Abstract
Background and significance
The A2B adenosine receptor (A2BAR) is the predominant adenosine receptor expressed in the colonic epithelia. We have previously shown that A2BAR mRNA and protein levels are upregulated during colitis. In this study we addressed the role of the A2BAR in the development of murine colitis and potential mechanism underlying its effects.
Methods
Dextran sodium sulfate (DSS), 2,4,6-trinitrobenzene sulfonic acid (TNBS) and Salmonella typhimurium were used to induce colitis in A2BAR null mice (A2BAR−/−). Colitis was determined using established clinical and histological scoring. keratinocyte derived chemokine (KC) measurements were performed using ELISA.
Results
Colonic inflammation induced by DSS, TNBS or S. typhimurium was attenuated in A2BAR−/− compared to their WT counterparts. Clinical features, histological score, myeloperoxidase activity were significantly decreased in A2BAR−/− mice. However, A2BAR−/− showed increased susceptibility to systemic Salmonella infection. Tissue levels of the neutrophil chemokine, KC was decreased in colitic A2BAR−/− mice. In addition, flagellin-induced KC levels were attenuated in A2BAR−/− mice. Neutrophil chemotaxis in response to exogenous IL-8 was preserved in A2BAR−/− mice, suggesting intact neutrophil migration in response to appropriate stimuli.
Conclusions
These data demonstrate, for the first time, that the A2BAR plays a pro-inflammatory role in colitis. A2B receptor antagonism may be an effective treatment for acute inflammatory intestinal diseases such as acute flare of inflammatory bowel disease.
Introduction
Adenosine, an endogenous purine nucleoside that is involved in a variety of physiological functions, is being increasingly recognized to modulate a wide variety of inflammatory/immune response1, 2. Following its release from cells or after being formed extracellularly during inflammation3–5 adenosine mediates its effect through one of the four receptors: A1, A2A, A2B and A3. Depending on the tissue or cell type, adenosine receptors mediate pro- or anti-inflammatory responses6. With respect to intestinal inflammation, the effect of adenosine mediated through the A2A adenosine receptor (A2AAR) has been the most studied. Multiple studies have demonstrated that A2AAR plays an anti-inflammatory role and the most potent anti-inflammatory and immunosuppressive effects of adenosine are generally attributed to occupancy of A2A receptors expressed on immune cells7, 8. A2AAR agonists have been demonstrated to suppress the expression of proinflammatory cytokines while sparing anti-inflammatory activity mediated by IL-10 and TGF-β9. A2A agonists also ameliorate bacterial colitis such as inflammation induced by C. difficile10. Similarly, A1 agonists have been shown to ameliorate intestinal inflammation11. Not much is known regarding the role of the A2BAR in intestinal inflammation.
A2BAR is expressed by immune cells as well as the intestinal epithelium. In contrast to immune cells that express multiple adenosine receptors6, 12, the A2BAR is the predominant adenosine receptor expressed in the colonic epithelium13. We have recently demonstrated that A2BAR mRNA and protein expression is upregulated during human and animal models of IBD and that TNF-α plays an important role in the upregulation of A2BAR14. In the colonic epithelium, A2BAR plays a prominent role in regulating vectorial electrogenic ion secretion, a secretory pathway that results in movement of isotonic fluid into the lumen13, 15, 16. An upregulation of ion secretion during inflammation is considered to be an important component of inflammation-associated diarrhea 17, 18. In addition to its effect on ion transport, A2BAR mediates IL-6 secretion by the colonic epithelial cells which is polarized to the luminal compartment and activates neutrophils5. Such neutrophil-epithelial interaction initiated by adenosine has been shown to be important for adhesion of Salmonella typhimurium19. Similarly, adenosine induces apically polarized fibronectin secretion, which potentiates adhesion, invasion of S .typhimurium through KC secretion20. Although these observations suggest a pro-inflammatory role for A2BAR in intestinal inflammation, the effect of A2BAR in the pathogenesis of intestinal inflammation is unknown. The current study addresses the role of A2BAR in colitis using three different models and examines the relative effects of systemic vs localized insults on the pathogenesis of intestinal inflammatory response. Finally, potential mechanism by which A2BAR mediates its effects is addressed.
Materials and Methods
Reagents
Dextran sodium sulfate (DSS, MP Biomedicals Inc, Aurora, OH), Trinitrobenzene sulfonic acid (TNBS, Sigma, St.Louis, MO), X-gal (5-bromo-4-chloro-3indolyl-b-D-galactopyranoside, Invitrogen) Myeloperoxidase, (MPO), SuperScript First strand synthesis system for RT–PCR (Invitrogen, CA), iQ SYBR Green Supermix (Biorad, Hercules, CA), KC Duoset ELISA kit, Recombinant Human IL-8 (R&D Systems Inc, Minneapolis, MN).
