Abstract
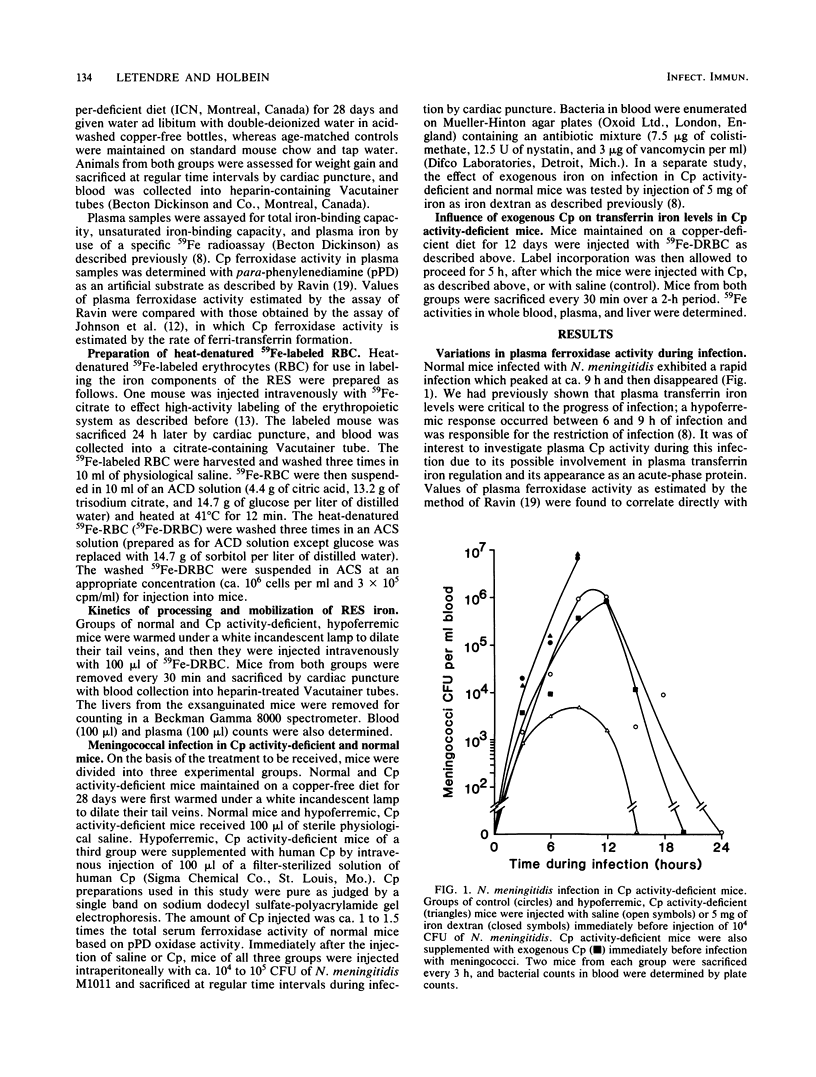
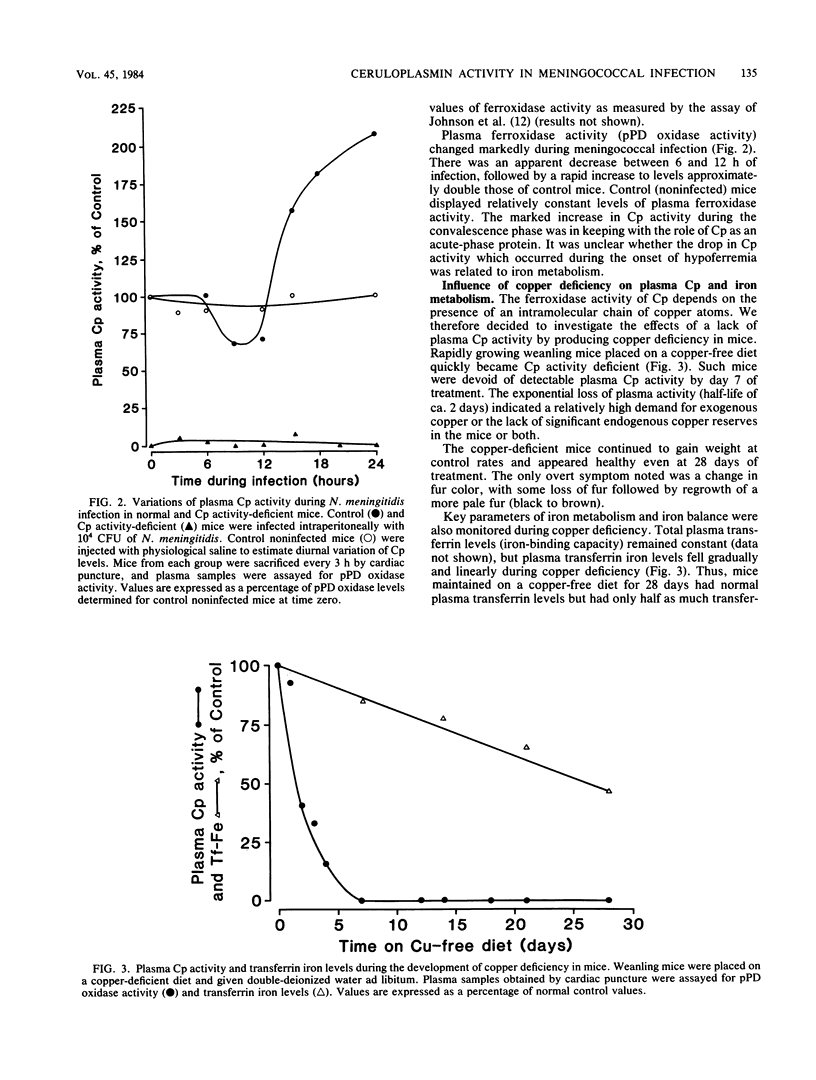
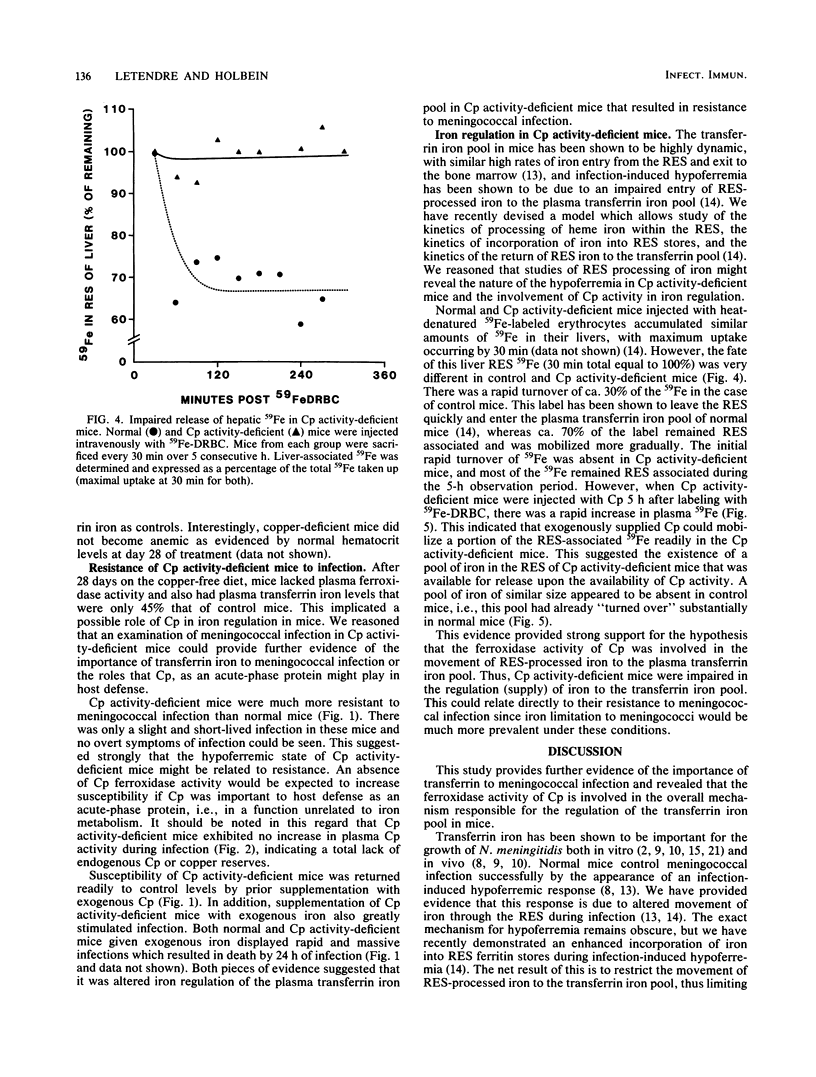
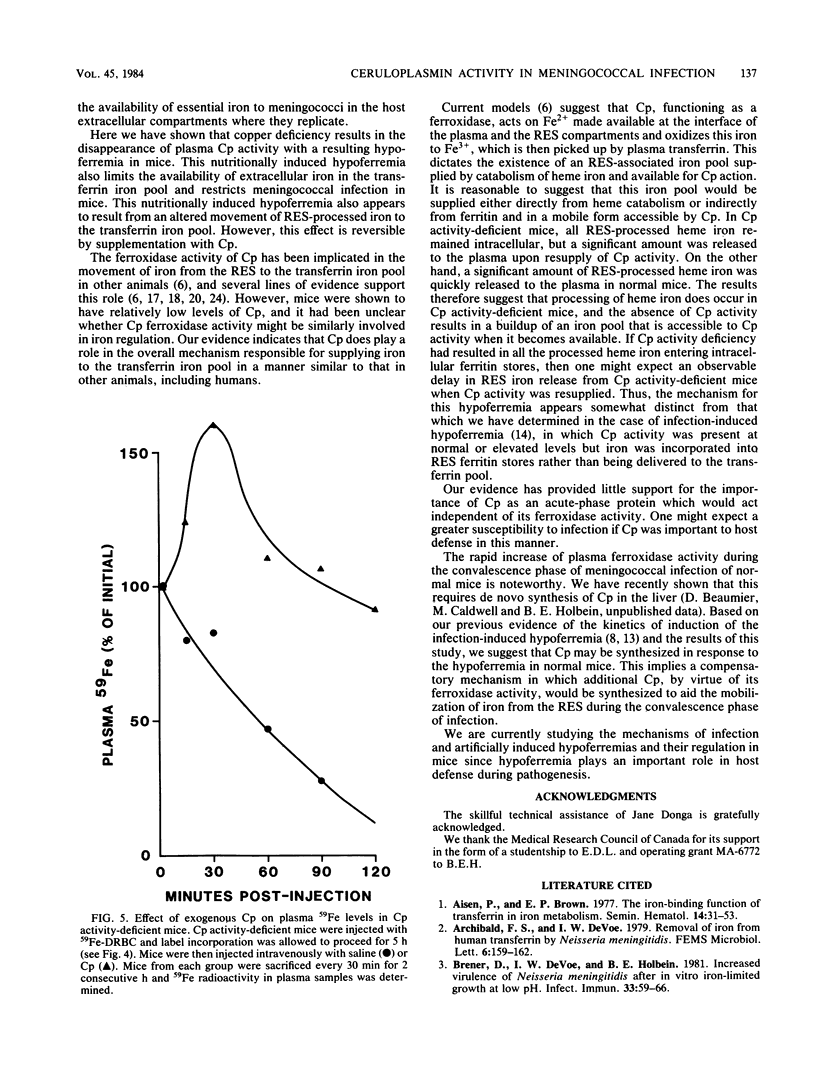
The role of ceruloplasmin (ferroxidase I; EC 1.16.3.1) in iron metabolism during experimental Neisseria meningitidis infection was investigated. Plasma ceruloplasmin activity was found to increase greatly in mice during the convalescence phase of iron-controlled infection and after a plasma hypoferremia had occurred. Ceruloplasmin activity-deficient animals became hypoferremic as a result of an impaired release of iron from the reticuloendothelial system as shown by impaired return of reticuloendothelial system-processed heme iron in these mice. Hypoferremia in ceruloplasmin activity-deficient mice was associated with an increased resistance to N. meningitidis infection, an effect reversed readily by ceruloplasmin supplementation or iron addition. This evidence implicated ceruloplasmin activity as an important component in