In his book The Black Death transformed,1 Samuel K Cohn claims that the epidemic disease described in western European historical sources from ad 1347 to the mid-seventeenth century under the names plague, pestis, pestilence, plagen and the like must have been a disease other than the modern plague that reached Hong Kong in May 1894 from other parts of China, and later spread first to India and then to all inhabited continents. Alexandre Yersin showed that the disease in Hong Kong was caused by a bacterium, later named Yersinia pestis. It was also Yersin who claimed that he had found not only the cause of plague in China, but also the cause of the medieval and early modern plague epidemics. Four years after the discovery of the bacillus, Paul-Louis Simond proposed the transmission route from the rat (Rattus rattus) via the flea (Xenopsylla cheopis) to humans, although the scientific community was not fully convinced until ten years later, since this hypothesis did not explain all observations. The problems with the hypothesis were forgotten, which is easy to understand when we remember that the doctors and epidemiologists who were working in India at that time were facing a worsening and very serious epidemic. The simple preventive message to public health workers and the public was: exterminate the rats.
The identification of medieval plague as the same disease as modern plague was accepted within thirty years, first by medical scientists and later by historians. Cohn writes, “Without argument, historians and scientists have taken the epidemiology of the modern plague and imposed it on the past, ignoring, denying, even changing contemporary testimony, both narrative and quantitative, when it conflicts with notions of how modern bubonic plague should behave.”2 I agree to some extent with Cohn's criticism of how historians have imposed a modern understanding of plague epidemics in India on historical epidemics, and especially how historians have invented large populations of rats in the medieval towns and countryside of northern Europe without any support from contemporary historical sources or archaeology.3 However, I strongly disagree with his main point, which is that the medieval and modern plague epidemics must have involved different diseases in medical and bacteriological terms. Thus, I argue that Yersinia pestis is the cause of both medieval bubonic plague and modern bubonic plague, and that the symptoms, signs, pathology and pathophysiology are very similar. On the other hand, the two series may have differed in speed of transmission, population mortality and some other epidemiological characteristics because of differences in climate, housing conditions, the availability and population density of flea species and other possible insect vectors, and the availability of susceptible mammals other than rats. Cohn's main arguments are as follows:
There is a lack of evidence of involvement of rats and fleas (Xenopsylla cheopis) in late-medieval/early-modern plagues.
The speed of transmission is different (medieval being rapid, modern being slow).
The mortality and the age and sex distribution of victims are different (e.g. high mortality in Europe in medieval times, low mortality in modern times in India).
Acquired immunity is different (long-lasting immunity in medieval times, no lasting immunity in modern times).
The signs and symptoms are different (e.g., boils mainly in the neck and armpits in medieval plagues, and in the groin in modern plague).
Cohn also claims that when Yersin and his contemporary medical doctors identified plague in Hong Kong with late medieval and early modern plague epidemics, no recent medical knowledge about plague was available to European doctors, and that the medical information available dated back to the last plague epidemic in London in 16654 or to earlier epidemics, even though it was generally known that there had been epidemics of plague in 1722 in Marseilles, in 1743 in Messina and in 1771–2 in Moscow.
Information
However, in addition to these three epidemics, there were repeated epidemics of plague in eastern Europe and in the eastern Mediterranean area in both the eighteenth and nineteenth centuries. Medical information from many of these epidemics was reported in great detail in Latin, in the main European languages, English, French and German, and in many other languages (Russian, Danish, Swedish, Polish and Hungarian). The following is a judicious selection of detailed descriptions of plague epidemics available to European doctors with academic training prior to 1894:
Johann Friedrich Schreiber, Observationes et cogitata de pestilentia, qvae annis 1738 et 1739 in Ukrainia grassata est, St Petersburg, 1740; Berlin, 1744.
Adam Chenot, Tractatus de peste, De origine, progressu, fatis, fine pestis in Daciæ Transilvanicæ quibusdam locis ab initio Octobris 1755, ad finem Januarii 1757, Vienna, 1766.
Charles de Mertens, Observationes de febribus putridis, de peste, Vienna, 1778; translated as Traité de la peste, contenant l'histoire de celle qui a régné à Moscou en 1771, Vienna, 1784; and as An account of the plague which raged at Moscow, in 1771, London, 1799.
Danilo S Samoïlowitz, Mémoire sur la peste, qui, en 1771, ravagea l'empire de Russie et surtout Moscou, Paris, 1781, and 1783.
Gustaf Orraeus, Descriptio pestis qvae anno 1770 in Jassia, et 1771 in Moscva grassata est, St Petersburg, 1784.
A Rafalovitch, ‘Lettres sur la peste d'Odessa de 1837’, Gaz. d. hôp., Par., 1847, 2nd series, 9: 13–15.
Frederik Vilhem Mansa, Pesten i Helsingør og Kiøbenhavn 1710 og 1711, Copenhagen, 1854.
A Rózsahegyi, ‘Ein in Wetljanka beobachteter Fall von Beulenpest’, Pest. med.-chir. Presse, Budapest, 1879, 15: 407–11.
John Netten Radcliffe, On the recent progress of Levantine plague and on quarantine in the Red Sea, Ninth annual report L.G.B. Supplement, London, 1881.
August Hirsch and Julius Heinrich Sommerbrodt, Mittheilungen über die Pest-Epidemie im Winter 1878–79 im russischen Gouvernement Astrachan, Berlin, 1880.