Experimental animals
The Animal Care Committee of the Emory University, Atlanta approved all procedures performed on animals. The generation of A2BAR−/− mice and its characterization has been described21. Mice lacking A2BAR exhibit a normal phenotype. The mice were on a C57BL/6 background as determined by the PCR-based strain detection method MAX (Charles River Labs, MA)21. In all experiments 8–10 week old C57BL/6 wild type (WT) and A2BAR−/− female mice were used. Colitis was induced by oral administration of DSS (3% wt/vol) in water ad libitum for 6 days. Age-matched WT and A2BAR−/− receiving regular water served as controls. Mice were observed daily and evaluated for changes in body weight and development of clinical symptoms. β-galactosidase staining in colonic tissue was done as described21. Gut-restricted and systemic S. typhimurium infection was induced as described previously22. Colitis was induced by Trinitrobrenzene sulfonic acid (TNBS) as described previously 23, 24.
Clinical and histological Score
Colitis was quantified with a clinical score, as described by Cooper et al, 25 by using the parameters of weight loss, stool consistency, and fecal blood. The length and weight of the colon were measured, and tissue obtained from each colon was processed for further assays. Colonic specimens obtained as described previously were fixed in formalin and coded for blind microscopic assessment of mucosal lesions (descending colon for DSS colitis and cecum for S typhimurium colitis). Sections were stained with haematoxylin and eosin for histological scoring as described by Cooper et al. Neutrophil infiltration into the colon was quantified by measuring myeloperoxidase (MPO) activity as described previously 26, 27
Measurement of cytokines and myeoloperoxidase assay
The pro-inflammatory cytokines were measured by real time PCR24. Total RNA was extracted from colonic tissue of WT and A2BAR−/− mice using TRIzol reagent. After quantification, a reverse transcription (RT) reaction was performed with 2 µg of each sample and oligo-dT primer, using the SuperScript First Strand Synthesis System for RT-PCR (Invitrogen, CA). The real-time iCycler sequence detection system (Bio-Rad) was used for real-time RT-PCR. In vivo KC levels were determined in the serum of WT and A2BAR−/− mice. Mice were given flagellin (1.0µg/mouse) intraperitoneally and serum was collected 90 minutes after the injection. The levels of KC in the serum samples were quantified by ELISA as described previously 28.Myeloperoxidase assay was performed as described previously 26. Colonic organ cultures and cytokines measurements from supernatants of organ culture were done as described previously29.
Intraperitoneal administration of IL-8
Recombinant Human IL-8 (R&D Systems) 1µg/100µl of 1% BSA/PBS was given intraperitoneally to age and gender matched WT and A2BAR−/− mice. Mice were sacrificed 4 hours after the administration of IL-8. 3.0 ml of HBSS solution (without calcium chloride, magnesium sulfate) was injected intraperitoneally and peritoneal fluid was collected through a catheter. Peritoneal fluid was centrifuged at 10,000 rpm for 10 minutes and cells were resuspended into 50 µl of HBSS and 10 µl of this suspension was smeared on the glass slide and the number of neutrophils were counted after Geimsa staining. MPO activity was measured in the remaining cells to assess the number of neutrophils.
Statistical analysis
The data are presented as mean±SE. Statistical analysis was conducted using Student’s t-test where p<0.05 was considered significant. ANOVA was used in experiments wherein multiple group comparison was involved.
Results
A2BAR expression is increased during colitis
Our previous studies have shown that A2BAR mRNA and protein expression is increased during colitis in both animal models as well as humans14. The A2BAR−/− mice used here were created by replacing exon 1 of the A2BAR gene with a reporter construct containing the gene encoding β-galactosidase (β-gal). In order to verify the upregulation of the A2BAR gene in the colonic epithelium during colitis, we determined β-gal expression before and after the induction of colitis in DSS fed mice. β-gal staining was undetectable in wild type (WT) mice. In contrast, colonic epithelial cells in A2BAR−/− mice showed positive staining for β-gal, indicating the expression of A2BAR in these cells (Figure 1). As shown in the lower right panel, β-gal expression levels were increased in A2BAR−/− DSS-treated mice (Figure 1).
Figure 1. A2B receptor expression is increased during colitis.
Six days after DSS or H2O administration, WT and A2BAR−/− mice were sacrificed and colonic tissues were stained for β-gal (blue) and photographed. Arrows point to staining localized to epithelial cells. Data are representative of 3 independent experiments.
A2BAR−/− mice are resistant to the development of DSS–induced colitis
To investigate the role of the A2BAR in the pathogenesis of colitis, we administered 3% DSS in drinking water to age- and sex- matched WT and A2BAR−/− mice. The mice were compared for clinical signs, including weight loss, stool consistency and occult blood. All WT mice that received DSS developed clinical signs of colitis after day 6. These mice developed weight loss, diarrhea and frank rectal bleeding with a total clinical score of 9.2±0.73 (Figure 2A). In contrast, A2BAR−/− mice did not exhibit weight loss at the end of the treatment period (Figure 2B). These mice had no diarrhea and significantly reduced rectal bleeding as compared to WT mice. Overall, A2BAR−/− mice showed protection from colitis as evaluated by their clinical score of 3±0.63 (*p<0.0002 Figure 2A). In addition, colon length, and other parameters of inflammation30 correlated with the clinical results. DSS administration to WT mice resulted in an 18% shortening of the colon length, compared with mice treated with water after the experimental period (WT DSS: 5.3±0.12, WT water 6.5±0.2 cm), However, DSS administration to the A2BAR−/− mice showed no effect on colon length (A2BAR−/− DSS 6.4±0.24 cm, A2BAR−/− water: 6.6±0.24 cm).
