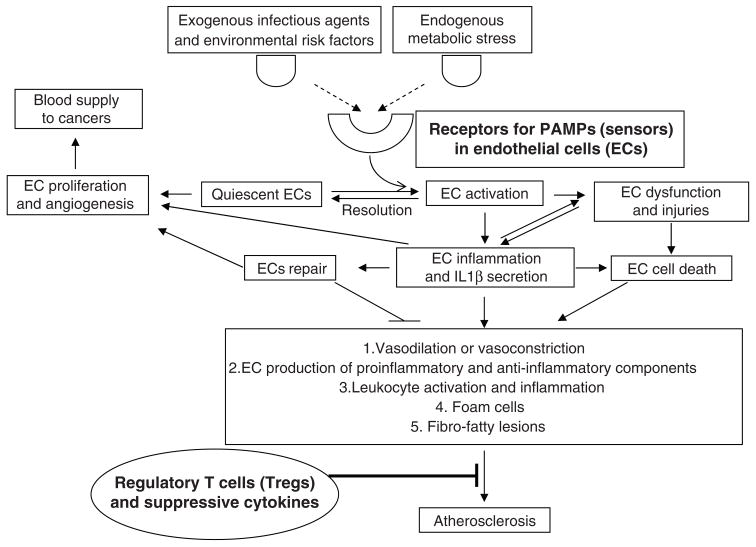
A typical multi-step picture in the atherogenic process has emerged [12]: first, atherosclerosis probably starts from endothelial cell (EC) inflammation, activation and dysfunction with the expression of adhesion molecules on the cell surface and secretion of proinflammatory cytokines. This step may be triggered by risk factors and metabolic stress signals. At the same time, lipids in the intima of arteries can accumulate. The low-density lipoproteins (LDL) are modified by enzymes and oxygen and are transformed into proinflammatory stimuli. Second, in response to the inflammation signals initiated in ECs, vascular smooth muscle cells (SMCs) release chemokines and chemoattractants, which act together with inflamed ECs in leading to the recruitment of monocytes and T cells into the arterial wall at specific sites. When the monocytes are translocated into the intimal layer and activated, they may differentiate into macrophages and then form foam cells by taking up lipid. Third, at this point, the inflammation has become chronic, and the fatty streak is now well on its way to becoming an atherosclerotic lesion. As the lesion matures, it becomes necrotic and calcified. Ultimately, the lesion may rupture, initiate a thrombus, block an artery, and cause a myocardial infarction or stroke[13]. The historical focus of immunological studies on regulation of atherogenesis has been on the functions of infiltrating macrophages and T cells. However, recent reports demonstrated that endothelial cells play a major role in the atherogenic initiation, changing their quiescence into activated phenotypes to support every phase of the inflammatory process[14,15]. In this review, we will focus on three related issues as we outlined in Fig. 1, (1) examining the recent progress in endothelial cell pathology, inflammation and their roles in atherosclerosis; (2) analyzing the roles of the receptors for pathogen-associated molecular patterns (PAMPs) in initiation of vascular inflammation and atherosclerosis; and (3) analyzing the advances in understanding of suppression of vascular inflammation and atherosclerosis by regulatory T cells. We apologize for not being able to include many valuable articles and reviews due to limited space.
Fig. 1.
Vascular inflammation and atherosclerosis are activated via receptors for PAMPs and suppressed by regulatory T cells.
2.Endothelial cell (EC) pathology and potential therapeutic targets
The ECs of all vascular beds form a single cell layer in vivo, which lines all blood and lymphatic vessels, and is located at the interface between circulation blood and the surrounding tissue. More than 1012 ECs in the human body cover a surface area of more than 1,000 m2. ECs have an elongated shape with an approximate size of 20 × 50 μm2 and the total weight of ECs is in excess of 100 g [16]. As such, the vascular endothelium can be considered a systemically disseminated “organ” system [17]. ECs serve a multitude of functions that help to maintain blood fluidity and thrombo-resistance, control vessel-wall permeability, and keep blood leukocytes and lymphocytes in a quiescent state. In pathological conditions, damaged, impaired, or dysfunctional ECs in these vascular beds contribute to the pathogenesis and complications of systemic and pulmonary hypertension, coronary heart disease, stroke, diabetes, kidney failure, and the major chronic diseases that constitute the leading causes of death and disability[18,19]. ECs are the organ that bridges several cardiovascular risk factors (e.g. a diet high in saturated fat, hypercholesterolemia, obesity, hyperglycemia, insulin resistance, hypertension, smoking[20], and congestive heart failure) and may serve as initiators in the development of vascular inflammation and atherosclerosis[21]. Proinflammatory cytokines, chemokines, and adhesion molecules that stimulate leukocytes also act on ECs [14] and promote EC inflammation. In order to better understand vascular EC inflammation, we outline the following aspects related to the EC pathology.
2.1. EC markers
Characteristic vascular EC markers include von Willebrand factor (factor VIII-related antigen), platelet endothelial cell adhesion molecule-1(PECAM1/CD31), CD34, CD105/endoglin, vascular-cell-adhesion molecule 1 (VCAM1/CD106), endothelin receptor B (ENDRB), P1H12/CD146, Tie 1, Tie 2, angiotensin converting enzyme (ACE), vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2 (KDR/Flk-1; kinase-insert domain receptor in humans, and fetal liver kinase-1 in mice)[22] and staining with Ulex europaeus lectin type 1 (for human cells). Of note, these EC markers are not universal (also see other sections) or consistent with every detection technique. In addition, cells at the afferent and efferent interfaces of lymph nodes (LNs) from all animals show differential expression of lymphatic endothelial cell (LEC) markers, with podoplanin, Prox-1, and vascular endothelial growth factor receptor 3 (VEGFR3) expressed in both microenvironments, but with lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) expressed only at the efferent interface. The chemokine CCL20 is uniquely expressed at the afferent interface by cells co-expressing podoplanin, and this expression is increased during infection with simian immunodeficiency virus or M. tuberculosis [23]. The common features of EC physiology include strong uptake of Dil-Ac-LDL and prostacyclin secretion. Moreover, ECs have specific morphology [16,24], which often adopt a typical “cobblestone” appearance at confluence and may form “tubes” in Matrigel (BD Matrigel™ Basement Membrane Matrix). Flow resistance of microvascular beds is higher than expected from in vitro studies of blood rheology, suggesting that there is a substantial layer on the luminal endothelial surface (endothelial surface layer, ESL) with a thickness in the range of 0.5–1 microm. In comparison, the typical thickness of the glycocalyx directly anchored in the endothelial plasma membrane amounts to only about 50–100 microm. The thick endothelial surface layer significantly impacts hemodynamic conditions, mechanical stresses acting on red cells in microvessels, oxygen transport, vascular control, coagulation, inflammation and atherosclerosis [25].
2.2. EC progenitors and circulating ECs
Endothelial progenitor cells are a circulating, bone marrow-derived cell population that appears to participate in both vasculogenesis and vascular homeostasis. This cell type may have tremendous therapeutic potential for a wide range of human diseases (see excellent review [26]). The endothelial progenitor cell population is defined by CD34+KDR+ (VEGFR2) double positive markers [22]. In addition, CD117 can also be an EC progenitor marker [27]. In certain disease conditions, circulating ECs (CECs) that have detached from affected blood vessels provide useful indicators for studying vascular injury, cardiovascular disease and progression of atherosclerosis [22]. CECs have also been detected in diverse endothelial damage conditions including acute coronary syndrome, coronary angioplasty, sickle cell anemia, thrombotic thrombocytopenic purpura, infection with Rickettsia conorii or cytomegalovirus, Behcet’s disease, systemic lupus erythematosus (SLE), and small vessel vasculitis. In addition, circulating endothelial cells (CECs) and circulating endothelial progenitors (CEPs) are promising surrogate biomarkers in oncology [28]. Three related circulating cell populations have been identified: (i) CECs are defined as CD45−, CD34+, and P1H12+; (ii) activated CECs are identified as CD45−. P1H12+, CD62+ or CD106+; and (iii) EC progenitors are defined as CD34+ and CD133+[29] or CD34+KDR+[22]. We and others have demonstrated that hyperhomocysteinemia (HHcy) acts as an independent risk factor in accelerating atherosclerosis [4–8]. We found that the peripheral EPC population is not significantly altered in HHcy mice. However, Hcy has a profound inhibitory effect on EC proliferation and migration at physiologically relevant concentrations and inhibits EC adhesion at concentrations of 200 μM and higher [6].
2.3 EC heterogeneity
There is significant heterogeneity in endothelial structure and function (see excellent reviews[30,31]) including (1) the heterogeneity in endothelial properties between species, organs, vessel classes and even within individual vessels and, (2) the differences in composition and role of the molecular layer on the luminal surface of endothelial cells. The endothelial lining of blood vessels in different organs differs with respect to morphology and permeability. Furthermore, the mediator release, antigen presentation or stress responses of endothelial cells vary between species, different organs and vessel classes. Finally, there are relevant differences even between adjacent endothelial cells, with some cells exhibiting specific functional properties, e.g. as pacemaker cells for intercellular calcium signals.
Organ-specific structural and functional properties of the endothelium are marked in the vascular beds of the lung and the brain. Pulmonary endothelium exhibits a high constitutive expression of adhesion molecules, which may contribute to the margination of the large intravascular pool of leucocytes in the lung. The pulmonary microcirculation is less permeable to protein and water flux as compared to large pulmonary vessels. Endothelial cells of the blood-brain barrier exhibit a specialized phenotype with no fenestrations, extensive tight junctions and sparse pinocytotic vesicular transport, which allows a strict control of exchange of solutes and circulating cells between the plasma and the interstitial space.
Arteries with different sizes or from different tissues vary in their sensitivities to inflammation and atherogenic stimuli. Human umbilical vein ECs (HUVECs) may be different from microvascular ECs in generating proinflammatory cytokines [32]. In addition, EC numbers in glomeruli and macrovessels are decreased in diabetes but EC numbers in retina are increased in diabetes [33]. Atherosclerosis predominantly affects large and mid-size elastic and muscular arteries at vessel bifurcations in heart, brain and extremities[3,34], which may result from the fact that aortic ECs located in lesion-prone regions are highly susceptible to injuries associated with atherosclerosis[35].
2.4 EC permeability
EC permeability is mediated by two pathways, transcellular and paracellular pathways – that is, solutes and cells can pass through (transcellular) or between (paracellular) ECs. In transcellular passage, cell fenestration or a complex system of transport vesicles can fuse and appear as channels that traverse single cell and allow the passage of leukocytes and solutes through ECs. In contrast, paracellular pathway is mediated by the coordinated opening and closure of EC cell-cell junctions. Several pharmacological strategies have been proposed in controlling EC permeability: (1) tyrosine kinase SRC inhibitors; (2) inhibitors blocking the association between VEGFR2 and VE cadherin; (3) inhibitors of VE-cadherin internalization; (4) inhibitors of metalloproteases and other lytic enzymes that can cleave extracellular domain of VE-cadherin; and (5) modulators that can modulate the activity of RAP1 or other small GTPases[36].
2.5 EC activation, inflammation and anti-inflammation targets
EC activation and EC inflammation are two interchangeable concepts in some reports. However, traditional EC activation suggests a status with upregulation of adhesion molecules and prothrombotic molecules; whereas EC inflammation has been defined as a status with generation of proinflammatory cytokines after stimulation.
