Abstract
To preserve the central nervous system (CNS) function after a traumatic injury, therapeutic agents must be administered to protect neurons as well as glial cells. Cell death in CNS injuries and diseases are attributed to many factors including glutamate toxicity and oxidative stress. We examined whether melatonin, a potent anti-oxidant and free radical scavenger, would attenuate apoptotic death of rat C6 astroglial cells under glutamate excitotoxicity and oxidative stress. Exposure of C6 cells to 500 µm l-glutamic acid (LGA) and 100 µm hydrogen peroxide (H2O2) for 24 hr caused significant increases in apoptosis. Apoptosis was evaluated by Wright staining and ApopTag assay. Melatonin receptor 1 appeared to be involved in the protection of these cells from excitotoxic and oxidative damage. Cells undergoing excitotoxic and oxidative stress for 15 min were then treated with 150 nm melatonin, which prevented Ca2+ influx and cell death. Western blot analyses showed alterations in Bax and Bcl-2 expression resulting in increased Bax:Bcl-2 ratio during apoptosis. Western blot analyses also showed increases in calpain and caspase-3 activities, which cleaved 270 kD α-spectrin at specific sites to generate 145 kD spectrin breakdown product (SBDP) and 120 kD SBDP, respectively. However, 15-min post-treatment of C6 cells with melatonin dramatically reduced Bax:Bcl-2 ratio and proteolytic activities, decreasing LGA or H2O2-induced apoptosis. Our data showed that melatonin prevented proteolysis and apoptosis in C6 astroglial cells. The results suggest that melatonin may be an effective cytoprotective agent against glutamate excitotoxicity and oxidative stress in CNS injuries and diseases.
Keywords: apoptosis, C6 astroglial cells, cytoprotective effects, glutamate, H2O2, melatonin
Introduction
Melatonin (N-acetyl-5-methoxytryptamine), the main hormone of the pineal gland, has ubiquitous actions as a direct as well as an indirect anti-oxidant and free radical scavenger. Besides directly detoxifying a variety of highly reactive molecules, melatonin also stimulates anti-oxidative enzymes [1, 2]. This ability to induce this ‘anti-oxidant cascade’ serves to increase melatonin’s effectiveness for resisting oxidative damage. The beneficial effects of melatonin on mitochondrial homeostasis may explain its protective properties for a number of degenerative conditions, including aging, Parkinson’s disease, Alzheimer’s disease, epilepsy, sepsis, and other injuries such as ischemia-reperfusion [3–5]. It has been shown to exert neuroprotection in models of brain and spinal cord trauma [6, 7], cerebral ischemia [8], and excitotoxicity [9]. Because acute oxidative stress is commonly involved in the progression of secondary tissue injury in these models, the neuroprotective effects of melatonin have been attributed to its activity as an antioxidant [10–12]. Indeed, numerous earlier in vitro studies provided evidence that melatonin acts as a direct scavenger of several reactive oxygen and nitrogen species [13, 14]. However, later studies failed to confirm the activity of melatonin as a potent direct chain-breaking anti-oxidant and suggested that in some circumstances it could function as a weak preventive anti-oxidant, presumably by acting as a weak metal ion chelator [15–17]. In the light of these reports, the present study was designed to assess the activity of melatonin as a cytoprotectant in a cell culture model. In an attempt to understand the mode of action of melatonin as a cytoprotective agent, we investigated the effects of melatonin against glutamate excitotoxicity and oxidative stress in C6 astroglial cells.
Rat C6 astroglial cells express both of the protein-coupled melatonin receptor subtypes, melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2), following treatment with melatonin at physiological or higher concentrations [16]. This finding indicated that MT1 and MT2 might be involved in melatonin-mediated cytoprotection. Recently, it has been reported that melatonin at pharmacological concentrations may modulate the growth of C6 cells both in vitro and in vivo [17]. It was shown earlier that apoptosis could be induced in C6 cells following exposure to glutamate excitotoxicity and oxidative stress [18, 19]. In both cases, increases in intracellular free [Ca2+] lead to the activation of the Ca2+-dependent proteases m-calpain and µ-calpain. Although the best characterized proteases in apoptosis are caspases, other proteases including calpains and cathepsins contribute to apoptosis in the presence or absence of caspase activation. Calpain substrates include membrane receptors and transporters, cytoskeletal proteins, and intracellular enzymes. A role for calpain has been demonstrated in cell death in the pathophysiology of diseases such as Alzheimer’s disease and demyelinating disease and central nervous system (CNS) injuries such as spinal cord injury and traumatic brain injury [20]. Also, calpain and caspases are known to ‘cross-talk’ for mediation of cell death in vitro as well as in vivo [20]. Apoptosis can occur via the mitochondrial pathway, which is controlled by the Bcl-2 family of proteins [21]. In most cases, the ratio of Bax to Bcl-2 ultimately plays a dominant role in determining whether cells are committed to apoptosis [21]. High levels of Bax correlate with a release of cytochrome c from mitochondria into the cytosol where cytochrome c, in conjunction with Apaf-1, is involved in the conversion of procaspase-9 to active caspase-9. Initiator caspases like caspase-9 can then go on to activate effector caspases such as caspase-3. Activated caspase-3 cleaves a number of key substrates, including cytoskeletal proteins, spectrin, and calpastatin (an endogenous inhibitor of calpain) in the CNS cells [20]. Caspase-3 cleaves α-spectrin at a specific site to produce the 120 kD spectrin breakdown product (SBDP), which is an indication of caspase-3 activity [21–23]. Also caspase-3 activation induces caspase-3-activated DNase activity to cause internucleosomal DNA fragmentation, the hallmark of apoptosis.
