Abstract
Cathelicidin production by human myeloid cells stimulated through toll like receptor (TLR) 2/1, the migration of human CD8+ T cells to inflamed skin sites, and the ability of murine dendritic cells (DCs) to migrate from skin sites of vaccination to mucosal lymphoid organs all occur via calcitriol-dependent mechanisms. Herein, we report that murine DCs exposed to TLR3/TLR4 ligands upregulate their expression of 1α-hydroxylase, the enzyme that converts circulating 25(OH)D3 to calcitriol, the active form of vitamin D3. TLR3/TLR4 ligands injected subcutaneously affect DC migration in vivo, allowing their trafficking to both draining and non-draining systemic and mucosal lymphoid organs. Subcutaneously delivered vaccines containing TLR3/TLR4 ligands and antigen stimulate the induction of both systemic and mucosal immune responses. Vaccines containing TLR9 ligands fail to stimulate 1α-hydroxylase protein expression, are incapable of redirecting DC migration into Peyer’s patches and do not induce mucosal immune responses. These findings support a hypothesis that active metabolites of vitamin D3 produced locally are able to affect various aspects of innate and acquired immune responses.
Keywords: dendritic cells, TLR ligands, mucosal immune response, calcitriol
1.0. Introduction
In mammals there are at least 12 members of the toll like receptor (TLR) family. These receptors not only recognize a number of specific components conserved among microorganisms, but are also capable of recognizing specific defensins as well as fragments of extracellular matrix proteins [1–7]. Since, the activation of macrophages or dendritic cells (DCs) through one or more of their TLRs enhances innate immunity and can modulate the subsequent development of antigen-specific adaptive immunity, some TLR ligands have been used as adjuvants in vaccine formulations administered parenterally to augment systemic immune responses [8–11]. Specific TLR ligands have been reported to promote the induction of both systemic and mucosal immune responses when co-administered with antigen intranasally [9–12].
It has recently been established that human monocyte-derived DC, activated with the TLR4 ligand lipopolysaccharide (LPS), are able to convert precursor form of vitamin D3 25(OH)D3 into its bioactive form (calcitriol, 1α25(OH)2D3) when expression of the vitamin D3 metabolizing enzyme, 25-hydroxy vitamin D3 1-α-hydroxylase (1α-hydroxylase) is upregulated [13]. It was further demonstrated that naïve CD8+ T cells acquire surface expression of CCR10 when activated in the presence of 1α25(OH)2D3 [14]. This chemokine receptor is necessary for effective T cell migration into inflamed epidermis, where the specific chemokine ligand CCL27 is actively produced [15]. Based on these data it has been concluded that human DCs, through an induced ability to endogenously produce 1α25(OH)2D3, are able to program activated T cell migration to inflamed skin [14].
There now exists a direct linkage between the anti-mycobacterial activity of human macrophages and their capacity to endogenously produce 1α25(OH)2D3 [16]. The expression levels of both the vitamin D receptor and 1α-hydroxylase become markedly upregulated in macrophages activated through TLR2/1, with the endogenously produced 1α25(OH)2D3 stimulating macrophage biosynthesis of the antimicrobial peptide cathelicidin [16]. Locally produced cathelicidin was further demonstrated to have bactericidal effects on M. tuberculosis leading to the conclusion that locally induced metabolism of circulating vitamin D3 precursors, generating the active hormone, plays an important role in innate immune defenses [16].
We have previously demonstrated that the subcutaneous or intradermal immunization of mature adult mice with vaccines containing the active form of vitamin D3 effectively induces the generation of both systemic and common mucosal immune responses [17, 18]. The mechanisms that allow both types of immune responses to be induced simultaneously have been characterized [19]. We now appreciate that the immunization of mice with vaccines containing 1α25(OH)2D3 alters the migratory properties of antigen-laden DCs that are mobilized from the tissue sites of immunization, allowing these antigen presenting cells to traffic beyond draining lymph nodes and localize within secondary lymphoid organs throughout the body, including the classical inductive sites of mucosal immunity [19].
Herein, we questioned whether the addition of select TLR ligands to vaccines administered parenterally would effectively promote the induction of antigen-specific common mucosal immune responses. We also questioned whether mucosal adjuvant properties of select TLR ligands are being mediated through mechanisms dependent upon locally generated 1α25(OH)2D3. Our findings demonstrate that the exposure of murine bone marrow derived DCs (BMDCs) to synthetic polyinosinic-polycytidylic acid double-stranded RNA (poly I:C) or E. coli LPS (TLR3 or TLR4 ligands), but not unmethylated CpG-containing synthetic oligonucleotides (CpG ODNs) (TLR9 ligand), induces their expression of 1α-hydroxylase. Local administration of TLR3 or TLR4 ligands to normal mice, similar to the local administration of 1α25(OH)2D3 itself, altered the migratory properties of DCs mobilized from skin injection sites, allowing their localization into multiple non-draining secondary lymphoid organs, including the Peyer’s patches (PPs). TLR3/4-mobilized DCs that had migrated to non-draining lymphoid organs were fully capable of processing and presenting antigen peptides to responsive CD4+ T cells. Finally, when TLR3 or TLR4 ligands were used as adjuvants for vaccines administered subcutaneously, they effectively stimulated the induction of both systemic and mucosal immune responses. The addition of ligands for TLR9 into vaccine formulations enhanced systemic immunity, but failed to elicit mucosal immune responses.
Our data is consistent with the hypothesis that calcitriol produced locally from 25(OH)D3 plays important roles in the regulation of both innate and adaptive immune processes in vivo. Furthermore, this natural hormone, as well as the inducible enzymes involved in its tissue-specific metabolism in vivo, could be readily amenable to exploitation for the rational design of systemically administered vaccine formulations, specifically tailored to provide both systemic and mucosal immune protection against infectious agents.
2.0. Materials and Methods
2.1. Animals
Female C3H/HeN and BALB/c mice (8–12 weeks old) obtained from Charles River Breeding Laboratory (Wilmington, MA) and DO11.10 T cell receptor transgenic mice were used in this study. DO11.10 T cell receptor transgenic mice on the BALB/c background were bred from animals originally purchased from Jackson Laboratories (Bar Harbor, ME). All mice were kept under specific pathogen free conditions at the Center of Comparative Medicine Animal Facility, University of Utah.
2.2. Tracking of bone marrow derived dendritic cells in vivo
Dendritic cells were generated from bone marrow of adult C3H/HeN mice as previously described [19]. At day 7 after culture initiation, CD11c+ BMDCs were isolated by positive selection using CD11c microbeads (Miltenyi Biotec Inc., Auburn, CA) and stimulated with 10ng/ml Escherichia coli LPS, strain 0111:B4, (Sigma, St. Louis, MO) in the presence or absence of 1α25(OH)2D3 (10−8 M, a kind gift of Milan Uskokovic, Hoffman-La Roche Inc., Nutley, NJ). In some experiments BMDCs were activated with 10ng/ml LPS in the presence or absence of the calcitriol precursor 25-hydroxycholecalcitriol (25(OH)D3) (10−7 M, Sigma, St. Louis, MO). After 24 hours, the DCs were exposed to 5μM carboxyfluoroscein succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) for 15 min at 37°C followed by extensive washing in PBS. CFSE stained cells (2×106 per mouse) were injected into the hind footpads of naïve syngeneic recipients. Forty-eight hours later, mice were sacrificed and individual lymphoid organs removed, single cell suspensions were prepared and analyzed for the presence of CFSE+ cells by FACScan.
2.3. Western Blot analysis for 1α-hydroxylase
Three million BMDCs/ml were stimulated with TLR ligands (20μg/ml poly I:C (Amersham Biosciences, Piscataway, NJ), 10ng/ml LPS or 20μg/ml CpG ODN (5′-TCC-ATG-ACG-TTC-CTG-ACG-TT-3′, synthesized by the University of Utah DNA core facility)) or left untreated and incubated for 24 hours at 37°C and 5% CO2. The cells were then treated with lysis buffer containing a cocktail of protease inhibitors. Protein samples were separated using 10% SDS-PAGE electrophoresis and transferred to polyvinylidene difluoride membranes and probed with sheep anti-murine 1α-hydroxylase antibody (The Binding Site, Birmingham, UK). Blots, after stripping (Restore™ Western Blot Stripping Buffer, Pierce), were then incubated with anti-β-actin antibodies (Sigma) to confirm comparable protein loading.
