Abstract
This paper presents a meta-analysis of high-resolution human leukocyte antigen (HLA) allele frequency data describing 497 population samples. Most of the datasets were compiled from studies published in eight journals from 1990 to 2007; additional datasets came from the International Histocompatibility Workshops and from the AlleleFrequencies.net database. In all, these data represent approximately 66,800 individuals from throughout the world, providing an opportunity to observe trends that may not have been evident at the time the data were originally analyzed, especially with regard to the relative importance of balancing selection among the HLA loci. Population genetic measures of allele frequency distributions were summarized across populations by locus and geographic region. A role for balancing selection maintaining much of HLA variation was confirmed. Further, the breadth of this meta-analysis allowed the ranking of the HLA loci, with DQA1 and HLA-C showing strongest balancing selection and DPB1 being compatible with neutrality. Comparisons of the allelic spectra reported by studies since 1990 suggest that most of the HLA alleles identified since 2000 are very-low-frequency alleles. The literature-based allele-count data, as well as maps summarizing the geographic distributions for each allele, are available online.
Introduction
The human major histocompatibility complex (MHC) contains over 200 genes in a 4 Mb region of chromosome 6. Approximately 40% of these genes have immunological functions, most notably the genes that encode the human leukocyte antigen (HLA) proteins. The HLA proteins are cell-surface antigens that present peptides to T-cell receptors, distinguishing self from non-self peptides. Additionally, some of them serve as ligands for killer-cell immunoglobulin-like receptors on natural killer cells and some T-cells. Extensive allele and haplotype diversity has been observed for the classical class I (HLA-A, -C, and -B) and class II (HLA-DRB1, -DQA1, - DQB1, -DPA1, and -DPB1) genes. Many hundreds of class I and DRB1 alleles have been observed globally and scores of alleles, many with relatively high frequencies, exist within any given population, making them the most polymorphic loci in the human genome. Specific polymorphisms at these loci have been associated with susceptibility to and protection from various auto-immune and infectious diseases, as well as certain cancers [1].
Because of their conspicuously high allelic diversity, HLA genes have been used for population studies, to study human demography, and to demonstrate trans-species polymorphism and balancing selection (the maintenance of novel and low frequency alleles at frequencies more evenly distributed than expected under models of neutral evolution). Population-level studies of HLA allelic diversity have provided insight into the selective forces that have acted to generate and maintain polymorphism in the human species [2, 3]. Historical and evolutionary relationships among modern human populations have been inferred from comparisons of HLA allele and haplotype frequency distributions [4–7]. Further, variation in the allelic diversity between populations, as well as comparisons of linkage disequilibrium (LD) patterns across the HLA region, have been used to infer demographic events such as bottlenecks and founder events [3, 8]. Finally, differences in allele and haplotype frequencies between populations belonging to different ethnic groups have been used to better understand associations between HLA and disease, in some cases illuminating mechanisms of disease progression and resistance [9, 10] and in other cases identifying the actual disease-predisposing locus due to high LD [11, 12].
Here, we present meta-analyses of allele frequency distribution data for the classical HLA loci in 497 population samples, representing 66,800 individuals in studies published over the last 17.5 years, describing measures of diversity and selection for each population at each typed locus in an attempt to summarize broad global patterns of allelic differentiation. These data and associated analyses are available in electronic form from http://www.pypop.org/popdata/.
Subjects and methods
Datasets
The data analyzed here were derived from three distinct sources (described below). Combined, these datasets constitute a collection of 497 globally well-distributed population samples, of at least 20 individuals each, representing allele frequency data for approximately 66,800 individuals in total. The 497 population samples were carefully screened for duplications (i.e., the same individuals represented in two datasets), but some populations are represented by more than one sample (e.g., at the HLA-B locus, there are five different samples of the Japanese population.)
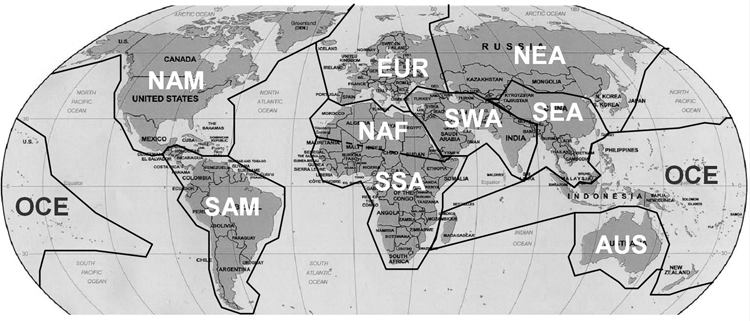
Each population data file was annotated with a three-letter abbreviation assigning it to one of the 10 world regions shown in Figure 1 [3, 13]: Sub-Saharan Africa (SSA), North Africa (NAF), Europe (EUR), Southwest Asia (SWA), Southeast Asia (SEA), Oceania (OCE), Australia (AUS), Northeast Asia (NEA), North America (NAM), South America (SAM) . These regional assignments were based on the origin of the population, in effect ignoring the last 1000 years of known human migration (e.g., people of European descent in the United States are assigned to the region EUR). Significantly admixed populations (those suspected to have origins in more than one of the 10 regions) were placed in a separate regional category: other (OTH). Each data file also includes the latitude and longitude of the population sample, inferred as accurately as possible from the information provided in the publication. The geographical coordinates of the sampling thus may not lie within the regional assignment.
Figure 1. Geographical categories for populations.
SSA = Sub-Saharan Africa; NAF = North Africa; EUR = Europe; SWA = Southwest Asia; SEA = Southeast Asia; OCE = Oceania; AUS = Australia; NEA = Northeast Asia; NAM = North America; SAM = South America. Admixed populations are assigned to the category other (OTH). The groupings are as recommended in references [3, 13].
Literature datasets
Datasets of HLA allele counts or frequencies were collected from a systematic review of the literature. For each of the following eight journals, every issue between January 1990 and August 2007 was surveyed for published allele frequency data: American Journal of Human Genetics, American Journal of Physical Anthropology, Human Immunology, Human Genetics, Immunogenetics, European Journal of Immunogenetics (later titled International Journal of Immunogenetics), Tissue Antigens, and Human Biology. In each issue, we identified articles describing allelic (as opposed to phenotypic) frequency data of a clearly defined population or subpopulation for one or more of the following classical HLA loci: A, C, B, DRB1, DQA1, DQB1, DPA1, and DPB1. Many of the datasets were taken from studies with an explicit anthropological focus, but control groups from case-control disease studies were also used, if the population samples were randomly selected. Nearly half of the datasets were found in Tissue Antigens. Four studies from other journals were brought to our attention and were also included.
For each article, allele frequency data were entered into a spreadsheet by hand or, when possible, copied and pasted directly from tables available via the online version of the journal article. Genotype and haplotype data (available in very few of the publications) were collapsed to allele-count data. A dual entry procedure was implemented for quality control purposes using different data entry personnel. If included in the publication, the reported allele counts were used, but more commonly, allele counts were calculated by multiplying the reported allele frequencies by the number of chromosomes (2n). If the sum of the allele frequencies did not equal 1, the data were rechecked against the original publication. In cases where erroneous frequencies were present in the original publication, obvious typographical errors were corrected, but in cases where the source of the error was not clear, the data were excluded from analysis. The allele-count data were then saved as flat, tab-delineated data files (described below). Fields describing the ethnicity of the population and bibliographic citation information were included in the data files. The year of publication was used as a proxy for the actual typing date of the population sample.
Publications were excluded from the literature datasets for the following reasons: more than 5% of the data were low resolution (i.e., typed at the two-digit “serological” level) or missing (e.g., “blank” alleles); the 2n sample size was less than 40 chromosomes; or the published data were available as part of the International Histocompatibility Workshops (IHW, described below).
The literature datasets describe 292 populations, or 35,063 individuals, with an average of 2.1 loci typed per population, and were obtained from a total of 185 publications [14–198]. The data files are available for download at http://www.pypop.org/popdata/.
International Histocompatibility Workshop datasets
Data from the 13th IHW Anthropology / Human Genetic Diversity component [199] and the 12th IHW Anthropology component [200] were also included in these analyses. Duplications between the workshop datasets and the literature datasets were carefully screened. Because they adhere to the same typing and reporting standards, the workshop datasets were used in preference to corresponding literature datasets in cases of duplication. Eight workshop population samples with a 2n size of less than 40 chromosomes were removed.
The final workshop datasets used for the present study describe 135 population samples, representing 17,480 individuals, with an average of 3.7 loci typed per population. Data from the 13th IHW are available from the dbMHC Web site (http://www.ncbi.nlm.nih.gov/mhc/). Data from the 12th and 13th IHW are available from the Gene[VA] Web site (http://geneva.unige.ch/IHWdata/).
Allelefrequencies.net datasets
To augment data obtained from the literature and the IHW, HLA data from the AlleleFrequencies.net database were downloaded on 9 December 2004 from http://www.allelefrequencies.net/. This public database contains researcher-submitted allele frequency data for HLA and other loci [201]. The data were published as printed tables in the 10 September 2004 issue of Human Immunology. In cases of duplication with population data in the literature or workshop datasets, those obtained from the AlleleFrequencies.net database were excluded. The same exclusion criteria described above for the literature datasets were also applied.
The AlleleFrequencies.net datasets that were selected for the present study describe 70 populations, representing 14,287 individuals, with an average of 2.1 loci typed per population. A list of these populations, with links to the AlleleFrequencies.net database, may be accessed at http://www.pypop.org/popdata/.