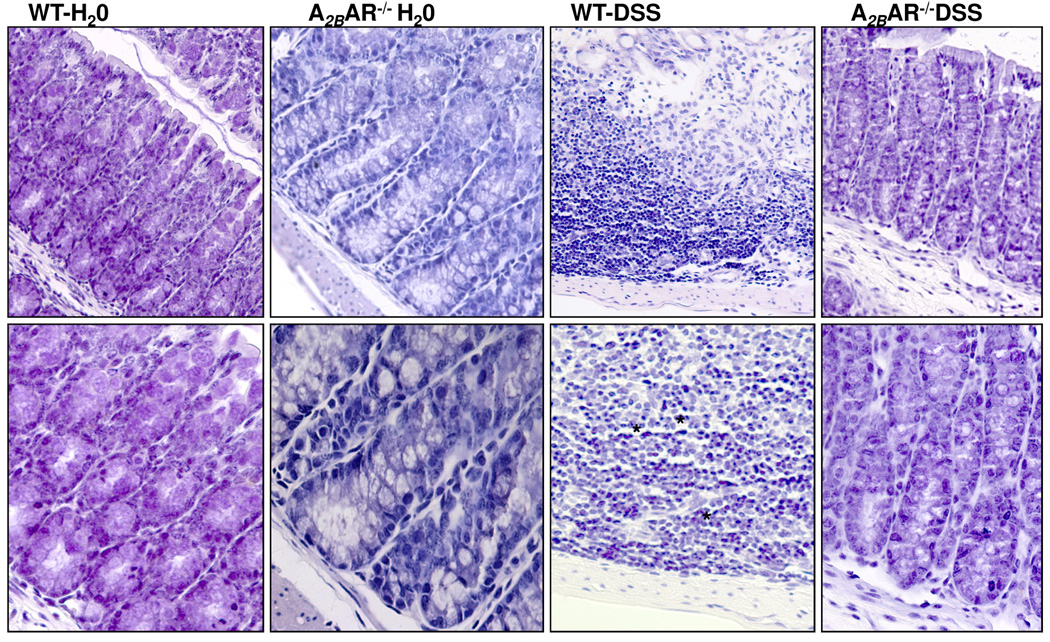
Figure 2. A2BAR mice are resistant to the development of dextran sodium sulfate– induced colitis.
Water or DSS treated mice were sacrificed 6 days after treatment. A. Disease severity is represented as clinical score. Results are expressed as Mean±SE, n=5 *p<0.002. B. Percent change in body weights *p<0.003. C. Representative histological sections of colon from each group are shown (n=5). * indicates neutrophil infiltrates. D. Histological assessment is represented as histological score. Data expressed as Mean±SE for the group compared (WT+DSS and A2BAR−/− + DSS), *p<0.007 E. Myeloperoxidase was measured as an index of neutrophil infiltration into the injured tissue as described in the Methods section. Each bar represents Mean±SE, n=5, *p<0.002. F. Colonic tissue culture supernatants were processed for KC secretion. KC levels in WT mice treated with DSS are significantly higher compared to A2BAR−/− treated with DSS (*p<0.01).
DSS-induced colitis is characterized by the presence of inflammation in the colon with marked crypt destruction, mucosal damage and epithelial erosions and infiltration of inflammatory cells into the mucosal tissue. Histological scores agreed with the clinical scores and confirmed the protective role of A2BAR gene deletion. As shown in Figure 2C, WT mice treated with DSS showed signs of inflammation and tissue damage. These mice had extensive crypt damage, epithelial erosion/ulceration, and infiltration of inflammatory cells in to the lamina propria and muscularis mucosa of colonic sections. In contrast, histological analysis of the sections from A2BAR−/− mice revealed significantly reduced inflammation and these mice were protected from DSS-associated tissue injury with fewer inflammatory infiltrates and ulcerations (Figure 2C). To confirm the histological finding we further determined granulocyte accumulation by MPO activity in the colonic tissue. As shown in Figure 2E, WT mice that received DSS had significantly increased MPO activity (1.76±0.3 U/mg protein) compared to A2BAR−/− that received DSS (0.26±0.14U/mg protein, *p< 0.003, n=5, Figure 2E). In addition, the levels of the keratinocyte derived chemokine (KC, a murine CXC chemokine, a functional homologue of interleukin (IL)-8 and an essential neutrophil chemoattractant31) were measured in organ culture as described in the Methods section. As shown in Figure 2F, WT mice showed significantly higher levels of KC (36.6±10.04 pg/ml media) compared to A2BAR−/− mice (12.59±2.9 pg/ml, *p<0.01,n=5). Taken together, these data demonstrate that mice with a targeted deletion of the A2BAR gene had significantly reduced severity of DSS-induced colitis.