In this study, we examined the cytoprotective effects of melatonin in rat C6 astroglial cells following exposure to l-glutamic acid (LGA) and hydrogen peroxide (H2O2). To clarify the potential role of melatonin receptors, we also examined the levels of expression of melatonin receptor subtypes, MT1 and MT2, in C6 cells following exposure to LGA or H2O2. Our data suggest that post-treatment of C6 cells with melatonin provides cytoprotection with involvement of melatonin receptors.
Materials and methods
Cell culture and treatments
Rat C6 cells were grown in monolayer to subconfluency in 75-cm2 flasks containing 10 mL of 1 × RPMI 1640 medium supplemented with 1% penicillin and streptomycin (GIBCO-BRL, Grand Island, NY, USA) and also 10% fetal bovine serum (FBS) in a fully humidified incubator containing 5% CO2 at 37°C. Prior to treatment with any agent, the cells were allowed to grow in 1 × RPMI 1640 in presence of 0.5% FBS for 24 hr. Dose–response studies were conducted to determine the suitable doses of the treatment agents used in the experiments. Just prior to treatment, stock solutions of 100 mm LGA and 100mm H2O2 were prepared in culture medium. Also, stock solutions of 10 mm melatonin were freshly prepared using dimethyl sulfoxide (DMSO) as vehicle and then diluted in culture medium for treatments. The final concentration of vehicle (<0.01% DMSO) did not affect C6 cells. We found that treatment with 150 nm melatonin alone for 24 hr did not induce apoptosis while treatment with 500 µm LGA and 100 µm H2O2 for 24 hr induced about 50% apoptotic death in C6 cells. Also, cells were treated with 500 µm LGA and 100 µm H2O2 for 15 min and then with 150nm melatonin for 24 hr. Cells from all treatment groups were used for determination of morphological and biochemical features of apoptosis, analysis of mRNA expression of the genes regulating apoptosis, and analysis of specific protein expression and activity by Western blotting.
Trypan blue dye exclusion test
Residual cell viability in the attached and detached cell populations was determined by trypan blue dye exclusion test, as we described in earlier reports [21–23]. At least 600 cells were counted in four different fields for determination of residual cell viability.
Wright staining and ApopTag assay
Cells from each treatment were sedimented onto the microscopic slide and fixed in methanol before examination of apoptosis by Wright staining and ApopTag assay [21–23]. Wright staining detected characteristic apoptotic features such as chromatin condensation, cell-volume shrinkage, and membrane-bound apoptotic bodies. ApopTag assay kit (Intergen, Purchase, NY, USA) was used for biochemical detection of DNA fragmentation in apoptotic cells. The nuclei containing DNA fragments were stained dark brown with ApopTag assay and were not counter-stained with methyl green that, however, stained normal nuclei pale to medium green. After ApopTag assay, cells were counted to determine the percentage of apoptosis.
Fura-2 assay
The fluorescence Ca2+ indicator fura-2/AM was used, as we described previously [21–23], for determination of intracellular free [Ca2+] in C6 cells. The value of Kd, a cell-specific constant, was determined experimentally to be 0.667 µm for the C6 cells, using standards of the Calcium Calibration Buffer Kit with Magnesium (Molecular Probes, Eugene, OR, USA).
Analysis of mRNA expression
Extraction of total RNA, reverse transcription-polymerase chain reaction (RT-PCR), and agarose gel electrophoresis were performed, as we described previously [22, 23], to determine the levels of mRNA expression of specific genes in C6 cells. All rat primers (Table 1) for the RT-PCR experiments were designed using Oligo software (National Biosciences, Plymouth, MN, USA). The level of mRNA expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene served as an internal control in the RT-PCR experiments.
Table 1.
Rat primers used in RT-PCR experiments for amplification of mRNA of the specific genes
| Gene | Primer sequences | Product size (bp) |
|---|---|---|
| GAPDH | Forward: 5′-TTC ACC ACC ATG GAG AAG GC-3′ | 237 |
| Reverse: 5′-GGC ATG GAC TGT GGT CAT GA-3′ | ||
| MT1 | Forward: 5′-TGC TAC ATT TGC CAC AGT CTC-3′ | 397 |
| Reverse: 5′-GAC CTA TGA AGT TGA GTG GGG-3′ | ||
| MT2 | Forward: 5′-TAC ATC AGC CTC ATC TGG CTT-3′ | 297 |
| Reverse: 5′- CAC AAA CAC TGC GAA CAT GGT-3′ | ||
| bax | Forward: 5′-GCA GAG AGG ATG GCT GGG GAG A-3′ | 352 |
| Reverse: 5′-TCC AGA CAA GCA GCC GCT CAC G-3′ | ||
| bcl-2 | Forward: 5′-GGA TGA CTT CTC TCG TCG CTA C-3′ | 255 |
| Reverse:5′-TGC AGA TGC CGG TTC AG-3′ |
RT-PCR, reverse transcription-polymerase chain reaction.
Antibodies
Monoclonal primary immunoglobulin (Ig)G antibody against β-actin (Sigma Chemical, St. Louis, MO, USA) was used to standardize cytosolic protein loading on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Primary IgG antibody against α-spectrin (Affiniti, Exeter, UK) was also used. All other primary IgG antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated goat anti-mouse secondary IgG antibody (ICN Biomedicals, Aurora, OH, USA) was used for detecting all primary IgG antibodies, and horseradish peroxidase-conjugated goat anti-rabbit secondary IgG antibody (ICN Biomedicals) was employed for detecting primary antibodies against calpain and α-spectrin.