Analysis
The PyPop population genetics software package
PyPop is an open-source software package designed to perform large-scale population genetic analyses on single-locus allele-count and multi-locus genotype data [202–205]. PyPop performs haplotype frequency estimation, tests for deviation from Hardy-Weinberg (H–W) equilibrium, and tests for balancing or directional selection, and computes the strength and significance of LD. The methods employed are described in [206, 207]. Although originally developed to analyze the highly polymorphic HLA region, PyPop has applicability to any multi-locus genetic data. PyPop was the primary analysis platform of the Anthropology component of the 13th and 14th International Histocompatibility Workshops [5, 13, 199, 204–210]. The PyPop input file format is an easily-parsed, tab-delimited plain-text file, and can accommodate both population allele-count data and individual genotype data.
The large number of alleles, along with the complexities of HLA nomenclature, demands certain pre-processing steps, especially for comparisons across different studies. PyPop has the ability to validate allele names by comparing them to an authoritative source, as defined by the user. For the HLA alleles examined in this study, allele names were checked against release 2.18 of the IMGT/HLA database [211, 212], downloaded on 29 Sept 2007 as MSF-formatted sequence alignment files from ftp://ftp.ebi.ac.uk/pub/databases/imgt/mhc/hla/. PyPop alerts the user to unrecognized allele names, which helps to catch data entry errors.
Pre-processing is also important when dealing with the nomenclature variation present across 17 years of published studies. Some HLA alleles are only detectable using a specific high-resolution genotyping method (e.g., sequence-based typing) and would be reported under a different four-digit name using lower-resolution methods [13, 209]. Other allele names have been changed or deleted entirely from the list of officially recognized alleles (see http://www.anthonynolan.com/HIG/lists/delnames.html). To achieve a common denominator of typing resolution, class I alleles are considered the same if their peptide sequences are identical across exons 2 and 3, whereas class II alleles are considered the same if they are identical across exon 2 [213]. For this study, all of the above nomenclature changes were encoded as a set of “binning rules” that PyPop applies before beginning population genetic analysis. This facilitates valid comparisons across datasets genotyped with different methods. These binning rules are available as part of the PyPop (version 0.7.0) package, downloadable at http://www.pypop.org/.
Ewens-Watterson homozygosity test of neutrality
The Ewens-Watterson (EW) homozygosity test of neutrality [214, 215] provides a method to detect the effect of selection using allele frequency data. Balancing selection maintains a number of alleles at relatively even frequencies, whereas directional selection favors one or a few alleles. The EW test uses the observed homozygosity statistic (Fobs , defined as the sum of the squared allele frequencies) and compares this with Fexp , the homozygosity expected under neutral evolution (random neutral mutations and genetic drift), for a given sample size (2n) and number of alleles (k), as described by Ewens [214].
When the null hypothesis of neutral evolution is rejected, this test suggests the effect of either balancing selection (when Fobs ≪ Fexp) or directional selection (when Fobs ≫ Fexp). An extreme demographic event in the history of the population (e.g., a founder effect or population bottleneck) may also increase Fobs relative to Fexp. Although it is possible to perform a one-sided test against the alternative hypothesis of balancing selection or directional selection (many software packages do report a one-sided test), here we report p-values for a two-sided test, which results in a more conservative test for loci at which balancing selection is suspected. The p-values are based on Slatkin’s [216, 217] implementation of a Monte-Carlo approximation to the EW homozygosity test of neutrality. It is also worth noting that while Fobs is calculated under the H–W assumptions, the EW test is not a test of H–W proportions.
The degree of deviation between Fobs and Fexp is described by the normalized deviate of homozygosity (Fnd), defined by Salamon and coworkers [218] as This statistic permits comparisons between population samples that have different numbers of alleles and sample sizes, such as those in the present study. Balancing selection results in negative Fnd values whereas directional selection, along with certain demographic events, leads to positive values.
For meta-analysis, the mean Fnd for groups of populations were tested using the t-test and the sign test. A two-tailed t-test can be used to test the null hypothesis that averaged over m different populations, is significantly different from zero [218], because under the null hypothesis of neutral evolution, is approximately normally distributed with a mean of zero and a variance of 1/m. Rejection of the null hypothesis suggests the effect of balancing selection ( ≪ 0), directional selection ( ≫ 0), or demographic events in the populations’ histories. In this latter case, selection can sometimes be distinguished from demographic effects by comparison of values (or individual Fnd values for a single population) at multiple loci, with the assumption that evidence for selection will be strongest at a specific locus, while a demographic effect should be evident at all loci. The sign test uses the expectation of equal proportions of negative and positive Fnd values, for a collection of populations, under neutral evolution. The sign test can be used to test the null hypothesis of equal proportions for the two categories [219].
Year of initial identification of HLA alleles
When available, the earliest year of publication of the article(s) describing each allele in the corresponding reference page on the Anthony Nolan Trust HLA Informatics Group Web site (http://www.anthonynolan.com/HIG/seq/refs/) or the year of creation of the record for each allele in the EMBL-EBI IMGT/HLA database (http://www.ebi.ac.uk/imgt/hla/) was used to represent the year in which that allele was first identified. After the truncation of allele names to four digits, the earliest such year was taken as the year of initial identification of that particular peptide sequence.
Genetic differentiation within and between regions
To compare the degree of genetic differentiation within and between different global regions, we used PHYLIP v3.6 [220] to calculate Nei’s Standard Genetic Distances between all pairs of populations at the HLA-A, -C, -B, -DRB1, and -DPB1 loci. We calculated the overall mean genetic distance for the populations at each locus, the mean genetic distance for each region (and certain subregions) at each locus. Infinite genetic distances were ignored for these calculations. Finally, each regional or subregional mean genetic distance value was divided by the overall mean genetic distance at each locus, generating the regional fraction of the overall genetic distance seen in each locus. Considered relative to one another, these fractions indicate the degree of differentiation in allele frequency distributions for the populations in each region and subregion.
Because data are available for different sets of populations at each locus, we restricted these calculations, such that we used only those populations common to individual pairs of loci, and only those pairs of loci with enough populations to compare (i.e., intraclass I comparisons, class I - DRB1 comparisons, and the DRB1 vs. DPB1 comparison).
Mapping and geographic allele frequency interpolation
Maps were plotted using the Generic Mapping Tools (GMT) package [221], of which the blockmean and surface functions were used to interpolate a two-dimensional allele frequency distribution from the available point estimates. GMT mapping scripts are available for download at http://www.pypop.org/popdata/.
Results
Number of Population Samples
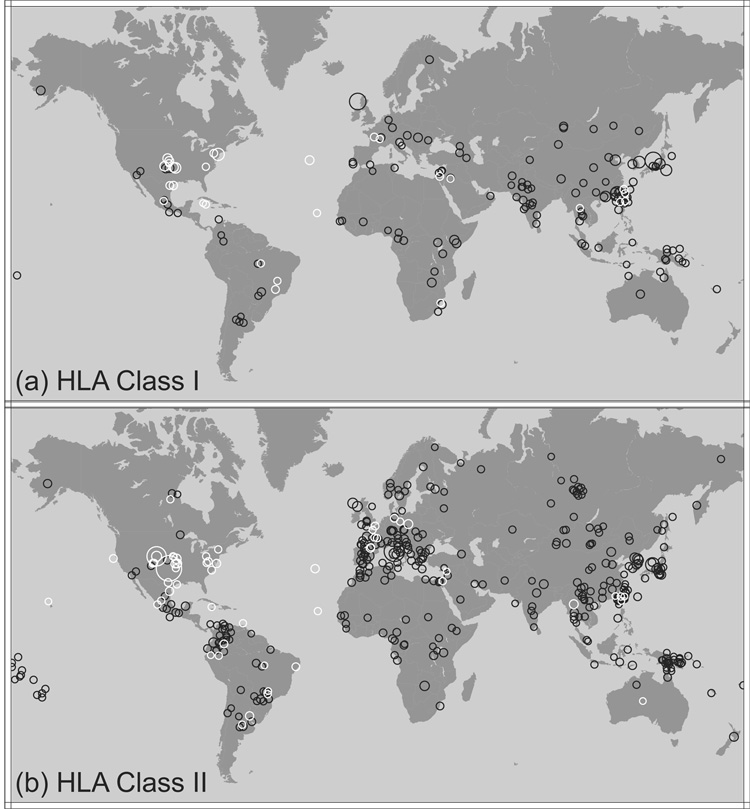
Table 1 summarizes the number of population samples (m) of the three meta-datasets, describing coverage by locus, by world region, and over all regions. The population samples are illustrated geographically in Figure 2, in which each population is plotted according to its sampling location (not necessarily the same as its regional assignment). Global representation of published HLA data is biased towards EUR and East Asia (SEA+NEA), with especially poor representation of Africa (SSA+NAF), SWA, and AUS; 128 East Asian and 117 EUR populations (representing approximately 18,400 and 24,700 sampled individuals, respectively) are included in the combined dataset, whereas only 55 African and four AUS populations (6,000 and 412 individuals, respectively) are included. The sampling bias is most pronounced for class II HLA loci, which were the first loci to be typed extensively and at high resolution; the earliest HLA molecular typing studies tended to sample EUR and East Asian populations, and populations from these regions represent 53% of the populations (and 68% of the individuals) with class II frequency data. In contrast, EUR and East Asian populations represent only 42% of the populations (and 58% of the individuals) with class I frequency data. Of the loci included in these analyses, DRB1 is best represented, with frequency data available for 259 populations (representing approximately 40,700 individuals), while the DPA1 locus is the least well-represented locus, with only 45 populations (3,800 individuals).
Table 1.