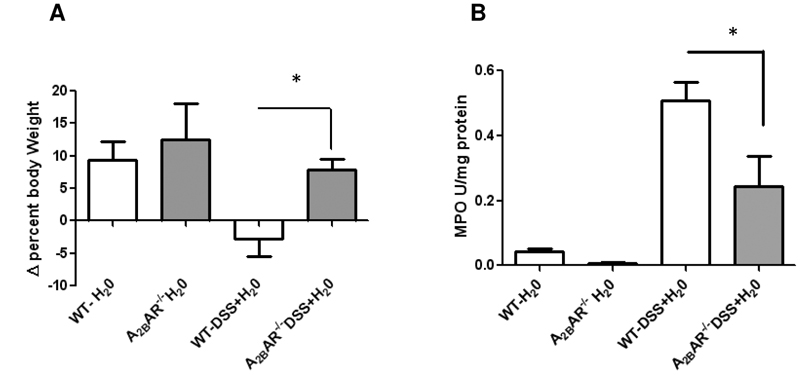
In the next set of experiments, we examined the effect of A2BAR gene deletion on recovery from DSS-induced colitis. Colitis was induced in WT or A2BAR−/− by the administration of DSS in the drinking water for 6 days (colitic phase), after which the mice were switched to regular drinking water for 6 additional days (recovery phase). As shown in Figure 3A, WT mice given DSS showed lower weight compared to water control (−2.8±2.6%), while A2BAR−/− gained weight (7.8±1.5%), demonstrating recovery from acute colitis. MPO measured at the end of the recovery period showed persistently elevated MPO activity in WT mice, while A2BAR−/− mice showed significantly less MPO (Figure 3B). These data demonstrate that the A2BAR−/− mice were not only protected from DSS-induced colitis but also showed improved recovery.
Figure 3. A2BAR−/− mice demonstrated improved recovery after the colitic phase.
Mice received water or DSS for seven days followed by water for another 6 days. A. Percent change in body weights in WT and A2BAR−/− during recovery phase Mean±SE, n=5, *p<0.01. B. Myeloperoxidase activity. Results are expressed as Mean ± SE., n=5, *p<0.049.
A2BAR−/− mice are resistant to oral Salmonella typhimurium–induced colitis
As an alternative model of colitis, we used oral infection with S. typhimurium where S typhimurium was administered after pretreatment of mice with streptomycin22. In this model, S. typhimurium induces clinical and histological features of enterocolitis that predominantly involve the cecum. We used this model since it resembles some of the clinical and histological features of human infection and acute flares of IBD, where epithelial enteric/pathogen interaction is thought to play an important role in the pathogenesis32 32, 33. Ceca of all the WT mice treated with S. typhimurium looked pale and were smaller compared to the A2BAR−/− mice, indicating protection against S. typhimurium-mediated effects (Figure 4A). As shown in Figure 4B, MPO activity was significantly higher in WT mice treated with S. typhimurium (2.5-fold) compared to control mice. In contrast, A2BAR−/− mice showed no increase in MPO activity. Histological analysis revealed that there was marked leukocyte infiltration with crypt damage in WT mice treated with S. typhimurium. In contrast, neutrophil infiltration was attenuated and crypt architecture was preserved in A2BAR−/− mice (Figure 4C & D). Together, these data demonstrate that A2BAR−/− mice are protected from S. typhimurium-induced colitis.
Figure 4. A2BAR−/− mice are protected from oral Salmonella Typhimurium–induced colitis.
Mice were pretreated with streptomycin (Strep) before the administration of S typhimurium, (Sal) and were sacrificed 48 hours after the administration of S .typhimurium. A. Cecum was dissected out from these animals and photographed. B. Cecum samples were processed for myeloperoxidase assay. Results are expressed as Mean±SE, n=6,*p<0.02. C. Representative histological sections of cecum from each group are shown. A predominant neutrophilic infiltrate is shown (*). D. Histological score. Bar graphs represent Mean±SE, *p< 0.001, n=6.
A2BAR−/− mice are resistant to the development of TNBS–induced colitis
We next examined whether our results would extend to a distinct model of colitis, namely that induced by the hapten TNBS24,23. WT and A2BAR−/− mice were randomized to receive ethanol or TNBS by rectal enema as described in the Methods section. As shown in Figure 5A, WT mice treated with TNBS showed signs of colon inflammation and tissue damage. These mice had extensive infiltration of inflammatory cells into the lamina propria and muscularis mucosa of colon. (top panel, Figure 5A). In contrast, histological analysis of the sections from A2BAR−/− mice revealed significantly reduced inflammation and these mice were protected from TNBS-associated tissue injury, with fewer inflammatory infiltrates and ulcerations (bottom panel Figure 5A). As noted in Figure 5B, WT mice that received TNBS showed significantly higher levels of MPO activity (Figure 5B) and KC mRNA compared to A2BAR−/− mice given TNBS (Figure 5C). These data are consistent with the protective effect of A2BAR gene deletion on the development of TNBS-induced colitis.