2.4. Chemotaxis assays
Chemotactic migration assays were performed as described elsewhere [20]. Briefly, CD11c+ BMDCs were treated overnight with 10ng/ml LPS, with or without 10−7 M 25(OH)D3 or 10−8 M 1α25(OH)2D3. After extensive washing, 5×105 BMDCs were placed into the upper chamber of Costar Transwell plates with 5μm diameter pores (Corning Costar, Cambridge, MA). Inserts were placed into wells containing 600μl of RPMI-1640/0.5% BSA with or without 100ng/ml of murine recombinant CCL21 (R&D System, Inc. Minneapolis, MN) or 1000nM of sphingosine-1-phosphate (S1P) (Sigma). After a 3-hour incubation at 37°C, migrated cells were collected from the bottom chamber, resuspended in 1ml PBS and quantitated by FACScan. Experimental groups were presented as mean ± SD of sample triplicates minus background migration (the number of cells migrated to the lower chamber with no added chemokine).
2.5. Tracking of mobilized dendritic cells in vivo
In vivo DC tracking assays were performed as described previously [19]. Briefly, 50μl of 0.2μm 0.25% fluoresbrite™ carboxy YG (green) latex microspheres (Polysciences, Inc., Warrington, PA) were injected into the right hind footpad of C3H/HeN mice in the presence or absence of 0.1μg 1α25(OH)2D3, 20μg of poly I:C, 10μg LPS, or 20μg CpG ODN). As an internal control, 50μl of 0.2μm 0.25% fluoresbrite™ carboxy NYO (red) latex microspheres were simultaneously injected into the left hind footpad of the same experimental animals. In some experiments the migratory properties of microsphere+ DCs were evaluated following the injection of different sized green microspheres (1.0μm vs. 0.2μm) into the footpads of experimental animals in the presence or absence of LPS. An injection of 0.2μm or 1.0μm 0.25% fluoresbrite™ carboxy NYO (red) microspheres into left footpad was used as an internal control. Forty-eight hours after microsphere injection, the experimental animals were sacrificed and individual secondary lymphoid organs were surgically excised and single cell suspensions prepared. FACScan analysis was made on microsphere+ cells.
In experiments where the phenotype of microsphere+ cells was examined, cell samples were additionally stained with fluorescent monoclonal antibodies directed against mouse CD11b (clone M1/70) and/or CD11c (clone HL3), CD8α (Ly-2) (clone 53-6.7), α4β7 (clone DATK32), CD62L (clone MEL-14) (BD Pharmingen, San Diego, CA), CD103 (clone 2E7), dendritic cells marker (clone 33D1) (eBioscience, San Diego, CA) or DEC205 (clone NLDC-145) (Miltenyi Biotec, Auburn, CA). Some cell samples were treated with CCL19-Fc (a fusion of mouse CCL19 and the Fc of human IgG1 (a gift of Dr. Jason Cyster, University of California, San Francisco, CA) for analysis of CCR7 expression followed by staining with goat anti-human Fcγ-PE. Samples were analyzed by FACScan.
2.6. DO11.10 TCR transgenic CD4+ T cell adoptive transfer experiments
DO11.10 TCR-transgenic CD4+ T cells, specific to the ovalbumin peptide 323–339 (OVA323–339) in association with I-Ad, were purified from the spleens and lymph nodes of naïve DO11.10 strain mice (10–12 weeks old) by positive selection using murine CD4+ T cell MicroBeads and AutoMacs (Miltenyi Biotec). Recipient BALB/c (10–12 week old) mice received an intravenous injection of 2×106 DO11.10 CD4+ T cells. After being rested for 24 hours, the DO11.10 T cell recipients were subcutaneously immunized with vaccine formulations containing 50μg of ovalbumin (OVA) in aluminum hydroxide (Alum) with or without 10μg LPS. Mice immunized with OVA/Alum in the presence of 0.1μg 1α25(OH)2D3 were used as a positive control. DO11.10 T cell recipients injected with 50μl PBS were included in this study as a negative control. Seventy-two hours later, individual secondary lymphoid organs were removed, single cell suspensions prepared, and the total cell population stained with KJ1-26 (idiotype-specific) and anti-CD69 (H1.2F3, BD Pharmingen) antibodies. Similar groups of animals were used to analyze proliferative responsiveness of transferred DO11.10 T cells. In these studies DO11.10 T cells were CFSE (5μM) stained for 15 min at 37°, washed and intravenously administered to naive BALB/c recipients. Twenty-four hours later, these animals were immunized with OVA as described above. Seven days later, the mice were sacrificed, individual popliteal lymph nodes (LNs), axillary LNs, spleen and Peyer’s patches collected and stained with anti-mouse CD4 (H129.19, BD Pharmingen) and analyzed by FACScan for the percentage of CD4+ T cells that were also CFSE+.
2.7. Animal vaccination
Lightly anesthetized C3H/HeN mice (10 weeks old, 5 animals per group) were subcutaneously immunized with 50μl of a vaccine formulation containing 1μg Diphtheria CRM 197 protein (DT, a kind gift of Wyeth-Ayerst Laboratories, Inc., Marietta, PA) plus Alum (275 μg/ml) in the presence or absence of 10μg LPS, 20μg poly I:C or 20μg CpG ODN. Mice immunized with DT and Alum in the presence of 0.1μg 1α25(OH)2D3 were used as a positive control. Serum, vaginal washes and stool samples were collected as previously described at 2, 3 and 4 weeks post primary immunization [17, 21]. In experiments where recall responses were evaluated, a secondary boost with 1μg DT in Alum with or without 10μg LPS, 20μg poly I:C, 20μg CpG or 0.1μg 1α25(OH)2D3 was given at 63 days post primary immunization. Serum, vaginal washes, fecal samples, nasal and lung lavages were collected 7 days later. All samples were stored at −70°C until antibody evaluation was performed. Levels of anti-DT specific antibodies in serum, vaginal washes, feces, lung lavages and nasal washes were quantitated by ELISA as previously described [22].
2.8. Statistical analysis
Data are shown as the mean ± the standard deviation (SD). Significant difference was evaluated by the unpaired Student t test with 2-tailed distributions. P values below 0.05 were considered to be statistically significant.
3.0 Results
3.1. LPS stimulation of murine BMDCs upregulates their endogenous expression of 1α-hydroxylase, alters their migratory properties following in vivo injection and modifies their chemotaxis toward the CCR7 ligand, CCL21
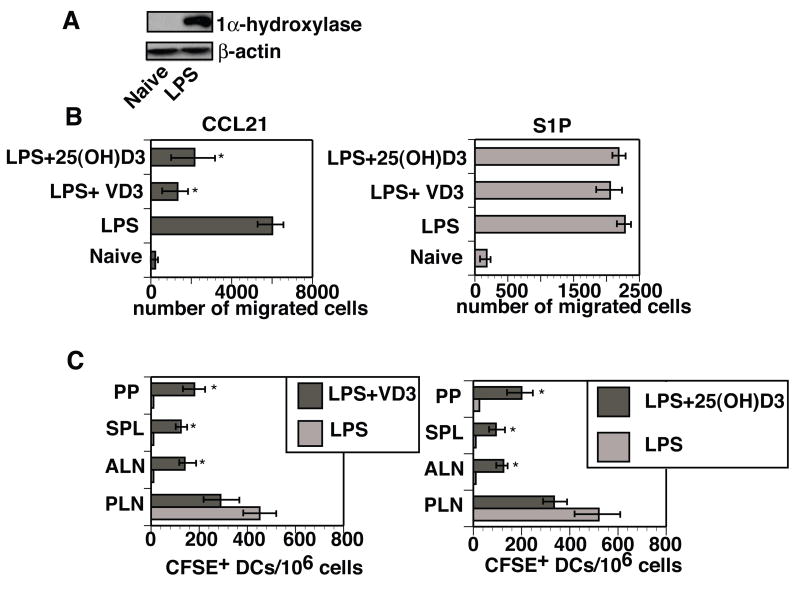
We initially questioned the ability of BMDCs to upregulate their expression of 1α-hydroxylase following an LPS stimulation. LPS treatment of BMDCs triggered a marked increase in 1α-hydroxylase protein expression when compared to control untreated BMDCs (Figure 1A). Based on data presented by others, an increased expression of 1α-hydroxylase in LPS stimulated BMDCs would allow 1α25(OH)2D3 to be endogenously produced from the circulating precursor 25(OH)D3 [13].