Results for all loci and geographic regions.
| reg 1 | m (ml,mw,ma) 2 | k̅, k sum3 | SE 4 | Fnd<0 5 | % sig6 | t-test p7 | sign test p7 | ||
|---|---|---|---|---|---|---|---|---|---|
| SSA | 13 (4,9,0) | 27, 66 | −1.05 | 0.11 | 100% | 38% | 3.0E−07 | 2.4E−04 | |
| NAF | 6 (2,4,0) | 24, 39 | −0.61 | 0.13 | 100% | 0% | 3.9E−03 | 3.1E−02 | |
| EUR | 18 (4,8,6) | 24, 66 | −0.14 | 0.08 | 67% | 0% | 9.6E−02 | 2.4E−01 | |
| SWA | 19 (7,9,3) | 20, 74 | −0.8 | 0.17 | 84% | 32% | 1.7E−04 | 4.4E−03 | |
| SEA | 30 (5,25,0) | 13, 65 | −0.09 | 0.14 | 63% | 0% | 5.4E−01 | 2.0E−01 | |
| OCE | 16 (8,8,0) | 8, 29 | −0.37 | 0.21 | 75% | 0% | 9.0E−02 | 7.7E−02 | |
| AUS | 4 (0,4,0) | 8, 12 | −0.42 | 0.45 | 75% | 0% | 3.6E−01 | 6.2E−01 | |
| NEA | 18 (9,9,0) | 22, 119 | −0.24 | 0.3 | 78% | 0% | 4.3E−01 | 3.1E−02 | |
| NAM | 9 (1,8,0) | 12, 34 | −0.28 | 0.31 | 78% | 0% | 3.7E−01 | 1.8E−01 | |
| SAM | 10 (6,3,1) | 9, 30 | −0.55 | 0.26 | 70% | 10% | 5.1E−02 | 3.4E−01 | |
| OTH | 11 (3,6,2) | 29, 63 | −0.66 | 0.24 | 82% | 27% | 1.5E−02 | 6.5E−02 | |
| ALL | 154 (49,93,12) | 18, 165 | −0.42 | 0.07 | 77% | 10% | 1.7E−09 | 2.2E−11 | |
| SSA | 10 (2,8,0) | 20, 35 | −1.06 | 0.13 | 100% | 40% | 1.6E−05 | 2.0E−03 | |
| NAF | 5 (3,2,0) | 17, 24 | −0.99 | 0.21 | 100% | 40% | 6.6E−03 | 6.2E−02 | |
| EUR | 11 (2,6,3) | 20, 33 | −1.21 | 0.06 | 100% | 55% | 7.1E−10 | 9.8E−04 | |
| SWA | 16 (5,7,4) | 20, 57 | −0.82 | 0.13 | 94% | 19% | 1.4E−05 | 5.2E−04 | |
| SEA | 25 (3,21,1) | 14, 46 | −0.89 | 0.14 | 88% | 20% | 1.2E−06 | 1.6E−04 | |
| OCE | 12 (8,4,0) | 11, 28 | −0.71 | 0.2 | 92% | 25% | 3.7E−03 | 6.3E−03 | |
| AUS | 4 (0,4,0) | 10, 16 | −1.04 | 0.2 | 100% | 25% | 8.8E−03 | 1.2E−01 | |
| NEA | 12 (8,4,0) | 19, 42 | −1.33 | 0.07 | 100% | 75% | 6.7E−10 | 4.9E−04 | |
| NAM | 7 (1,6,0) | 14, 30 | −0.51 | 0.26 | 71% | 14% | 7.6E−02 | 4.5E−01 | |
| SAM | 5 (2,3,0) | 6, 10 | −0.89 | 0.32 | 80% | 20% | 3.5E−02 | 3.8E−01 | |
| OTH | 7 (1,4,2) | 24, 44 | −0.91 | 0.15 | 100% | 29% | 6.1E−04 | 1.6E−02 | |
| ALL | 114 (35,69,10) | 16, 77 | −0.94 | 0.05 | 93% | 32% | 1.1E−35 | 5.7E−23 | |
| SSA | 13 (4,9,0) | 36, 108 | −0.97 | 0.11 | 100% | 23% | 6.1E−07 | 2.4E−04 | |
| NAF | 5 (1,4,0) | 30, 54 | −1.09 | 0.17 | 100% | 40% | 2.0E−03 | 6.2E−02 | |
| EUR | 19 (5,8,6) | 39, 120 | −0.75 | 0.12 | 89% | 21% | 7.0E−06 | 7.3E−04 | |
| SWA | 18 (4,9,5) | 29, 132 | −0.82 | 0.11 | 94% | 11% | 7.5E−07 | 1.4E−04 | |
| SEA | 30 (5,24,1) | 26, 128 | −0.46 | 0.14 | 80% | 7% | 1.9E−03 | 1.4E−03 | |
| OCE | 13 (8,5,0) | 17, 65 | −0.59 | 0.21 | 92% | 8% | 1.1E−02 | 3.4E−03 | |
| AUS | 4 (0,4,0) | 14, 27 | −0.72 | 0.27 | 100% | 25% | 5.4E−02 | 1.2E−01 | |
| NEA | 16 (9,7,0) | 36, 178 | −0.7 | 0.33 | 94% | 38% | 4.1E−02 | 5.2E−04 | |
| NAM | 10 (2,8,0) | 21, 79 | −0.27 | 0.17 | 70% | 0% | 1.2E−01 | 3.4E−01 | |
| SAM | 8 (4,3,1) | 22, 67 | −0.51 | 0.18 | 88% | 0% | 1.7E−02 | 7.0E−02 | |
| OTH | 10 (3,6,1) | 48, 117 | −0.97 | 0.11 | 100% | 20% | 5.1E−06 | 2.0E−03 | |
| ALL | 146 (45,87,14) | 30, 295 | −0.68 | 0.06 | 90% | 16% | 2.2E−23 | 2.7E−24 | |
| SSA | 9 (3,5,1) | 23, 68 | −0.62 | 0.18 | 89% | 22% | 5.7E−03 | 3.9E−02 | |
| NAF | 15 (5,9,1) | 27, 62 | −0.63 | 0.1 | 93% | 0% | 8.6E−06 | 9.8E−04 | |
| EUR | 55 (26,13,16) | 29, 110 | −0.76 | 0.06 | 93% | 7% | 2.3E−18 | 2.0E−11 | |
| SWA | 9 (4,5,0) | 25, 51 | −0.92 | 0.13 | 100% | 22% | 8.5E−05 | 3.9E−03 | |
| SEA | 39 (14,23,2) | 20, 76 | −0.88 | 0.08 | 90% | 23% | 2.2E−13 | 3.4E−07 | |
| OCE | 43 (37,6,0) | 13, 50 | −0.42 | 0.15 | 72% | 12% | 8.0E−03 | 5.4E−03 | |
| AUS | 2 (0,2,0) | 17, 24 | −0.15 | 0.88 | 50% | 0% | 8.5E−01 | 1.00E+00 | |
| NEA | 41 (31,10,0) | 24, 135 | −0.87 | 0.17 | 93% | 39% | 5.0E−06 | 1.0E−08 | |
| NAM | 14 (5,9,0) | 14, 44 | −0.32 | 0.21 | 79% | 7% | 1.3E−01 | 5.7E−02 | |
| SAM | 19 (8,7,4) | 13, 42 | −0.37 | 0.16 | 74% | 11% | 3.3E−02 | 6.4E−02 | |
| OTH | 12 (4,3,5) | 36, 66 | −1.25 | 0.06 | 100% | 50% | 4.9E−10 | 4.9E−04 | |
| ALL | 258 (137,92,29) | 22, 189 | −0.70 | 0.05 | 87% | 18% | 3.1E−37 | 1.7E−35 | |
| SSA | 10 (2,4,4) | 7, 8 | −1.33 | 0.05 | 100% | 50% | 2.7E−10 | 2.0E−03 | |
| NAF | 7 (2,5,0) | 7, 8 | −1.33 | 0.09 | 100% | 29% | 4.0E−06 | 1.6E−02 | |
| EUR | 61 (37,11,13) | 7, 8 | −1.4 | 0.04 | 100% | 61% | 1.5E−44 | 8.7E−19 | |
| SWA | 9 (4,2,3) | 7, 8 | −1.44 | 0.05 | 100% | 67% | 2.6E−09 | 3.9E−03 | |
| SEA | 7 (4,1,2) | 8, 8 | −1.48 | 0.04 | 100% | 100% | 2.3E−08 | 1.6E−02 | |
| OCE | 18 (11,4,3) | 6, 8 | −0.9 | 0.1 | 100% | 6% | 6.7E−08 | 7.6E−06 | |
| AUS | 2 (0,2,0) | 6, 7 | −1.44 | 0.19 | 100% | 50% | 6.0E−02 | 5.0E−01 | |
| NEA | 31 (22,5,4) | 7, 8 | −1.08 | 0.08 | 97% | 29% | 1.7E−14 | 3.0E−08 | |
| NAM | 15 (3,9,3) | 5, 7 | −0.96 | 0.12 | 93% | 0% | 8.4E−07 | 9.8E−04 | |
| SAM | 21 (8,7,6) | 5, 7 | −0.9 | 0.12 | 100% | 10% | 1.8E−07 | 9.5E−07 | |
| OTH | 10 (4,2,4) | 7, 8 | −1.46 | 0.06 | 100% | 80% | 1.4E−09 | 2.0E−03 | |
| ALL | 191 (97,52,42) | 7, 8 | −1.21 | 0.03 | 99% | 41% | 7.6E−96 | 1.2E−53 | |
| SSA | 8 (1,5,2) | 13, 19 | −0.95 | 0.13 | 100% | 12% | 1.3E−04 | 7.8E−03 | |