Figure 5. A2BAR receptor−/− mice are resistant to the development of TNBS–induced colitis.
Both WT and A2BAR−/− mice were administered ethanol or TNBS (150 mg/kg body weight) intrarectally. A. Representative histological sections of colon from each group are shown (n=5), * indicates neutrophil infiltrates. B. Myeloperoxidase activity is represented as fold increase over control. C. mRNA levels of KC were determined by real time RT-PCR. Data is represented as Mean±SE, n= 5.
A2BAR is not required for systemic S. typhimurium sepsis
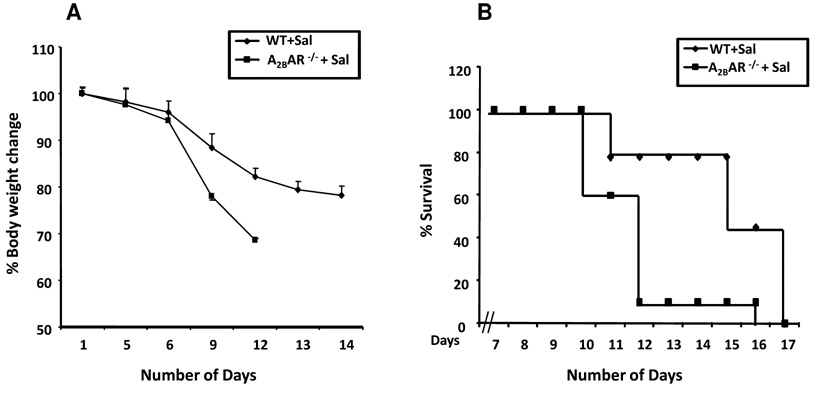
The attenuated colonic inflammation in response to DSS, S. typhimurium or TNBS might result from a systemic defect in immune response, or limited to inflammatory response in the colon in response to luminal insults. We explored these possibilities in the next set of experiments. To determine whether the immune response to S. typhimurium was impaired in A2BAR−/− mice, we examined the response to orally administered S. typhimurium without pretreatment with streptomycin. S. typhimurium, under these circumstances, leads to systemic invasion through jejunum and ileum34 resulting in the colonization of liver and spleen with progression to generalized sepsis. Mice succumb to S. typhimurium sepsis and die within 10–14 days after the administration of S. typhimurium. WT and A2BAR−/− were administered S. typhimurium (SL3201; 106 colony-forming units per mouse by gavage). Mice were weighed daily and observed for clinical signs of sepsis and for mortality. As shown in Figure 6A, 90% of A2BAR−/− mice died within 10 days compared to 20% of WT mice. Consistent with the mortality data, A2BAR−/− mice showed signs of weight loss earlier than WT mice. However, overall mortality was comparable in both groups at the end of the experiment (Figure 6B). These data demonstrate that immune response to systemic Salmonella infection was actually heightened in A2BAR−/− mice.
Figure 6. A2BAR is not required for systemic S. typhimurium sepsis.
WT and A2BAR−/− mice were administered S. typhimurium (SL3201; 104 colony-forming units per mouse, gavage). Mice were weighed daily and were observed for clinical signs of sepsis and for mortality, n=10.
A2BAR−/− mice exhibit defective chemokine response
The foregoing data suggest that attenuation of inflammatory response as a result of A2BAR gene deletion was limited to the colon. We hypothesized that colonic epithelial A2BAR may regulate inflammatory response through secretion of the chemokine, KC. Consistent with this notion is the reduced level of IL-8-like chemokine (KC) neutrophilic infiltrate, and MPO activity in the A2BAR−/− mice compared to WT mice in all three models of acute colitis. To determine whether A2BAR is required to mediate KC secretion, we administered flagellin, a potent inducer of epithelial KC response35 to WT and A2BAR−/− mice. A2BAR−/− mice showed significantly lower levels of KC (60±23.3 ng/ml), compared to WT mice treated with flagellin (153.3±22.42 ng/ml, *p<0.004, n=3, Figure 7A).
Figure 7. Flagellin-induced KC levels were attenuated in A2BAR−/− mice.
WT and A2BAR−/− mice were injected with vehicle or flagellin intraperitoneally. Serum samples were collected 2 hours after administration. A. KC Mean±SE. B. Intraperitoneal administration of IL-8 rescued neutrophil migration in A2BAR−/− mice: WT and A2BAR−/− mice received vehicle or IL-8 (1µg/mouse) intraperitoneally. Peritoneal fluid was collected 4 hours after administration and processed for MPO. Data represents Mean±SE, n=6, P<0.63.
A2BAR is not required for neutrophil migration
We next determined whether A2BAR is required for neutrophil migration in the presence of appropriate chemotactic stimulus, i.e. IL-8. WT and A2BAR−/− mice were given recombinant human IL-8 (1.0 µg) intraperitoneally as described in the Methods section. Four hours after the administration of IL-8, mice were sacrificed and neutrophils in the peritoneal fluid were determined by MPO activity. As shown in Figure 7B, the administration of IL-8 induced in a brisk neutrophil migration into the peritoneum in the WT mice (302.5±133.2 U/mg protein) which was similar in A2BAR−/− mice (217±105.7, n=4).