C Zuber, ‘Rapport sur une mission médicale en Russie. La peste du gouvernement d'Astrakhan’, Rec. d. trav. Comité consult. d'hyg. pub. de France, Par., 1880, 9: 87–167.
Thirty articles, letters and annotations in the Lancet, 1828–1888. The medically most interesting, because they contain descriptions of patients and symptoms, are:
‘On the plague in Alexandria’ [1815], 1828–9, i: 390–1;
Dr E di Woolmar, ‘On the plague’ (review of his book, Abhandlung über die
Pest [1827]) [Egypt, 1800], 1829–30, i: 55–6, 1829;
James Laidlaw, ‘Ulceration of the iliac artery from a plague carbuncle’ [Alexandria 1835], 1838–9, i: 613–15;
‘The plague’ [Baghdad 1867], 1867, ii: 111;
‘The plague in Russia’ [Astrakhan 1879], 1879, i: 173–5.
Other Lancet articles report epidemics of plague in Constantinople, Jerusalem, Malta, Persia, South China (1882) and elsewhere. There are also long and heated discussions about the effectiveness of quarantine during plague epidemics and about the contagiousness or otherwise of plague.
During the plague epidemic in Astrakhan in 1879, the Royal College of Physicians held a meeting in London “to discuss the measures which it is desirable should be taken in the public interest in relation to plague now prevailing in the East”.5 Both the French and the British governments dispatched medical commissions to Astrakhan during the epidemic, and Germany and Austria sent a joint commission.6 All these commissions reported in writing to their respective governments7 and to the academic medical communities at home.
There is also convincing evidence that the medical community in Hong Kong was familiar with at least some of the recent literature on plague before the arrival of Shibasaburo Kitasato and Yersin in 1894. Kitasato arrived in Hong Kong on 12 June and Yersin on 15 June. Kitasato was met by Dr James A Lowson, Acting Superintendent of the Government Civil Hospital, and in his first publication on plague he thanks “Dr. Lowson for his kind assistance”.8 However, as early as 18 May, the Governor of Hong Kong had reported to London that the city was
… infected with bubonic plague… . That the disease was really and indeed bubonic plague was indubitable; the excellent, though necessarily succinct and abbreviated, reports furnished by the Government medical officers at Hong-Kong left no room for doubt on this head. As regards its origin, it appears that an epidemic of fatal disease was depopulating certain quarters of the city of Canton towards the end of March, and, according to a communication of April 26th, the nature of the disease then prevalent in Canton and causing considerable mortality was declared to be identical with plague. The first case seen in Hong-Kong by Dr. Jas. A. Lowson—who had only just returned from Canton, whither he had gone to ascertain the actual nature of the epidemic prevailing there—was on May 8th …9
On the same page of the Lancet there is a reference to a report of the British Council at Pakhoi to the Colonial Secretary on “the prevalence of plague at that place … for several years past”. The Lancet first reported plague in Hong Kong on 16 June:
… the disease … bears a remarkable resemblance to the plague … The disease is said to be sudden in its onset and attended with high fever, followed by swellings of the glands in the groin, axilla, neck, and other parts, and in many cases to prove fatal in from twenty-four to fifty-six hours, death being preceded by comatose symptoms.10
Dr Lowson must have known the publication by Netten Radcliffe listed above since he had submitted information to it. In this article, Netten Radcliffe lists a number of areas where plague was known to exist, including many places in the Middle East, southern Siberia and China (including Pakhoi). Kitasato writes, “the disease almost seemed to have vanished from the face of the earth. This, however, was not so. In China it has existed to this day, especially in Yun Nan, where it occurs every year in an endemic form. From the latter place it was imported to Canton.”11 Similarly, in his first paper on plague, Yersin writes, “The disease has been endemically rife for a long time on the high plateaux of Yunnam and from time to time there have been outbreaks very close to the frontier with our Indo-Chinese possessions, in Mong-tzé, in Lang-Tcheou and in Pakhoï.”12 Both Kitasato and Yersin must have received information about plague epidemics in China from Lowson, if they did not have it already before they arrived. I assume that the British medical doctors in Hong Kong also read the Lancet, although there would have been a considerable postal delay.
Notes and correspondence on plague giving similar descriptions to those in the Lancet also appeared in the two leading German medical journals of the period (i.e. before August 1894) the Centralblatt für Bakteriologie and the Deutsche Medicinische Wochenschrift. Robert Koch certainly knew of the plague epidemic in Canton and was convinced that the disease was caused by a bacterium before the epidemic reached Hong Kong. It is reasonable to assume that the imperial Japanese expedition to China was planned before the epidemic reached Hong Kong, since Kitasato and the pathologist Aoyama, together with a number of laboratory technicians and a good deal of equipment, arrived in Hong Kong less than a month after the first plague case was reported there.
My conclusions from all of this are that the academic medical communities in England, France and Germany had detailed and, for the time, modern knowledge of the disease “bubonic plague” itself and of the nineteenth-century epidemics and some of those in the eighteenth century. The series of nineteenth-century epidemics was a direct continuation of the similar eighteenth-century series of epidemics in eastern Europe and the seventeenth-century and earlier series in eastern and western Europe, and these in turn were a direct continuation of the medieval plague series. Doctors who were working in the emerging scientific field of bacteriology suspected that bubonic plague was a communicable disease and were eager to investigate it. They had detailed information about the epidemic in Canton before it spread to Hong Kong, and all of this information was available to Dr Lowson and his colleagues in Hong Kong before the arrival of the epidemic. A sentence such as this from the Lancet (16 June 1894), which stated under the headline ‘Plague in Hong-Kong’ that “the disease … bears a remarkable resemblance to the plague which, some 230 years ago, visited London”13 was written for didactic reasons, not because the writer did not have more recent and much better information, and was aimed at the ordinary British doctor and the British public.