Two types of EC activation have been identified. Type I activation is mediated by ligands that bind to heterotrimeric G-protein-coupled receptors. Type II activation is a more sustained inflammatory response mediated by IL-1β and TNF-α [14]. Upregulation of EC activation marker von Willebrand factor (vWF) levels is generally accepted as an indicator of EC injury [37]. Several other molecules are also important for EC activation. Vascular endothelial (VE)-cadherin is a strictly endothelial specific adhesion molecule located at junctions between endothelial cells. VE-cadherin is of vital importance for the maintenance and control of ECs contacts, vascular permeability and leukocyte extravasation. In addition to its adhesive functions, VE-cadherin regulates various cellular processes such as cell proliferation, apoptosis and modulates VEGF receptor functions[38]. PECAM-1 (or CD31) is a molecule expressed on all cells within the vascular compartment, being expressed to different degrees on most leukocyte sub-types, platelets, and on endothelial cells where its expression is largely concentrated at junctions between adjacent cells. As well as exhibiting adhesive properties, PECAM-1 is an efficient signaling molecule and is known to have diverse roles in vascular biology including roles in angiogenesis, platelet function, and thrombosis, mechano-sensing of EC response to fluid shear stress, and regulation of multiple stages of leukocyte migration through venular walls [39].
Significant progress has been made in identifying potential therapeutic targets for suppressing atherogenic inflammation, as outlined in Table 1[40–60] (also see a review by Rader and Daugherty[61]). Recent report shows that selective or combined inhibitors of cyclooxygenase (COX)-1, COX-2 and 5-lipooxygenase (5-LOX) might be beneficial in the treatment of atherosclerosis [58]. In addition, peroxisome proliferator-activated receptor (PPAR) α is a nuclear receptor activated by natural ligands such as fatty acids as well as by synthetic ligands such as fibrates currently used to treat dyslipidemia. PPARα improves markers for atherosclerosis and insulin resistance and inhibits inflammation [59].
Table 1.
Potential therapeutic interventions may inhibit ECs activation, inflammation and dysfunction,
| Potential therapeutics | Reference |
|---|---|
| ACE inhibitors | Anderson et al, 2000;Mancini et al, 1996 |
| Acyl-coA: cholesterol acyltransferase | Kharbanda et al, 2005 |
| Angiotensin II receptor antagonists | Ghiadoni et al, 2000 |
| Antioxidative Vitamins | Gilligan et al, 1994;McSorley et al, 2003 |
| β blockers | Kalinowski et al, 2003 |
| Calcium channel blockers | Taddei et al, 1997 |
| COX inhibitors | Chenevard et al, 2003 |
| Endothelin Antagonists and vasopeptidase inhibitors | Szabo et al, 1998 |
| HDL | Spieker et al, 2002 |
| Immune modulation therapy | Torre-Amione et al, 2004 |
| Intravenous immunoglobulin (IVIg) | Xu et al, 1998 |
| Metformin and rosiglitazone | Jensterle et al, 2008 |
| Pentoxifylline | Bruynzeel et al, 1997 |
| Peroxisome proliferator-activated receptor (PPAR) | Zandbergen et al, 2007 |
| Selective or combined inhibitors of cyclooxygenase (COX)-1, COX-2 and 5-lipooxygenase (5-LOX) | De Gaetano et al, 2003 |
| Soluble TNF receptors/anti-TNF-α antibodies | Aukrust et al, 2005 |
| Statins | Skaletz-Rorowski et al,2003 |
| Sonic hedgehog (Shh) | Agouni et al, 2007 |
| Tetrahydrobioperin and L- Arginine | Fukuda et al, 2002 |
2.6 Vasomotor dysfunction and EC injuries
EC injuries involve membrane damage, increased permeability, swelling and necrosis. EC dysfunction is characterized by impaired vasomotor response (reduced vasodilation and increased EC-dependent contraction), cell proliferation, platelet adhesion/aggregation, vascular permeability and leukocyte-endothelial interactions that participate in vascular inflammation [21]. EC’s vasodilator dysfunction and injuries precede the establishment of the earliest lesions of atherosclerosis [18], which thus is a common precursor and denominator of various pathologic conditions, such as stroke, myocardial infarction, hypertension and atherosclerosis [62]. There are different techniques to evaluate the EC functional activity, which can be classified into EC-dependent and EC-independent based on the amount of nitric oxide (NO) produced and the vasodilation effect. EC vasomotor function/dysfunction is assessed on a pressure myograph as the vessel myogenic response to acetylcholine [63,64], which remains the gold standard[62]. In addition, several EC dysfunction markers have been proposed, including elevated circulating levels of von Willebrand factor, plasminogen activator inhibitor-1, some adhesion molecules, isoprostane and thrombomodulin[62]. Nearly all stimuli elicit vasodilation via nitric oxide, which is a volatile gas, biologically active, presents in all tissues. NO is produced by the action of nitric oxide synthases (NOS) on L-arginine amino acid. Both constitutive and inducible NOS (iNOS) are expressed in ECs [65]. EC specific NOS (eNOS) is a point of convergence of signaling pathways responsible for the execution of hemodynamic adaption in physiologic and pathologic conditions. eNOS is stimulated by fluid shear stress, cyclic strain, acetylcholine, endothelins, angiotensins II and IV, bradykinin, estrogens, VEGF, insulin-like growth factor-1 (IGF-1), opiates, cannabinoids, L-arginine and TGF-β. In contrast, eNOS is inhibited by high NO output from iNOS, high CO output from heme oxygenase-1, superoxide anions, oxidized LDL, asymmetric dimethylarginine (ADMA), advanced glycation end products (AGEs), hyperglycemia, erythropoietin and TNF-α [62]. EC-derived NO activates the guanylate cyclase of vascular smooth muscle cells to promote cGMP-dependent vasodilation [66]. A risk factor for cardiovascular disease, hyperhomocystinemia (HHcy), is associated with EC dysfunction. We found that arterial relaxation in response to the EC-dependent vessel relaxant, acetylcholine or the NOS activator (A23187), is significantly impaired in cystathionine beta-synthase null (CBS(−/−)) mice, a HHcy mouse model. eNOS activity is significantly reduced in mouse aortic endothelial cells (MAECs) of CBS(−/−) mice, as well as in Hcy-treated mouse and human aortic endothelial cells (HAECs). Ultimately, a protein kinase C (PKC) inhibitor, GF109203X (GFX), reverses Hcy-mediated eNOS inactivation and threonine 495 phosphorylation in HAECs. These data suggest that HHcy impairs endothelial function and eNOS activity, primarily through PKC activation[5]. EC dysfunction promotes vascular inflammation by inducing the production of vasoconstrictor agents, adhesion molecules and growth factors[21]. Considering the role of the endothelium in the initiation and propagation of vascular wall injury, there is a need for the discovery of validated biomarkers to serve as a predictor of activation of inflammatory cascades in the development of vascular injury[67].
2.7 EC responses to proinflammatory cytokines
As outlined in Table 2[68–83], proinflammatory cytokines and anti-inflammatory cytokines play critical roles in modulation of atherogenesis (also see a comprehensive review[84]). Mechanistically, locally released proinflammatory cytokines and chemokines activate ECs to upregulate soluble adhesion molecules, activate neutrophils and generate reactive oxygen species, which serve to amplify the initial inflammation leading to dysregulated apoptosis, secondary necrosis and overt vascular injury lesions [67]. By releasing substances via autocrine, paracrine or endocrine manners, ECs modulate (i) the vascular smooth muscle cells and exercise vasodilation or vasoconstriction; (ii) production of prothrombotic and antithrombotic components, and fibrinolytics and antifibrinolytics; (iii) EC proliferation; (iv) EC migration; and (v) leukocyte activation and inflammation [65]. During inflammation, cell surface adhesion molecules guide the adhesion and migration of circulating leukocytes across the EC lining of the blood vessels to access the site of injury. In addition, oxygen-derived free radicals mediate the disruption of the EC surface layer and increase vascular wall adhesiveness by ox-LDL [85]. Of note, EC denudation is not an absolute prerequisite to allow platelet attachment to arterial wall[86].
Table 2.
Pro-inflammatory cytokines and anti-inflammatory cytokines play critical roles in regulation of atherogenesis.
| cytokines | Animal Genotype | Genomic background | Effect on atherogenesis | Effect on plasma cholesterol | Reference |
|---|---|---|---|---|---|
| Anti-inflammatory | |||||
| IL-10 | ApoE−/− 10−/− | C57BL/6 | increase | no change | Caligiuri et al, 2003 |
| APOE*3-LeidenIL10 overexpression | C57BL/6 | reduce | lower | Eefting et al, 2007 | |
| IL-2 | ApoE−/− IL-2 injection | C57BL/6J | increase | Upadhya et al, 2004 | |
| ApoE−/− anti-IL-2 injection | C57BL/6J | reduce | Upadhya et al, 2004 | ||
| Il-5 | LDLR−/− IL-5 bone marrow transplantation | C57BL/6 | increase | no change | Binder et al, 2004 |
| TGF-β | ApoE−/− anti-TGF-β injection | C57BL/6J | increase | no change | Mallat et al, 2001;Robertson et al, 2003 |
| Pro-inflammatory | |||||
| IL-1 β | ApoE−/−/IL-1 β −/− | C57BL/6J | reduce | no change | Kirii et al, 2003 |
| IL-4 | LDLR−/− IL-4−/− | C57BL/6 | no change | no change | King et al, 2007 |
| ApoE−/− IL-4−/− | C57BL/6 | reduce | no change | Davenport et al,2003 | |
| IL-12 | ApoE−/− IL-12−/− | C57BL/6 | reduce | no change | Davenport et al,2003 |
| IL-18 | ApoE−/−IL-18−/− | C57BL/6J | reduce | higher | Elhage et al, 2003 |
| IL-1Ra | ApoE−/− IL-1Ra overexpression | 50% C57BL/6J and 50% DBA/2 | reduce | no change | Merhi-Soussi et al,2005 |
| IL-6 | ApoE−/− IL-6 injection | C57Bl/6 | increase | no change | Huber et al, 1999 |
| ApoE−/−IL-6−/− | C57BL/6 | increase | higher | Schieffer et al, 2004 | |
| IL-33 | ApoE−/− IL-33 injection | C57BL/6 | reduce | Miller et al, 2008 | |
| TNF-α | ApoE−/− TNF-α −/− | 75%C57BL/6J and 25% 129S | reduce | higher | Branen et al, 2004 |
| IFN-γ | LDLR−/−IFN-γ −/− | C57BL/6 | reduce | no change | Buono et al, 2003 |
EC responses can be different to the same group of inflammation-regulating cytokines. Proinflammatory cytokine tumor necrosis factor-α (TNF-α) selectively diminish the ability of arterial rings to relax to the EC-dependent vasodilator acetylcholine, indicating that cytokine activity may be associated with endothelial vasodilator dysfunction as judged by arterial vascular tone. The cytokine granulocyte colony stimulating factor (G-CSF) and erythropoietin induce ECs relaxed, whereas proinflammatory cytokines TNF-α, interleukin-6 (IL-6) and suppressive cytokine IL-10 induce contraction of human arterial segments, suggesting a complex picture for relexation/contraction during inflammation. Cytokine induced relaxation or constriction are inhibited by blockers of endothelial-derived nitric oxide (NO) and endothelin, respectively. Reports in patients with congestive heart failure have shown a correlation between plasma levels of IL-6 and TNF-α and impaired endothelium-dependent vasodilation of the brachial artery and vein. The proinflammatory cytokines TNF-α and IL-1β (but not IL-6) induce transient and reversible EC dysfunction in humans. Importantly, TNF-α activates nuclear factor-κB (NF-κB) with subsequent upregulation of EC adhesion molecules. Adhesion and transmigration of different leucocyte subsets are stimulated after TNF treatment of ECs. In this regard, TNF-α enhances EC adhesion and vascular invasion of dendritic cells, the most potent antigen presenting cell type. Therefore, TNF-α might decrease endothelial NO bioactivity by several pathways. Importantly, ECs transfected with eNOS (endothelial NOS) reduce monocyte-endothelial binding after TNF-α stimulation. Finally, TNF-α-mediated oxidative stress may directly cause apoptotic cell death of the endothelium and up-regulates the death signaling protein, Fas/apo-1/CD95, a cell surface-borne protein belonging to the TNFR (TNF receptor) superfamily. A recent report showed that mildly oxidized LDL (low-density lipoprotein)-induced apoptosis of human coronary ECs involves TNFRs. These data suggest that ECs are the major targets of cytokine signals derived from different vascular cells [87].