the regulation of the plasma transferrin iron pool and suggested that an important role of additional ceruloplasmin as an acute-phase protein might be related to the requirement of additional transferrin iron. This study also provided further evidence of the importance of transferrin iron and host hypoferremia in bacterial infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Brown E. B. The iron-binding function of transferrin in iron metabolism. Semin Hematol. 1977 Jan;14(1):31–53. [PubMed] [Google Scholar]
- Brener D., DeVoe I. W., Holbein B. E. Increased virulence of Neisseria meningitidis after in vitro iron-limited growth at low pH. Infect Immun. 1981 Jul;33(1):59–66. doi: 10.1128/iai.33.1.59-66.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden E., Hsieh H. S. The biological role of ceruloplasmin and its oxidase activity. Adv Exp Med Biol. 1976;74:505–529. doi: 10.1007/978-1-4684-3270-1_43. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Holbein B. E. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981 Oct;34(1):120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980 Sep;29(3):886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E., Jericho K. W., Likes G. C. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect Immun. 1979 May;24(2):545–551. doi: 10.1128/iai.24.2.545-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Osaki S., Frieden E. A micromethod for the determination of ferroxidase (ceruloplasmin) in human serums. Clin Chem. 1967 Feb;13(2):142–150. [PubMed] [Google Scholar]
- Letendre E. D., Holbein B. E. Mechanism of impaired iron release by the reticuloendothelial system during the hypoferremic phase of experimental Neisseria meningitidis infection in mice. Infect Immun. 1984 May;44(2):320–325. doi: 10.1128/iai.44.2.320-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre E. D., Holbein B. E. Turnover in the transferrin iron pool during the hypoferremic phase of experimental Neisseria meningitidis infection in mice. Infect Immun. 1983 Jan;39(1):50–59. doi: 10.1128/iai.39.1.50-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The mobilization of iron from the perfused mammalian liver by a serum copper enzyme, ferroxidase I. J Biol Chem. 1971 May 10;246(9):3018–3023. [PubMed] [Google Scholar]
- RAVIN H. A. An improved colorimetric enzymatic assay of ceruloplasmin. J Lab Clin Med. 1961 Jul;58:161–168. [PubMed] [Google Scholar]
- Ragan H. A., Nacht S., Lee G. R., Bishop C. R., Cartwright G. E. Effect of ceruloplasmin on plasma iron in copper-deficient swine. Am J Physiol. 1969 Nov;217(5):1320–1323. doi: 10.1152/ajplegacy.1969.217.5.1320. [DOI] [PubMed] [Google Scholar]
- Roeser H. P., Lee G. R., Nacht S., Cartwright G. E. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970 Dec;49(12):2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Lee G. R., Cartwright G. E. Ferroxidase activity of rat ceruloplasmin. Am J Physiol. 1974 Nov;227(5):1094–1097. doi: 10.1152/ajplegacy.1974.227.5.1094. [DOI] [PubMed] [Google Scholar]