Clinical Symptoms
Contrary to Cohn, my impression of the limited number of historical documents containing detailed descriptions of the clinical disease is that they give a clinical picture very similar to descriptions of untreated modern bubonic plague and sometimes of untreated pneumonic plague. I give five examples:
Louis Heyligen, Avignon, 1347:14
It is said that the disease takes three forms. In the first people suffer an infection of the lungs, which leads to breathing difficulties. Whoever has this corruption or contamination to any extent cannot escape, but will die within two days. Anatomical examinations, in which many corpses were opened, were carried out … to discover the origins of this disease, and it was found that all those who died suddenly had infected lungs, and had been coughing up blood. And this form … is the most contagious, for when one infected person dies everyone who saw him during his illness, visited him, had any dealings with him, or carried him to burial, immediately follows him, without any remedy. There is another form of the disease which exists alongside the first one, in which boils erupt suddenly in the armpits … And there is also a third form, which again coexists with the other two, but takes its own course, and in this people of both sexes are attacked in the groin …
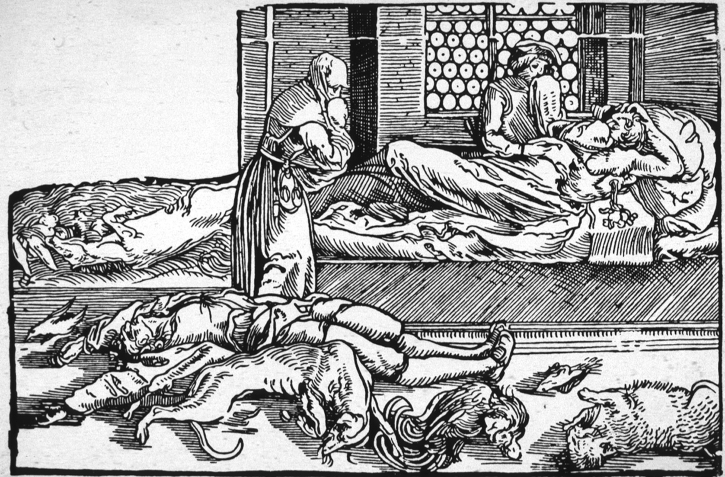
Figure 1:
‘La peste’, wood engraving by Hans Weiditz the Younger, for Francesco Petrarca's Trostspiegel im Glüuck und Unglüuck, Augsburg, 1535. The victims include wild and domestic animals as well as humans. The person on the bed has a bubo in the axilla. (Wellcome Library, London.)
Michele da Piazza, Messina, Sicily, October 1347:15 [The illness] caused the eruption of a sort of boil, the size of a lentil, on the thigh or arm, which so infected and invaded the body that the victims violently coughed up blood, and after three days’ incessant vomiting, for which there was no remedy, they died… . Not just one person in a house died, but the whole household, down to the cats and the livestock, followed their master to death.
Monastery annals from Novgorod : 1364:16 There was a great plague in Nizhniy Novgorod and the entire district. People coughed up blood and others became diseased in their glands. This lasted one, two or three days, and few could endure it, and died… . In the same year, there was plague in Pereslavl. The disease developed like this: first, it is as if one had been hit with a pike behind the shoulder blade, or under the breast near the heart, or between the shoulders. A person who falls ill in this way begins to cough up blood and dies rapidly. 1417:17 First it is as if one is struck by a pike and a gland becomes visible, or else one starts to cough up blood, after which a trembling begins and a fever burns over the whole body.
Charles de Mertens, Moscow, 1771:18 [The plague in general] is ushered in by head-ach, stupor, resembling intoxication, shiverings, depression of spirits, and loss of strength; these are followed by some degree of fever, together with nausea and vomiting…. They feel an itching or pain in those parts of the body where buboes and carbuncles are about to appear. During the height of the plague, many of the infected die on the second or third day, before these tumours have time to come out, and with no other external marks except petechiæ or purple spots, which appear a short time before death; in some these spots are altogether wanting. The buboes and carbuncles generally come out on the second or third day, seldom on the fourth.
We recognize the symptoms of modern bubonic plague in all five descriptions, and possibly also pneumonic plague in three of them. Other examples from northern Europe could well be added.19 They may be compared with the following two quotations from a modern textbook of internal medicine and a textbook of pathology.
American College of Physicians Medicine:20 Clinical manifestations: The usual incubation periods for bubonic plague and pneumonic plague are 2 to 7 days and 2 to 3 days, respectively (range, 1 to 14 days). Plague generally presents as an acute illness characterized by the abrupt onset of fever, chills, headache, gastrointestinal symptoms, and local pain, followed within hours by the development of a painful, swollen mass of lymph nodes [buboes] in the groin or axilla. Skin overlying the buboes is usually red-purple in color. Buboes are initially tense and hard but rapidly become fluctuant. Spontaneous rupture and drainage can occur. Buboes do not develop in patients with septicemic plague; instead, these patients have gastrointestinal signs and symptoms: nausea, vomiting, diarrhea, and abdominal pain. Severe pharyngitis, severe diarrhea, and cough with or without hemoptysis may also be early clinical manifestations of plague.