Western blot analysis
Protein extraction, SDS-PAGE, and Western blot analysis were performed as we described previously [21–23]. The autoradiograms were scanned using Photoshop software (Adobe Systems, Seattle, WA, USA) and the optical density (OD) of each band was determined using Quantity One software (BioRad, Hercules, CA, USA).
Statistical analysis
Results obtained from different treatments were analyzed using StatView software (Abacus Concepts, Berkeley, CA, USA). Data were expressed as mean ± standard error of mean (S.E.M.) of separate experiments (n ≥ 3) and compared by one-way analysis of variance (ANOVA) followed by the Fisher posthoc test. Significant difference (P < 0.05) between control (CTL) and LGA or H2O2 was indicated by *. Significant difference (P < 0.05) between LGA or H2O2 treatment and LGA or H2O2 treatment + melatonin post-treatment was indicated by #.
Results
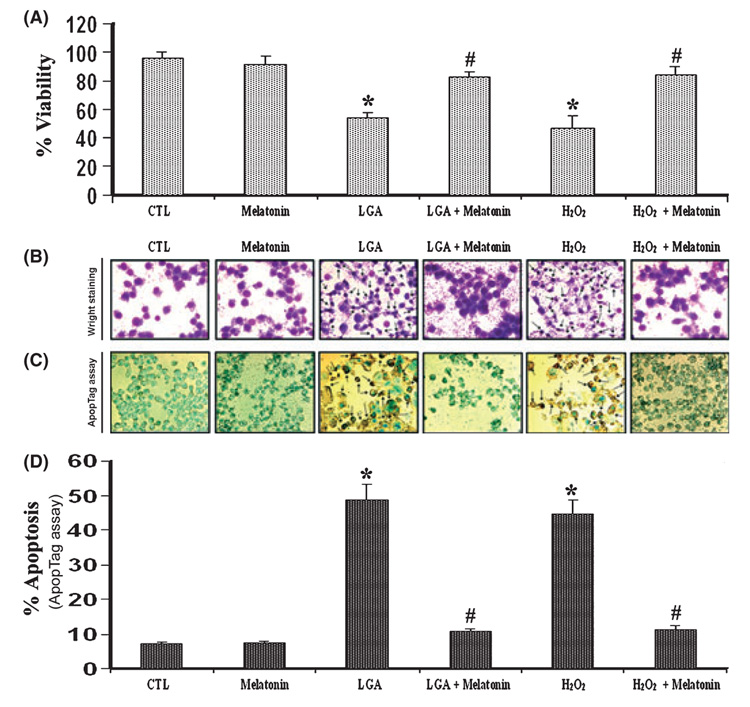
The viability of C6 cells was evaluated under a light microscope using Trypan blue exclusion test (Fig. 1). Melatonin post-treatment of cells in presence of LGA or H2O2 restored cell viability (Fig. 1A). Morphological features of apoptosis were detected following Wright staining (Fig. 1B). Apoptotic death was confirmed based on the characteristic morphological features such as condensation of the nucleus and cytoplasm, cytoplasmic blebbing, and the formation of apoptotic bodies. Results obtained from Wright staining were further supported by the ApopTag assay (Fig. 1C). Both control (CTL) and melatonin-treated cells showed little or no brown color, confirming almost absence of ApopTag-positive cells or apoptosis. All treatment groups were examined under the light microscopy and cells were counted to determine the amount of apoptotic cells (Fig. 1D). Compared with CTL cells, cells treated with 500 µm LGA or 100 µm H2O2 showed an increase (P < 0.05) in (more than 50%) apoptotic cells (Fig. 1A,D). At 15-min post-treatment of cells with 150 nm melatonin, there was a decrease in 500 µm LGA or 100 µm H2O2-induced apoptosis by 3-fold, compared with treatment of cells with 500 µm LGA or 100 µm H2O2 only.
Fig. 1. Determination of apoptosis in C6 cells after the treatments.
(A) Melatonin post-treatment prevented l-glutamic acid (LGA) or H2O2-mediated decrease in C6 cell viability. The trypan blue dye exclusion test was used to assess cell viability. (B) Wright staining showing representative cells from each treatment group. The arrows indicate apoptotic cells. (C) ApopTag assay showing representative cells from each treatment group. The arrows indicate apoptotic cells. (D) Bar graphs indicating the percentage of apoptotic cells (based on ApopTag assay). Treatment groups (panels A–D): control (CTL); 150 nm melatonin (24 hr); 500 µm LGA (24 hr); 500 µm LGA (15 min) + melatonin post-treatment (24 hr); 100 µm H2O2 (24 hr); and 100 µm H2O2 (15 min) + melatonin post-treatment (24 hr).