| NAF | 13 (5,8,0) | 14, 21 | −0.64 | 0.1 | 100% | 8% | 2.7E−05 | 2.4E−04 | |
| EUR | 54 (31,13,10) | 13, 19 | −1.08 | 0.04 | 100% | 33% | 9.1E−31 | 1.1E−16 | |
| SWA | 7 (3,3,1) | 13, 18 | −1.1 | 0.1 | 100% | 29% | 2.8E−05 | 1.6E−02 | |
| SEA | 22 (15,5,2) | 13, 24 | −1.04 | 0.11 | 95% | 50% | 2.1E−09 | 1.1E−05 | |
| OCE | 23 (17,6,0) | 10, 17 | −0.77 | 0.13 | 87% | 17% | 4.8E−06 | 4.9E−04 | |
| AUS | 2 (0,2,0) | 9, 12 | −0.78 | 0.12 | 100% | 0% | 6.8E−02 | 5.0E−01 | |
| NEA | 33 (24,6,3) | 11, 20 | −0.8 | 0.13 | 82% | 36% | 3.4E−07 | 3.2E−04 | |
| NAM | 13 (4,9,0) | 7, 17 | −0.41 | 0.17 | 69% | 0% | 2.5E−02 | 2.7E−01 | |
| SAM | 19 (10,7,2) | 6, 15 | −0.61 | 0.15 | 79% | 5% | 4.0E−04 | 1.9E−02 | |
| OTH | 8 (4,2,2) | 12, 17 | −1.11 | 0.06 | 100% | 38% | 3.0E−07 | 7.8E−03 | |
| ALL | 202 (114,66,22) | 11, 30 | −0.87 | 0.04 | 91% | 26% | 1.9E−58 | 7.7E−36 | |
| SSA | 7 (6,1,0) | 5, 8 | −1.36 | 0.12 | 100% | 29% | 1.8E−05 | 1.6E−02 | |
| NAF | 0 (0,0,0) | NA | NA | NA | NA | NA | NA | NA | |
| EUR | 10 (9,1,0) | 3, 7 | −0.09 | 0.26 | 60% | 0% | 7.1E−01 | 7.5E−01 | |
| SWA | 1 (1,0,0) | 5, 5 | −0.48 | NA | 100% | 0% | NA | NA | |
| SEA | 2 (2,0,0) | 4, 5 | −1.02 | 0.68 | 100% | 0% | 2.8E−01 | 5.0E−01 | |
| OCE | 10 (4,5,1) | 4, 5 | −0.67 | 0.17 | 80% | 0% | 2.4E−03 | 1.1E−01 | |
| AUS | 0 (0,0,0) | NA | NA | NA | NA | NA | NA | NA | |
| NEA | 4 (1,2,1) | 4, 5 | −0.6 | 0.47 | 75% | 0% | 2.4E−01 | 6.2E−01 | |
| NAM | 5 (0,2,3) | 3, 4 | 0.93 | 0.07 | 0% | 0% | 1.2E−04 | 6.2E−02 | |
| SAM | 5 (4,1,0) | 2, 3 | −1.06 | 0.14 | 100% | 0% | 1.2E−03 | 6.2E−02 | |
| OTH | 1 (1,0,0) | 5, 5 | −1.27 | NA | 100% | 0% | NA | NA | |
| ALL | 45 (28,12,5) | 4, 9 | −0.53 | 0.13 | 73% | 4% | 1.1E−04 | 2.5E−03 | |
| SSA | 13 (8,3,2) | 16, 43 | 0.13 | 0.35 | 69% | 8% | 7.1E−01 | 2.7E−01 | |
| NAF | 3 (2,1,0) | 17, 22 | −0.38 | 0.17 | 100% | 0% | 1.2E−01 | 2.5E−01 | |
| EUR | 41 (32,5,4) | 16, 52 | 0.29 | 0.09 | 32% | 0% | 1.6E−03 | 2.8E−02 | |
| SWA | 3 (2,1,0) | 15, 26 | 0.77 | 0.44 | 0% | 0% | 1.6E−01 | 2.5E−01 | |
| SEA | 17 (15,2,0) | 14, 43 | −0.08 | 0.23 | 59% | 6% | 7.4E−01 | 6.3E−01 | |
| OCE | 20 (14,5,1) | 9, 31 | 0.02 | 0.19 | 60% | 0% | 9.1E−01 | 5.0E−01 | |
| AUS | 2 (0,2,0) | 8, 10 | −0.05 | 0.31 | 50% | 0% | 8.5E−01 | 1.00E+00 | |
| NEA | 13 (5,5,3) | 12, 25 | −0.53 | 0.09 | 92% | 0% | 7.6E−05 | 3.4E−03 | |
| NAM | 7 (0,7,0) | 7, 14 | 0.56 | 0.51 | 43% | 0% | 2.8E−01 | 1.00E+00 | |
| SAM | 21 (16,4,1) | 5, 19 | −0.39 | 0.21 | 67% | 5% | 7.9E−02 | 1.9E−01 | |
| OTH | 7 (6,1,0) | 17, 25 | −0.09 | 0.2 | 57% | 0% | 6.4E−01 | 1.00E+00 | |
| ALL | 147 (100,36,11) | 12, 67 | 0.01 | 0.07 | 55% | 2% | 8.5E−01 | 2.5E−01 | |
See text for three-letter abbreviations of world regions. ALL indicates all populations.
m: Total number of populations sampled (ml: number of populations from the literature dataset; mw: number of populations from the workshop dataset; ma:number of populations from the AlleleFrequencies.net dataset).
k̅ : Average number of observed distinct alleles in each region. ksum: Total number of observed distinct alleles across all populations in an entire region. A “distinct allele” is defined as having a distinct peptide sequence across exons 2 and 3 (for class I) or exon 2 (for class II). For comparison, by this definition, the total number of known distinct alleles in release 2.18 of the IMGT/HLA database is as follows: A, 481; C, 250; B, 806; DRB1, 418; DQA1, 14; DQB1, 63; DPA1, 14; DPB1, 113.
Standard error of mean Fnd estimate.
Percentage of populations with a negative Fnd value.
Percentage of populations with a significantly negative Fnd value, as tested by Slatkin’s implementation of the Monte Carlo approximation of the Ewens-Watterson exact test, using a two-tailed test (p < 0.05) of the null hypothesis of neutrality.
p-values less than 0.01 are in boldface.
Figure 2. Population samples plotted on global map.
(a) Populations with class I HLA data; (b) Populations with class II HLA data. Circle size is relative to 2N sample size. Black symbols are native populations, which are assigned to the geographic region in which they were sampled. White symbols are populations that have migrated in the last 1000 years; when considered to be admixed, these populations are assigned to the OTH category, otherwise they are assigned to their region of origin (see Subjects and methods for details).
In total, there are 416 population samples with class II HLA data and 171 population samples with class I HLA data. Whereas data for fewer population samples and sampled individuals are available for the class I loci, more populations have data across multiple class I loci than across multiple class II loci; the mean number of class I loci typed per population with class I data is 2.43, whereas the mean number of class II loci typed per population with class II data is 2.03. Thus, although there are fewer class I data overall, interlocus comparisons are more likely to pertain to the same population samples for class I than for class II.