In addition to classical proinflammatory cytokines, a few adipocytokines from fat tissue have recently been identified including leptin, adiponectin and resistin. Both leptin and resistin are pro-inflammatory. Leptin induces endothelial dysfunction, increase blood pressure and atherosclerosis. Resistin activates NF-κB-dependent cytokine release and adhesion molecule expression. In contrast, adiponectin is anti-inflammatory and vasculo-protective, which prevents atherosclerosis [88].
2.8 EC proliferation, angiogenesis and repair
Endothelial cell proliferation and replication are associated with vessel proliferation and angiogenesis, which are new therapeutic targets in diseases, such as cancer and muscular dystrophy[89]. Vascular morphogenesis encompasses a temporally regulated set of morphological changes that endothelial cells undergo to generate a network of interconnected tubules. The formation of a new capillary involves multiple steps such as EC activation, migration, alignment, proliferation, tube formation, branching, anastomosis, and maturation of intercellular junctions and the surrounding basement membrane, etc. Each of these stages is either known or suspected to fall under the influence of VEGF, notch, and transforming growth factor-β (TGF-β)/bone morphogenetic protein signaling pathways[90].
Blood vessels, either in insufficient numbers or in excess, contribute to the pathogenesis of many diseases, thus, agents that stimulate angiogenesis can improve blood flow in patients with ischemic diseases. In contrast, anti-angiogenic agents are used to treat disorders ranging from macular degeneration to cancer. Tumor vascular endothelium is characterized by its increased permeability, abnormal morphology, disorganized vascular networks and variable density. There are two types of angiogenesis inhibitors: first, antiangiogenesis agents include the monoclonal antibody bevacizumab; and tyrosine kinase inhibitors sorafenib and sunitinib that disrupt EC survival and block new tumor blood supply, second, vascular disrupting agents that include those that disrupt the already established vasculature[91].
The repair of the endothelium after inflammatory injury is essential to maintaining homeostasis. Using a mouse model of carotid injury linked re-endothelialization, we have observed impaired re-endothelialization and increased neointimal formation in severe hyperhomocysteinemia mice. The capacity of homocysteine to inhibit proliferation and migration of EC may be responsible for impaired re-endothelialization and contribute to arteriosclerosis in hyperhomocysteinemia[6]. We also reported previously that homocysteine (Hcy) inhibits EC growth by transcriptional inhibition of the cyclin A gene via a hypomethylation-related mechanism[92]. The mRNA levels of cyclin A are significantly suppressed by Hcy and a DNA methyltransferase inhibitor. Hcy inhibits DNA methyltransferase 1 (DNMT1) activity by 30%. Adenovirus-transduced DNMT1 gene expression reverses the inhibitory effect of Hcy on cyclin A expression and EC growth inhibition[8].
Endotoxins are a set of complex lipopolysaccharide (LPS), which are an integral component of gram-negative bacteria such as E. coli [93]. Loss of the normal EC structure is observed in the aorta of the animals treated with LPS[94]. Erythropoietin is found to inhibit LPS-induced EC apoptosis. EC injury activates the mobilization of circulating angiogenic cells, thus completing EC repair[94].
Microparticles (MPs) are small fragments generated from the plasma membrane after stimulation. Among the proteins harbored by MPs, sonic hedgehog (Shh) is present in MPs generated from activated/apoptotic T lymphocytes[95]. Shh carried by MPs induces NO release from ECs and decreases production of reactive oxygen species. When the phosphoinositide-3 kinase (PI3-kinase) and extracellular-signal-regulated kinase (ERK) signaling are specifically inhibited, the effects of MPs are reversed. In vivo injection of MPs in mice is also able to improve EC function by increasing NO release, and it reverses EC dysfunction after ischemia/reperfusion, suggesting that Shh represents a new therapeutic approach against endothelial dysfunction during acute severe endothelial injury[60].
2.9 Immunogenicity of ECs
Even though ECs are not listed as a type of antigen presenting cell, the important role of ECs in antigen-presentation and immunogenicity in vascular inflammation and autoimmune responses have been recognized. ECs represent a highly heterogeneous population of cells with the ability to modulate the function of immune cells [96]. Matrix-embedding confers a quiescent EC state with reduced expression of chemokines, adhesion, co-stimulatory, and major compatibility complex molecules. Matrix-embedded ECs induce an immune-inhibitory phenotype of dendritic cells and regulatory T cells to a greater extent than non-embedded ECs[34]. However, liver sinusoidal endothelial cells (LSEC) are microvascular endothelial cells with a unique phenotype reminiscent of dendritic cells and a unique function as APCs for CD4+ T cells[97]. All the microvascular and small vessel endothelial cells are ‘constitutively’ positive for MHC class II antigens.
Immunogenicity of ECs is significantly upregulated when ECs are activated. Activated ECs express major histocompatibility complex (MHC) class II and T cell co-stimulators such as CD80 and CD86[14,34,98,99], and select the migration of antigen-specific lymphocytes into the site of inflammation [100]. Unlike bone marrow-derived antigen presenting cells, which utilize B7/CD28 interactions, human ECs utilize lymphocyte function antigen 3 (LFA3)/CD2 pathways to stimulate T cells. ECs activate a CD45RO + B7-independent subpopulation of T cells. Release of MHC and non-human leukocyte antigens (HLA) from ECs stimulates an alloantibody and autoimmune response leading to chronic transplant rejection [101]. In vivo studies suggest that aortic ECs can acquire and cross-present exogenous antigen (Ag) on MHC class I. The process is most efficient for intact proteins rather than degraded peptide fragments[102].
The ET(B)R inhibitor BQ-788 increases T cell adhesion to human ECs in vitro, an effect countered by intercellular adhesion molecule-1 (ICAM-1) blockade or treatment with NO donors[103]. In addition, EC auto-antigens are recognized by anti-endothelial cell antibodies (AECA), which are found in various diseases and recognize several auto-antigen determinants. Some AECA activate ECs, resulting in increased leucocyte adhesiveness, activation of vascular thrombosis[104]. Moreover, oligomerization of the chemokine interferon-γ (IFN-γ)-inducible protein of 10 kDa (IP-10; CXCL10) plays an important role in the recruitment of activated T lymphocytes via transendothelial migration into sites of inflammation by interacting with CXCR3 [105].
In searching for the mechanism underlying the generation of autoantigens and autoantibodies, we found that alternative splicing occurs in 100% of the autoantigen RNA RNA transcripts associated with various autoimmune diseases. This is significantly higher than the approximately 42% rate of alternative splicing observed in the 9554 randomly selected human gene transcripts (p<0.001). In addition, the product of a transcript that does not undergo alternative splicing is unlikely to be a target antigen in autoimmunity[106]. We also found that TNF-α downregulates expression of prototypic alternative splicing factor ASF/SF2, which correlates with our finding of reduced expression of ASF/SF2 in inflamed cells from patients with autoimmune disease[107], suggesting that proinflammatory cytokines upregulate untolerized auto-antigens via modulation of expression of ASF/SF2. Based on these data, we proposed a new model of stimulation-responsive splicing for the generation of autoantigens and self antigens[108].
2.10. EC death
Programmed cell death (PCD) plays an essential role in the EC homeostasis and pathology. Eight forms of cell death have been identified in various types of cells, including two well-characterized forms, apoptosis and autophagy [109,110], and six atypical forms, including paraptosis, calcium-mediated cell death, apoptosis-inducing factor (AIF)/poly-(ADP-ribose) polymerase (PARA)-dependent cell death, oncosis[111], caspase-1-dependent inflammatory cell death pyroptosis[112] and caspase-1-indepednent inflammatory cell death pyronecrosis[113]. It is unclear whether all of the forms of cell death play a role in regulation of EC homeostasis. EC dysfunction is associated with increased rates of EC apoptosis. The presence of severe EC dysfunction and increased apoptotic rates of circulating ECs are associated with a high incidence of cardiovascular events in patients with severe hypertension, coronary heart disease, atherosclerosis, allograft vasculopathy, heart failure, diabetic retinopathy and scleroderma[114–116]. EC death favors thrombosis[117]. The inducers of apoptosis in atherosclerosis include Oxidized LDL (oxLDL) [118], oxysterols, reactive oxygen species, reactive nitrogen species (NO at high levels), X-, γ, and UV radiation, heat, cytokines and Fas ligand; whereas the inhibitors of apoptosis in atherosclerosis include shear stress, NO at low levels, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), cowpox virus CremA, baculovirus protein p35, the inhibitors of apoptosis (IAP) protein family and microorganism [115]. Overexpression of the integrin-linked kinase(ILK) gene significantly prevents oxLDL-induced cell death[118]. In addition, mechanistically, Fas ligand (FasL) can induce apoptosis in cells bearing the Fas receptor. Overexpression of endothelial FasL is anti-inflammatory and inhibit atherosclerotic lesion by 49% under hypercholesterolemic conditions, suggesting that EC apoptosis induced by attack of Fas-expressing cells (lymphocytes and inflammatory cells) contributes to atherogenesis [119].
3. Receptors for pathogen-associated molecular patterns (PAMPs) and vascular cell inflammation
In addition to being targets during the secondary wave of vascular inflammation, ECs are capable of expressing a broad spectrum of pro- inflammatory cytokines including IL-1β, IL-3, IL-5, IL-6, IL-8, IL-11, IL-15, TNF-α (tumour necrosis factor), chemokines [IL-8, monocyte chemotactic protein-1 (MCP-1) and RANTES], CSFs (colony-stimulating factors), GM-CSF(granulocyte/macrophage CSF), PDGF, VEGF and FGF, and anti-inflammatory cytokines including IL-1α, IL-10, IL-13 and TNF-β[87], suggesting an essential role of ECs in initiating the first wave of vascular inflammation. The question regarding how risk factors, metabolic stress and danger signals trigger vascular endothelial cell inflammation remain unknown. An exciting area of progress is the identification of the roles of PAMP recognition receptors (PRRs) in the pathogenesis of atherosclerosis. Four major families of PRRs have been identified as important components of innate immunity, participating in the sensory systems for host defense against the invasion of infectious agents and uncontrolled metabolic stress (danger signals). The Toll-like receptors (TLRs) recognize a variety of conserved microbial PAMPs derived from bacteria, viruses, protozoa and fungi. TLRs work in synergy with the three cytosolic sensing receptor families including NLRs [NOD (nucleotide binding and oligomerization domain)-like receptors] (which sense bacteria), RLRs [RIG-I (retinoic acid-inducible gene 1)-like receptors] (which sense viruses) and CLRs (C-type lectin receptors) (which sense fungi). All of these receptor families signal an upregulation of a range of immune and inflammatory genes. Elucidation of detailed mechanisms underlying how receptors for PAMPs bridge risk factors to vascular cell inflammation and atherosclerosis holds great therapeutic potential. Unlike existing anti-inflammatory drugs, new drugs based on characterization of PRRs should be capable of inhibiting specific components of innate immunity and inflammation, leaving sufficient redundant function to avoid issues of immunosuppression[120].