Figure 1. The activation-induced upregulation of 1α-hydroxylase in murine dendritic cells alters their trafficking properties in vivo and their chemotaxis in vitro.
(A) CD11c+ BMDCs were stimulated with LPS (10ng/ml) for 24 hours at 37°C or left untreated. Cell lysates were then prepared and analyzed for the presence of 1α-hydroxylase by Western Blot. Proteins were visualized by ECL Plus Western Blotting Detection System. The blot was later stripped and reprobed with antibodies against β-actin to ensure an equal loading of protein. Results are representative of five experiments. (B) CD11c+ BMDCs were matured in vitro for 24 hours in the presence of LPS (10ng/ml) with or without 1α25(OH)2D3 (VD3, 10−8 M) or 25(OH)D3 (10−7 M). The BMDCs were then washed and evaluated for chemotactic responses toward CCL21 (100ng/ml) and S1P (1000nM) using Transwells (5μm pores). After a 3-hour incubation at 37°C, cells that migrated to the bottom chamber were collected and counted. Results are presented as mean of triplicates ± SD. *- difference between numbers of LPS matured DCs in the presence of LPS in the presence of 25(OH)D3 or 1α25(OH)2D3 that migrated into the bottom chamber and numbers of LPS only treated BMDCs that migrated to the bottom chamber was statistically significant (p<0.02-0.009). This experiment was repeated twice with similar results. (C) CD11c+ BMDCs were treated with 10ng/ml of LPS in the presence or absence of 10 8M 1α25(OH)2D3 or 10−7M 25(OH)D3. After a 24-hour incubation at 37°C, the BMDCs were stained with CFSE and subcutaneously injected into naive recipients (three mice per group). Forty-eight hours later, mice were sacrificed and single cell suspensions from individual lymphoid organs (popliteal (PLN), axillary (ALN) lymph nodes, spleens (SPL) and Peyer’s patches (PP)) were prepared and analyzed by FACScan. A total of 400,000 events were collected. Results are presented as mean ± SD. * - difference between numbers of CFSE+ DCs detected in various secondary lymphoid organs of C3H/HeN mice that received a subcutaneous injection of BMDCs exposed to the influences of LPS in the presence of 25(OH)D3 or 1α25(OH)2D3 and numbers of CFSE+ DCs detected in various secondary lymphoid organs of mice that received a subcutaneous injection of BMDCs treated LPS alone was statistically different (p<0.05-0.01).
Murine BMDCs matured in vitro with LPS and added 1α25(OH)2D3 exhibit altered migratory patterns to lymphoid organs in vivo and a reduced chemotaxis toward a CCR7 ligand (CCL21) in vitro [19]. We, therefore, questioned whether BMDCs matured with LPS in the presence of the 1α-hydroxylase substrate 25(OH)D3, would exhibit altered chemotaxis similar to BMDCs directly exposed to 1α25(OH)2D3. BMDCs matured with LPS in the presence of 25(OH)D3 or 1α25(OH)2D3 had a diminished capacity to migrate toward a CCL21 gradient (~67% and 81% inhibition, respectively) when compared to BMDCs matured with LPS alone (Figure 1B). Interestingly, BMDCs from these same treatment groups were able to effectively chemotax toward sphingosine-1-phosphate (S1P) (Figure 1B). It has been reported that chemotaxis toward S1P is important for lymphocyte egress from secondary lymphoid organs and tissues and is CCR7 independent [23].
We have previously reported that 1α25(OH)2D3-exposed BMDCs gain an ability to bypass draining lymph nodes (LN) and localize to multiple non-draining secondary peripheral and mucosal lymphoid organs following their subcutaneous injection into naïve syngeneic recipients [19]. This led us to investigate whether the migratory properties of BMDCs could be affected by endogenously produced 1α25(OH)2D3. When BMDCs were matured with LPS in the presence of either 1α25(OH)2D3 or 25(OH)D3 and injected subcutaneously, they were able to migrate from the injection sites to the draining as well as non-draining secondary lymphoid organs (Figure 1C). The vast majority of mobilized BMDCs matured in vitro with LPS without added hormone localized to the draining LN (Figure 1C).
Experiments presented above indicate that murine BMDCs stimulated with LPS in the presence of 25(OH)D3, similar to 1α25(OH)2D3-exposed BMDCs, are able to avoid sequestration to the draining LNs and localize to numerous non-draining secondary lymphoid organs, including inductive sites of mucosal immunity.
3.2. LPS exposure is able to mobilize DCs from the skin and allow their localization to multiple non-draining secondary lymphoid organs
We recently reported that DCs can become mobilized from the skin in response to a subcutaneous latex microsphere injection, bypass the draining LN and enter numerous non-draining secondary lymphoid organs, including PPs, when 1α25(OH)2D3 is added to the microsphere inoculum [19]. We therefore questioned whether LPS, co-administered with latex microspheres, would affect the trafficking pattern of mobilized DCs. C3H/HeN mice were injected into the right hind footpad with 0.2μm green fluorescent microspheres in the presence of 10μg LPS and with red fluorescent microspheres (0.2μm) alone into left hind footpad. Control animals were injected with 0.2μm green fluorescent microspheres in the presence of 0.1μg 1α25(OH)2D3 in their right hind footpads and 0.2μm red fluorescent microspheres alone in their left hind footpads. In both groups of mice after 48 hours, mobilized red microsphere+ cells predominantly localized to the draining LNs (left popliteal LN (PLN)) (Figure 2A). However, under the influence of LPS, similar to 1α25(OH)2D3, DCs containing green fluorescent microspheres localized to multiple draining and non-draining secondary lymphoid organs, including the PPs (Figure 2A). Unlike the co-administration of 1α25(OH)2D3 with 0.2μm microspheres, LPS co-administration with latex microspheres resulted in a 2 fold increase in cellularity of the draining LNs, while the cellularity of other secondary lymphoid organs was unaffected by LPS co-administration (data not shown).
Figure 2. Dendritic cells leaving a subcutaneous site of LPS injection are capable of trafficking to multiple secondary lymphoid organs throughout the body.
(A) Green fluorescent latex microspheres (0.2μm) were injected into the right hind footpads of mature adult C3H/HeN mice in the presence or absence of 1α25(OH)2D3 (0.1μg) or LPS (10μg). Red fluorescent latex microspheres were injected alone into the left hind footpad of the same animals. After forty-eight hours, individual lymphoid tissues were analyzed for the presence of microsphere+ DCs. A total of 400,000 events were collected. Data is presented as mean ± SD. *- differences in the numbers of green microsphere+ DCs in a particular lymphoid organ from animals exposed to the influences of LPS or 1α25(OH)2D3 and the numbers of red microsphere+ DCs was statistically significant (p<0.01-0.003). Results are representative of five independent experiments. (B) Mature adult C3H/HeN mice were injected with 0.2μm green microspheres into the right thigh and 0.2μm red microspheres into the right abdominal area. Both injection sites drain into the same inguinal LN. Forty-eight hours post injection, the draining inguinal LN was collected and single cell suspensions were prepared. Samples were analyzed by FACScan for the presence of green or red microspheres+ DCs. A total of 400,000 events were collected. Experiment was repeated three times with similar results. (C) Green fluorescent microspheres (1.0μm or 0.2μm) were injected in the presence of LPS (10μg) into the right hind footpads of normal mice (three animals per group). Red fluorescent microspheres (1.0μm or 0.2μm) were injected alone into the left hind footpads of the same mice. After forty-eight hours, individual lymphoid organs were removed (popliteal lymph node (PLN), Peyer’s patch (PP)) and single cell suspensions were prepared. Samples were analyzed by FACScan for the presence of microsphere+ DCs. Data presented as mean ± SD. *- difference between numbers of green microsphere+ DCs found in PLN and PPs of animals that received 1.0μm (or 0.2μm) microspheres plus LPS and numbers of red microsphere+ DCs in PLN and PPs of mice that were injected with 1.0μm (or 0.2μm) microspheres alone was statistically significant (p<0.02-0.007). Results are representative of two experiments.