Number of distinct alleles (k)
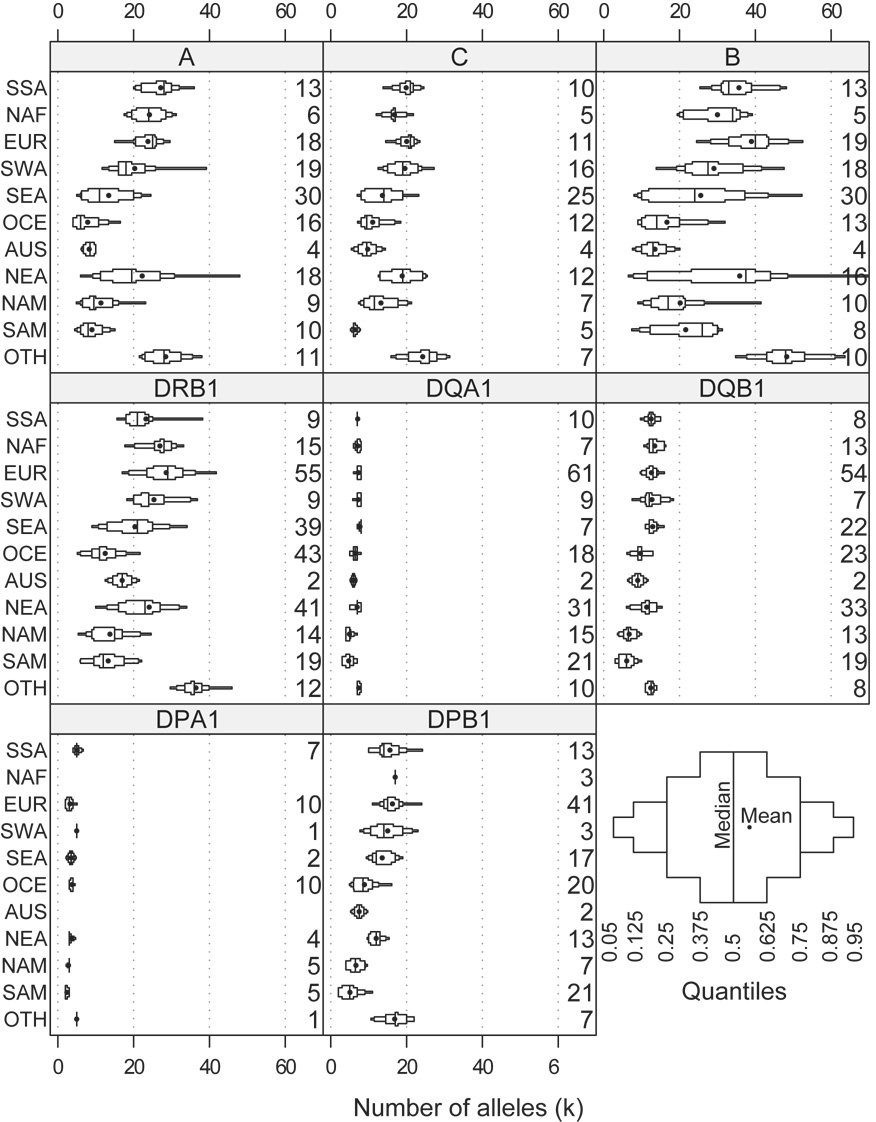
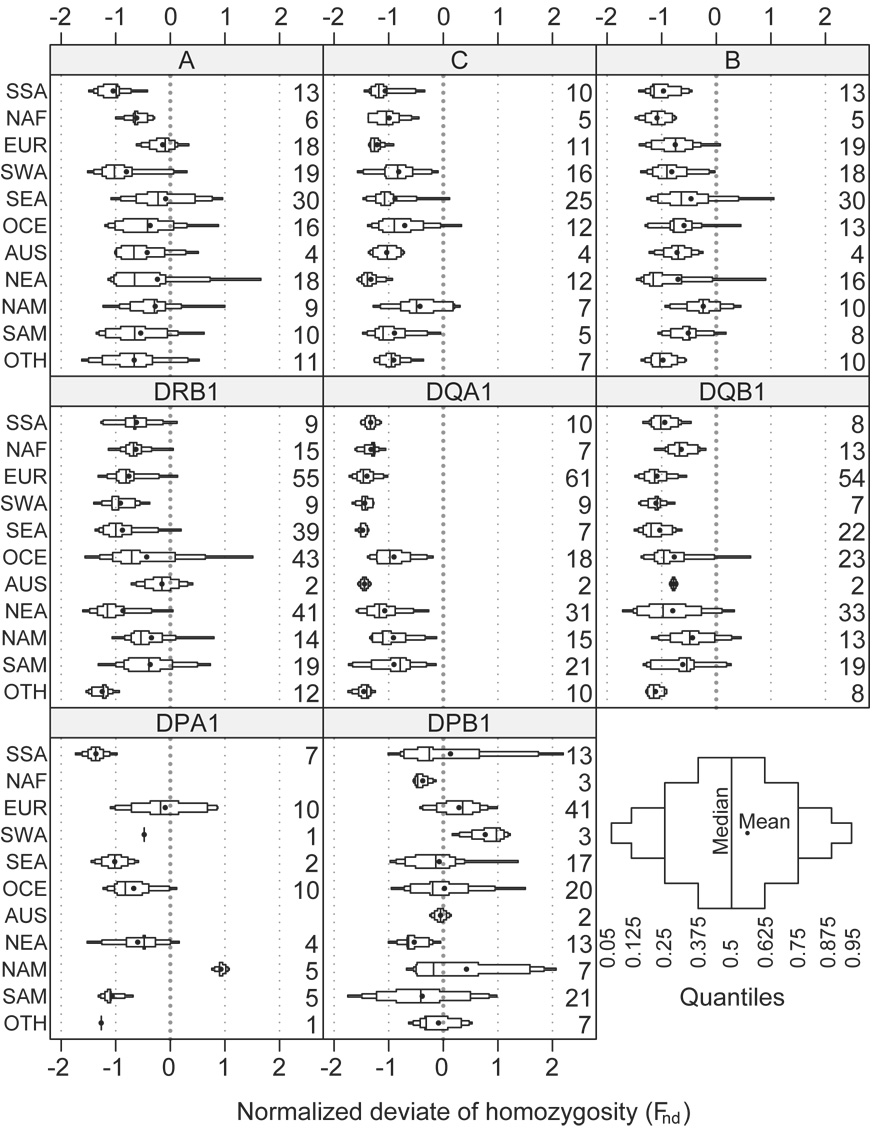
The number of distinct four-digit alleles was counted for each population and locus. The mean number of alleles (k̅) is shown in Table 1, tabulated by locus, by world region, and over all regions. The distribution of k in each region is shown graphically in Figure 3. In general, the geographic regions SSA, NAF, EUR, SWA, and NEA have the highest values of k across all HLA loci compared to other world regions. However, the non-geographic grouping OTH, which includes populations that are significantly admixed, with ancestry deriving from two or more geographic regions, often has even more alleles, as expected; this is especially prominent at HLA-B and -DRB1. Compared with the other loci, DQA1 and DQB1 have the smallest variance in k, while HLA-B has the highest variance.
Figure 3. Modified box plot illustrating the distribution of allele number (k) by locus and region.
The median value is represented by the middle vertical line and the mean value by the point. The number on the right of each subplot represents the number of populations for each locus and region combination. The panel in the bottom right illustrates the interpretation of quantiles.
Of course, the number of alleles detected in a given population (k) strongly depends upon its sample size (2n). However, this meta-analysis was restricted to sample sizes of 2n ≥ 40 chromosomes. Furthermore, the overall mean sample size for the datasets presented here was 256 chromosomes, and minimum regional mean (for OCE) was 128 chromosomes. At this sampling level, many loci do not exhibit any k versus 2n dependency. However, the highly polymorphic loci HLA-B and -DRB1 do exhibit a slight relationship. This may bias the k̅ estimate for OCE at these loci.
Number of alleles versus year of publication
We also examined the relationship between k, the year of initial identification of each allele, and the year of publication of each dataset. We combined recent (i.e., since 2002) population samples from within selected regions (EUR, SEA, and Africa [SSA+NAF]) in order to estimate regional allele frequencies and ranked these regional frequencies by the year in which each identified allele was first described. Within each of these regions, approximately 95% of regionally prevalent (i.e., allele frequency > 0.02) alleles had already been described by 1990 for class II and by 1995 for class I, with an average of 99.7% of the reported alleles falling into the “Common and Well Documented” (CWD) allele category of Cano et al. [213] (details are available at http://www.pypop.org/popdata/). Given the dramatic increases in the numbers of novel alleles that have been identified in the last seven years, it might be expected that datasets published in the 1990s would be deficient in terms of the number of distinct alleles identified when compared with more recently published datasets, potentially confounding meaningful comparative analyses. However, when values of k in datasets published in the 1990s are compared with datasets published in the 2000s, and when alleles identified in each group of datasets are assigned to CWD or non-CWD categories, it becomes clear that the overwhelming majority of recently reported population-level allelic diversity is consistent with that reported in the 1990s, suggesting that population samples from both time periods are comparable.
Observed homozygosity (Fobs)
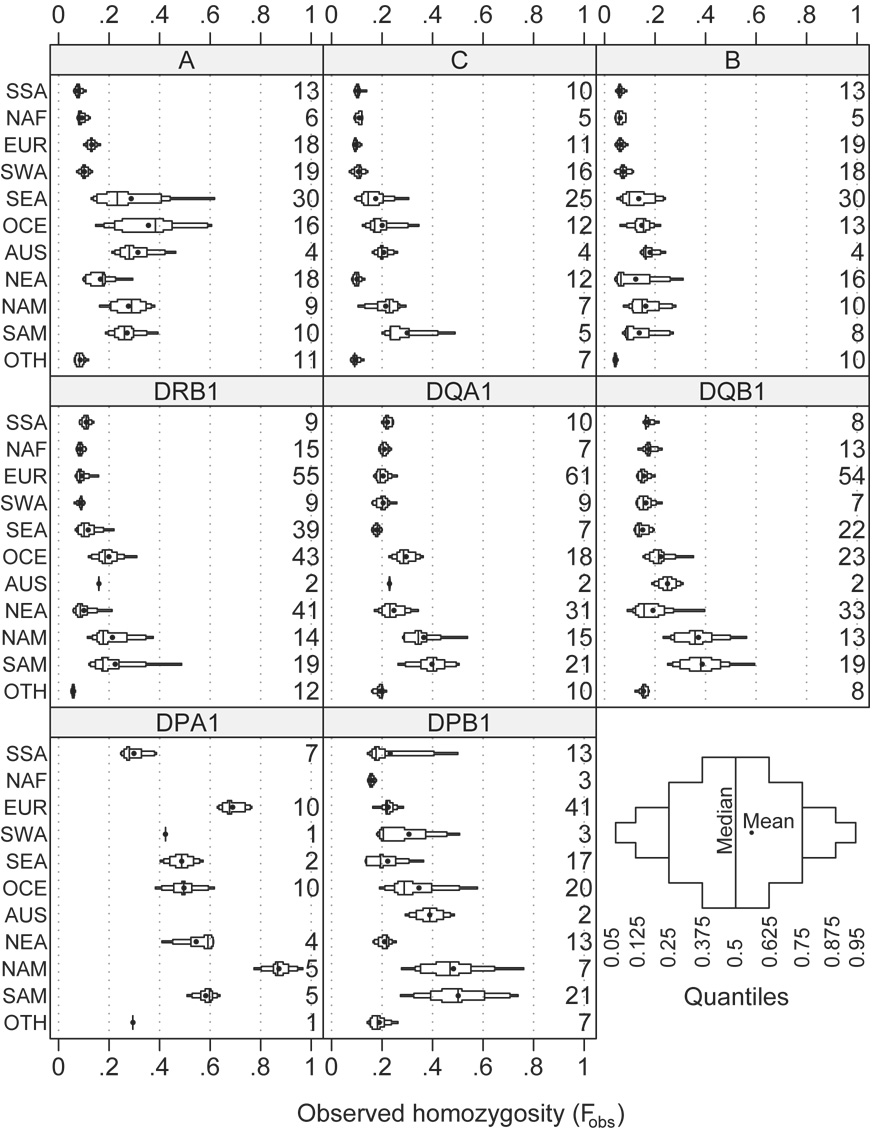
The distribution of the observed homozygosity (Fobs) is illustrated in Figure 4. Generally, Fobs is inversely proportional to k, as expected: the lowest Fobs values are found in SSA, NAF, EUR, SWA, NEA, and OTH, whereas the highest Fobs values are often found in NAM and SAM. The variance of Fobs tends to be much smaller than that of k (e.g., compare these two distributions for SSA at the B locus.) This is because the difference in k in these diverse populations is primarily due to low-frequency alleles that have little effect on Fobs.