Discussion
In this study we demonstrate that A2BAR plays a pro-inflammatory role in the intestine. Using three mouse models of colitis that have distinct inciting factors, we demonstrate that the A2BAR gene deletion ameliorates inflammatory response in the colon. DSS elicits an acute mucosal inflammatory response that mimics several features of acute colitis and flares of inflammatory bowel disease 25,36. In the injury phase, mice develop weight loss, blood in stool and diarrhea similar to the symptoms in acute flares of inflammatory bowel disease (IBD). Our data demonstrate that A2BAR−/− mice develop significantly reduced clinical symptoms, including weight loss, blood in stool and diarrhea associated with the administration of DSS. The reduced severity of colitis is also reflected in a reduced histological score wherein A2BAR−/− mice showed less inflammatory infiltrates, ulcers and epithelial damage. When DSS treatment was terminated after the first week, most wild type mice are unable to heal injury as reflected by continued weight loss and persistently high inflammatory infiltrate, while A2BAR−/− mice showed weight gain indicating mucosal healing and recovery. The efficient healing of A2BAR−/− mice likely reflects reduced DSS-induced injury in the colitic phase. However, it is also possible that A2BAR receptor may play a role in inhibiting wound healing. Our data also show that the A2BAR gene deletion protects against the development of tiffilitis induced by S. Typhimurium a in streptomycin-pretreated mice as well as colitis induced by TNBS. Together, these data demonstrate that the A2B receptor mediates a pro-inflammatory response in the intestine. Based on our previous data that showed upregulation of A2BAR mRNA and protein expression in human and animal models of IBD14, A2BAR antagonism may be an effective treatment for acute flares of IBD and infectious colitis.
While A2BAR−/− mice were resistant to an inflammatory response to luminally administered toxins or bacteria (DSS, S. typhimurium, or TNBS) in the colon, they were highly susceptible to systemic challenge S. typhimurium when administered without antibiotic pretreatment. This resulted in systemic invasion, likely through its entry through jejunal and ileal mucosa and colonization of mesenteric lymph node, spleen and liver leading to sepsis and mortality within two weeks of infection37 . Interestingly, our data show that systemic immune response to Salmonella is heightened in A2BAR−/− mice leading to rapid weight loss and death significantly earlier than their WT counterparts. These data are consistent with the increased susceptibility of A2BAR−/− mice to lipopolysaccharide (LPS)21. This study also found that the A2BAR−/− mice displayed a mild but significant increase in the level of the proinflammatory cytokine, TNF-α, under baseline conditions. These data were taken to suggest that the A2BAR plays a systemic anti-inflammatory role. Another explanation for the increased susceptibility of A2bAR−/− mice to systemic Salmonellosis is the lack of neutrophil recruitment in the absence of A2BAR. This notion is supported by a recent study which demonstrated that neutrophil depletion increased bacterial translocation 38 Other studies have corroborated with the anti-inflammatory effects of A2BAR in some tissues39–41. However, the role of A2BAR in mediating effects on macrophage TNF-α was clarified by another study wherein the investigators demonstrated that deletion of the A2BAR gene increases TNF-α, but the receptors do not have a direct role in the macrophage TNF-α response. Rather, A2BAR mediates a pro-inflammatory response through secretion of cytokines such as IL-6 from macrophages 42, 43. This is consistent with other studies that showed a pro-inflammatory role for A2BAR in some tissues 20, 43–48. Such disparate effects of adenosine receptors, depending on the tissue, may be explained by its signaling partners in the tissue. We and others have shown that the A2BAR may exist in a multiprotein complex49, 50 in the lung and intestinal epithelia, and interaction with its partners may determine its functional effects. Further, the A2BAR activates other signaling pathways through Gq coupling51, 52 and similarly, the signaling pathway it activates may also play a role in its function.
A common denominator of the three models of colitis used in this study is the predominant neutrophilic infiltrate that characterize the histology and as evident by MPO. KC is the major neutrophil chemoattractant secreted by the epithelium in response to a variety of luminal agents, including Salmonella 53,54,35. Our data suggest that one of the underlying mechanisms for decreased inflammatory infiltrates in the A2BAR−/− mice is the reduced ability of the epithelia to secrete KC. Indeed, our data show that KC secretion in response to flagellin is attenuated in A2BAR−/− mice. With respect to S. typhimurium, KC has been demonstrated to play a critical role in the inflammatory response elicited by this pathogen54,55 . The neutrophil emigration into the peritoneum in response to the administration of IL-8 in A2BAR−/− mice is consistent with the notion that neutrophil migration into the colon may be impaired in the A2BAR−/− mice due to defective KC secretion by the colonic epithelia. Although A2BAR−/−-mediated KC secretion may play an important role in the development of colonic inflammation, inflammatory response is complex and other factors may contribute to the A2BAR receptor-mediated pro-inflammatory response. Additional studies, such as microarray analysis of colonic mucosa from WT versus A2BAR−/− mice may give further insight into additional pro-inflammatory pathways triggered by the activation of A2BAR in intestinal inflammation.