Analysis of the surface phenotype of microsphere+ cells that had localized within the draining LNs determined that the majority, regardless of co-administered 1α25(OH)2D3, LPS, or alone, were CD11c+CD11b+, with remaining cells being CD11c−CD11b+ (data not shown). CD11b (MAC-1) is a marker expressed on all myeloid lineage cells and CD11c is a marker expressed by all types of murine DCs [24, 25]. Approximately 45–50% of the microsphere+ DCs within the draining LN of LPS treated animals phenotyped 33D1+CD8α−, while 20–25% of 1α25(OH)2D3-exposed microsphere+ DCs had this phenotype. 33D1+CD8α− DCs are believed to be involved in initiation of humoral immune responses [26]. A smaller percentage of the DCs from both groups were DEC205+CD8α+, which is indicative of cells capable of stimulating cell-mediated immune responses [26, 27] (Table 1). In contrast, virtually all microsphere+ cells that bypassed draining LN sequestration and had localized to non-draining secondary lymphoid organs from both the 1α25(OH)2D3 and LPS treatment groups phenotyped CD11c+CD11b+33D1+CD8α− (Table 1).
Table 1.
Surface phenotype of the microsphere+ DCs within draining and non-draining secondary lymphoid organs following a subcutaneous injection of 0.2μm latex microspheres with 1α25(OH)2D3 or LPS.
| Secondary lymphoid organs | Additions to microsphere inoculuma | Phenotype of microsphere+ cellsb | |
|---|---|---|---|
| CD8α−33D1+ (%) | CD8α+DEC205+ (%) | ||
| Popliteal LN (draining) | None | 68.1±3.2 | 12.2±1.3 |
| 1α25(OH)2D3 | 21.3± 3.5c | 31.1±2.8c | |
| LPS | 47.5±4.3c | 16.3±0.9 | |
| Axillary LN (non-draining) | None | N/Dd | N/D |
| 1α25(OH)2D3 | 98.5±0.3 | 1.9±0.2 | |
| LPS | 95.4±1.7 | 2.1±0.3 | |
| PP (non-draining, mucosal) | None | N/D | N/D |
| 1α25(OH)2D3 | 97.8±1.1 | 2.0±0.1 | |
| LPS | 96.5±1.5 | 1.8±0.3 | |
Green 0.2μm fluorescent microspheres (50μl of a 0.25% solution) were injected into the hind footpads of C3H/HeN mice (3 per group) in the presence of 0.1μg 1α25(OH)2D3 or 10μg LPS. The injection of microspheres alone served as the control.
Single cell suspensions were prepared from multiple secondary lymphoid organs after 48 hours. Cells were stained with antibodies directed against CD8α, DEC205 and 33D1 and analyzed by FACScan. Phenotype analysis was done on microsphere+ cells. At least 1000 microsphere+ events were analyzed.
Differences in the percentages of LPS or 1α25(OH)2D3-exposed microsphere+ DCs in the draining LNs of experimental mice and the percentage of microsphere+ DCs in the draining LNs of mice that received microspheres only were statistically significant (p<0.05-0.002)
N/D – not detected
Although most of microsphere+ DCs that localized in the draining LNs of untreated animals or in animals treated with 1α25(OH)2D3 or LPS expressed CCR7, the expression levels were much lower (50% reduction) in both of the experimental groups compared to CCR7 expression levels on the microsphere+ DCs in the draining LNs following microsphere only injection (data not shown). Virtually all the microsphere+ DCs that localized in the non-draining axillary LNs and PP expressed high levels of CD62L, α4β7 and CCR7 (data not shown), consistent with what we previously reported [19].
Most microsphere+ DCs that localized to the draining LNs from the LPS and 1α25(OH)2D3 treated groups expressed elevated levels of α4β7 when compared to the control group (200–600% increase) (data not shown and [19]). Although, α4β7 is an integrin important for the localization of lymphocytes to mucosal tissues, the influences of this integrin on the surface of DCs in currently unknown [28, 29].
While 30–50% of the DCs that localized to the draining LNs expressed positive levels of the CD103 marker, regardless of group, virtually all microsphere+ DCs that had migrated to non-draining secondary lymphoid organs expressed high levels of CD103 (data not shown). CD103+ DCs have recently been found at a high frequency in the gut-associated tissues [30, 31].
Collectively, the phenotype analysis of migrating DCs led us to conclusion that some differences were observed in the phenotypes of the microsphere+ DCs that had localized to the draining lymphoid organs from both the control and experimental groups, while the phenotype of microsphere+ DCs that had bypassed sequestration in the draining LNs and migrated to non-draining secondary lymphoid organs were quite similar between both experimental groups.
To establish that subcutaneously administered 0.2μm microspheres are being delivered to LNs inside of DCs, and do not access the LNs in a cell-free form, C3H/HeN mice were injected with 0.2μm green fluorescent microspheres and 0.2μm red fluorescent microspheres into two distinct skin sites that drain into the same inguinal LN (ILN). After 48 hours, it was determined that individual microsphere+ DCs in the ILNs contained only single colored microspheres, supporting the conclusion that the injected latex microspheres are taken up at the site of injection and delivered to draining LNs inside of DCs (Figure 2 B).
Our findings concerning the effects of LPS treatment on DC migration in vivo seemed to contradict some previously published observations, reporting that the migration of DCs from skin sites injected with 1.0μm microspheres was completely inhibited when the microspheres were co-administered with LPS [32]. This suggested that the migratory properties of mobilized DCs might depend upon the size of the microspheres used experimentally. C3H/HeN mice were subcutaneously injected with 1.0μm green microspheres in the presence of LPS into the right hind footpad and 1.0μm red microspheres alone into the left hind footpad to test this possibility. A parallel group of mice was injected in an identical manner, except 0.2μm green microspheres in the presence of LPS and red microspheres alone were used. After forty-eight hours, green 0.2μm microsphere+ DCs were detected in draining and non-draining secondary lymphoid organs as we observed earlier, and red 0.2μm microsphere+ DCs localized mainly to the draining LN (left PLN) (Figure 2A and 2C). In mice that received green 1.0μm microspheres in the presence of LPS, practically no green microsphere+ DCs were observed in the draining LN (right PLN), while red 1.0μm microsphere+ DCs were detectable in the draining LN (left PLN) (Figure 2C). Our experimental results, therefore, confirmed the previously reported observations that the migration of myeloid cells containing 1.0μm microspheres was blocked under the influence of LPS [32]. Most importantly, the findings from this experiment also support our observations that DCs containing 0.2μm microspheres are capable of migrating from the skin sites of inoculation to both draining and non-draining secondary lymphoid organs under the influence of LPS.
This finding led us to question whether distinct subsets of cells might be involved in the peripheral uptake of 0.2μm or 1.0μm microspheres. When C3H/HeN mice were subcutaneously injected with either 0.2μm or 1.0μm green microspheres without added adjuvant, the majority (>75%) of DCs containing 0.2μm microspheres in the draining LN phenotyped CD11c+CD11b+ (Table 2). Most of these DCs also phenotyped as 33D1+CD8α− and a small subpopulation phenotyped DEC205+CD8α+ (data not shown). In contrast, a much smaller percentage of DCs containing 1.0μm microspheres phenotyped CD11c+CD11b+ (~30%) with the majority (~62%) phenotyping CD11c−CD11b+ (Table 2).