Figure 4. Modified box plot illustrating the distribution of observed homozygosity (Fobs) by locus and region.
For an explanation of the plot elements, see the legend to Figure 3.
Normalized deviate of homozygosity (Fnd)
For each population, the normalized deviate of homozygosity (Fnd) and the corresponding two-sided p-value was calculated. Negative Fnd values result when Fobs has lower than expected homozygosity (Fexp). Significantly negative Fnd values imply balancing selection and/or high levels of gene flow, whereas significantly positive values imply directional selection and/or extreme demographic effects as a result of genetic drift.
The mean Fnd () values, tabulated by locus and geographic region, are illustrated in Table 1. is negative in all geographic regions for loci A, C, B, DRB1, DQA1, and DQB1. Only DPA1 and DPB1 show positive values in some regions; DPB1 in particular has values very close to zero for most regions. The distribution of Fnd is illustrated in Figure 5. DQA1 displays the strongest evidence for balancing selection, and the least variance in Fnd, while the DPB1 Fnd distribution is compatible with neutral expectations and displays the greatest variance both within and between regions.
Figure 5. Modified box plot illustrating the distribution of the normalized deviate of homozygosity (Fnd) by locus and region.
For an explanation of the plot elements, see the legend to Figure 3.
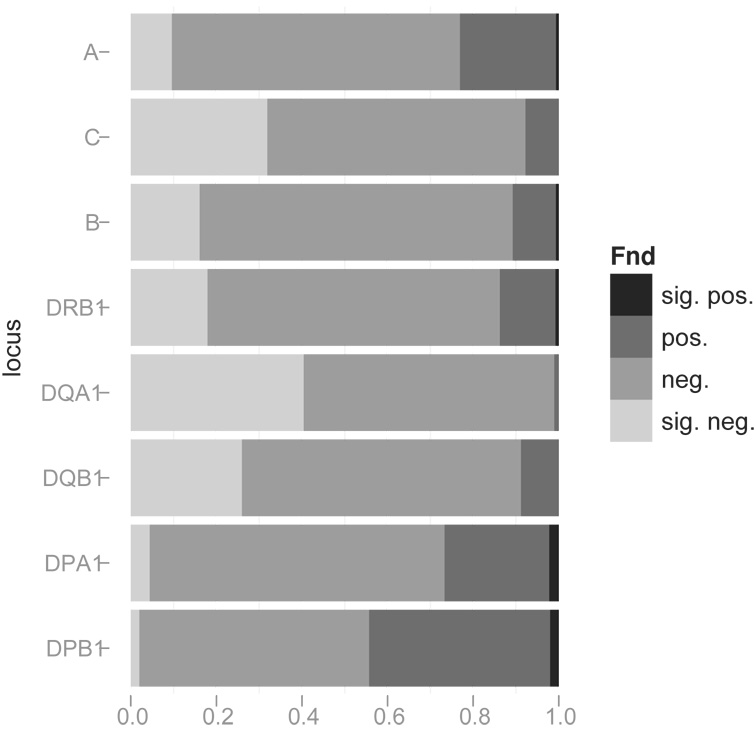
The percentage of populations with negative (Fnd < 0) and significantly negative (Fnd ≪ 0) values of Fnd is summarized in Table 1 (by locus, by region, and over all regions, and including statistical significance) and illustrated in Figure 5 (by locus and region) and in Figure 6 (by locus and indicating significance across all regions and including significantly positive Fnd results). Loci DQA1, C, and DQB1, in that order, have the greatest percentage of populations with significantly negative Fnd values, whereas DPA1 and DPB1 have the lowest percentage.
Figure 6. Bar plot of Fnd results.
Shading indicates proportions of populations with significantly negative (suggestive of balancing selection and/or gene flow), negative, positive, and significantly positive (suggestive of directional selection and/or genetic drift) values of the normalized deviate of homozygosity (Fnd), as tested by Slatkin’s implementation of the Monte Carlo approximation of the Ewens-Watterson exact test, using a two-tailed test (p < 0.05) of the null hypothesis of neutrality.
The distribution of Fnd values may also serve as the basis for statistical tests. p-values for the t-test (based on and the central limit theorem) and the sign test (based on the relative proportions of negative and positive Fnd values) are presented in Table 1 by locus and geographic region. In most cases, the t-test and the sign test are in fairly close agreement, across regions and overall, although the t-test has more statistical power. Using both tests, significantly negative values (or Fnd distributions) are found at class I loci B and C and at class II loci DQA1, DQB1, and DRB1 in the majority of world regions (p ≪ 0.01, indicated in boldface in Table 1). The other loci, A, DPA1 and DPB1, fail to demonstrate significance for most world regions. This lack of significance may be due to Fnd distributions that are close to the neutral expectation of zero, as is the case for A and DPB1, or it may be due to the low power of these statistical tests at loci with few population samples, as in the case for DPA1.
When all world regions are considered together, all HLA loci except DPB1 and DPA1 demonstrate highly significantly negative values (or Fnd distributions) by both the t-test and sign test (p < 10−8; see Table 1). DPA1 also demonstrates signs of balancing selection, despite the small number of population samples (p < 10−4, t-test). DPB1, however, is strikingly compatible with neutral expectations.
Ranking the relative strengths of selection across loci
To better understand the relative strength of balancing selection (as evidenced by the Fnd statistic), the distribution of Fnd values between each pair of loci was examined with a permutation test. The resulting relative rank order of values is DQA1 ≪ C < DQB1 ≪ DRB1 < B ≪ A ≪ DPB1, where ≪ indicates a significant difference between (p<0.01, asymptotic 2-sample permutation test). DPA1 has a value between that of HLA-B and A, but it is not included because the small number of populations sampled at this locus minimizes the statistical power of the permutation test.
With respect to the strength of selection evident among the class I and class II loci, the differences cannot be solely attributed to k, the number of alleles per locus; the B and DRB1 loci are the most polymorphic, yet they fall in the middle of the rank order of strength of selection.
Regional differences of Fnd
Considering the results by region, SSA, EUR, SWA, NEA, and OTH have the greatest number of loci (six) with significantly negative values (p < 0.01, t-test and sign test). This cannot be solely attributed to the number of population samples per region: SEA and OCE, for example, are also well-sampled regions.
NAM, on the other hand, shows a trend of values elevated relative to other regions, at most loci. Very few NAM populations have significantly negative Fnd values. The most striking example is DPA1, where NAM has a significantly positive Fnd distribution (p ≤ 0.0001, t-test).
The overall (“ALL”) summary for each locus was calculated by averaging across all population samples in all regions (Table 1). We also calculated averages of the regional averages, and the results were nearly identical to that observed using the overall averaging method (results not shown).
Genetic differentiation within and between regions
To compare the degree of differentiation in allele frequency distribution in areas that have been densely sampled to the degree of differentiation over an entire region, we calculated the fraction of the overall mean Nei’s standard genetic distance for populations in each region for which data were available for both loci in all pairs of loci. In addition, we calculated these fractions for several densely sampled and well-defined subregions. Data for 13 aboriginal populations from Taiwan (TWN) were available for the class I and DRB1 loci; three of these populations are admixed and 10 are aboriginal; the set of all 13 populations is denoted as SEA-TWN, and the set of 10 populations is denoted as TWN. Data for eight populations from Papua New Guinea (OCE-PNG) were available for the class I loci, and data for four were available for the class I loci. These were further subdivided into PNG Highland (PNGH) and PNG Lowland (PNGL) categories.