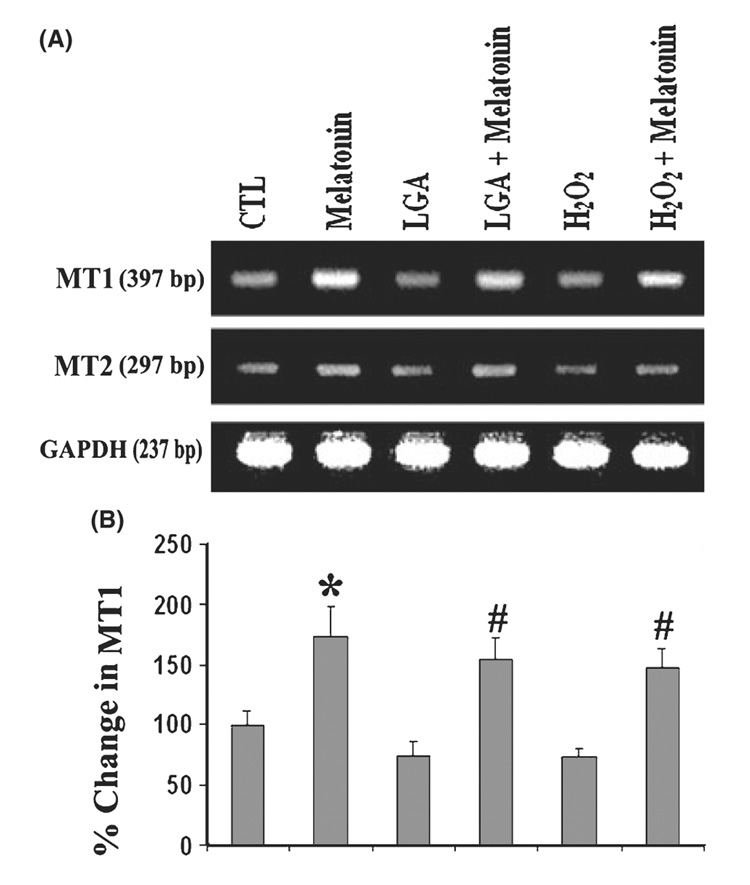
To ascertain whether the C6 cells used in these experiments expressed melatonin receptors (MT1 and MT2) at the mRNA level, we performed RT-PCR experiments using the rat-specific primers (Table 1). We examined mRNA expression of specific genes (Fig. 2). When the RNA samples from cells treated with 100 nm melatonin was processed without reverse transcription, no amplified products were detected indicating RT-PCR specificity and the absence of DNA contamination. Our results showed the presence of MT1 and MT2 in C6 cells, but the expression level of MT1 was greater than that of MT2 in melatonin-treated cells (Fig. 2A). The level of expression of MT2 was negligible. The level of GAPDH gene expression served as an internal control. We also detected higher level of expression of MT1 in melatonin alone treated cells than post-treatment of cells with melatonin (Fig. 2). LGA or H2O2 treatment of cells reduced the levels of MT1 significantly by 2-fold, compared with untreated cells. Post-treatment with melatonin restored MT1 expression level in C6 cells exposed to LGA or H2O2. Our result suggested that post-treatment of cells with melatonin decreased LGA or H2O2-induced apoptosis with an increase in expression of melatonin receptor, particularly MT1, at mRNA level.
Fig. 2. Reverse transcription-polymerase chain reaction (RT-PCR) experiments for examining levels of expression of melatonin receptors in C6 cells.
(A) Representative agarose gel pictures to show levels of mRNA expression of melatonin receptor 1 (MT1) and melatonin receptor 2 (MT2). (B) Bar graphs indicating the percent change in mRNA expression of MT1. Treatment groups (panels A and B): control (CTL); 150 nm melatonin (24 hr); 500 µm LGA (24 hr); 500 µm l-glutamic acid (LGA) (15 min) + melatonin post-treatment (24 hr); 100 µm H2O2 (24 hr); and 100 µm H2O2 (15 min) + melatonin post-treatment (24 hr).
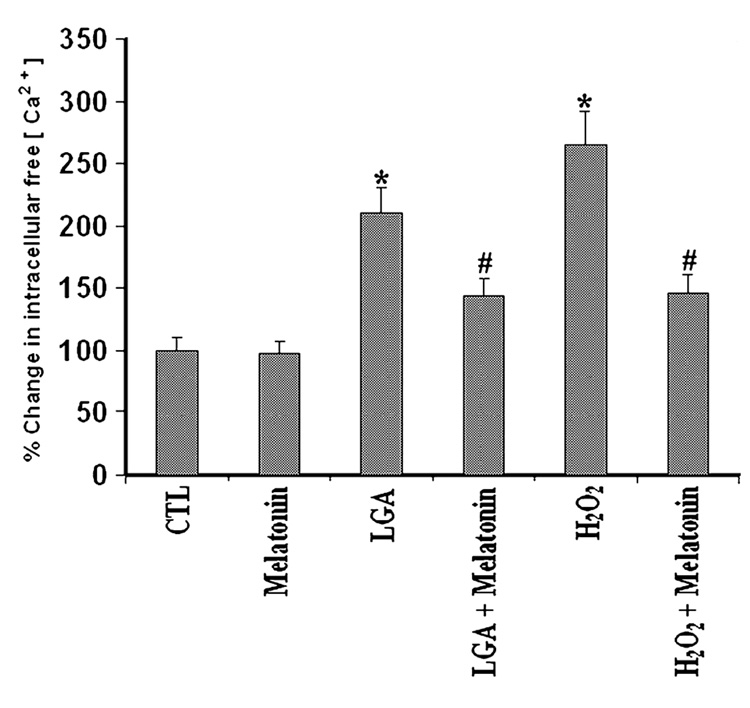
Intracellular free [Ca2+] was determined in all treatment groups using fura-2 assay (Fig. 3). Cells treated with 500 µm LGA or 100 µm H2O2 for 24 hr had a significant increase (P = 0.001), more than 2- to 2.5-folds, in intracellular [Ca2+] level when compared with CTL cells. This increase was 70% attenuated (P = 0.01) by post-treatment with melatonin. Furthermore, there was no significant difference (P = 0.3126) between intracellular [Ca2+] in CTL cells and those cells treated with LGA or H2O2 and melatonin (Fig. 3). No significant difference (P = 0.88) was seen between CTL cells and cells treated with melatonin alone. Compared with CTL cells, treatment of cells with LGA or H2O2 alone caused significant increases (P = 0.015) by more than 2-fold in intracellular free [Ca2+] (Fig. 3), suggesting the possibility of activation of the Ca2+-dependent protease calpain in course of cell death.