Table 2.
The majority of 0.2μm microsphere+ DCs that localize within the draining LNs phenotype as CD11c+CD11b+, while the majority of cells containing 1.0μm microspheres phenotype as CD11c−CD11b+
| Experimental groupsa | Phenotype of microsphere+ cells residing in the draining LNb | |
|---|---|---|
| CD11c+CD11b+ (%) | CD11c−CD11b+ (%) | |
| 0.2μm microspheres | 76.3±1.1 | 13.8±0.8 |
| 1.0μm microspheres | 30.5±2.8c | 62.8±1.4c |
1.0μm or 0.2μm green fluorescent microspheres (50μl of a 0.25% solution) were injected into hind footpads of C3H/HeN mice (3 per group).
Single cell suspensions were prepared from the draining popliteal lymph nodes after 48 hours. Cells were stained with antibodies specific for CD11c and CD11b and analyzed by FACScan. Phenotype analysis was done on microsphere+ cells. At least 1000 microsphere+ events were analyzed.
Differences between the percentage of 0.2μm microsphere+ DCs and the percentage of 1.0μm microsphere+ DCs were significant (p<0.001-0.005)
A phenotypic analysis of DCs containing either high or low numbers of microspheres revealed that most of 0.2μm microsphere+ DCs from both subpopulations (with high or low numbers of microspheres per cell) expressed both CD11c and CD11b. The subpopulation of 1.0μm microsphere+ cells containing a high number of ingested microspheres per cell expressed only the CD11b marker, while 1.0μm microsphere+ cells containing low numbers of microspheres expressed both CD11b and CD11c (data not shown). These data suggest that cells that take up 1.0μm or 0.2μm microspheres at the skin sites of injection and migrate to the draining LNs could be distinct subpopulations of mobilized cells.
3.3. Protein loaded DCs emigrating from LPS-exposed skin sites can effectively present antigen peptides to naïve CD4+ T cells residing in multiple secondary lymphoid organs
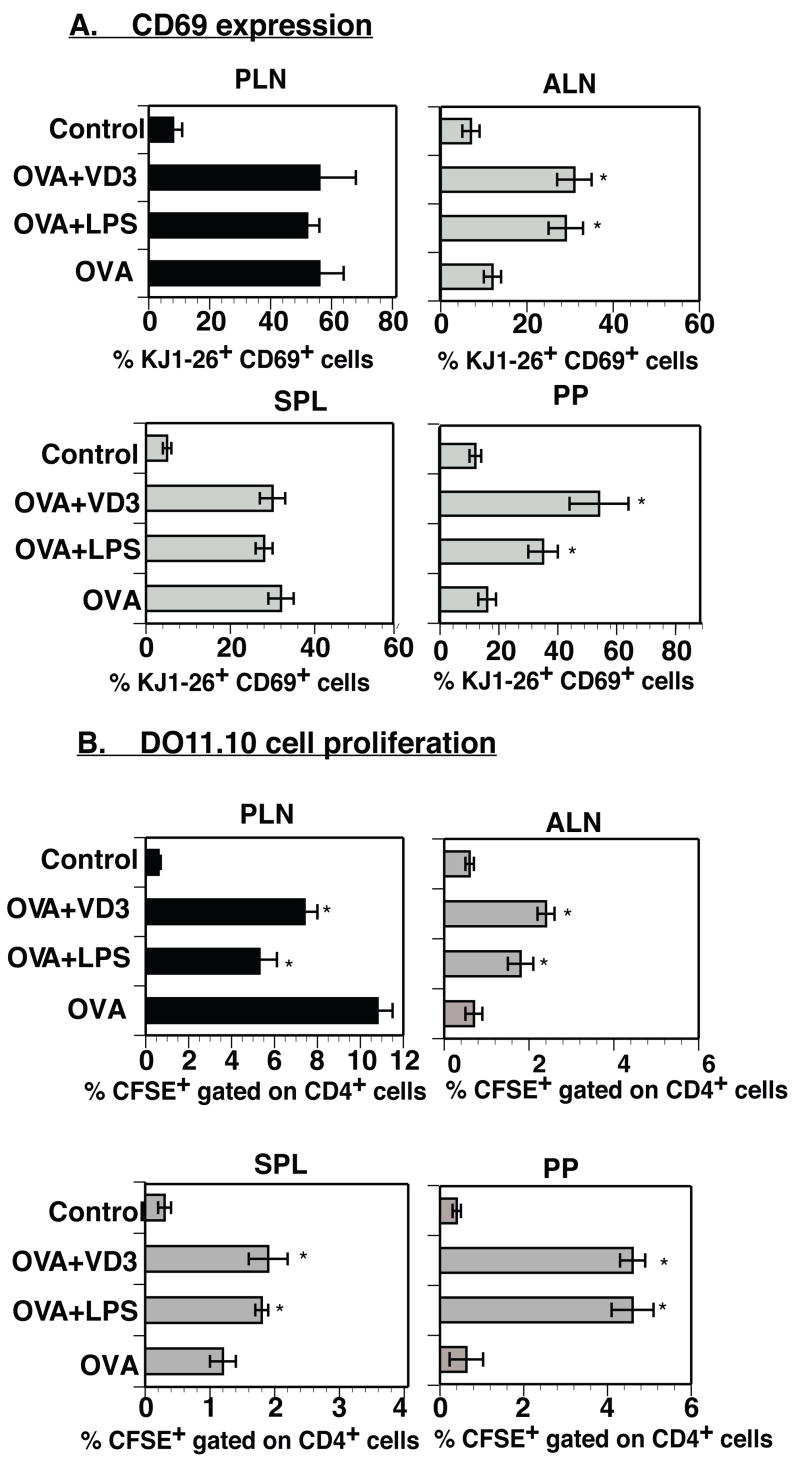
We questioned whether the migratory DCs possess the capacity to effectively present antigen to naïve CD4+ T cells and stimulate their clonal expansion. The immunization of DO11.10 CD4+ T cell recipients with OVA in the presence of LPS or 1α25(OH)2D3 resulted in an increased percentage of KJ1-26+ CD69+ cells in all tested draining and non-draining secondary lymphoid organs except the spleen (54–58% in the draining popliteal LNs, 31–34% in the axillary LNs, and 36–50% in the PPs) (Figure 3A). Only the draining LN of adoptive recipients that were immunized with OVA alone contained an increased percentage of double positive cells (58%) (Figure 3A). The expression of CD69 by DO11.10 CD4+ T cells in non-draining lymphoid organs of mice immunized with OVA in the presence of LPS or 1α25(OH)2D3 suggests that these cells were being activated locally by antigen-laden DCs that had migrated from the subcutaneous site of antigen administration.
Figure 3. Mobilized antigen-laden DCs that traffic to non-draining lymphoid organs are fully capable of productively initiating an antigen-specific immune response.
(A) BALB/c mice received 2×106 CD4+ T cells from DO11.10 donors. After a 24-hour rest, the DO11.10 T cell recipients were subcutaneously immunized with 50μg OVA in Alum in the presence or absence of 0.1μg 1α25(OH)2D3 or 10μg LPS. Recipients were sacrificed 72 hours post-immunization and their secondary lymphoid organs were analyzed for KJ1-26+CD69+ T cells. Data presented as mean ± SD. *- difference between the percentage of KJ1-26+ cells co-expressing CD69 in tested lymphoid organs of animals immunized with OVA and added LPS or 1α25(OH)2D3 and the percentage of KJ1-26+CD69+ cells detected in various lymphoid organs of animals immunized with OVA alone was statistically different (p<0.04-0.02). The results presented are representative of two independent experiments. (B) BALB/c recipients of DO11.10 CD4+ T cells stained with CFSE were subcutaneously immunized with vaccine formulations containing 50μg OVA in Alum with or without 0.1μg 1α25(OH)2D3 or 10μg LPS. After 7 days, individual secondary lymphoid organs were removed, and single cell suspensions were prepared for analysis by FACScan. Data presented as mean ± SD. *- difference between percentage of CFSE+CD4+ cells in various lymphoid organs of mice immunized with OVA plus LPS or 1α25(OH)2D3 and percentage of CFSE+CD4+ cells detected in various lymphoid organs of animals immunized with OVA alone was statistically different (p<0.05-0.01). Results were obtained from two independent experiments.