3.1. PAMP receptor features
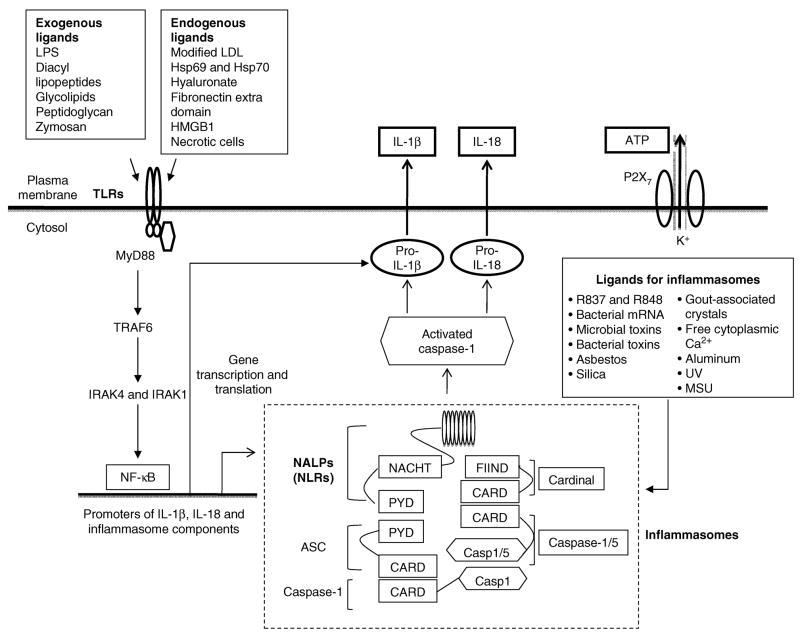
All PAMPs-recognition receptors (PRRs) share the following characteristics: PRRs are (1) germline-encoded; (2) they are expressed in a wide variety of host tissues and cells including ECs; (3) they share some of seven conserved domains including the LRR (leucine-rich repeat) domain, the TIR [Toll/IL (interleukin)-1 receptor] domain, the NBS (nucleotide-binding site), the CARD (caspase recruitment domain), the PYD (pyrin domain), the helicase domain and the CTLD (C-type lectin domain)[121]; and (4) they are capable in recognizing a limited numbers of evolutionally conserved molecules. PRRs have three major functions: (i) transcriptionally activating nuclear factor-κB (NF-κB)-dependent gene expression, such as the expression of proinflammatory cytokines pro-IL-1β, pro-IL-18 and TNF-α; (ii) transcriptionally inducing the expression of type I interferons (IFN-β) and IFN-dependent genes including inducible nitric oxide synthase; and (iii) post-translationally activating inflammasome-related caspase-1, secretion of IL-1β and IL-18, as outlined in Fig. 2. In addition, another class of receptors are considered as PRRs, which are scavenger receptors (SRs) expressed mostly in macrophages but also in endothelial cells (SREC I, SRECII and SR-PSOX). These receptors recognize distinct “epitopes” on the modified low-density lipoprotein (LDL) and participate in internalization of LDL particles and phagocytic clearance. Some SRs may also function as co-receptors of TLRs, involved in macrophage activation [15].
Fig. 2. TLRs and NLRs signaling pathways in the generation of proinflammatory cytokines IL-1β and IL-18.
Abbreviations: ASC, apoptosis-associated speck-like protein containing a CARD; CARD domain, caspase recruitment domain; FIIND, F-interacting domain of Cardinal protein; HMGB1, High mobility group box 1; Hsp60, heat shock protein 60; IRAK4 and IRAK1, IL-1 receptor (IL-1R)–associated kinases 4 and 1; IL-1β, interleukin-1β; LDL, low density lipoproteins; LPS, lipopolysaccharides; MSU, monosodium urate; MyD88, Myeloid differentiation primary response gene (88); NACHT domain, a 300 to 400 residue predicted nucleoside triphosphatase (NTPase) domain present in NAIP, CIITA, HETA and TP1; NALP, NACHT domain, LRR domain, and pyrin domain- containing protein; NF-κB, nuclear factor-κB; NLRs, Nod-like receptors; P2X7, a purinergic receptor; PYD, pyrin domain; R837 and R848, imidazoquinoline compounds that serve as inflammasome activating agents and TLR7 and TLR8 agonists; TLRs, toll-like receptors; TRAF6, TNF receptor-associated factor 6; UV, Ultraviolet light.
3.2. TLRs and their roles in atherosclerosis
TLRs represent a primary line of defense against invading pathogens in mammals, plants and insects. TLRs recognize the structures of lipids, carbohydrates, nucleic acids and various proteins, which are collectively referred to as pathogen associated molecular patterns (PAMPs)[122]. Eleven TLRs have been identified in humans while 13 TLRs can be encoded by mouse genome. TLRs 1–9 are conserved between humans and mouse[123]. TLRs are primary sensors in response against PAMPs[124]. TLRs are also important for induction of adaptive immune responses, but misguided responses can lead to autoimmune pathology[124]. Of note, TLRs 1, 2, 4, 5, and 6 are located in the plasma membrane and recognize bacterial wall components. In comparison, TLRs 3, 7, 8, and 9 are preferentially expressed in intracellular compartments, such as endosomes, and recognize nucleic acid structures[123]. TLR repertoire can be further expanded in the following two manners: (1) formation of heterodimers, TLR2/TLR1 and TLR2/TLR6; and (2) cooperation with other PRRs[15]. TLR signaling shares IL-1 receptor pathway, activates the NF-κB (Fig. 2), mitogen-activated protein kinase (MAPK), and interferon (IFN)-regulatory factor 3 (IRF3), and releases proinflammatory cytokines[15,125]. Several IKK [inhibitors of NF-κB (IκB) kinase] and NF-κB inhibitors are under development[126]. How these inhibitors will affect TLR signaling, vascular inflammation and atherosclerosis remains to be seen. Co-stimulation and interplay of TLR4 or TLR3 with TLR7 (stimulated by anti-viral compound R848) has been reported to induce secretion of mature IL-1β, implying the TLR synergy activates an inflammasome efficiently. However, R848, the TLR7/8 ligand, can also activate NALP3 inflammasomes (see next section). It is not clear if the effective mature IL-1β production induced upon cotreatment of LPS or poly (I:C) with R848 is derived from TLR3/4 and TLR7 synergy, TLR3/4 and NALP3 interplay, or both[127].
In addition to exogenous ligands including LPS[128], an envelope glycoprotein encoded by mouse mammary tumor virus[129], diacyl lipopeptides, glycolipids, peptidoglycan and zymosan[15], TLRs can also be activated by host-derived stress-associated molecular patterns (SAMPs), including oxidized low density lipoprotein (oxidized LDL), high mobility group B1 (HMGB1), biglycan, hyaluronic acid fragments, heat shock protein (HSP)70, HSP60, fibronectin extra domain [15], serum amyloid A, necrotic cells and possibly advanced glycation end-products[3,15]. Proatherogenic TLR2 responses to unknown endogenous or unknown endemic exogenous agonists are mediated by non-bone marrow derived cells including endothelial cells[3]. Researchers have identified at least 15 different negative regulators of the TLRs including MyD88s (a short form of MyD88), IRAKM, SOCS1, NOD2, PI3K and TOLLIP [130]. TLR4 antagonists, such as CRX-526, E5531 and E5564, have been under development as potential therapeutics [129].
3.3. NLRs and inflammasomes
NOD-like receptors (NLRs; also known as CATERPILLAR proteins) are defined by their tripartite domain structure containing a variable C-terminus, a middle nucleotide-binding domain (NBD, also known as the NACHT domain), and a leucine rich repeat (LRR) domain. The mid-NBD domain exhibits ATPase activity, and regulates oligomerization. The LRR domain mediates autoregulation, protein-protein interaction, and/or PAMP sensing. NLRs are highly conserved through evolution. The NLR system comprises 22 cytoplasmic proteins that include 5 members of the NOD (Nucleotide-binding oligomerization domain) subfamily, 14 NALP (NACHT-, LRR- and pyrin-domain-containing proteins) members, IPAF, NAIP and CIITA. The putative function of the majority of 22 human NLRs in activating caspase-1 has not been confirmed except IPAF, NALP1, NALP2 and NALP3[19]. Stimulation of NOD1 and NOD2, two prototypic NLRs, results in the activation of MAPK and NF-κB. On the contrary, a different set of NLRs induces caspase-1 activation through the assembly of a protein complex termed the inflammasome[131]. Three inflammasomes have been characterized[132]: NALP1 inflammasome, NALP3 inflammasome and IPAF inflammasome. NALP1 inflammasome consists of four components, NALP1, PYCARD (ASC)[133], caspase-1 and caspase-5, and functions as primary mediator of susceptibility to anthrax lethal toxin[134]; NALP3 inflammasome consists of three components, NALP3, PYCARD and caspase-1, and is capable in sensing a broad range of stimuli including anti-viral compounds R837 and R848, bacterial mRNA, UVB irradiation-induced increase in free cytoplasmic Ca++ [135], gout-associated uratic acid crystals, aluminium adjuvants[136], asbestos and silica[137], bacterial toxins derived from Listeria M., staphylococcus A. and shigella F, etc[138]; IPAF inflammsome consists of three components, IPAF[139], NAIP and caspase-1 and functions to sense flagellin derived from Legionella P., Salmonella T., pseudomonas A. and shigella F[138]. A database search has found as many as 203 putative NLRs in the sea urchin genome[138], suggesting that additional mammalian inflammasomes may be found. An important function of NLRs is in the regulation of caspase-1 activation and proinflammatory cytokine interleukin-1 (IL-1β) processing. Caspase-1 is responsible for conversion of pro-IL-1β to mature IL-1β (also known as IL-1β-converting enzyme (ICE)) (Fig. 2). In addition, two other related cytokine precursors, pro-IL-18 and pro-IL-33 are also cleaved by caspase-1[138]. ASC is markedly expressed at the site of vascular injury. Neointimal formation was significantly attenuated in ASC−/− mice after injury. IL-1β and IL-18 are expressed in the neointimal lesion in wild-type mice but show decreased expression in the lesion of ASC−/− mice. These findings suggest that ASC is critical for neointimal formation after vascular injury and identify ASC as a novel therapeutic target for atherosclerosis and restenosis[140].
In addition to NLRs-containing inflammasomes, the cytoskeleton-organizing protein PSTPIP1 requires the familial Mediterranean fever protein termed pyrin to assemble the ASC pyroptosome, a molecular platform that recruits and activates caspase-1. Pyrin is a cytosolic receptor for PSTPIP1. Ligation by PSTPIP1 activates pyrin, thereby allowing it to interact with ASC and facilitate ASC oligomerization into an active ASC pyroptosome, which is a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation [141]. Therefore, constitutive ligation and activation of pyrin by mutant PSTPIP1 proteins explain the autoinflammatory phenotype observed in pyogenic sterile arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome [142]. Whether this autoinflammatory phenotype contributes to vascular inflammation remains to be addressed.