Robbins’ Textbook of pathology:21 [Robbins distinguishes three clinical forms: bubonic, pneumonic and septicaemic.] Bubonic form: The bubonic pattern of plague is the most common variant. It usually begins abruptly with high temperature, chills, tachycardia and headache, soon followed by mental confusion, delirium and prostration. The site of entry of the organisms may be unaffected, but at times is marked by a vesicle, pustule or small necrotizing ulceration. The regional nodes of drainage are usually involved and characteristically become enlarged, sometimes to the extreme diameter of 5 cm. [A detailed description of the further development of the buboes follows.] In many cases the nodes throughout the body develop localized areas of necrosis similar to those in the regional nodes. In the more fulminating cases, the bacteremia induces widespread, disseminated hemorrhage and necrosis in the skin, spleen, liver, mucous membranes of the body, and linings of the alimentary, respiratory and urinary tracts. Often these hemorrhagic necroses create ecchymotic or large map-like areas. Sometimes small secondary foci of ulcerative necrosis develop within these lesions… . Hemorrhages and necrotic lesions may also be found in the serous cavities and the endocardium and epicardium.
Table 1.
Comparison of symptoms
| Medieval and early modern plague | Plague after 1894 |
|---|---|
| Plague: | Bubonic plague: |
| Fever | Fever |
| Shivering | Chills |
| Local pain in armpit or groin | Local pain followed by swollen |
| Buboes in armpits or groin | lymph nodes in groin or axilla |
| Headache | Headache |
| Nausea and vomiting | Gastrointestinal symptoms |
| High case mortality, but some survive | Case mortality 40–60% |
| Severe plague: | Septicaemic plague: |
| Cough | Cough with or without |
| Cough up blood | Haemoptysis |
| Incessant vomiting | Severe vomiting and diarrhoea |
| Petechiae and purple skin | Large necrotic lesions in the skin |
| Carbuncles | Secondary foci of ulcerative necrosis |
| Patient dies within two to three days | Patient dies within two to three days |
Cohn also considers the number of buboes found in each patient to be important. He claims that while medieval sources often describe multiple buboes in individual patients, it was rare for more than one bubo per patient to be found in India. It is true that multiple buboes were infrequent in Hong Kong and India, 2 per cent in one investigation.22 However, as stated by Robbins, this is dependent on the virulence of the bacterial strain. In addition, many of the other necrotic skin lesions may have been described as buboes (or carbuncles) in the historical documents.23 Cohn also attaches great importance to small differences in the localization of buboes, with boils mainly in the neck and armpits in medieval times and in the groin in modern plague. In William Hunter's investigation from Hong Kong, about 60 per cent of the buboes were in the femoral or inguinal region, the rest in the neck or in the axilla.24 The groin is certainly mentioned in many medieval documents—see, for example, the quote from Louis Heyligen above—but it is true that the axilla is mentioned more frequently. All authors agree that the infection in bubonic plague enters the human body through a bite by an infected flea (or perhaps by another insect vector). Differences in distribution over the body surface may only reflect when and in what circumstances people are bitten by fleas. For instance, bites during the day may more frequently be on the legs, while bites in bed during the night may be more evenly distributed over the body surface.
My conclusion is that the descriptions of symptoms and clinical development found in medieval sources are very similar to descriptions found in modern medical texts, the latter mostly based on observations from epidemics of plague in Vietnam, Madagascar and Peru. Table 1 summarizes the similarities.
Immunity
Cohn claims that acquired immunity is different in medieval and modern plague, with long-lasting immunity being typical of medieval epidemics, but no lasting immunity in modern plague. There is, however, very little evidence to support this assertion. It is difficult to identify the conditions under which a person who has been exposed to Yersinia pestis acquires immunity and for how long acquired immunity lasts. This issue has been under discussion for at least eighty years. One fact is certain: vaccination with killed bacteria gives only short-lived protection. This, combined with the fact that there are well-documented cases both from historical plague epidemics and from modern epidemics of people who have caught the disease more than once, has led some people to conclude that acquired immunity is short-lived and that there is no protection against a second attack. However, as pointed out by Cohn, this conclusion conflicts with observations from many of the historical plague epidemics. The fact that people who have recovered from plague during previous epidemics are unlikely to contract the infection again has been known for a very long time. For instance, people who had survived the disease (the so-called “mortis”) were preferentially employed as attendants in plague wards in some periods. One reason why the long-lasting immunity in humans observed during medieval epidemics has not been confirmed in modern epidemics is, of course, that in modern times very few people have been exposed to plague infection more than once. However, immunity acquired from vaccination cannot be compared to immunity acquired from surviving a fulminate infection. Different vaccines may even give very different levels of immunity.25 In recent years, Yersinia species have been used as model species for the study of infectious mechanisms, and large numbers of papers on virulence factors and pathogenic processes have been, and are being, published. One possible mechanism of permanent cellular immunity is that the plague bacterium is taken up by host cells and continues to live inside cells in some tissues after the acute illness, giving lifelong immunity.26 People who have been treated with antibiotic drugs may not acquire this long-lasting cellular immunity. Because of the threat to humans that would be posed by the use of Yersinia pestis as a weapon by terrorists, work has recently begun on the development of a vaccine that will give long-lasting protection.27 A live attenuated vaccine is now available. Whilst this vaccine is effective, it retains some virulence, and in most countries it is not considered to be suitable for use in humans. A recombinant subunit plague vaccine has recently been developed. Preliminary studies with this vaccine in human clinical trials suggest that the vaccine is safe, well tolerated and immunogenic.28
In my opinion, many questions remain open as regards immunity to plague, but it is clear that acquired immunity consists of both humoral immunity, which is not very effective or long-lasting, and cellular immunity, which is much more effective, especially against pneumonic plague, and which is of longer duration and may give lifelong protection. There is also the possibility that natural (non-acquired) immunity to Yersinia pestis may play a role.29
Mortality
Cohn argues that there is a considerable difference in mortality between medieval and modern plagues, with high mortality in Europe in the medieval and early modern period, and low mortality in modern epidemics in India. However, it is important to distinguish between case mortality and mortality in a population during an epidemic. Case mortality may differ from one epidemic to another depending on the virulence of the particular strain of Yersinia pestis, just as with other bacterial diseases.30 In modern epidemics of plague, case mortality has been between 40 and 60 per cent in the bubonic form and close to 100 per cent in the septicaemic and pneumonic form before the introduction of treatment with antibiotics.31 We have limited information about case mortality in medieval plague epidemics. It may have been higher than in modern epidemics in some cases, but we have good evidence that at least some patients survived the bubonic illness. Population mortality was certainly much higher in some of the medieval and early modern epidemics than in India. But population mortality is strongly dependent on the proportion infected, which again is dependent on factors such as the density of fleas and people's living conditions.