The expression of CD69 on adoptively transferred DO11.10 CD4+ T cells correlated with their ability to undergo clonal expansion since immunization of DO11.10 T-cell recipients with OVA in the presence of LPS or 1α25(OH)2D3 resulted in expanded numbers of D011.10 T cells in draining as well as non-draining secondary lymphoid organs (Figure 3B). DO11.10 CD4+ T cell expansion was primarily restricted to the draining LNs when the DO11.10 T cell recipients were immunized with OVA alone (Figure 3B). These findings provide evidence that antigen-laden DCs that migrate to non-draining secondary lymphoid organs are able to effectively present antigen peptides to antigen-responsive CD4+ T cells residing in these lymphoid tissues and promote their clonal expansion.
3.4. Subcutaneously administered TLR ligands that induce increases in 1α-hydroxylase protein expression in DCs are able to alter their migratory patterns in vivo and promote the induction of mucosal immune responses to co-administered antigens
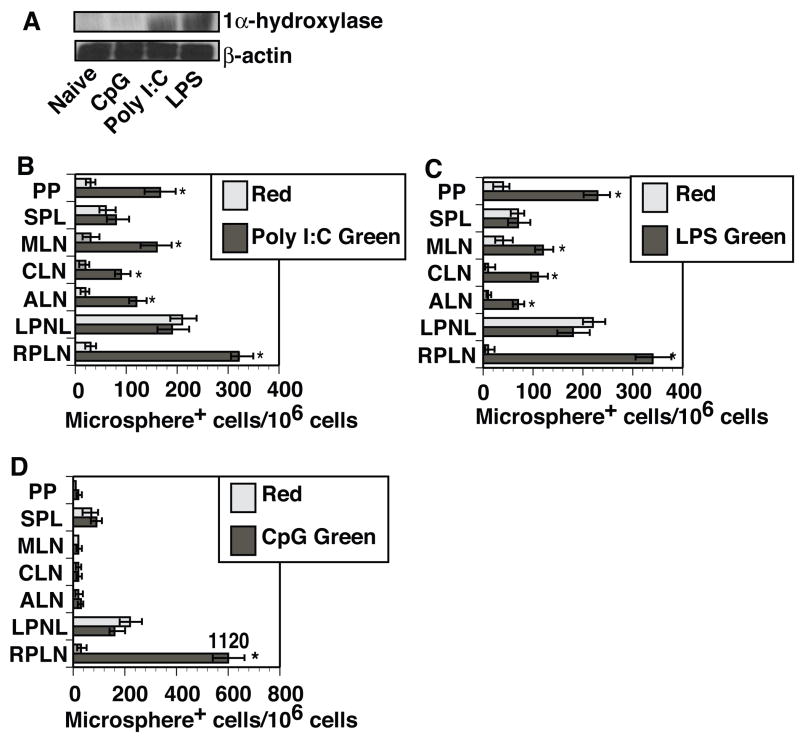
Major targets of most TLR ligands are the myeloid cells, including macrophages and DCs [33–36]. It has been reported that several distinct TLR ligands are capable of inducing the expression of 1α-hydroxylase in human monocytes and macrophages [13, 16]. This led us to question whether 1α-hydroxylase protein could be expressed following murine DCs exposure to various TLR ligands. Western blot analysis determined that BMDCs exposed to LPS (TLR4) or poly I:C (TLR3) showed an increased expression of 1α-hydroxylase, while BMDC activation with CpG ODNs (TLR9) failed to increase expression of this enzyme (Figure 4A).
Figure 4. TLR ligands that upregulate the expression of 1α-hydroxylase in DCs are able to alter their migratory properties following activation-induced mobilization.
(A) CD11c+ BMDCs were activated with CpG ODN (20μg/ml), poly I:C (20μg/ml), LPS (10ng/ml) or left untreated. Cells were harvested 24 hours post activation, lysed and analyzed for 1α-hydroxylase protein expression by Western Blot analysis. The blot was later stripped and reprobed with antibodies against β-actin to ensure an equal loading of protein. Results presented are representative of three experiments. (B-D) Green fluorescent microspheres (0.2μm) in the presence of (B) 20μg poly I:C, (C) 10μg LPS, or (D) 20μg CpG ODNs were injected into the right hind footpads of C3H/HeN mice (three per group). Red fluorescent microspheres (0.2μm) were then injected alone into the left hind footpads of the same animals. After forty-eight hours, individual lymphoid organs (right popliteal (RPLN), left popliteal (LPLN), axillary (ALN), cervical (CLN) and mesenteric (MLN) lymph nodes, spleens (SPL) and Peyer’s patches (PP)) were removed. Single cell suspensions were prepared and analyzed by FACScan. Data presented as mean ± SD. *- difference between numbers of green microsphere+ DCs in animals co-injected with microspheres plus poly I:C, LPS or CpG ODN found in various secondary lymphoid organs and numbers of red microsphere+ DCs in the lymphoid organs analyzed from the same mice was statistically significant (p<0.01-0.003). The data presented in Figure 4B–D are representative of three independent experiments.
To evaluate the capacity of distinct TLR ligands to influence DC trafficking in vivo, C3H/HeN mice were injected subcutaneously into the right hind footpad with 0.2μm green microspheres in the presence of either poly I:C, LPS or CpG ODNs. The test animals also received an injection of 0.2μm red microspheres alone into their left hind footpads as an internal control. FACScan analysis 48 hours post injection determined that DCs containing red microspheres were found to predominantly localize to the draining LN (left PLN) in all experimental groups (Figure 4B–D). Green microsphere+ DCs from animals co-injected with microspheres in the presence of LPS or poly I:C were found in both draining and non-draining secondary lymphoid organs (Figure 4B–C), while the co-injection of microspheres with CpG ODNs resulted in an increased localization of green microsphere+ DCs to the draining LNs only (Figure 4D). These data demonstrate that TLR ligands capable of upregulating 1α-hydroxylase expression in DCs also promoted the migration of microsphere+ DCs to non-draining secondary lymphoid organs.