3.4. Inflammatory caspases and inflammatory cytokines
Inflammatory caspases and inflammasomes are newly-identified master sensors and switches of inflammation initiation. Inflammatory caspases (also known as group 1 caspases) are encoded by three main genes in humans, caspase-1, caspase-4 and caspase-5 and three main genes in mouse, caspase-1, caspase-11, and caspase-12. These caspases are characterized by the presence of a CARD domain at the N-terminus [132]. Three human caspases, caspase-1, caspase-4 and caspase-5, are inflammatory caspases since the main caspase-1 substrates are precursors of proinflammatory cytokines pro-IL-1β, pro-IL-18 (Fig. 2), and pro-IL-33. In addition to these three cytokines, caspase-1 cleaves 41 protein substrates including proteins along the glycolysis pathway [143](also see http://origin.bic.nus.edu.sg/casbase-bin/squery_browse.pl). Caspase-1 mediates serum-withdrawal-induced apoptosis of endothelial cells, independently of reactive oxygen species production[144]. Tripeptidyl peptidase II inhibitor (AAF-cmk), proteosome inhibitors lactacystin[145] and MG132 [146]inhibit maturation of caspase-1 and Shigella-induced or anthrax lethal toxin-mediated apoptosis. Caspase-1-mediated myocardial apoptosis plays an important role in the progression of heart failure [147]. Studies in caspase-12 deficient mice suggest that this caspase is important for endoplasmic reticulum (ER) stress-induced apoptosis[148]. However, caspase-12 deficient mice clear bacterial infection more efficiently than wild-type littermates and have an enhanced production of the proinflammatory cytokines IL-1β and IL-18 but not TNF-α and IL-6. Thus, caspase-12 is considered to be a decoy caspase that blocks caspase-1 activation resulting in enhanced vulnerability to bacterial infection and septic mortality[132,149].
3.5. IL-1 family
Three cytokines, pro-IL-1β, pro-IL-18, and pro-IL-33 belong to the IL-1 family[150] and play essential roles in inflammation[132]. IL-1 cytokines IL-1β, IL-1α, IL-1 receptor antagonist (IL-1Ra) induce expression of many effector proteins, e.g. cytokines/chemokines, adhesion molecules, VCAM-1, ICAM-1, MCP-1[68], NOS, matrix metalloproteinases (MMPs) and prothrombotic function [151]. IL-1β is the most potent proinflammatory cytokine among all proinflammatory cytokines including tumor necrosis factor-α, interferon-γ and IL-6. Eleven members of IL-1β family have been identified[150]. IL-1β does not have a signal peptide, thus IL-1β is secreted via a nonclassical secretory pathway, which can be inhibited by methylamine, low temperature, or serum-free medium, and can be enhanced by raising culture temperature, or by the presence of calcium ionophores, dinetrophenol, carbonyl cyanide chlorophenylhydrazone, or the classical secretory pathway inhibitors brefeldin A and monensin[152]. Purinergic receptor P2X7, that senses for ATP released from injured cells, triggers IL-1β secretion[153]. Histone deacetylase inhibitors prevent exocytosis in IL-1β-containing secretory lysosomes via disrupting microtubules[154]. IL-18 is a key player in models of atherosclerosis, lupus erythematosus, graft-versus-host disease, hepatitis, appetite control and obesity. The IL-18 binding protein, a naturally occurring and specific inhibitor of IL-18, neutralizes IL-18 activities. Other therapeutic options for reducing IL-18 activities are inhibitors of caspase-1, human monoclonal antibodies to IL-18, soluble IL-18 receptors, and anti-IL-18 receptor monoclonal antibodies [155].
4. The roles of the receptors for PAMPs and proinflammatory cytokines in pathogenesis of atherosclerosis
4.1. Mouse models of atherosclerosis
Mouse models have been extensively used in characterizing atherogenesis and vascular inflammation. Normal mice are short-lived and resistant to cardiovascular diseases, a number of genetic mutant mouse models have been developed via either spontaneous mutation or gene manipulation[156]. ApoE−/− deficient mouse is the most widely used mouse model with total plasma cholesterol concentration of 11 mM, compared to 2 mM for the parent wild-type C57BL/6 mice[157] and it exhibits advanced intimal lesions that are largely confined to the aortic root area. To mimic widely-distributed atherosclerosis that is seen in humans, a high cholesterol diet with the induction of plasma cholesterol concentrations (approaching 30 mM) is required[156]. Apolipoprotein E (ApoE) targets IL-1 receptor-associated kinase-1 (IRAK-1) activation and thereby interrupts IL-1β and IL-18 signaling in vascular smooth muscle cells (VSMCs), which represents a novel antiatherogenic activity of ApoE[158]. The second widely-used atherosclerosis model is low density lipoprotein receptor (LDLR) −/− deficient mice. However, these mice have mildly elevated cholesterol levels on a chow diet (5.8 versus 3.1 mM for homozygous and wild-type). When fed a 1.25% cholesterol diet, homozygotes have extremely high plasma cholesterol concentrations in the 41 mM range [156]. In the case of normal chow diet-fed LDLR−/− mice, there is the minimal amount of atherosclerotic lesions present, even after 9–12 months [159].
Other mouse models of atherosclerosis include the following: (1) high density lipoprotein (HDL) scavenger receptor class B type 1 deficient mice with ApoE−/− deficiency that are sensitive to high fat/high cholesterol diet and have total cholesterol levels in the range of 40 mM, which develop severe coronary artery disease; (2) the db/db mouse strain carries a spontaneous mutation of the leptin-ObR system that is analogous to the cp gene. Several studies showed impaired endothelial and vascular function, abnormal cardiac function, retinal damage, and glomerular sclerosis consistent with frank diabetes; (3) the ob gene is a mutation resulting in the production of a defective leptin that does not bind to the ObR. There are cardiac and vascular dysfunctions, but there are no reports of atherosclerosis and ischemic lesions[156].
4.2. TLRs, inflammasome and atherosclerosis
The depletion of TLR2 in LDLR−/− deficient mice leads to a significant reduction (50%) of lesion size in both aortic sinus and the aorta of mice fed with hypercholesterolemic diet[160]. TLR2 expression is significantly upregulated in the lesser curvature region of the aorta within 1–2 weeks of feeding LDLR−/− mice a high fat diet[3]. Neointimal hyperplasia is markedly suppressed in TLR-2KO mice compared with WT mice after vascular injury [161]. Similarly, the depletion of TLR4 in ApoE−/− deficient mice results in significant reduction in atherosclerotic lesion size and macrophages in lesion [162]. The synthetic lipoprotein Pam3CSK4 and LPS induced TLR2 and TLR4 expression at mRNA and protein levels are inhibited by candesartan, an antihypertensive drug angiotensin II type-1 receptor blocker, both in vitro and in vivo[163].
4.3. IL-1β pathway and atherosclerosis
IL-1β is among the dominant cytokines expressed in human atherosclerotic plaques[164]. In addition, IL-1β levels are significantly higher in patients with unstable angina as compared to patients with stable angina[151]. Lack of IL-1β decreases the severity of atherosclerosis in ApoE deficient mice[68]. In contrast, since IL-1 receptor antagonist (IL-1Ra) is an endogenous inhibitor of IL-1, atherosclerotic lesion size in IL-1Ra+/−/ApoE−/− mice is significantly increased by 30% compared with IL-1Ra+/+/ApoE−/− mice[165]. Based on these results, a recombinant human IL-1 receptor antagonist, Anakinra, has been used to inhibit inflammatory apoptosis in experimental acute myocardial infarction [166]. In addition, the data from IL-18 deficient ApoE−/− mice showed reduced atherosclerosis in spite of increased serum cholesterol, which support a proatherogenic role for IL-18 [69]. IL-33 is a novel IL-1-like cytokine. IL-1 Receptor Accessory Protein and ST2 comprise the IL-33 Receptor Complex[167]. In contrast to the roles of IL-1β and IL-18 in proatherogenesis, IL-33 can reduce atherosclerosis development in ApoE−/− mice on a high-fat diet. This protective role of IL-33 in the development of atherosclerosis results from the induction of IL-5 and ox-LDL antibodies [72].
5. The roles of CD4+CD25high regulatory T cells (Tregs) in inhibition of vascular inflammation and atherogenesis
5.1. Tregs
Among several regulatory T cell subsets identified, Tregs are the best characterized[168–172]. Comprising 5–10% of peripheral CD4+ T cells, Tregs exhibit potent immunosuppressive functions[173], and play an important role in the regulation of autoimmunity and pathogenesis of atherosclerosis. Of note, CD25high has proved to be by far the most useful surface marker for nTregs (see our recent reviews for details)[174,175]. Recently, Roncarolo and Battaglia summarized the difference between mouse and human Tregs[176].
5.2. Treg suppression of innate immune responses
Interestingly, the suppressive effects of these cells are not restricted to the adaptive immune system including CD4+ T cells, CD8+ T cells[177], natural killer (NK) cells[178], and B cells[179], but can also affect the activation and function of innate immune cells (monocytes, macrophages, dendritic cells)[180] and neutrophils[181]. Treg effects on innate immune cells are less well known. Alternatively activated macrophages (AAM) are cells with strong antiinflammatory potential involved in immune regulation, tissue remodeling, parasite killing, and tumor promotion. Treg co-cultured monocytes/macrophages display typical features of AAM, including up-regulated expression of CD206 (macrophage mannose receptor) and CD163 (hemoglobin scavenger receptor), an increased production of CCL18, and an enhanced phagocytic capacity. In addition, the monocytes/macrophages have reduced expression of HLA-DR and a strongly reduced capacity to respond to lipopolysaccharide (LPS) in terms of production of proinflammatory mediators [IL-1β, IL-6, IL-8, macrophage inflammatory protein 1α (MIP-1α), TNF-α], NF-κB activation, and tyrosine phosphorylation. Mechanistic studies reveal that Tregs produce IL-10, IL-4, and IL-13 and that these cytokines are the critical factors involved in the suppression of the proinflammatory cytokine response. In contrast, the Treg-mediated induction of CD206 is entirely cytokine-independent, whereas the up-regulation of CD163, CCL18, and phagocytosis are (partly) dependent on IL-10 but not on IL-4/IL-13. Together these data demonstrate a previously unrecognized function of Tregs, namely their ability to induce alternative activation of monocytes/macrophages. Moreover, the data suggest that the Treg-mediated induction of AAM partly involves a novel, cytokine-independent pathway[182].
5.3. Treg suppression of atherosclerosis and vascular inflammation
Tregs, as an active mechanism of immune suppression, have been targeted due to their tremendous therapeutic potentials to prevent autoimmune diseases and atherosclerosis[174]. Tregs are also powerful inhibitors of atherosclerosis in several mouse models [183,184]. These results provide new insights into the immunopathogenesis of atherosclerosis and could lead to new therapeutic approaches that involve immune modulation using Tregs[183]. Treg numbers are reduced in atherosclerotic compared with nonatherosclerotic ApoE-KO mice. The suppressive properties of Tregs from ApoE-KO mice are compromised in comparison with those from their C57BL/6 littermates. Oxidation of low-density lipoprotein (LDL) and the subsequent processing of oxidized LDL (oxLDL) by macrophages results in activation of specific T cells, which contributes to the development of atherosclerosis. Thus, oxLDL attenuates the suppressive properties of Tregs from C57BL/6 mice and more so in ApoE-KO mice. Transfer of Tregs from age-matched ApoE-KO mice results in significant attenuation of atherosclerosis compared with transfer of CD4+CD25+/− T cell controls or phosphate-buffered saline. These results suggest that Tregs may play a protective role in the progression of atherosclerosis and could be considered a therapeutic tool if results from human studies can solidify observations in murine models[185].