Fig. 3. Determination of intracellular free [Ca2+] using fura-2.
The data were from C6 cells grown in phenol-red free medium, treated for 24 hr, and then exposed to fura-2. Treatment groups: control (CTL); 150 nm melatonin (24 hr); 500 µm l-glutamic acid (LGA) (24 hr); 500 µm LGA (15 min) + melatonin post-treatment (24 hr); 100 µm H2O2 (24 hr); and 100 µm H2O2 (15 min) + melatonin post-treatment (24 hr).
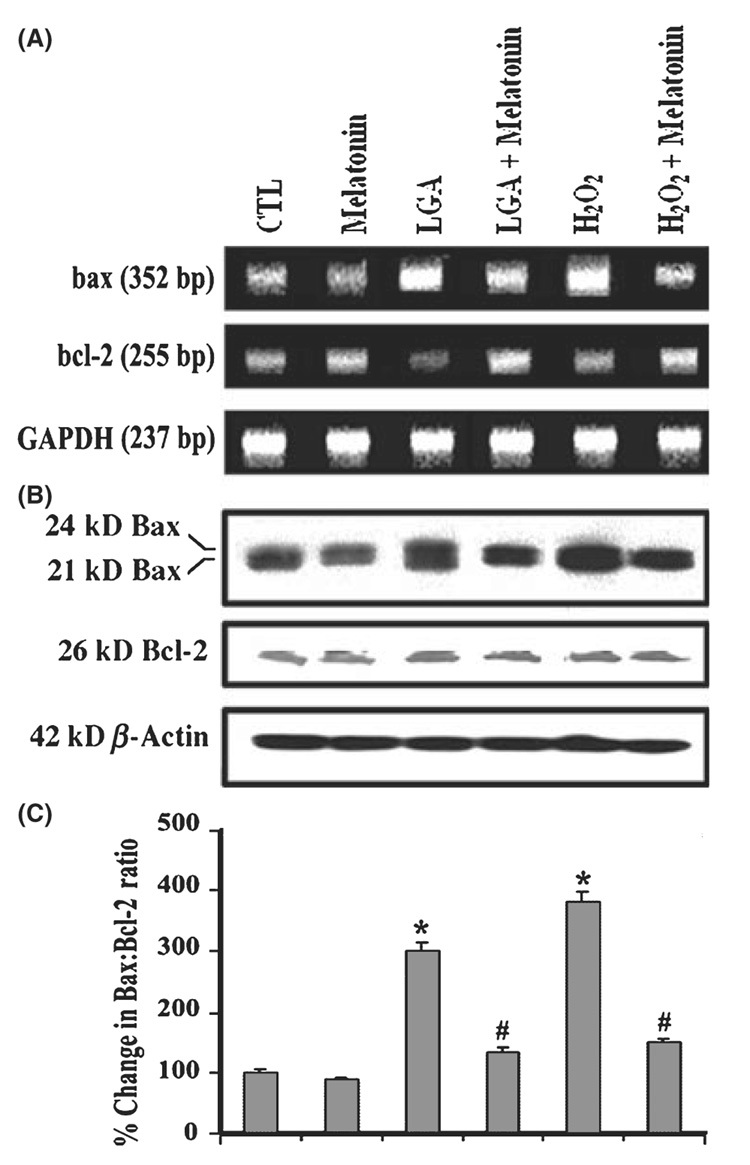
The commitment of C6 cells to apoptosis was measured by examining any increase in the ratio of Bax expression to Bcl-2 expression (Fig. 4). Examination of mRNA levels by RT-PCR showed that LGA or H2O2 treatment increased bax gene expression and decreased bcl-2 gene expression while mRNA levels of GAPDH gene remained uniform in all treatments (Fig. 4A). There was no change in bax and bcl-2 gene expression in melatonin treated cells, compared with CTL cells (Fig. 4A). We used a monoclonal antibody that recognized both 21 kD Baxa and 24 kD Baxβ isoforms, and another antibody detected 26 kD Bcl-2 on the Western blots. Treatment of C6 cells with LGA or H2O2 increased total Bax expression (Fig. 4B), resulting in an increase in Bax:Bcl-2 ratio (Fig. 4C) to promote mitochondrial release of pro-apoptotic factors. Cells treated with LGA or H2O2 showed significant increase (P = 0.004) in the Bax:Bcl-2 ratio (2- to 4-folds), compared with CTL cells (Fig. 4C). There was a significant difference (P = 0.043) in Bax:Bcl-2 ratio between cells treated with LGA or H2O2 alone and those cells post-treated with melatonin. There was no significant difference (P = 0.710) between CTL cells and cells exposed to LGA or H2O2 and then post-treated with melatonin. Melatonin treatment alone did not significantly (P = 0.635) alter the Bax:Bcl-2 ratio. Again, level of β-actin was monitored to ensure that equal amount of protein was loaded in each lane.
Fig. 4. Alterations in expression of Bax and Bcl-2 at mRNA and protein levels.