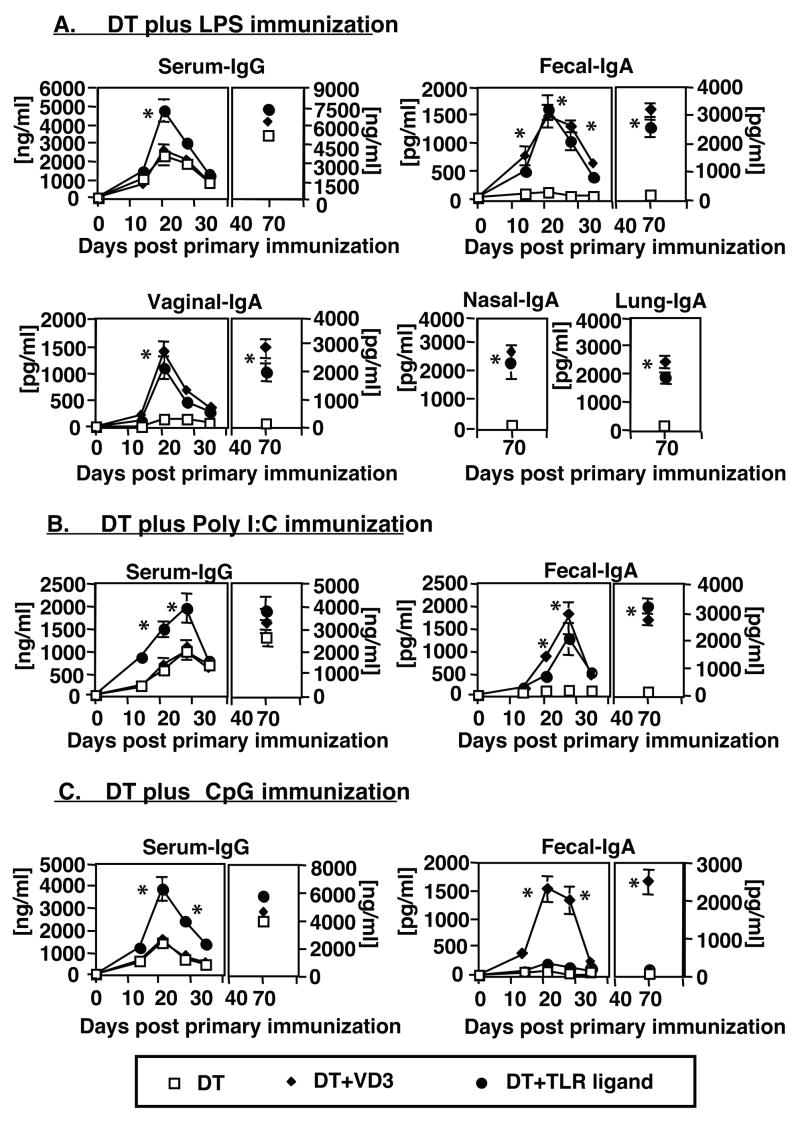
We previously reported that the ability of 1α25(OH)2D3 to induce mucosal immune responses in vivo was directly correlated with its ability to promote the migration of antigen-laden DCs from the skin to non-draining secondary lymphoid organs, especially to the PPs [18]. Our data now indicates that LPS and poly I:C are both able to promote DC trafficking to known inductive sites of mucosal immunity, thereby suggesting that these TLR ligands might function as effective mucosal adjuvants for subcutaneously administered antigens. All of the animals vaccinated with Diphtheria CRM 197 protein (DT) in the presence of LPS, poly I:C or CpG ODNs mounted enhanced anti-DT serum IgG responses when compared to immune responses of mice immunized with DT/Alum alone (Figure 5A–C). However, only the mice immunized with vaccines containing LPS or poly I:C mounted significant levels of mucosal anti-DT IgA antibody responses following primary and booster immunizations (Figure 5A–B and data not shown). In contrast, no anti-DT IgA antibodies were detected in the mucosal secretions of mice immunized with DT/Alum alone or DT in the presence of CpG ODNs (Figure 5C and data not shown).
Figure 5. Vaccine formulations, containing LPS or poly I:C as adjuvants are able to promote the induction of both systemic and mucosal immune responses.
Groups of mature adult C3H/HeN mice (five per group) were subcutaneously immunized with a vaccine formulation containing 1μg diphtheria CRM 197 protein (DT) and added (A) LPS (10μg), (B) poly I:C (20μg) or (C) CpG ODN (20μg). With each of the TLR ligands being tested, parallel groups were added that were immunized with DT alone, or with DT and 0.1μg 1α25(OH)2D3. Nine weeks after immunization, all groups of animals were subcutaneously reimmunized as described above. Serum and mucosal samples were collected at various time points post vaccination and analyzed for the presence of DT-specific antibodies by ELISA. Results are reported as the mean value of anti-DT antibodies detected in the serum and/or mucosal secretions of five mice per group (±SD). *- difference between levels of anti-DT antibodies in serum or mucosal secretions of mice immunized with DT in the presence of 1α25(OH)2D3 or TLR ligand and levels of anti-DT antibodies in serum or mucosal secretions of mice immunized with DT only were statistically significant (p< 0.004-0.001)
Our results indicate that vaccines containing TLR ligands capable of promoting the migration of DCs beyond draining LNs (LPS and poly I:C), like 1α25(OH)2D3, support the induction of mucosal immune responses. CpG ODNs added to vaccines, however, augmented the magnitude of systemic immune responses without any observed induction of mucosal immunity.
4.0 Discussion
We and others have previously reported that both systemic and mucosal antigen-specific immune responses can be induced in mice and other animal species following parenteral immunization with protein vaccines containing small quantities of 1α25(OH)2D3 [17–19, 21, 22, 37, 38]. The mucosal antibody responses induced by these systemically administered vaccines were established to reflect local production by IgA-secreting B cells residing in the lamina propria, and the levels of antigen-specific IgA antibodies present in the mucosal secretions were determined to be independent of serum antibody titers [18].
We hypothesized that vaccines containing TLR ligands able to stimulate 1α-hydroxylase expression in DCs should be capable of initiating common mucosal immune responses to co-administered antigens. We found that murine DCs were induced to express 1α-hydroxylase following their stimulation with LPS, suggesting that 1α25(OH)2D3 was being locally produced in vivo in response to stimulation through TLR4 BMDCs matured in vitro with LPS in the presence of the precursor 25(OH)D3 were able to traffic beyond the draining LN and localize into various non-draining secondary lymphoid organs, including the PPs following an adoptive transfer into naïve C3H/HeN recipients. This finding suggested that alterations to the migratory properties of BMDCs, matured with LPS in the presence of 25(OH)D3 in vitro, were probably due to effects mediated by newly synthesized 1α25(OH)2D3.
The co-injection of LPS and latex microspheres into the skin of experimental mice, similar to the co-injection of 1α25(OH)2D3 and microspheres, resulted in the localization of microsphere+ DCs into numerous non-draining secondary lymphoid organs, including the PPs. This was only observed with small 0.2μm microspheres, since the migration of DCs containing 1.0μm microspheres was completely inhibited by co-administration of LPS. This finding, along with observed differences in the phenotypes of 0.2μm and 1.0μm microsphere-containing DCs, suggest that the size of antigen particles employed may represent a component of vaccine design that needs additional consideration.
Our analysis of the surface phenotype of the migratory 0.2μm microsphere+ DCs determined that the virtually all DCs mobilized from LPS injection sites that ultimately trafficked to non-draining lymphoid organs were CD11c+CD11b+, as previously reported (data not shown and [19]). A substantial percentage of the microsphere+ DCs that localized into the draining LNs, and practically all microsphere+ DCs that had localized into non-draining lymphoid tissues phenotyped as 33D1+. It has been shown that 33D1+ DCs are capable of effectively initiating humoral immune responses [26, 27]. This data suggests that 1α25(OH)2D3- or LPS-mobilized DCs leaving cutaneous sites of vaccine administration should be capable of effectively supporting the development of humoral immune responses subsequent to their localization to peripheral or mucosal lymphoid organs.
The mobilized microsphere+ DCs that migrated beyond the draining LNs were also found to express CD103. TGFβ is known to stimulate the expression of CD103 on CD8+ T cells [39, 40], and we have recently found (unpublished data) that murine DCs can be induced to express large quantities of bioactive TGFβ following their activation in vitro with LPS in the presence of either calcitriol or its precursor hormone 25(OH)D3. Whether the expression of CD103 on DCs leaving sites of calcitriol exposure is a TGFβ-dependent event, however, is currently unknown. Recently, it has been shown that the co-culture of CD103+ DCs with purified CD4+ or CD8+ T cells stimulates the expression of both α4β7 and CCR9 on lymphocytes, two molecules that effectively guide their localization into mucosal tissues [39, 40]. CD103+ DCs might be programmed to produce the retinal dehydrogenases needed to metabolize vitamin A to the active metabolite, since all trans retinoic acid is now known to confer intestinal homing capabilities to both T and B lymphocytes via its ability to promote expression of α4β7 and CCR9 [41, 42].
Our experiments evaluated possible mechanisms by which LPS (a TLR4 ligand) promotes an alteration in the migratory properties of mobilized DCs, leading to the induction of mucosal immune responses. Stimulation of responsive cells through TLR4 activates two known signaling pathways, the MyD88- and the TRIF-dependent pathways [1, 43]. CD11c+ DCs generated from the bone marrow of adult mice expressed 1α-hydroxylase following their activation with poly I:C (TLR3 ligand, TRIF-dependent pathway) but not following their activation with CpG ODNs (TLR9 ligand, MyD88-dependent pathway). When naïve mice were subcutaneously injected with fluorescent microspheres plus poly I:C or CpG ODNs, microsphere+ DCs could only be found in the non-draining lymphoid organs of mice that received the TLR ligand capable of upregulating 1α-hydroxylase expression (poly I:C). A co-administration of both CpG ODNs and poly I:C with microspheres resulted in an increase in the number of microsphere+ DCs that localized to non-draining secondary lymphoid organs and the PPs of experimental mice (data not shown). These data indicate that stimulation of both TLR-dependent signaling pathways is important to achieve optimal DC mobilization from inflammatory skin sites. Stimulation through the TRIF-dependent pathway alone, however, appears to be sufficient and necessary for mobilized DCs to bypass sequestration in the draining lymph nodes and traffic to non-draining secondary lymphoid organs.