Several approaches have been developed to increase Tregs and inhibit development of atherosclerosis. Tolerance to oxLDL and malondialdehyde-treated LDL (MDA-LDL) is induced in LDL receptor−/− mice fed a Western-type diet by oral administration of oxLDL or MDA-LDL before the induction of atherogenesis. Oral tolerance to oxLDL results in a significant attenuation of the initiation (30% to 71%; P<0.05) and progression (45%; P<0.05) of atherogenesis. Tolerance to oxLDL induces a significant increase in Tregs in spleen and mesenteric lymph nodes, and these Tregs specifically respond to oxLDL with increased TGF-β production. Tolerance to oxLDL also increases the mRNA expression of Foxp3, CTLA-4, and CD25 in the plaque. In contrast, tolerance to MDA-LDL does not affect atherogenesis. OxLDL-specific T cells, present in LDL receptor−/− mice and important contributors in the immune response leading to atherosclerotic plaque, can be counteracted by oxLDL-specific Tregs activated via oral tolerance induction to oxLDL. These results suggest that the induction of oral tolerance to oxLDL may be a promising strategy to modulate the immune response during atherogenesis and a new way to treat atherosclerosis[186]. In addition, heat shock protein 60 (HSP60)-specific T cells contribute to the development of the immune responses in atherosclerosis. To explore the effect of oral tolerance induction to HSP60 and the peptide HSP60 (253 to 268) on atherosclerosis, HSP60 and HSP60 (253 to 268) are administered orally to LDLR−/− mice before induction of atherosclerosis. This tolerance results in a significant 80% reduction in plaque size in the carotid arteries and in a 27% reduction in plaque size at the aortic root. Reduction in plaque size correlates with an increase in Tregs in several organs and in an increased expression of Foxp3, CD25, and CTLA-4 in atherosclerotic lesions of HSP60-treated mice. The production of IL-10 and TGF-β by lymph node cells in response to HSP60 is observed after tolerance induction. Oral tolerance induction to HSP60 and a small HSP60-peptide leads to an increase in the number of Tregs, resulting in a decrease in plaque size as a consequence of increased production of IL-10 and TGF-β. These beneficial results of oral tolerance induction to HSP60 and HSP60 (253 to 268) may provide new therapeutic approaches for the treatment of atherosclerosis[187].
Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are in widespread use due to their LDL reducing properties and concomitant improvement of clinical outcome in patients with and without preexisting atherosclerosis. Immune mediated mechanisms play a dominant role in the beneficial effects of statins. Atorvastatin, but not mevastatin nor pravastatin, treatment of human peripheral blood mononuclear cells (PBMCs) increases the number of Tregs. These Tregs, induced by atorvastatin, express high levels of Foxp3, which correlate with an increased regulatory potential. Furthermore, co-culture studies reveal that atorvastatin induces Tregs are derived from peripheral CD4+CD25− cells. Simvastatin and pravastatin treatment in hyperlipidemic patients increases the number of Tregs. In C57BL/6 mice, however, no effect of statins on Tregs is evident. In conclusion, statins appear to significantly influence the peripheral pool of Tregs in humans. This finding may shed light on the mechanisms governing the plaque stabilizing properties of statins[188].
TLR2 can sense endogenous insultsincluding vascular injury induced by cuff-placement around the femoral artery, and induces inflammatory genes expression such as TNF-α, IL-1β, IL-6, and MCP-1 [161]. It remains unknown whether Tregs can inhibit acute vascular inflammation in addition to its inhibition on chronic vascular inflammation and atherosclerosis. Our recent report showed that Tregs inhibit cuff-induced vascular inflammation presumably by suppressing innate immune responses[189].
5.4. Treg apoptosis pathways are targets for modulation of vascular inflammation
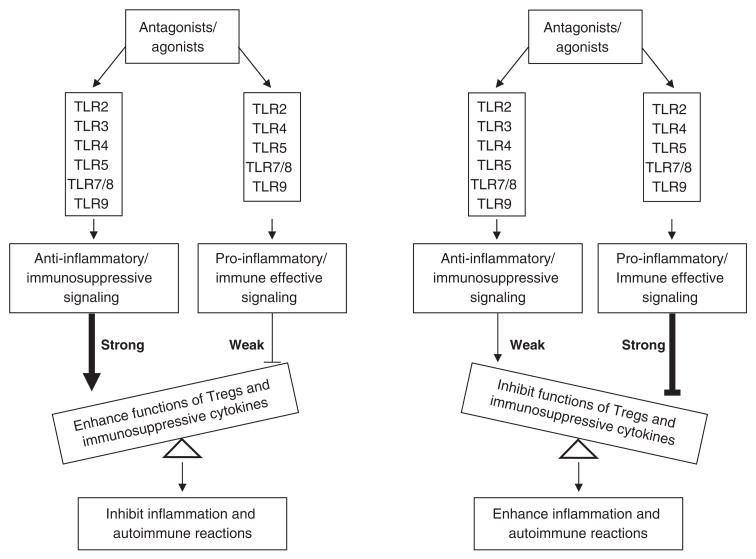
In the last thirteen years, substantial molecular differences between homeostasis of Tregs and that of other subsets of T cells and some factors specific in regulation of Treg survival have been characterized. In our recent review[174], we examined 91 factors, pathways and drugs, both well-characterized and newly defined, regarding the survival and homeostasis of Tregs, suggesting that Treg homeostasis and apoptosis pathways are targets for development of new therapeutics for atherosclerosis and autoimmune diseases. In addition, recent reports[190] suggest that TLRs play important roles in modulating the function of Tregs and immunosuppressive cytokines IL-10 and TGF-β as outlined in Fig. 3. We recently reported that removal of Tregs via a pro-apoptotic protein Bax-dependent apoptotic pathway significantly enhances anti-self antigen immune responses[191]. In several studies, we reported that Tregs have an interleukin-2 (IL-2) withdrawal-triggered apoptosis pathway, in which the upregulation of Bax expression in Tregs is induced[189]. By using transgenic mouse models, we found that modulation of Treg apoptosis pathway, by enhanced Bax expression[189] or decreased expression of anti-apoptotic protein translationally controlled tumor protein (TCTP)[192–194], accelerates development of vascular inflammation presumably via decreasing the striking threshold of vascular inflammation. Our results suggest that vascular inflammation is induced by cuff-placement presumably via necrotic cell-triggered TLRs’ signaling (Fig. 2). Our results have also demonstrated the proof of principle that the modulation of Tregs apoptosis/survival could be used as a new therapeutic approach for inflammatory cardiovascular diseases[189].
Fig. 3.
Interaction between agonists/antagonists with TLRs can either enhance or inhibit functions of Tregs and immunosuppressive cytokines, which leads to inhibition or enhancement of inflammation and autoimmune reactions. TLR: toll-like receptor.
Acknowledgments
I am very grateful to Prof. B. Ashby for critical reading,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou X, et al. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102(24):2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis. In: Wyngaarden J, Smith LH, Bennett JC, editors. Cecil Textbook of Medicine. W.B. Saunders Company; 1992. pp. 293–298. [Google Scholar]
- 3.Tobias PS, Curtiss LK. Toll-like receptors in atherosclerosis. Biochem Soc Trans. 2007;35(Pt 6):1453–1455. doi: 10.1042/BST0351453. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. Hyperhomocysteinemia accelerates atherosclerosis in cystathionine beta-synthase and apolipoprotein E double knock-out mice with and without dietary perturbation. Blood. 2003;101(10):3901–3907. doi: 10.1182/blood-2002-08-2606. [DOI] [PubMed] [Google Scholar]
- 5.Jiang X, et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25(12):2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan H, et al. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc Res. 2006;69(1):253–262. doi: 10.1016/j.cardiores.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao D, et al. Hyperhomocysteinemia decreases circulating high-density lipoprotein by inhibiting apolipoprotein A-I Protein synthesis and enhancing HDL cholesterol clearance. Circ Res. 2006;99(6):598–606. doi: 10.1161/01.RES.0000242559.42077.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamaluddin MD, et al. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin Agene. Blood. 2007;110(10):3648–3655. doi: 10.1182/blood-2007-06-096701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Q. Infections, heat shock proteins, and atherosclerosis. Curr Opin Cardiol. 2003;18(4):245–252. doi: 10.1097/00001573-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Stoll LL, et al. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(12):2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 11.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 12.Hallenbeck JM, et al. Immunology of ischemic vascular disease: plaque to attack. Trends Immunol. 2005;26(10):550–556. doi: 10.1016/j.it.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Tobias P, Curtiss LK. Thematic review series: The immune system and atherogenesis. Paying the price for pathogen protection: toll receptors in atherogenesis. J Lipid Res. 2005;46(3):404–411. doi: 10.1194/jlr.R400015-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 15.Yan ZQ, Hansson GK. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007;219:187–203. doi: 10.1111/j.1600-065X.2007.00554.x. [DOI] [PubMed] [Google Scholar]
- 16.Manconi F, et al. Culturing endothelial cells of microvascular origin. Methods Cell Sci. 2000;22(2–3):89–99. doi: 10.1023/a:1009895723488. [DOI] [PubMed] [Google Scholar]
- 17.Augustin H. Preface---Methods in Endothelial Cell Biology. In: Augustin H, editor. Methods in Endothelial Cell Biology. Springer; 2004. pp. VII–IX. [Google Scholar]
- 18.Mensah GA. Healthy endothelium: the scientific basis for cardiovascular health promotion and chronic disease prevention. Vascul Pharmacol. 2007;46(5):310–314. doi: 10.1016/j.vph.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Opitz B, et al. Extra- and intracellular innate immune recognition in endothelial cells. Thromb Haemost. 2007;98(2):319–326. [PubMed] [Google Scholar]
- 20.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 21.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112(7):375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 22.Fadini GP, et al. Technical notes on endothelial progenitor cells: ways to escape from the knowledge plateau. Atherosclerosis. 2008;197(2):496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 23.Pegu A, et al. Afferent and efferent interfaces of lymph nodes are distinguished by expression of lymphatic endothelial markers and chemokines. Lymphat Res Biol. 2007;5(2):91–103. doi: 10.1089/lrb.2007.1006. [DOI] [PubMed] [Google Scholar]
- 24.Walter-Yohrling J, et al. Murine endothelial cell lines as models of tumor endothelial cells. Clin Cancer Res. 2004;10(6):2179–2189. doi: 10.1158/1078-0432.ccr-03-1013. [DOI] [PubMed] [Google Scholar]
- 25.Pries AR, Kuebler WM. Normal endothelium. Handb Exp Pharmacol. 2006;(176 Pt 1):1–40. doi: 10.1007/3-540-32967-6_1. [DOI] [PubMed] [Google Scholar]
- 26.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 27.Reiss YaEB. FACS analysis of endothelial cells. In: Augustin H, editor. Methods in endothelial cell biology. Springer-Verlag; Berlin Heidelberg: 2004. pp. 157–165. [Google Scholar]
- 28.Bertolini F, et al. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discov Today. 2007;12(19–20):806–812. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Del Papa N, et al. Circulating endothelial cells as a marker of ongoing vascular disease in systemic sclerosis. Arthritis Rheum. 2004;50(4):1296–1304. doi: 10.1002/art.20116. [DOI] [PubMed] [Google Scholar]
- 30.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 31.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 32.Nilsen EM, et al. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42(5):635–642. doi: 10.1136/gut.42.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naruse KaKGL. Effect of diabetes on endothelial function. In: AJMaV, editor. Contemporary cardiology: Diabetes and cardiovascular Disease. Humana Press, Inc; 2001. [Google Scholar]
- 34.Methe H, et al. Endothelial immunogenicity--a matter of matrix microarchitecture. Thromb Haemost. 2007;98(2):278–282. [PubMed] [Google Scholar]
- 35.Clarke M, et al. Cell death in the cardiovascular system. Heart. 2007;93(6):659–664. doi: 10.1136/hrt.2006.088203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejana E, et al. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121(Pt 13):2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- 37.Reinhart K, et al. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med. 2002;30(5 Suppl):S302–312. doi: 10.1097/00003246-200205001-00021. [DOI] [PubMed] [Google Scholar]
- 38.Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28(2):223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 39.Woodfin A, et al. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27(12):2514–2523. doi: 10.1161/ATVBAHA.107.151456. [DOI] [PubMed] [Google Scholar]
- 40.Skaletz-Rorowski A, Walsh K. Statin therapy and angiogenesis. Curr Opin Lipidol. 2003;14(6):599–603. doi: 10.1097/00041433-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Anderson TJ, et al. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35(1):60–66. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 42.Mancini GB, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation. 1996;94(3):258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 43.Ghiadoni L, et al. Effect of the angiotensin II type 1 receptor blocker candesartan on endothelial function in patients with essential hypertension. Hypertension. 2000;35(1 Pt 2):501–506. doi: 10.1161/01.hyp.35.1.501. [DOI] [PubMed] [Google Scholar]