Another similarity between medieval and modern plague epidemics is that both series of epidemics involved a number of different mammalian species and sometimes birds32 in addition to humans. This is a crucial point, since very few infectious diseases are so species non-specific.
Based on all these similarities, I find it extremely unlikely that medieval plague and modern plague are different diseases in the bacteriological and medical sense. Further DNA analyses of bacterial remains from skeletons in medieval or early modern plague graveyards, similar to those already carried out by Michel Drancourt, Didier Raoult and their collaborators,33 will hopefully settle this question once and for all in the near future.
Rats and Fleas
Samuel Cohn is not the only author who has argued that the medieval plagues and the modern plague are different diseases. Susan Scott and Christopher Duncan (2001) have suggested that the historical plagues were a disease related to Ebola,34 Graham Twigg (1984) has suggested anthrax,35 and John Findlay D Shrewsbury (1970) that many of the medieval plagues in Britain were typhus.36 Only Cohn (2002) has no specific disease to suggest.37 However, all these authors agree that most or all of the historical plagues in northern Europe must have involved a disease different from modern bubonic plague. All these authors share one assumption, which explains most of their arguments, however different the details may be. They take it for granted that Simond's infection model, black rat → rat flea → human, which was developed to explain the spread of plague in India, is the only possible way an epidemic of Yersinia pestis infection could spread. From Shrewsbury to Cohn, these authors have argued that for climatic reasons, black rats and the Xenopsylla cheopis flea could not have lived in northern European towns and countryside in medieval times, at least not in the numbers necessary to maintain an epidemic of plague. I agree with this, but not with the assumption that rats and Xenopsylla cheopis fleas are necessary for a human plague epidemic to spread.
Simond's model certainly explained to a large extent what happened in India. But the main reason why this hypothesis was not accepted at once was that it did not explain all the observations. According to Simond's hypothesis, the spread of infection from one village to the next should be slow, because the rat population has to become infected and die before rat fleas will seek humans as a source of blood to any great extent. This process would take at least two weeks, sometimes four. Such a delay was indeed observed and reported from districts where rats and rat fleas were the main mode of transmission.38 However, the Indian plague commission also reported cases where transmission was saltatory (one infected person travelled, and the epidemic spread rapidly from him at the new location), cases where no rats, dead, sick or healthy, could be found, and cases where plague was transmitted by merchandise (mainly grain and clothes). The following are a few relevant quotations from a report by the Indian Plague Commission.39
VII.—Conveyance of Plague from Infected to Uninfected Places: The important problem [is] … whether the infection was imported by a patient who was already infected elsewhere, or by infected rats, or indirectly by means of infected clothes or merchandise… . In villages [in India] the conditions for the discovery of the mode of infection are comparatively favourable, and in many cases the mode of infection was actually ascertained.
VII. (1).—Introduction of Infection by Human Communication: The evidence which has been placed before us shows, in the clearest manner, that in many cases plague has been introduced into a place by a person arriving from a plague-infected locality. [Twelve detailed examples given.]
VII. (2).—Introduction of Infection by Rats: … the rat infection in the village to which plague is imported has preceded the outbreak of disease there among men. [Many examples given.]
VII. (3).—Introduction of Infection by Means of Clothes: That plague can be conveyed by clothes to distant places is, however, placed entirely beyond the reach of reasonable doubt by the fact that two Goanese stewards, on their arrival in London in September 1896, contracted plague in a fatal form from wearing clothes which they had brought with them from Bombay, and kept in a box until their arrival in England.
VII (4)—Introduction of Plague by Means of Merchandise: [Some examples of infected grain and rice.]
E H Hankin, who worked at the government laboratory in Agra during this period, was quite clear in his conclusions:40 “Rats [are] not a necessary cause or agent in the spread of plague.” And he gives many examples of which I shall quote two: “In Garhwal out of forty outbreaks investigated by Planck, a rat mortality was only observed in eight”, “When the disease spread through the town [Hulbi], despite careful search, dead rats were never observed.” In 1900, a ship carried plague to Glasgow. In the period from the beginning of August to the end of September, 36 cases of plague (16 fatal) occurred in thirteen different houses near the docks, but far apart.