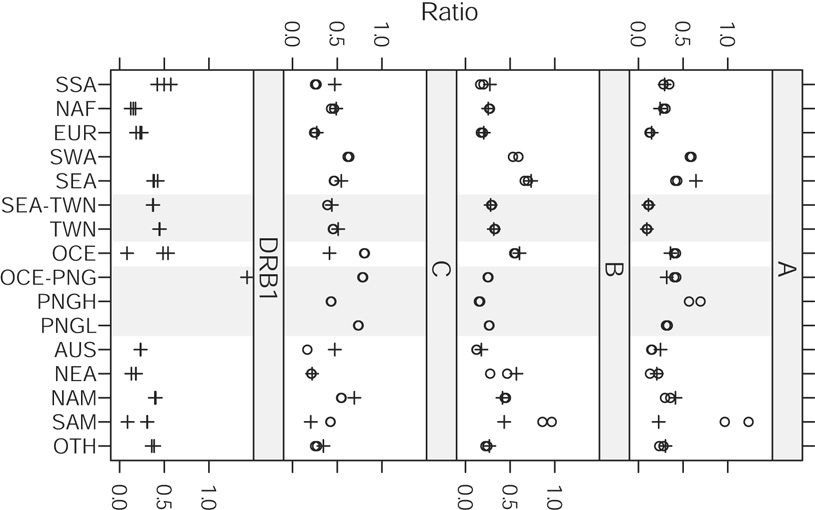
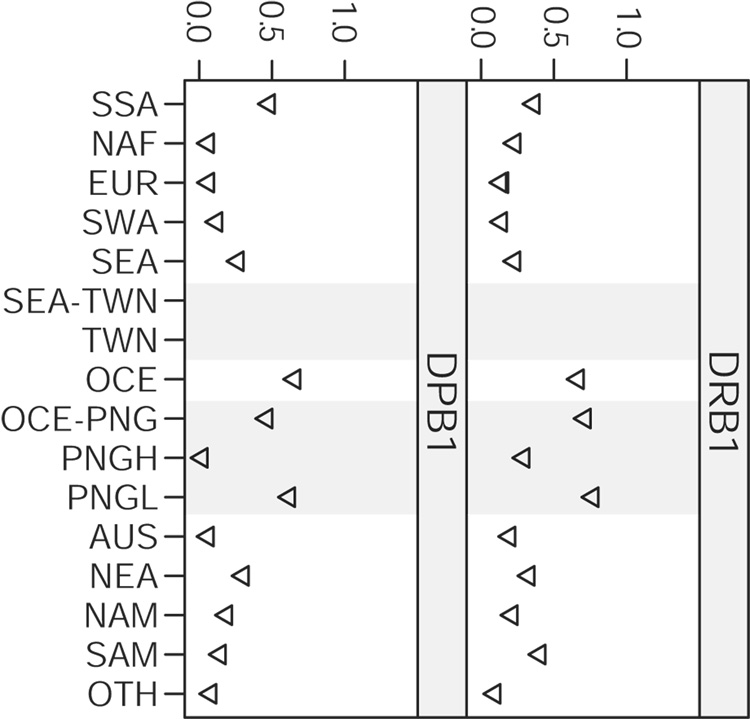
These fractions are shown in Figure 7 for comparisons between pairs of class I loci and between class I loci and the DRB1 locus, and in Figure 8 for comparisons between the DRB1 and DPB1 loci. More than 100 populations were shared among the class I loci (135 between A and B, and 105 between both A and C and B and C), whereas fewer were shared in comparisons of the class I loci with DRB1 (79 between A and DRB1, 74 between B and DRB1, and 61 between C and DRB1), and 66 were shared between DRB1 and DPB1. Very few populations were shared in comparisons between the class I loci and the DPB1 locus (26 between A and DPB1, 20 between B and DPB1, and 18 between C and DPB1) and these comparisons are not presented. It follows that there will be little overlap in the set of populations common to the DRB1 and DPB1 loci and the sets of common populations in comparison between the various class I and DRB1 loci; because of this, the figures for these comparisons are presented separately.
Figure 7. Genetic differentiation present in world regions and subregions.
Comparisons of the same subsets of populations are illustrated with circles (intraclass I) or crosses (class I - DRB1).
Figure 8. Genetic differentiation present in world regions and subregions.
The regional fraction of the overall Nei’s standard genetic distance is greater than 1 in some cases (e.g., SAM at HLA-A and OCE-PNG at DRB1) as a result of the extremely large degree of differentiation between populations in these regions at these loci. Many of the values for a given region are the same or very similar for the intraclass I loci comparisons, as a result of HLA-A, -C, and -B data being available for the same population in the largest number of cases.
As has been noted previously [5, 7], the degree of differentiation for SSA, NAF, and EUR populations is generally lower than that for SEA, OCE, NAM and SAM populations, especially at the class I loci. Here, we can see that the degree of differentiation in densely sampled subregions is comparable in many cases with (or even greater than) that for SSA, NAF, and EUR. In particular, the 10 non-admixed aboriginal Taiwanese populations demonstrate greater differentiation than SSA, NAF, and EUR at the HLA-B and –C loci and greater differentiation than NAF and EUR at the DRB1 locus. At the DRB1 and HLA-C loci, TWN (which constitutes approximately half of all SEA populations in these comparisons) displays levels of differentiation that are comparable to SEA. Similar patterns are seen for the PNGH and PNGL subregions at all loci. In most instances, differentiation in PNGH is observed at levels comparable with entire regions. At the HLA-A locus, PNGH populations display greater differentiation than any region other than SAM, and at HLA-B, PNGL populations display greater differentiation than any region other than SEA.
Discussion
The large quantity and broad scope of data available in published papers are the motivation for this meta-analysis of HLA allele-count data. Although working with these data poses challenges typical of meta-analytic studies (e.g. data entry errors and problems inherent in making comparisons between studies published over 17 years), such challenges have been minimized in the current study through the combination of human error-checking of the primary data and the use of PyPop to validate and bin allele names to analytically relevant common denominators. The literature-based data compiled here are available for public download and may prove useful for future studies of anthropology and HLA diversity, transplant registry analyses, and the validation of control populations in disease studies.
The large number of populations included here provides an opportunity to compare the distributions of summary statistics of allele frequencies for populations from different regions of the world. Although some of the results here are similar to those of previous meta-analyses (discussed below), the breadth and depth of the datasets assembled for this study permit statistical tests among some genetic loci and world regions that may not have been previously possible. In terms of typing schemes, sample sizes, and perhaps quality control, there is a fair amount of heterogeneity between and among the three meta-datasets assembled for this study. However, there were no substantive differences in allelic diversity among them when compared by region and locus. Certain regions of the world (e.g., EUR and SEA) are better represented than others, which might bias the overall average summary statistics presented in Table 1. However, when the overall averages were compared with the average of regional averages, results were nearly identical.
In light of the ever-growing list of known HLA alleles (a list that continues to grow dramatically every year), the lack of correlation between k and dataset year is perhaps a surprising result. However, the alleles identified in recent years tend to be very-low-frequency (and even unique) alleles and so do not greatly affect the results of older studies. This, in turn, suggests that the bulk of recently detected allelic polymorphism may not significantly affect meta-analyses of HLA diversity spanning decades because the vast majority of alleles present in most populations had been described by 1995 for class I loci and by 1990 for class II loci. However, an understanding of the distribution of these low-frequency and rare alleles is still important for disease studies and for determining the chance of finding a suitable transplant donor.
The EW homozygosity test of neutrality has proven to be a useful tool for detecting selection at HLA loci [3, 7, 209, 218, 219, 222–231], making it an appropriate method for these meta-analyses. This meta analysis reveals an extremely strong signal of balancing selection at DQA1, HLA-C, and DQB1. The result for HLA-C is interesting because comparative population genetic data at this locus have been scarce until recently; allele-level HLA class II typing methods were developed much earlier than those for class I, and HLA-C genotyping is still less common than -A and -B typing. Data for DPA1, the least typed of the eight classical loci, are also collected here in quantity for the first time, although there are insufficient population samples at this locus to draw conclusions with high statistical power.
It is important to keep in mind that just because the EW test does not demonstrate evidence of balancing selection (e.g., at DPB1), this does not mean selection is not operating. At the same time, the strikingly different patterns observed between different loci (e.g., DPB1 compared with other loci) cannot be explained by demographic forces alone. Further, the consistent and differing patterns between the loci argue for a major role of selection at these loci, combined, of course, with demographic effects.
The observation that evidence for balancing selection in this study is strongest at the DQA1 locus is interesting. Although the DQA1 and DQB1 loci display low levels of diversity relative to extremely polymorphic loci like DRB1, it should be noted that the diversity of DQ proteins may be comparable to that for DR proteins. The seven DQA1 alleles and 11 DQB1 alleles observed on average in each population can form potentially 77 distinct DQ proteins at the population level (although not all DQA1-DQB1 combinations will result in functional molecules). In contrast, the 22 DRB1 alleles observed on average in each population can form, at most, 44 distinct DR proteins, because there are only two unique DRA alleles to form the alpha subunit. The strong balancing selection seen at the DQA1 and DQB1 loci may reflect selection for increased diversity of DQ proteins, as opposed to individual DQA1 or DQB1 alleles. Although balancing selection is clearly at work at the DRB1 locus, this locus may be under stronger selection for allelic diversity than for protein diversity.
Locus-level comparisons with previous studies
A subset of the literature data presented here was previously analyzed by Tsai and Thomson [219]. This analysis, encompassing approximately half of the present literature dataset, provided a tantalizing picture of locus-to-locus variation and a convincing argument for the value of meta-analysis of literature data. As in the current study, the authors reported the greatest evidence for balancing selection at DQA1, followed by DQB1, DRB1, B and A, with very little evidence at DPA1 and DPB1. Sanchez-Mazas [7] also recently performed EW tests using the data from the 12th and 13th IHW. The present results are compatible with that study, in which the DPB1 locus did not exhibit any sign of balancing selection, HLA-A exhibited almost no evidence, and DQA1 exhibited the strongest evidence, followed by HLA-C (according to the percentage of populations demonstrating significant rejection). The current analysis is able to extend the results of these two analyses because of the large number of additional studies considered. As such, there is now adequate data to make useful comparisons at HLA-C and also to stratify the results by world regions.
Meyer et al. [3] examined class I loci in 20 populations and class II loci in 17 populations (all of which are included in the workshop dataset of the current study), chosen to represent a global sampling of human populations. The values presented here are very similar at all loci except DRB1 (Meyer et al. find , not significant, as compared to −0.70, significant [p = 3.1*10−37, t-test], in the present study.) The difference is most likely due to the smaller number of populations being averaged and the greater influence of outlier populations such as East Timorese and Guarani-Nandewa in the Meyer et al. study. Although we also observe several exceptionally high DRB1 Fnd values among populations from OCE, NAM, and SAM, the bulk of these regional distributions lies more or less in line with other world regions, with the preponderance of populations having negative Fnd values (Figure 5).