- 44.Kalinowski L, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107(21):2747–2752. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- 45.Taddei S, et al. Lacidipine restores endothelium-dependent vasodilation in essential hypertensive patients. Hypertension. 1997;30(6):1606–1612. doi: 10.1161/01.hyp.30.6.1606. [DOI] [PubMed] [Google Scholar]
- 46.Szabo G, et al. Endothelin-A and -B antagonists protect myocardial and endothelial function after ischemia/reperfusion in a rat heart transplantation model. Cardiovasc Res. 1998;39(3):683–690. doi: 10.1016/s0008-6363(98)00165-5. [DOI] [PubMed] [Google Scholar]
- 47.Chenevard R, et al. Selective COX-2 inhibition improves endothelial function in coronary artery disease. Circulation. 2003;107(3):405–409. doi: 10.1161/01.cir.0000051361.69808.3a. [DOI] [PubMed] [Google Scholar]
- 48.Fukuda Y, et al. Tetrahydrobiopterin restores endothelial function of coronary arteries in patients with hypercholesterolaemia. Heart. 2002;87(3):264–269. doi: 10.1136/heart.87.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aukrust P, et al. Inflammation in coronary artery disease: potential role for immunomodulatory therapy. Expert Rev Cardiovasc Ther. 2005;3(6):1111–1124. doi: 10.1586/14779072.3.6.1111. [DOI] [PubMed] [Google Scholar]
- 50.Bruynzeel I, et al. Pentoxifylline inhibits human T-cell adhesion to dermal endothelial cells. Arch Dermatol Res. 1997;289(4):189–193. doi: 10.1007/s004030050179. [DOI] [PubMed] [Google Scholar]
- 51.Xu C, et al. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. Am J Pathol. 1998;153(4):1257–1266. doi: 10.1016/S0002-9440(10)65670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensterle M, et al. Improvement of endothelial function with metformin and rosiglitazone treatment in women with polycystic ovary syndrome. Eur J Endocrinol. 2008 doi: 10.1530/EJE-08-0507. [DOI] [PubMed] [Google Scholar]
- 53.Spieker LE, et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation. 2002;105(12):1399–1402. doi: 10.1161/01.cir.0000013424.28206.8f. [DOI] [PubMed] [Google Scholar]
- 54.Kharbanda RK, et al. Systemic Acyl-CoA:cholesterol acyltransferase inhibition reduces inflammation and improves vascular function in hypercholesterolemia. Circulation. 2005;111(6):804–807. doi: 10.1161/01.CIR.0000155236.25081.9B. [DOI] [PubMed] [Google Scholar]
- 55.Gilligan DM, et al. Effect of antioxidant vitamins on low density lipoprotein oxidation and impaired endothelium-dependent vasodilation in patients with hypercholesterolemia. J Am Coll Cardiol. 1994;24(7):1611–1617. doi: 10.1016/0735-1097(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 56.Torre-Amione G, et al. Effects of a novel immune modulation therapy in patients with advanced chronic heart failure: results of a randomized, controlled, phase II trial. J Am Coll Cardiol. 2004;44(6):1181–1186. doi: 10.1016/j.jacc.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 57.McSorley PT, et al. Vitamin C improves endothelial function in healthy estrogen-deficient postmenopausal women. Climacteric. 2003;6(3):238–247. [PubMed] [Google Scholar]
- 58.de Gaetano G, et al. Prevention of thrombosis and vascular inflammation: benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol Sci. 2003;24(5):245–252. doi: 10.1016/S0165-6147(03)00077-4. [DOI] [PubMed] [Google Scholar]
- 59.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007;1771(8):972–982. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agouni A, et al. Sonic hedgehog carried by microparticles corrects endothelial injury through nitric oxide release. Faseb J. 2007;21(11):2735–2741. doi: 10.1096/fj.07-8079com. [DOI] [PubMed] [Google Scholar]
- 61.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451(7181):904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 62.Goligorsky MS. Endothelial cell dysfunction and nitric oxide synthase. Kidney Int. 2000;58(3):1360–1376. doi: 10.1046/j.1523-1755.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- 63.Bolz SS, et al. Intact endothelial and smooth muscle function in small resistance arteries after 48 h in vessel culture. Am J Physiol Heart Circ Physiol. 2000;279(3):H1434–1439. doi: 10.1152/ajpheart.2000.279.3.H1434. [DOI] [PubMed] [Google Scholar]
- 64.Bakker EN, et al. Organoid culture of cannulated rat resistance arteries: effect of serum factors on vasoactivity and remodeling. Am J Physiol Heart Circ Physiol. 2000;278(4):H1233–1240. doi: 10.1152/ajpheart.2000.278.4.H1233. [DOI] [PubMed] [Google Scholar]
- 65.Esper RJ, et al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giannotti G, Landmesser U. Endothelial dysfunction as an early sign of atherosclerosis. Herz. 2007;32(7):568–572. doi: 10.1007/s00059-007-3073-1. [DOI] [PubMed] [Google Scholar]
- 67.Tesfamariam B, DeFelice AF. Endothelial injury in the initiation and progression of vascular disorders. Vascul Pharmacol. 2007;46(4):229–237. doi: 10.1016/j.vph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Kirii H, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 69.Elhage R, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59(1):234–240. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 70.Eefting D, et al. The effect of interleukin-10 knock-out and overexpression on neointima formation in hypercholesterolemic APOE*3-Leiden mice. Atherosclerosis. 2007;193(2):335–342. doi: 10.1016/j.atherosclerosis.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 71.Merhi-Soussi F, et al. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res. 2005;66(3):583–593. doi: 10.1016/j.cardiores.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Miller AM, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205(2):339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King VL, et al. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171(6):2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huber SA, et al. Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1999;19(10):2364–2367. doi: 10.1161/01.atv.19.10.2364. [DOI] [PubMed] [Google Scholar]
- 75.Upadhya S, et al. Atherogenic effect of interleukin-2 and antiatherogenic effect of interleukin-2 antibody in apo-E-deficient mice. Angiology. 2004;55(3):289–294. doi: 10.1177/000331970405500308. [DOI] [PubMed] [Google Scholar]
- 76.Schieffer B, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110(22):3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 77.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163(3):1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Binder CJ, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114(3):427–437. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Branen L, et al. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24(11):2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 80.Mallat Z, et al. Inhibition of transforming growth factor-beta signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ Res. 2001;89(10):930–934. doi: 10.1161/hh2201.099415. [DOI] [PubMed] [Google Scholar]
- 81.Buono C, et al. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23(3):454–460. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 82.Robertson AK, et al. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112(9):1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caligiuri G, et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9(1–2):10–17. [PMC free article] [PubMed] [Google Scholar]
- 84.von der Thusen JH, et al. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55(1):133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 85.Vink H, et al. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation. 2000;101(13):1500–1502. doi: 10.1161/01.cir.101.13.1500. [DOI] [PubMed] [Google Scholar]
- 86.Gawaz M, et al. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115(12):3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kofler S, et al. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (Lond) 2005;108(3):205–213. doi: 10.1042/CS20040174. [DOI] [PubMed] [Google Scholar]
- 88.Guzik TJ, et al. Adipocytokines - novel link between inflammation and vascular function? J Physiol Pharmacol. 2006;57(4):505–528. [PubMed] [Google Scholar]
- 89.Vannini N, et al. Endothelial cell aging and apoptosis in prevention and disease: E-selectin expression and modulation as a model. Curr Pharm Des. 2008;14(3):221–225. doi: 10.2174/138161208783413248. [DOI] [PubMed] [Google Scholar]
- 90.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102(6):637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 91.Cooney MM, et al. Drug insight: vascular disrupting agents and angiogenesis--novel approaches for drug delivery. Nat Clin Pract Oncol. 2006;3(12):682–692. doi: 10.1038/ncponc0663. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, et al. Cyclin A transcriptional suppression is the major mechanism mediating homocysteine-induced endothelial cell growth inhibition. Blood. 2002;99(3):939–945. [PMC free article] [PubMed] [Google Scholar]
- 93.Cardoso LS, et al. Polymyxin B as inhibitor of LPS contamination of Schistosoma mansoni recombinant proteins in human cytokine analysis. Microb Cell Fact. 2007;6:1. doi: 10.1186/1475-2859-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nogueras S, et al. Coupling of endothelial injury and repair: an analysis using an in vivo experimental model. Am J Physiol Heart Circ Physiol. 2008;294(2):H708–713. doi: 10.1152/ajpheart.00466.2007. [DOI] [PubMed] [Google Scholar]
- 95.Martinez MC, et al. Transfer of differentiation signal by membrane microvesicles harboring hedgehog morphogens. Blood. 2006;108(9):3012–3020. doi: 10.1182/blood-2006-04-019109. [DOI] [PubMed] [Google Scholar]
- 96.Danese S, et al. Immune regulation by microvascular endothelial cells: directing innate and adaptive immunity, coagulation, and inflammation. J Immunol. 2007;178(10):6017–6022. doi: 10.4049/jimmunol.178.10.6017. [DOI] [PubMed] [Google Scholar]
- 97.Limmer A, Knolle PA. Liver sinusoidal endothelial cells: a new type of organ-resident antigen-presenting cell. Arch Immunol Ther Exp (Warsz) 2001;49(Suppl 1):S7–11. [PubMed] [Google Scholar]
- 98.Satoh S, et al. Glomerular endothelium exhibits enhanced expression of costimulatory adhesion molecules, CD80 and CD86, by warm ischemia/reperfusion injury in rats. Lab Invest. 2002;82(9):1209–1217. doi: 10.1097/01.lab.0000029620.13097.19. [DOI] [PubMed] [Google Scholar]
- 99.Yang XF. Immunology of stem cells and cancer stem cells. Cell Mol Immunol. 2007;4(3):161–171. [PubMed] [Google Scholar]
- 100.Marelli-Berg FM, Jarmin SJ. Antigen presentation by the endothelium: a green light for antigen-specific T cell trafficking? Immunol Lett. 2004;93(2–3):109–113. doi: 10.1016/j.imlet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 101.Rose ML. Endothelial cells as antigen-presenting cells: role in human transplant rejection. Cell Mol Life Sci. 1998;54(9):965–978. doi: 10.1007/s000180050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bagai R, et al. Mouse endothelial cells cross-present lymphocyte-derived antigen on class I MHC via a TAP1- and proteasome-dependent pathway. J Immunol. 2005;174(12):7711–7715. doi: 10.4049/jimmunol.174.12.7711. [DOI] [PubMed] [Google Scholar]
- 103.Buckanovich RJ, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14(1):28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- 104.Belizna C, et al. Antiendothelial cell antibodies in vasculitis and connective tissue disease. Ann Rheum Dis. 2006;65(12):1545–1550. doi: 10.1136/ard.2005.035295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Campanella GS, et al. Oligomerization of CXCL10 is necessary for endothelial cell presentation and in vivo activity. J Immunol. 2006;177(10):6991–6998. doi: 10.4049/jimmunol.177.10.6991. [DOI] [PubMed] [Google Scholar]
- 106.Ng B, et al. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J Allergy Clin Immunol. 2004;114(6):1463–1470. doi: 10.1016/j.jaci.2004.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xiong Z, et al. Alternative splicing factor ASF/SF2 is down regulated in inflamed muscle. J Clin Pathol. 2006;59(8):855–861. doi: 10.1136/jcp.2005.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang F, et al. Model of stimulation-responsive splicing and strategies in identification of immunogenic isoforms of tumor antigens and autoantigens. Clin Immunol. 2006;121(2):121–133. doi: 10.1016/j.clim.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Rubinsztein DC, et al. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6(4):304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 110.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115(10):2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bredesen DE, et al. Cell death in the nervous system. Nature. 2006;443(7113):796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Willingham SB, et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2(3):147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonetti PO, et al. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 115.Geng YJ. Molecular signal transduction in vascular cell apoptosis. Cell Res. 2001;11(4):253–264. doi: 10.1038/sj.cr.7290094. [DOI] [PubMed] [Google Scholar]