In summary, we demonstrate that A2BAR−/− mice develop reduced inflammation in response to luminal toxins (DSS, TNBS) and Salmonella. We present data that suggest that attenuated KC secretion by the colonic epithelium may play a role in the decreased inflammatory response seen in A2BAR−/− mice. Taken together, our study focuses on the A2BAR as a therapeutic target for treatment of acute intestinal inflammatory disorders such as acute flares of IBD.
Acknowledgement
This work was supported by a Ruth L. Kirstein National Research Service Award for Individual Postdoctoral Fellows (F32) (V.L.K), M.V.K is a recipient of Research fellowship from CCFA, National Institute of Diabetes and Digestive and Kidney Diseases Grants, DK06411 (S.V.S), DK 02831 (D.M), and Digestive Disease Research Center Grant, 5R24DK064399-02, KR NIH HL13262. We thank Tracy Obertone for careful editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
"No conflicts of interest exist"
References
- 1.Fredholm B. International Union of Pharmacology. XXV. Nomenclature and classification of Adenosine Receptors. The American Society for Pharmocology and Experimental Therapeutics. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. doi: 10.1016/s0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 3.Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5'-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320–2325. doi: 10.1172/JCI116462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitaraman SV, Merlin D, Wang L, Wong M, Gewirtz AT, Si-Tahar M, Madara JL. Neutrophil-epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic receptors in gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G401–G410. doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 7.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 8.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M, Watanabe S, Cominelli F. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26–33. doi: 10.1053/j.gastro.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 10.Cavalcante IC, Castro MV, Barreto AR, Sullivan GW, Vale M, Almeida PR, Linden J, Rieger JM, Cunha FQ, Guerrant RL, Ribeiro RA, Brito GA. Effect of novel A2A adenosine receptor agonist ATL 313 on Clostridium difficile toxin A-induced murine ileal enteritis. Infect Immun. 2006;74:2606–2612. doi: 10.1128/IAI.74.5.2606-2612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozacmak VH, Sayan H. Pretreatment with adenosine and adenosine A1 receptor agonist protects against intestinal ischemia-reperfusion injury in rat. World J Gastroenterol. 2007;13:538–547. doi: 10.3748/wjg.v13.i4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolachala VL, Bajaj R, Chalasani M, Sitaraman SV. Purinergic Mechanisms in Gastrointestinal Inflammation. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00454.2007. [DOI] [PubMed] [Google Scholar]
- 13.Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- 14.Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobbins JW, Laurenson JP, Forrest JN., Jr Adenosine and adenosine analogues stimulate adenosine cyclic 3', 5'-monophosphate-dependent chloride secretion in the mammalian ileum. J Clin Invest. 1984;74:929–935. doi: 10.1172/JCI111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett KE, Cohn JA, Huott PA, Wasserman SI, Dharmsathaphorn K. Immune-related intestinal chloride secretion. II. Effect of adenosine on T84 cell line. Am J Physiol. 1990;258:C902–C912. doi: 10.1152/ajpcell.1990.258.5.C902. [DOI] [PubMed] [Google Scholar]
- 17.Binder HJ, Foster ES, Budinger ME, Hayslett JP. Mechanism of electroneutral sodium chloride absorption in distal colon of the rat. Gastroenterology. 1987;93:449–455. doi: 10.1016/0016-5085(87)90905-x. [DOI] [PubMed] [Google Scholar]
- 18.Halm DR, Rechkemmer GR, Schoumacher RA, Frizzell RA. Apical membrane chloride channels in a colonic cell line activated by secretory agonists. Am J Physiol. 1988;254:C505–C511. doi: 10.1152/ajpcell.1988.254.4.C505. [DOI] [PubMed] [Google Scholar]
- 19.Wall DM, Nadeau WJ, Pazos MA, Shi HN, Galyov EE, McCormick BA. Identification of the Salmonella enterica serotype Typhimurium SipA domain responsible for inducing neutrophil recruitment across the intestinal epithelium. Cell Microbiol. 2007;9:2299–2313. doi: 10.1111/j.1462-5822.2007.00960.x. [DOI] [PubMed] [Google Scholar]
- 20.Walia B, Castaneda FE, Wang L, Kolachala V, Bajaj R, Roman J, Merlin D, Gewirtz AT, Sitaraman SV. Polarized fibronectin secretion induced by adenosine regulates bacterial-epithelial interaction in human intestinal epithelial cells. Biochem J. 2004 doi: 10.1042/BJ20040021. Pt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barthel M, Hapfelmeier S, Quintanilla-Martinez L, Kremer M, Rohde M, Hogardt M, Pfeffer K, Russmann H, Hardt WD. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foligne B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci. 2006;51:390–400. doi: 10.1007/s10620-006-3143-x. [DOI] [PubMed] [Google Scholar]