TLR ligands that allowed DCs leaving skin sites of vaccine administration to traffic into non-draining secondary lymphoid organs, positively correlated with their ability to function as adjuvants for the stimulation of systemic and mucosal immune responses. The addition of LPS or poly I:C to subcutaneously administered vaccines was capable of stimulating the simultaneous induction of both systemic and mucosal immune responses, while the use of CpG ODNs as an adjuvant augmented the generation of only systemic immunity to subcutaneously administered antigens. It will be of interest to determine whether the immune responses elicited against bacterial or viral pathogens containing TLR ligands can promote the effective development of mucosal immunity, especially under conditions where the primary site of infection/vaccination is peripheral and not mucosal.
Data presented in this manuscript demonstrate that activation of the TRIF-dependent signaling pathway in DCs entering sites of vaccine delivery, stimulates the endogenous production 1α25(OH)2D3 in vivo, ultimately expanding the types of adaptive immune effector responses elicited. This calcitriol-dependent mechanism should provide a broader range of immune protection to vaccinated hosts.
It is now well appreciated that antigens administered intranasally, along with appropriate adjuvants, are able to simultaneously stimulate the generation of both antigen-specific systemic and mucosal immune responses [44, 45]. The skin, while representing a major portal of entry for infection, is also an immunologically important tissue. Recent data supports the concept that antigen presentation through the skin, like antigen presentation via the nasal mucosa, can effectively stimulate the generation of both systemic and mucosal immune effector responses. We believe that this process is dependent upon locally produced 1α25(OH)2D3 and is mediated through calcitriol ability to alter the migratory properties of DCs mobilized from the sites of antigen administration/infection, allowing them to localize and present antigen in classical inductive sites of mucosal immunity. Our present finding that DC activation by TLR ligands through the MyD88 independent pathway leads to an upregulated expression of 1α-hydroxylase indicates that the innate responses by some microbial pathogens should be able to stimulate effective adaptive mucosal defenses. Therefore, the immune modulatory influences of locally produced calcitriol should now be expanded to include the stimulation of cathelicidin production, the homing of effector T cells to the skin and promoting the migration of antigen-laden DCs from a skin site of vaccination to numerous secondary lymphoid organs, including the PPs, for the effective generation of both adaptive systemic and mucosal immune responses.
Acknowledgments
This work was supported by National Institute of Health grant DK 55491 and AI 059242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167(5):2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 3.Su SL, Tsai CD, Lee CH, Salter DM, Lee HS. Expression and regulation of Toll-like receptor 2 by IL-1beta and fibronectin fragments in human articular chondrocytes. Osteoarthritis Cartilage. 2005;13(10):879–86. doi: 10.1016/j.joca.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 6.Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006;84(4):333–41. doi: 10.1111/j.1440-1711.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 8.Jiang ZH, Koganty RR. Synthetic vaccines: the role of adjuvants in immune targeting. Curr Med Chem. 2003;10(15):1423–39. doi: 10.2174/0929867033457340. [DOI] [PubMed] [Google Scholar]
- 9.McCluskie MJ, Weeratna RD, Payette PJ, Davis HL. The potential of CpG oligodeoxynucleotides as mucosal adjuvants. Crit Rev Immunol. 2001;21(1–3):103–20. [PubMed] [Google Scholar]
- 10.Ichinohe T, Watanabe I, Ito S, Fujii H, Moriyama M, Tamura S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005;79(5):2910–9. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloat BR, Cui Z. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm Res. 2006;23(6):1217–26. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 12.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, et al. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006;74(1):694–702. doi: 10.1128/IAI.74.1.694-702.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha, 25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102(9):3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 14.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8(3):285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 15.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194(10):1541–7. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 17.Daynes RA, Enioutina EY, Butler S, Mu HH, McGee ZA, Araneo BA. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect Immun. 1996;64(4):1100–9. doi: 10.1128/iai.64.4.1100-1109.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enioutina EY, Visic D, McGee ZA, Daynes RA. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine. 1999;17(23–24):3050–64. doi: 10.1016/s0264-410x(99)00147-4. [DOI] [PubMed] [Google Scholar]
- 19.Enioutina EY, Bareyan D, Daynes RA. Vitamin D3-mediated alterations to myeloid dendritic cell trafficking in vivo expand the scope of their antigen presenting properties. Vaccine. 2007;25(7):1236–49. doi: 10.1016/j.vaccine.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134(1):255–66. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enioutina EY, Visic D, Daynes RA. The induction of systemic and mucosal immune responses to antigen-adjuvant compositions administered into the skin: alterations in the migratory properties of dendritic cells appears to be important for stimulating mucosal immunity. Vaccine. 2000;18(24):2753–67. doi: 10.1016/s0264-410x(00)00059-1. [DOI] [PubMed] [Google Scholar]
- 22.Enioutina EY, Visic DM, Daynes RA. The induction of systemic and mucosal immunity to protein vaccines delivered through skin sites exposed to UVB. Vaccine. 2002;20(16):2116–30. doi: 10.1016/s0264-410x(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 23.Czeloth N, Bernhardt G, Hofmann F, Genth H, Forster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175(5):2960–7. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- 24.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171(5):1753–71. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L, Dakic A. Development of dendritic cell system. Cell Mol Immunol. 2004;1(2):112–8. [PubMed] [Google Scholar]
- 26.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 27.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farstad IN, Halstensen TS, Kvale D, Fausa O, Brandtzaeg P. Topographic distribution of homing receptors on B and T cells in human gut-associated lymphoid tissue: relation of L-selectin and integrin alpha 4 beta 7 to naive and memory phenotypes. Am J Pathol. 1997;150(1):187–99. [PMC free article] [PubMed] [Google Scholar]
- 29.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152(7):3282–93. [PubMed] [Google Scholar]
- 30.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–42. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 31.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202(8):1063–73. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotta G, Edwards EW, Sangaletti S, Bennett C, Ronzoni S, Colombo MP, et al. Lipopolysaccharide or whole bacteria block the conversion of inflammatory monocytes into dendritic cells in vivo. J Exp Med. 2003;198(8):1253–63. doi: 10.1084/jem.20030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12(32):4247–54. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 35.Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–35. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 36.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589(1):1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 37.Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J Infect Dis. 2006;193(4):598–600. doi: 10.1086/499970. [DOI] [PubMed] [Google Scholar]
- 38.Van der Stede Y, Cox E, Van den broeck W, Goddeeris BM. Enhanced induction of the IgA response in pigs by calcitriol after intramuscular immunization. Vaccine. 2001;19(15–16):1870–8. doi: 10.1016/s0264-410x(00)00440-0. [DOI] [PubMed] [Google Scholar]
- 39.Lim SP, Leung E, Krissansen GW. The beta7 integrin gene (Itgb-7) promoter is responsive to TGF-beta1: defining control regions. Immunogenetics. 1998;48(3):184–95. doi: 10.1007/s002510050422. [DOI] [PubMed] [Google Scholar]
- 40.Ling KL, Dulphy N, Bahl P, Salio M, Maskell K, Piris J, et al. Modulation of CD103 expression on human colon carcinoma-specific CTL. J Immunol. 2007;178(5):2908–15. doi: 10.4049/jimmunol.178.5.2908. [DOI] [PubMed] [Google Scholar]
- 41.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21(4):527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314(5802):1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 44.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25(30):5467–84. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Kiyono H, Fukuyama S. NALT- versus Peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4(9):699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]