The experience of Glasgow as regards the association of plague with rats is an exception to what has been the experience elsewhere, e.g. Bombay, Alexandria, Sydney, &c. For although rats were not unplentiful in the infected area, no sickness was observed among them either before, during, or after the outbreak in 1900. From the end of August to November, 236 rats were caught within the infected area, many of them in and about the infected houses, but in none of them could bacteriologists, who specially made search, find any trace of the plague microbe.41
Similar observations have been made in many later modern epidemics.42
Thus, Simond's hypothesis could not explain how someone who was infected with bubonic plague through a flea bite could transport the disease to a different district. Nor could it explain why the disease was often confined to a particular household, infecting all its members, while neighbouring households were free of plague, since the territories of groups of rats, both black and brown, are known to be larger than a single urban house.43 However, there are well-documented examples of saltatory transmission and confinement to households from many of the modern epidemics, and there are also apparently reliable descriptions from historical epidemics.
If rats are not involved in a particular epidemic of plague, it is unlikely that the rat flea Xenopsylla cheopis is involved. Indeed, there are many examples of modern epidemics where no Xenopsylla cheopis fleas have been found in spite of thorough searches. Since the bubonic and septicaemic forms of plague need a vector organism (most likely an insect) to transmit the infection from one mammal to another, an important question is whether there are candidate insect species that could have been responsible for transmission during medieval times in northern Europe. Many species of insects, lice,44 ticks and in particular various species of fleas, have been shown to be capable of transmitting plague between different animal species and from different animals to humans.45 Of these, only the human flea Pulex irritans and the human body louse Pediculus humanus humanus could possibly have been present in all European countries and in sufficient numbers to be real candidates. The body louse is known to be able to transmit typhus between human subjects, but there is little evidence of its involvement in the spread of plague. In the report from the plague in Glasgow in 1900 quoted above we can read, “The homes (of the plague victims) consisted of a single room kept in a dirty, unventilated and overcrowded state, and swarming with vermin.”46 In most modern epidemics where no Xenopsylla cheopis have been identified, large numbers of the human flea Pulex irritans have been found instead.47 Thus, in an official WHO publication on plague in 1960, Robert Pollitzer concluded, “Pulex irritans plays the main role in the spread of human plague.”48
In 1914, A W Bacot and C J Martin published their famous observation describing how the stomach and proventriculus of infected Xenopsylla cheopis fleas were sometimes filled with a gelatinous mass of blood and plague bacteria that blocked the alimentary canal.49 They also showed that such fleas are particularly liable to transmit plague between rats. Later, many investigators have tried to determine whether other species of fleas would “block” when infected by Yersinia pestis. One early finding, which is important for Cohn in his argumentation, is that Pulex irritans does not block, and therefore presumably is far less efficient at transmitting Yersinia pestis than Xenopsylla cheopis. However, there are weaknesses in the experimental design of these early investigations, and the results are therefore difficult to interpret. Firstly, most of the experiments were designed to investigate transmission of Yersinia pestis between rats (or sometimes mice, rabbits or guinea-pigs) by the particular species of fleas under investigation. Secondly, only a limited combination of all the relevant factors (temperature and humidity of the air, strain of the flea species, time interval between the flea feeding on a sick animal and the potentially infectious feeding on a healthy animal, duration of the exposure window, etc.) was investigated in each experiment. One of the last, and in my opinion the best, of the experiments in this early series of investigations was carried out by Burroughs and published in 1947. For some of the flea species, Burroughs obtained results which differed considerably from those obtained by previous investigators. One of his conclusions was that “the vector efficiency of a species may vary considerably for different strains of the species collected from widely separated geographical areas”.50 His strain of Pulex irritans was obtained from deer and manifested vector capacity between guinea-pigs. It was also capable of blocking. It has recently been shown that some flea species become immediately infectious and transmit “efficiently for at least four days postinfection … and may remain infectious for a long time, because the fleas do not suffer block-induced mortality”.51 My conclusion on this point is that we at present know too little about the vector capability of Pulex irritans, in particular its vector capability between humans and in the environmental conditions found in a bed.
Conclusions
Most (or all) late medieval and early modern “plague” epidemics were caused by Yersinia pestis (as were the Justinian plagues (ad 542–767)52 and possibly the plague epidemic in about 1100 bc described in 1 Samuel53).
Most (or all) of the historical European plague epidemics did not involve rats as intermediate hosts. The mode of transmission was from human to human via an insect vector.
Pulex irritans may have been the most important arthropod vector in Europe prior to the late nineteenth century, but other ectoparasites (other fleas, lice, etc.) could also have been involved.54
In China before 1894 and in Hong Kong, in many (but not all) areas of India, and in cities like Bombay, Colombo, Alexandria and Sydney, the most important arthropod vector was Xenopsylla cheopis, and the mode of transmission was from Rattus rattus (or Rattus norvegicus) to man.
Acknowledgments
I would like to thank the Librarians at the Library of Medicine and Health Science, University of Oslo, for help with old books and documents, and Ms Alison Coulthard for correcting my English.
Footnotes
1 Samuel K Cohn, The Black Death transformed: disease and culture in early Renaissance Europe, London, Arnold, 2002. Cohn's more recent views are expressed in ch. 4 of this volume, ‘Epidemiology of the Black Death and successive waves of plague’, pp. 74–100.
2 Cohn, The Black Death transformed, op. cit., note 1 above, p. 2.
3 For example, Ole J Benedictow, Plague in the late medieval Nordic countries: epidemiological studies, Oslo, Middelalderforlaget, 1992.