(A) Representative agarose gels to show mRNA levels of bax, bcl-2, and GAPDH genes (reverse transcription-polymerase chain reaction). (B) Representative Western blots to show protein levels of Bax, Bcl-2, and GAPDH (Western blotting). (C) Densitometric analysis showing the Bax:Bcl-2 ratio in six treatment groups. Treatment groups (panels A–C): control (CTL); 150 nm melatonin (24 hr); 500 µm l-glutamic acid (LGA) (24 hr); 500 µm LGA (15 min) + melatonin post-treatment (24 hr); 100 µm H2O2 (24 hr); and 100 µm H2O2 (15 min) + melatonin post-treatment (24 hr).
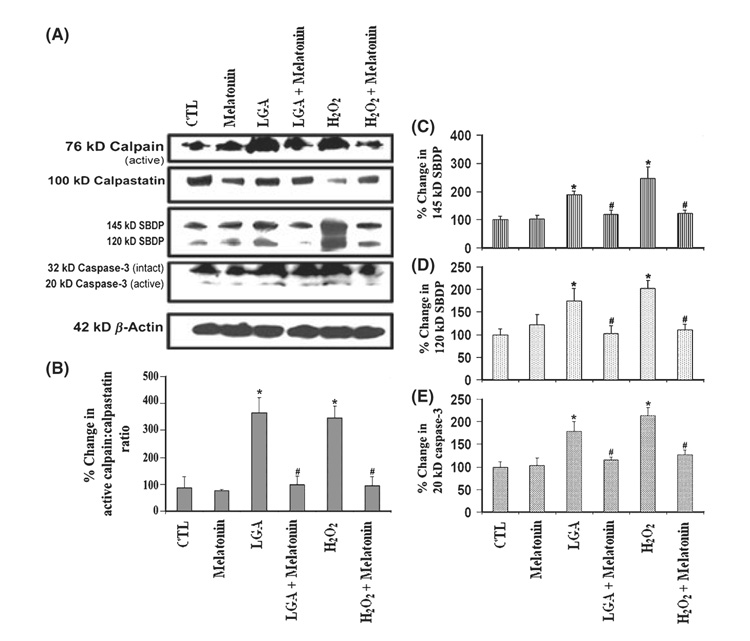
Western blotting showed increase in the production of active calpain fragment and degradation of calpastatin (an endogenous calpain inhibitor), and also, increase in active caspase-3 fragment very prominently following treatment of cells with LGA or H2O2 alone (Fig. 5). Because high level of calpastatin prevented calpain-mediated proteolysis [22], a reduction in calpastatin level would leave calpain activity unregulated. We detected calpain activation in the formation of 76 kD calpain active fragment and calpain activity in the generation of calpain-specific 145 kD SBDP, whereas caspase-3 activation in formation of 20 kD caspase-3 active fragment and caspase-3 activity in the formation of caspase-3 specific 120 kD SBDP (Fig. 5A). Cells treated with melatonin showed attenuation of the 76 kD calpain active fragment, 145 kD SBDP, 120 kD SBDP, and 20 kD caspase-3 active fragment. Also, our results showed significant increase (P = 0.0171) in calpain active fragment:calpastatin ratio in cells treated with LGA or H2O2 when compared with CTL cells (Fig. 5A,B). The calpain active fragment:calpastatin ratio was significantly reduced in melatonin post-treated cells (Fig. 5A,B). We also observed that post-treatment with melatonin significantly (P ≤ 0.05) decreased the amounts of 145 kD SBDP (Fig. 5A,C), 120 kD SBDP (Fig. 5A,D), and 20 kD caspase-3 active fragment (Fig. 5A,E) in the LGA or H2O2 treated cells. The difference in proteolytic activities between CTL cells and cells treated with LGA or H2O2 and then exposed to melatonin was nonsignificant (P ≥ 0.6), demonstrating that melatonin was fully capable of controlling both calpain and caspase-3 activities (Fig. 5). Taken together, the results suggested that melatonin provided cytoprotection to C6 astroglial cells during glutamate excitotoxicity and oxidative stress.
Fig. 5. Determination of activation and activity of calpain as well as of caspase-3.
(A) Representative Western blots to show levels of 76-kD calpain active fragment, 100 kD calpastatin (endogenous calpain inhibitor), 145 kD spectrin breakdown product (SBDP), 120 kD SBDP, 32 kD caspase-3 inactive fragment and 20 kD caspase-3 active fragment, and β-actin. Densitometric analysis to show percent change (B) in active calpain: calpastatin ratio, (C) in calpain-specific 145 kD SBDP, (D) in caspase-3-specific 120 kD SBDP, and (E) in 20 kD caspase-3. Treatment groups (panels A–E): control (CTL); 150 nm melatonin (24 hr); 500 µm l-glutamic acid (LGA) (24 hr); 500 µm LGA (15 min) + melatonin post-treatment (24 hr); 100 µm H2O2 (24 hr); and 100 µm H2O2 (15 min) + melatonin post-treatment (24 hr).
Discussion
The pineal gland hormone melatonin is an important agent that has multiple actions such as anti-oxidant and free radical scavenging properties. Its efficacy has been tested in experimental neurodegenarative diseases and neurotrauma models. Melatonin has been shown to attenuate glutamatemediated Ca2+-influx and inflammation by inhibiting the level of pro-inflammatory cytokines [8–15, 24]. Melatonin has the ability to inhibit apoptosis in brain cells [24] as well in other tissues [25]. Based on the results obtained from our current study, we suggest that cytoprotective action of melatonin is due to inhibition of calpain and caspase-3 proteolytic pathways involved in cell death (Fig 1–Fig 5). Our results support a direct relationship between cytoprotection and inhibition of proteolytic activities of calpain and caspase-3.