- 116.Wassmann S, et al. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res. 2006;99(8):e74–83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 117.Bombeli T, et al. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89(7):2429–2442. [PubMed] [Google Scholar]
- 118.Zhang X, et al. Protection against oxidized low-density lipoprotein-induced vascular endothelial cell death by integrin-linked kinase. Circulation. 2001;104(23):2762–2766. doi: 10.1161/hc4801.100792. [DOI] [PubMed] [Google Scholar]
- 119.Yang J, et al. Endothelial overexpression of Fas ligand decreases atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24(8):1466–1473. doi: 10.1161/01.ATV.0000134402.94963.2f. [DOI] [PubMed] [Google Scholar]
- 120.Erridge C. The roles of pathogen-associated molecular patterns in atherosclerosis. Trends Cardiovasc Med. 2008;18(2):52–56. doi: 10.1016/j.tcm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Palsson-McDermott EM, O’Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007;35(Pt 6):1437–1444. doi: 10.1042/BST0351437. [DOI] [PubMed] [Google Scholar]
- 122.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 123.Dunne A, O’Neill LA. Adaptor usage and Toll-like receptor signaling specificity. FEBS Lett. 2005;579(15):3330–3335. doi: 10.1016/j.febslet.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 124.Pasare C, Medzhitov R. Toll-like receptors: balancing host resistance with immune tolerance. Curr Opin Immunol. 2003;15(6):677–682. doi: 10.1016/j.coi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Stehlik C, Dorfleutner A. COPs and POPs: Modulators of Inflammasome Activity. J Immunol. 2007;179(12):7993–7998. doi: 10.4049/jimmunol.179.12.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 127.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 128.Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 129.Leon CG, et al. Discovery and Development of Toll-Like Receptor 4 (TLR4) Antagonists: A New Paradigm for Treating Sepsis and Other Diseases. Pharm Res. 2008;25(8):1751–1761. doi: 10.1007/s11095-008-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liew FY, et al. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5(6):446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 131.Shaw MH, et al. NOD-like receptors (NLRs): bona fide intracellular microbial sensors. Curr Opin Immunol. 2008 doi: 10.1016/j.coi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14(1):10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 133.Masumoto J, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem. 1999;274(48):33835–33838. doi: 10.1074/jbc.274.48.33835. [DOI] [PubMed] [Google Scholar]
- 134.Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38(2):240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- 135.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 136.Eisenbarth SC, et al. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008 doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20(1):3–9. doi: 10.1016/j.coi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Poyet JL, et al. Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J Biol Chem. 2001;276(30):28309–28313. doi: 10.1074/jbc.C100250200. [DOI] [PubMed] [Google Scholar]
- 140.Yajima N, et al. Critical role of bone marrow apoptosis-associated speck-like protein, an inflammasome adaptor molecule, in neointimal formation after vascular injury in mice. Circulation. 2008;117(24):3079–3087. doi: 10.1161/CIRCULATIONAHA.107.746453. [DOI] [PubMed] [Google Scholar]
- 141.Fernandes-Alnemri T, et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu JW, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28(2):214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shao W, et al. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J Biol Chem. 2007;282(50):36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 144.King AR, et al. A role for caspase-1 in serum withdrawal-induced apoptosis of endothelial cells. Lab Invest. 2003;83(10):1497–1508. doi: 10.1097/01.lab.0000093096.62765.85. [DOI] [PubMed] [Google Scholar]
- 145.Hilbi H, et al. Tripeptidyl peptidase II promotes maturation of caspase-1 in Shigella flexneri-induced macrophage apoptosis. Infect Immun. 2000;68(10):5502–5508. doi: 10.1128/iai.68.10.5502-5508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Squires RC, et al. Proteasomes control caspase-1 activation in anthrax lethal toxin-mediated cell killing. J Biol Chem. 2007;282(47):34260–34267. doi: 10.1074/jbc.M705687200. [DOI] [PubMed] [Google Scholar]
- 147.Merkle S, et al. A role for caspase-1 in heart failure. Circ Res. 2007;100(5):645–653. doi: 10.1161/01.RES.0000260203.55077.61. [DOI] [PubMed] [Google Scholar]
- 148.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403(6765):98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 149.Saleh M, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440(7087):1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 150.Barksby HE, et al. The expanding family of interleukin-1 cytokines and their role in destructive inflammatory disorders. Clin Exp Immunol. 2007;149(2):217–225. doi: 10.1111/j.1365-2249.2007.03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Simon AD, et al. Circulating levels of IL-1beta, a prothrombotic cytokine, are elevated in unstable angina versus stable angina. J Thromb Thrombolysis. 2000;9(3):217–222. doi: 10.1023/a:1018758409934. [DOI] [PubMed] [Google Scholar]
- 152.Rubartelli A, et al. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. Embo J. 1990;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22(3):364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- 154.Carta S, et al. Histone deacetylase inhibitors prevent exocytosis of interleukin-1beta-containing secretory lysosomes: role of microtubules. Blood. 2006;108(5):1618–1626. doi: 10.1182/blood-2006-03-014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dinarello CA. Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol. 2007;27(1):98–114. doi: 10.1016/j.semnephrol.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 156.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15(6):318–330. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 157.Zhang SH, et al. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 158.Kawamura A, et al. Apolipoprotein E interrupts interleukin-1beta signaling in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27(7):1610–1617. doi: 10.1161/ATVBAHA.106.129957. [DOI] [PubMed] [Google Scholar]
- 159.Veniant MM, et al. Lipoprotein size and atherosclerosis susceptibility in Apoe(−/−) and Ldlr(−/−) mice. Arterioscler Thromb Vasc Biol. 2001;21(10):1567–1570. doi: 10.1161/hq1001.097780. [DOI] [PubMed] [Google Scholar]
- 160.Mullick AE, et al. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115(11):3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shishido T, et al. Central role of endogenous Toll-like receptor-2 activation in regulating inflammation, reactive oxygen species production, and subsequent neointimal formation after vascular injury. Biochem Biophys Res Commun. 2006;345(4):1446–1453. doi: 10.1016/j.bbrc.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 162.Michelsen KS, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101(29):10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dasu MR, et al. Candesartan inhibits Toll-like receptor expression and activity both in vitro and in vivo. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galea J, et al. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16(8):1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 165.Isoda K, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24(6):1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 166.Abbate A, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117(20):2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 167.Chackerian AA, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179(4):2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 168.Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6(4):327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 169.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6(4):331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 170.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 171.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 172.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114(9):1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Horwitz DA, et al. The potential of human regulatory T cells generated ex vivo as a treatment for lupus and other chronic inflammatory diseases. Arthritis Res. 2002;4(4):241–246. doi: 10.1186/ar414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang XF. Factors regulating apoptosis and homeostasis of CD4+CD25highFOXP3+ regulatory T cells are new therapeutic targets. Front Biosci. 2008;13:1472–1499. doi: 10.2741/2775. [DOI] [PubMed] [Google Scholar]
- 175.Ke XY, et al. Roles of CD4+CD25high FOXP3+ Tregs in lymphomas and tumors are complex. Front Biosci. 2008;13:3986–4001. doi: 10.2741/2986. [DOI] [PubMed] [Google Scholar]
- 176.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 177.Camara NO, et al. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T cell activation. Eur J Immunol. 2003;33(12):3473–3483. doi: 10.1002/eji.200323966. [DOI] [PubMed] [Google Scholar]
- 178.Wolf AM, et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9(2):606–612. [PubMed] [Google Scholar]
- 179.Janssens W, et al. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171(9):4604–4612. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 180.Taams LS, Akbar AN. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr Top Microbiol Immunol. 2005;293:115–131. doi: 10.1007/3-540-27702-1_6. [DOI] [PubMed] [Google Scholar]
- 181.Lewkowicz P, et al. Lipopolysaccharide-Activated CD4+CD25+ T Regulatory Cells Inhibit Neutrophil Function and Promote Their Apoptosis and Death. J Immunol. 2006;177(10):7155–7163. doi: 10.4049/jimmunol.177.10.7155. [DOI] [PubMed] [Google Scholar]
- 182.Tiemessen MM, et al. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A. 2007;104(49):19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ait-Oufella H, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12(2):178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 184.George J. Mechanisms of disease: the evolving role of regulatory T cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2008;5(9):531–540. doi: 10.1038/ncpcardio1279. [DOI] [PubMed] [Google Scholar]
- 185.Mor A, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(4):893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 186.van Puijvelde GH, et al. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114(18):1968–1976. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 187.van Puijvelde GH, et al. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(12):2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- 188.Mausner-Fainberg K, et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis. 2008;197(2):829–839. doi: 10.1016/j.atherosclerosis.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 189.Xiong Z, et al. Higher expression of Bax in regulatory T cells increases vascular inflammation. Front Biosci. 2008;13:7143–7155. doi: 10.2741/3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Conroy H, et al. TLR ligand suppression or enhancement of Treg cells? A double-edged sword in immunity to tumours. Oncogene. 2008;27(2):168–180. doi: 10.1038/sj.onc.1210910. [DOI] [PubMed] [Google Scholar]
- 191.Yan Y, et al. HLA-A2.1-restricted T cells react to SEREX-defined tumor antigen CML66L and are suppressed by CD4+CD25+ regulatory T cells. Int J Immunopathol Pharmacol. 2007;20(1):75–89. doi: 10.1177/039463200702000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xiong Z, et al. Expression of TCTP antisense in CD25(high) regulatory T cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang Y, et al. An N-terminal region of translationally controlled tumor protein is required for its antiapoptotic activity. Oncogene. 2005;24(30):4778–4788. doi: 10.1038/sj.onc.1208666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yan Y, Xiong Z, Zhang S, Song J, Huang Y, Thornton AM, Wang H, Yang X-F. CD25high T cells with a prolonged survival inhibit development of diabetes. International Journal of Immunopathology and Pharmacology. 2008;21(4) doi: 10.1177/039463200802100401. [DOI] [PMC free article] [PubMed] [Google Scholar]