4 For example, Daniel Defoe, A journal of the plague year, London, 1722.
5 Lancet, 1879, i: 281–2 , p. 281.
6 Ibid.; ‘Report on the French medical commission on plague in Astrakhan, 1878–91’, Lancet, 1880, ii: 634–5.
7 Lancet, 1879, i: 637–8.
8 Shibasaburo Kitasato, ‘The bacillus of bubonic plague’, Lancet, 1894, ii: 428–30, p. 430.
9 ‘The plague at Hong-Kong’, Lancet, 1894, ii: 269–70, p. 270.
10 ‘The plague in Hong-Kong’, Lancet, 1894, i: 1518.
11 Kitasato, op. cit., note 8 above, p. 429.
12 “La maladie sévissait depuis très longtemps, à l’état endémique, sur les hauts plateaux du Yunnam et avait fait, de temps à autre, quelques apparitions tout près de la frontière de nos possessions indo-chinoises, à Mong-tzé, à Lang-Tcheou et à Pakhoï.” A E J Yersin, ‘La peste bubonique à Hong Kong’, Annales de l'Institut Pasteur, 1894, 8: 662–7.
13 Lancet, 1894, i: 1518.
14 Louis Heyligen, ‘The plague in Avignon’, in Rosemary Horrox (trans. and ed.), The Black Death, Manchester University Press, 1994, pp. 41–5, on pp. 42–3.
15 Michele da Piazza, ‘Chronicle, 1347–1361’, in Horrox (trans. and ed.), op. cit., note 14 above, pp. 35–6.
16 Translated from Matthias Akiander, Utdrag ur Ryska Annaler (Excerpts from Russian Annals), Helsinki, Simelii, 1849, p. 107.
17 Ibid., p. 138.
18 Charles de Mertens, An account of the plague which raged at Moscow, in 1771, London, 1799, pp. 42–3.
19 Lars Walløe, ‘Pest og folketall 1350–1750’, Historisk Tidsskrift, 1982, 61: 1–45; also published as: Lars Walløe, Plague and population: Norway 1350–1750, Avhandlinger (Norske videnskaps-akademi), new series, No. 17, Oslo, University of Oslo, Department of Physiology, 1995, pp. 1–48.
20 ACP Medicine, New York, WebMD, 2007 (http://acpmedicine.com/cgi-bin/publiccgi.pl) section 7, ch. XI, W Conrad Liles, ‘Infections due to brucella, francisella, Yersinia pestis, and bartonella’, rev 9/07, pp. 7–9, and section 8, ch. V, Jeffrey Duckin, ‘Bioterrorism’, rev 6/04, pp. 13–14.
21 Stanley L Robbins, Textbook of pathology, 2nd ed., Philadelphia, Saunders, 1962, pp. 283–4.
22 William Hunter, ‘Buboes and their significance in plague’, Lancet, 1906, ii: 83–6.
23 Robbins, op. cit., note 21 above, pp. 283–4.
24 Hunter, op. cit., note 22 above.
25 Richard W Titball and E Diane Williamson, ‘Second and third generation plague vaccines’, ch. 30 in Mikael Skurnik, José Antonio Bengoechea, and Kaisa Granfors (eds), The genus Yersinia, New York, Kluwer, 2003.
26 Ralph R Isberg and Guy T Van Nhieu, ‘Two mammalian cell internalization strategies used by pathogenic bacteria’, Annual Rev. Genet., 1994, 28: 395–422.
27 Richard W Titball and E Diane Williamson, ‘Vaccination against bubonic and pneumonic plague’, Vaccine, 2001, 19: 4175–84.
28 D S Reed and M J Martinez, ‘Respiratory immunity is an important component of protection elicited by subunit vaccination against pneumonic plague’, Vaccine, 2006, 24: 2283–89; S R Morris, ‘Development of a recombinant vaccine against aerosolized plague’, Vaccine, 2007, 25: 3115–17.
29 T H Chen and K F Meyer, ‘Susceptibility and antibody response of Rattus species to experimental plague’, J. Infect. Dis., 1974, 129, suppl: S62–S71.
30 Robbins, op. cit., note 21 above, pp. 283–4.
31 Liles, op. cit., note 20 above, pp. 7–9.
32 For example, in current epizootics in Kazakhstan, according to a lecture by Sergey Pole at a plague conference in Oslo in 2005.
33 Didier Raoult, Gérard Aboudharam, Eric Crubézy, Georges Larrouy, Bertrand Ludes, and Michel Drancourt, ‘Molecular identification by “suicide PCR” of Yersinia pestis as the agent of medieval Black Death’, Proc. Natl. Acad. Sci. USA, 2000, 97 (23): 12800–803; Michel Drancourt and Didier Raoult, ‘Molecular insights into the history of plague’, Microbes Infect., 2002, 4: 105–9; Michel Drancourt, Véronique Roux, La Vu Dang, Lam Tran-Hung, Dominique Castex, Viviane Chenal-Francisque, Hiroyaki Ogata, Pierre-Edouard Fournier, Eric Crubézy, Didier Raoult, ‘Genotyping, Orientalis-like Yersinia Pestis, and plague pandemics’, Emerg. Infect. Dis., 2004, 10 (9): 1585–92; Ingrid Wiechmann and Gisela Grupe, ‘Detection of Yersinia pestis DNA in two early medieval skeletal finds from Aschheim (Upper Bavaria, 6th century A.D.)’, Am. J. Phys. Anthropol., 2005, 126: 48–55.
34 Susan Scott and Christopher Duncan, Biology of plagues: evidence from historical populations, Cambridge University Press, 2001, pp. 384–9.