- 24.Dalmasso G, Charrier-Hisamuddin L, Thu Nguyen HT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–178. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 26.Castaneda FE, Walia B, Vijay-Kumar M, Patel NR, Roser S, Kolachala VL, Rojas M, Wang L, Oprea G, Garg P, Gewirtz AT, Roman J, Merlin D, Sitaraman SV. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996;270:L836–L845. doi: 10.1152/ajplung.1996.270.5.L836. [DOI] [PubMed] [Google Scholar]
- 28.Gewirtz AT, Simon PO, Jr, Schmitt CK, Taylor LJ, Hagedorn CH, O'Brien AD, Neish AS, Madara JL. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J Clin Invest. 2001;107:99–109. doi: 10.1172/JCI10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 31.Parks WC. Matrix metalloproteinases in lung repair. Eur Respir J Suppl. 2003;44:36s–38s. doi: 10.1183/09031936.03.00001203. [DOI] [PubMed] [Google Scholar]
- 32.Sartor RB. Innate immunity in the pathogenesis and therapy of IBD. J Gastroenterol. 2003;38 Suppl 15:43–47. [PubMed] [Google Scholar]
- 33.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Deng SX, Cheng AC, Wang MS, Cao P. Gastrointestinal tract distribution of Salmonella enteritidis in orally infected mice with a species-specific fluorescent quantitative polymerase chain reaction. World J Gastroenterol. 2007;13:6568–6574. doi: 10.3748/wjg.v13.i48.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gewirtz AT, Rao AS, Simon PO, Jr, Merlin D, Carnes D, Madara JL, Neish AS. Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-kappaB pathway. J Clin Invest. 2000;105:79–92. doi: 10.1172/JCI8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–5593. doi: 10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ. Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 2001;3:1335–1344. doi: 10.1016/s1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 38.Kuhl AA, Kakirman H, Janotta M, Dreher S, Cremer P, Pawlowski NN, Loddenkemper C, Heimesaat MM, Grollich K, Zeitz M, Farkas S, Hoffmann JC. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–1892. doi: 10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 39.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xaus J, Mirabet M, Lloberas J, Soler C, Lluis C, Franco R, Celada A. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol. 1999;162:3607–3614. [PubMed] [Google Scholar]
- 41.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 42.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on adenosine-dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther. 2008;324:694–700. doi: 10.1124/jpet.107.131540. [DOI] [PubMed] [Google Scholar]
- 43.Ryzhov S, Solenkova NV, Goldstein AE, Lamparter M, Fleenor T, Young PP, Greelish JP, Byrne JG, Vaughan DE, Biaggioni I, Hatzopoulos AK, Feoktistov I. Adenosine Receptor Mediated Adhesion of Endothelial Progenitors to Cardiac Microvascular Endothelial Cells. Circ Res. 2007 doi: 10.1161/CIRCRESAHA.107.158147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feoktistov I, Polosa R, Holgate ST, Biaggioni I. Adenosine A2B receptors: a novel therapeutic target in asthma? Trends Pharmacol Sci. 1998;19:148–153. doi: 10.1016/s0165-6147(98)01179-1. [DOI] [PubMed] [Google Scholar]
- 45.Zhong H, Belardinelli L, Maa T, Feoktistov I, Biaggioni I, Zeng D. A(2B) adenosine receptors increase cytokine release by bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2004;30:118–125. doi: 10.1165/rcmb.2003-0118OC. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Handy DE, Loscalzo J. Adenosine-dependent induction of glutathione peroxidase 1 in human primary endothelial cells and protection against oxidative stress. Circ Res. 2005;96:831–837. doi: 10.1161/01.RES.0000164401.21929.CF. [DOI] [PubMed] [Google Scholar]
- 47.Kolachala VL, Bajaj R, Wang L, Yan Y, Ritzenthaler JD, Gewirtz AT, Roman J, Merlin D, Sitaraman SV. Epithelial-derived fibronectin expression, signaling, and function in intestinal inflammation. J Biol Chem. 2007;282:32965–32973. doi: 10.1074/jbc.M704388200. [DOI] [PubMed] [Google Scholar]
- 48.Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726–734. [PubMed] [Google Scholar]
- 49.Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem. 2002;277:33188–33195. doi: 10.1074/jbc.M202522200. [DOI] [PubMed] [Google Scholar]
- 50.Huang P, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci U S A. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linden J, Auchampach JA, Jin X, Figler RA. The structure and function of A1 and A2B adenosine receptors. Life Sci. 1998;62:1519–1524. doi: 10.1016/s0024-3205(98)00100-3. [DOI] [PubMed] [Google Scholar]
- 52.Linden J. Cell biology. Purinergic chemotaxis. Science. 2006;314:1689–1690. doi: 10.1126/science.1137190. [DOI] [PubMed] [Google Scholar]
- 53.Zhong W, Kolls JK, Chen H, McAllister F, Oliver PD, Zhang Z. Chemokines orchestrate leukocyte trafficking in inflammatory bowel disease. Front Biosci. 2008;13:1654–1664. doi: 10.2741/2789. [DOI] [PubMed] [Google Scholar]
- 54.Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]