The exact mechanism of melatonin-mediated cytoprotection is not well understood, but melatonin receptormediated actions have been implicated. Our present data indicate that both melatonin receptors (MT1 and MT2) are expressed in C6 cells, but involvement of MT2 in cytoprotection is negligible. The increase in expression of MT1 at mRNA level in melatonin-treated cells was substantial (Fig. 2). Previous studies have reported that physiological levels of melatonin are cytoprotective in cells expressing melatonin receptors [16, 17, 26]. In contrast, our studies showed that pharmacological concentrations of melatonin are needed to protect C6 cells from LGA or H2O2-induced cell death indicating that protection rendered by melatonin could be via receptor-mediated pathway. Pharmacological levels of melatonin are always required to prevent oxidative damage in presence of such massive amounts of oxidants. Under these circumstances, physiological levels of antioxidants are always inadequate.
Our findings support a relationship between cell death (Fig. 1) and an increase in intracellular free [Ca2+] levels following exposure of C6 cells to LGA or H2O2 alone (Fig. 3). Melatonin post-treatment of C6 cells exposured to LGA or H2O2 showed a decrease in intracellular [Ca2+]. One of the mechanisms of the cytoprotective effect of melatonin against glutamate excitotoxicity or oxidative stress may be due to reduction in intracellular free [Ca2+] and subsequent Ca2+-dependent events. While the exact mechanism of action of melatonin for attenuating Ca2+ influx in C6 cells exposed to LGA or H2O2 is not known, there are several examples in other cell types showing melatonin’s suppressive influence on intracellular free [Ca2+] and neuroprotective effects by inhibiting both mitochondrial and endoplasmic reticulum pathways of cell death [27] or by direct binding to calreticulin [28].
Because changes in expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 control the mitochondrial pathway of apoptosis [29], we examined the expression of Bax and Bcl-2 at the mRNA and proteins levels. An increase in Bax opens the mitochondrial transition pore, allowing cytochrome c release into the cytosol for activation of caspases leading to cell death [29]. Our results showed that melatonin could protect apoptotic cells by inhibiting the alternations in mRNA and protein levels of Bax and Bcl-2 so as to maintain basal Bax:Bcl-2 ratio (Fig. 4). Therefore, apart from protecting the cells from Ca2+-dependent events, melatonin may provide cytoprotection by inhibiting the rise in Bax:Bcl-2 ratio and mitochondria-dependent caspase cascades. Certainly, melatonin has been shown to have a variety of beneficial actions on mitochondria for normal function [3–5, 24].
The observation that treatment with LGA or H2O2 alone leads to increases in calpain and caspase-3 activities is in agreement with the results of our previous studies [18, 19]. Moreover, treatment with melatonin has been shown to decrease calpain and caspase-3 activities in the spinal cord injury in rats [30]. Pro-apoptotic Bax action is thought to be upstream of the caspases in the mitochondria-mediated apoptotic death pathway. From our own observations, reduction in the Bax:Bcl-2 ratio (Fig. 4) correlates well with reduction in calpain and caspase-3 activities (Fig. 5). These findings, taken together, supported the notion that melatonin provided cytoprotection due, in part, to inhibition of the mitochondria-dependent apoptotic pathway [31].
In conclusion, our results indicate that melatonin post-treatment effectively prevents excitotoxicity and oxidative damage in astroglial cells. Further, melatonin post-treatment can be associated with an increase in MT1 expression. The cytoprotective effects of melatonin may be due, in part, to reduction in Ca2+ influx, Bax:Bcl-2 ratio, and calpain and caspase-3 activities during glutamate excitotoxicity and oxidative stress. Although there are several protease inhibitors that protect cells possibly by preventing protein degradation, a multi-active agent like melatonin may be more useful in protecting cells from many destructive pathways. Our results imply that the multi-active agent melatonin may have promising therapeutic potential for cytoprotection in CNS diseases and injuries.
Acknowledgments
This investigation was supported in part by the R01 grants from the NINDS (NS-31622, NS-41088, NS-45967, and NS-57811) and NCI (CA-91460) of the National Institutes of Health (Bethesda, MD).