35 Graham Twigg, The Black Death: a biological reappraisal, London, Batsford, 1984, pp. 201, 219–21.
36 J F D Shrewsbury, A history of bubonic plague in the British Isles, Cambridge University Press, 1970, pp. 148–52.
37 Cohn, op. cit., note 1 above, p. 247.
38 C J Martin, ‘Discussion on the spread of plague’, Br. med. J., 1911, ii: 1249–63.
39 Report of the Indian Plague Commission, volume V, 1898–99, London, 1901, pp. 106, 108, 112, 113.
40 E H Hankin, ‘On the epidemiology of plague’, J. Hygiene, 1905, 5: 48–83, p. 66.
41 Report of the Indian Plague Commission, volume V, 1898–99, Reports from commissioners, inspectors, and others: 1901, ‘Local Government Board (Scotland)’, London, HMSO, 1901, pp. 55–62.
42 For example, W A Lethem, ‘The epidemiology of bubonic plague in Great Britain, with special reference to its spread by Pulex irritans’, J. State Med., 1923, 31: 508–15; C R Eskey, ‘Chief etiological factors of plague in Equador and the antiplague campaign’, Public Health Reports, 1930, 45: 2077–155.
43 David E Davis, John T Emlen and Allan W Stokes, ‘Studies on home range in the brown rat’, J. Mammol., 1948, 29: 207–25; H-J Telle, ‘Beitrag zur Kenntnis der Verhaltungsweise von Ratten, vergleichend dargestellt bei Rattus norvegicus und Rattus rattu's’, Zeitschrift für angevandte Zoologie, 1966, 53: 129–96; R F Ewer, ‘The biology and behaviour of a free-living population of black rats (Rattus rattus)’, Animal Behaviour Monographs, 1971, 4: 125–74.
44 Linda Houhamdi, Hubert Lepidi, Michel Drancourt, Didier Raoult, ‘Experimental model to evaluate the human body louse as a vector of plague’, J. Infect. Dis., 2006, 194: 1589–96.
45 Norman Gratz, ‘Rodent reservoirs and flea vectors of natural foci of plague’, in David T Dennis, Kenneth L Gage, Norman Gatz, Jack D Pound, Evgueni Tikhomirov, Plague manual: epidemiology, distribution, surveillance and control, Geneva, World Health Organization, 1999, pp. 63–96; Robert Pollitzer, ‘A review of recent literature on plague’, Bull. World Health Organ., 1960, 23: 313–400, pp. 357–62.
46 Reports from Commissioners, op. cit., note 41 above, pp. 55–62.
47 Lethem, op. cit., note 42 above; Eskey, op. cit., note 42 above; M Delanoë, ‘L'importance de la puce de l'homme, Pulex irritans, dans les épidémies de peste au Maroc’, Bulletin de la Société de Pathologie Exotique, 1932, 25: 958–60; G Blanc and M Baltazard, ‘Recherches expérimentales sur la peste: l'infection de la puce de l'homme, Pulex irritans L.’, C. R. Acad. Sci. Paris, 1941, 213: 813–16; A Laudisoit, H Leirs, R H Makundi, S Van Dongen, S Davis, S Neerinckx, J Deckers, R Libois, ‘Plague and the human flea, Tanzania’, Emerg. Infect. Dis., 2007, 13 (5): 687–93.
48 Robert Pollitzer, ‘Plague’, Bull. World Health Organ., 1960, 23: 313–400, p. 360.
49 A W Bacot and C J Martin, ‘Observations on the mechanism of the transmission of plague by fleas’, J. Hygiene, 1914, 13 (suppl 3): 423–39.
50 Albert L Burroughs, ‘Sylvatic plague studies: the vector efficiency of nine species of fleas compared with Xenopsylla cheopis’, J. Hygiene, 1947, 45: 371–96, p. 394.
51 R J Eisen, S W Bearden, A P Wilder, J A Montenieri, M F Antolin, and K L Gage, ‘Early-phase transmission of Yersinia pestis by unblocked fleas as a mechanism explaining rapidly spreading plague epizootics’, Proc. Natl. Acad. Sci. USA, 2006, 103 (42): 15380–85, on p. 15380; R J Eisen, J L Lowell, J A Montenieri, S W Bearden, K L Gage, ‘Temporal dynamics of early-phase transmission of Yersinia pestis by unblocked fleas’, J. med. Entomol., 2007, 44: 672–7.
52 J C Russell, The control of late ancient and medieval population, Philadelphia, The American Philosophical Society, 1985, pp. 111–38; J R Maddicott, ‘Plague in seventh-century England’, Past and Present, 1997, 156: 7–54; D Stathakopoulos, ‘The Justinianic plague revisited’, Byzantine and Modern Greek Studies, 2000, 24: 256–76; Wiechmann and Grupe, op. cit., note 33 above; Drancourt, et al., ‘Genotyping’, op. cit., note 33 above. Cf. Lester K Little (ed.), Plague and the end of Antiquity: the pandemic of 541–750, Cambridge University Press, 2007.
53 Lars Walløe, ‘Was the disruption of the Mycenaean world caused by repeated epidemics of Bubonic plague?’, Opuscula Atheniensia, 1999, 24: 121–6.
54 Cf. Frédérique Audoin-Rouzeau, Les chemins de la peste: le rat, la puce et l'homme, Paris, Tallandier, 2007, arguing for the importance of Nosopsyllus fasciatus in northern Europe as a more likely vector than Xenopsylla cheopis.