References
- 1.Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 2.M Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms NO+, *NO and HNO (protonated NO−) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J Pineal Res. 2007;43:382–388. doi: 10.1111/j.1600-079X.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- 3.Mesenge C, Margaill I, Verrecchia C, et al. Protective effect of melatonin in a model of traumatic brain injury in mice. J Pineal Res. 1998;25:41–46. doi: 10.1111/j.1600-079x.1998.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 4.León J, Acuña-Castroviejo D, Escames G, et al. Melatonin mitigates mitochondrial malfunction. J Pineal Res. 2005;38:1–9. doi: 10.1111/j.1600-079X.2004.00181.x. [DOI] [PubMed] [Google Scholar]
- 5.Jou MJ, Peng TI, Yu PZ, et al. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43:389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaptanoglu E, Tuncel M, Palaoglu S, et al. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J Neurosurg. 2000;93:77–84. doi: 10.3171/spi.2000.93.1.0077. [DOI] [PubMed] [Google Scholar]
- 7.Borlongan CV, Yamamoto M, Takei N. Glial cell survival is enhanced during melatonin-induced neuroprotection against cerebral ischemia. FASEB J. 2000;14:H1307–H1317. doi: 10.1096/fj.14.10.1307. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera J, Reiter RJ, Tan DX. Melatonin reduces oxidative neurotoxicity due to quinolinic acid: In vitro and in vivo findings. Neuropharmacology. 2000;39:507–514. doi: 10.1016/s0028-3908(99)00128-8. [DOI] [PubMed] [Google Scholar]
- 9.Tan DX, Chen LD, Poeggeler B, et al. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr J. 1993;1:57–60. [Google Scholar]
- 10.Pieri C, Marra M, Moroni F, et al. Melatonin: a peroxyl radical scavenger more efficient than vitamin E. Life Sci. 1994;55:271–276. doi: 10.1016/0024-3205(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 11.Tan DX, Manchester LC, Reiter RJ, et al. Melatonin directly scavenges hydrogen peroxide: a potentially metabolic pathway of melatonin biotransformation. Free Radic Biol Med. 2000;29:1177–1185. doi: 10.1016/s0891-5849(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 12.Reiter RJ, Tan DX, Manchester LC, et al. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 13.Livrea MA, Tesoriere L, D’Arpa D, et al. Reaction of melatonin with lipoperoxyl radicals in phospholipid bilayers. Free Radic Biol Med. 1997;23:706–711. doi: 10.1016/s0891-5849(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 14.Li XJ, Gu J, Lu SD, Sun FY. Melatonin attenuates MPTP-induced dopaminergic neuronal injury associated with scavenging hydroxyl radical. J Pineal Res. 2002;32:47–52. doi: 10.1034/j.1600-079x.2002.10831.x. [DOI] [PubMed] [Google Scholar]
- 15.Fowler M, Daroszewska M, Ingold KU. Melatonin does not “directly scavenge hydrogen peroxide”: demise of another myth. Free Radic Biol Med. 2003;34:77–83. doi: 10.1016/s0891-5849(02)01186-3. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong KJ, Niles LP. Induction of GDNF mRNA expression by melatonin in rat C6 glioma cells. Neuroreport. 2002;13:473–475. doi: 10.1097/00001756-200203250-00023. [DOI] [PubMed] [Google Scholar]
- 17.Martín V, Herrera F, Carrera-Gonzalez P, et al. Intracellular signaling pathways involved in the cell growth inhibition of glioma cells by melatonin. Cancer Res. 2006;66:1081–1088. doi: 10.1158/0008-5472.CAN-05-2354. [DOI] [PubMed] [Google Scholar]
- 18.Sribnick EA, Ray SK, Banik NL. Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neuroscience. 2006;137:197–209. doi: 10.1016/j.neuroscience.2005.08.074. [DOI] [PubMed] [Google Scholar]
- 19.Sur P, Sribnick EA, Wingrave JM, et al. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003;971:178–188. doi: 10.1016/s0006-8993(03)02349-7. [DOI] [PubMed] [Google Scholar]
- 20.Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- 21.Das A, Sribnick EA, Wingrave JM, et al. Calpain activation in apoptosis of ventral spinal cord 4.1 (VSC4.1) motoneurons exposed to glutamate: calpain inhibition provides functional neuroprotection. J Neurosci Res. 2005;81:551–562. doi: 10.1002/jnr.20581. [DOI] [PubMed] [Google Scholar]
- 22.Das A, Banik NL, Ray SK. Differentiation decreased telomerase activity in rat glioblastoma C6 cells and increased sensitivity to IFN-gamma and taxol for apoptosis. Neurochem Res. 2007;32:2167–2183. doi: 10.1007/s11064-007-9413-y. [DOI] [PubMed] [Google Scholar]
- 23.Das A, Banik NL, Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110:1083–1095. doi: 10.1002/cncr.22888. [DOI] [PubMed] [Google Scholar]
- 24.Jou MJ, Peng TI, Reiter RJ, et al. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res. 2004;37:55–70. doi: 10.1111/j.1600-079X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 25.Radogna F, Paternoster L, Albertini MC, et al. Melatonin antagonizes apoptosis via receptor interaction in U937 monocytic cells. J Pineal Res. 2007;43:154–162. doi: 10.1111/j.1600-079X.2007.00455.x. [DOI] [PubMed] [Google Scholar]
- 26.Castro LM, Gallant M, Niles LP. Novel targets for valproic acid: up-regulation of melatonin receptors and neurotrophic factors in C6 glioma cells. J Neurochem. 2005;95:1227–1236. doi: 10.1111/j.1471-4159.2005.03457.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin AM, Fang SF, Chao PL, et al. Melatonin attenuates arsenite-induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res. 2007;43:163–171. doi: 10.1111/j.1600-079X.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 28.Macías M, Escames G, Leon J, et al. Calreticulin-melatonin. An unexpected relationship. Eur J Biochem. 2003;270:832–840. doi: 10.1046/j.1432-1033.2003.03430.x. [DOI] [PubMed] [Google Scholar]
- 29.Polster BM, Kinnally KW, Fiskum G. BH3 death domainpeptide induces cell type-selective mitochondrial outer membrane permeability. J Biol Chem. 2001;276:37887–37894. doi: 10.1074/jbc.M104552200. [DOI] [PubMed] [Google Scholar]
- 30.Samantaray S, Sribnick EA, Das A, et al. Melatonin attenuates calpain upregulation, axonal damage, and neuronal death in spinal cord injury. J Pineal Res. 2007 doi: 10.1111/j.1600-079X.2007.00534.x. (in press – PMID: 18086148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andrabi SA, Sayeed I, Siemen D, et al. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004;18:869–871. doi: 10.1096/fj.03-1031fje. [DOI] [PubMed] [Google Scholar]