Salamon et al. [218] examined three class II loci (DRB1 and DQB1 in 23 populations and DPB1 in 14 populations) using data from published literature and found the most negative at DQB1 ( compared to −0.88 in the present study [both results significant, p ≤ 0.0001, t-test]). However, differences with the present study are again seen at DRB1 (, not significant, compared to −0.70, significant, in the present study) and also at DPB1 ( compared to 0.01 in the present study, although neither significantly different from zero). Again, the differences can be explained by the small number of populations being averaged and the correspondingly large influence of outliers (such as the Javanese and Indonesian populations) in the Salamon et al. study. Mack et al. [4] concluded that the high Fnd values observed in these OCE outlier populations were the result of selection in response to a local pathogen. Valdes et al. [226] examined variation of class II loci, considered at the locus and amino acid level, in 22 populations from the 12th IHW, with similar results to those of the Salamon et al. study.
On a smaller scale, Hedrick and Thomson [222] examined HLA-A and -B, using serological data (i.e., two-digit allele names) in 22 populations, most of which were European or of European descent. They found 11 populations at HLA-A and 14 at HLA-B to have significantly lower homozygosity than expected under neutrality. Although the present study reported less evidence for balancing selection at HLA-A, when we reanalyzed the data at the two-digit level (results not shown), the results were closer to those of Hedrick and Thomson [222]. Indeed, most loci show more negative Fnd values when analyzed at the two-digit level versus the four-digit (amino acid) level.
Regional comparisons
The results presented here agree with the observation that relatively high genetic diversity (i.e., lowest Fobs values) is commonly found among African populations [232]. High diversity of class I loci in NEA populations was also recently described [3], but there were insufficient data to draw conclusions at class II loci. NEA populations are particularly well-represented among the literature datasets, and we observe mean Fobs for NEA populations similar to the low values found in SSA and EUR, especially at class II loci.
OCE populations appear to have a greater range of Fnd values at DRB1 than other loci and regions. This result may be due in part to the large number of OCE populations included in the literature dataset at this locus, but likely also reflects heterogeneity in the demographic histories of the islands that constitute this region, because the OCE populations included in this meta-analysis extend from Indonesia across the Pacific into Polynesia (see Figure 1). Populations in western OCE might be expected to display allele spectra similar to SEA populations, whereas Polynesian populations in eastern OCE are likely to have experienced successive founder effects over the last 1000–1500 years, as the Polynesian islands were discovered and colonized.
For individual populations, comparisons of Fnd results are not always in concert across different loci. For example, a Javanese population has a conspicuously high Fnd of +2.94 at DRB1 (p < 0.05, two-tailed EW test of neutrality). However, the Fnd value at the DPB1 locus in this same Javanese population is negative (−0.6769) and consistent with neutral expectations. Similarly, the of NAM at DPA1 (m=5 populations) is far above the mean for the rest of the world (0.93 versus −0.72). Although NAM has among the highest across other loci as well, the result for DPA1 are exceptionally elevated relative to the rest of the world.
It is difficult to disentangle the complex interplay of selection, demography, and genetic linkage creating these patterns. Population bottlenecks may be responsible for the elevated Fnd values seen at some loci in isolated populations, even while those same populations maintain a large part of their ancestral diversity at other HLA loci, presumably as a result of the influence of balancing selection, linkage disequilibrium, or a combination of these two forces. The ability of balancing selection to maintain diversity in MHC genes despite the loss of genetic diversity in other genes during a population bottleneck has been described in another mammalian species [233].
Mack and Erlich [5] and Sanchez-Mazas et al. [234] noted a high level of differentiation in the HLA-B and -C allelic frequency distributions of SEA populations, and in particular of aboriginal Taiwanese populations (relative to other regions of the world). Mack and Erlich [5] speculated that densely sampled populations in other areas of the world might display comparable levels of differentiation. This has significance for anthropology and diversity studies, as well as applications that rely on such studies (e.g., selecting a control population for a disease association study or selecting a target population for transplant registry donor recruitment) as it challenges the assumption that a broad, diffuse sampling of populations is sufficient to obtain an adequate understanding of the global distribution of HLA polymorphism.
To test this, we calculated the mean pairwise Nei’s standard genetic distances at the HLA-A, -B, -C, -DRB1, and -DPB1 loci for populations in each global region, as well as for aboriginal Taiwanese populations and populations from Papua New Guinea. These comparisons demonstrate considerable differentiation among populations from relatively small areas. The area of Taiwan is 35,801 square kilometers, and the area of Papua New Guinea is 462,840 square kilometers (although the individual Highland and Lowland regions are necessarily smaller). In comparison, Europe has an area of more than 10 million square kilometers, and Africa over 30 million. It may be the case that these island populations have been isolated from one-another for considerable time, but if the pattern of significant differentiation in populations from relatively small areas proves to be the norm around the globe, then higher density sampling methods may be required in order to more accurately assess global patterns of polymorphism distribution.
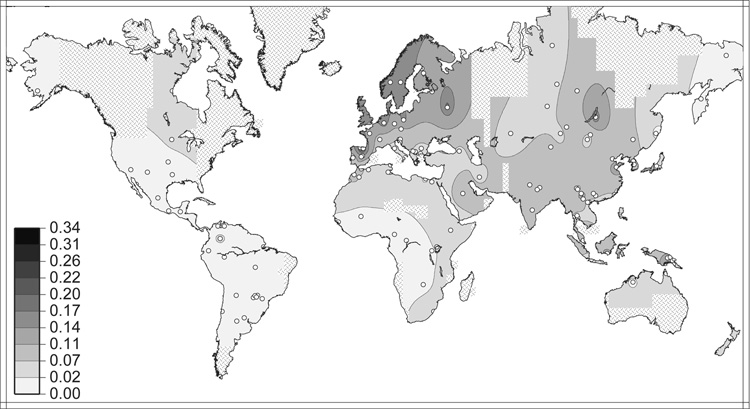
The meta-dataset presented in the current study can be used to estimate the geographic distribution for each allele. Figure 9 shows the frequency distribution of the DRB1*1501 allele, as interpolated from 225 non-migrant populations. (Additional maps for each observed HLA allele at all eight classical loci are available online at http://www.pypop.org/popdata.) These maps are valuable as first approximations of the global distributions of common alleles. However, they should not be overinterpreted; the frequency clines were generated from the primary data by an algorithm that does not take into account geographic barriers (cf., discussion by Liu et al. [235]). It is also important to note that these maps are constructed from allele frequency data, not summary measures (e.g., principal components), which are often used to map geographic trends between populations. However, as such, these maps still provide a useful allele frequency reference tool and also allow some interesting comparisons with previously described population genetic patterns.
Figure 9. Global frequency distribution of the DRB1*1501 allele.
Distribution was inferred from 224 population samples, indicated by the plotted points. The shaded regions indicate interpolated allele frequency; crosshatched areas are too far from a sampled population to permit meaningful frequency estimation. Only non-migrant populations were used to generate this map. Frequency maps for all alleles at all loci are available at http://www.pypop.org/popdata/.
For example, recent studies have investigated clines in allele frequencies for KIR and their HLA ligands [236, 237] as well as clines in genetic diversity for non-MHC data [238, 239]. Single et al. [237] observed a decreasing trend in the frequencies for HLA-B Bw4 and HLA-C C-group2 alleles with distance from Africa. These same clinal patterns are evident for some allele frequency results from the current meta-analysis (e.g., C-group1 alleles: Cw*0303, *0304, *0702, *0801; C-group2 alleles: *0501, *0602, *1701), but not for others (e.g., C-group1 alleles: *0701, *0802, *1601; C-group2 allele: *1502). Individual maps may also reveal allele-specific patterns of global diversification. For example the B*4002 allele is seen at low frequencies in SSA, NAF, and EUR, but at high frequencies in NEA, NAM and SAM.
Acknowledgments
We thank Derek Middleton for making HLA frequency data available to the research community, as well as all laboratories that contributed data to the 12th and 13th IHW. Diogo Meyer, Kristie Mather, and Mark Nelson provided early guidance for data-gathering and analysis. The authors appreciate the help of Andrea Cabello, Kitty Deng, Jacob Gunn-Glanville, Jonathan Najman, and Neha Prakash, who assisted in compiling the literature dataset. This work was supported by NIH Grant GM35326 (G.T., R.S., A.L., Y.T.) and NIH Grant GM40282 (O.S.), the Vermont Advanced Computing Center under NASA grant NNG-05GO96G (R.S.), FNS (Switzerland) Grants 31.49771.96 and 3100A0-112651 (A.S.-M.), and the University of California Leadership Excellence through Advanced Degrees (UC LEADS; Y.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Works cited
- 1.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 2.Meyer D, Thomson G. How selection shapes variation of the human major histocompatibility complex: a review. Ann Hum Genet. 2001;65:1. doi: 10.1046/j.1469-1809.2001.6510001.x. [DOI] [PubMed] [Google Scholar]
- 3.Meyer D, Single RM, Mack SJ, Erlich HA, Thomson G. Signatures of demographic history and natural selection in the human major histocompatibility complex Loci. Genetics. 2006;173:2121. doi: 10.1534/genetics.105.052837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mack SJ, Bugawan TL, Moonsamy PV, Erlich JA, Trachtenberg EA, Paik YK, Begovich AB, Saha N, Beck HP, Stoneking M, Erlich HA. Evolution of Pacific/Asian populations inferred from HLA class II allele frequency distributions. Tissue Antigens. 2000;55:383. doi: 10.1034/j.1399-0039.2000.550501.x. [DOI] [PubMed] [Google Scholar]
- 5.Mack SJ, Erlich HA. Population relationships as inferred from classical HLA genes. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 747. [Google Scholar]
- 6.Sanchez-Mazas A. HLA genetic differentiation of the 13th IHWC population data relative to worldwide linguistic families. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 758. [Google Scholar]
- 7.Sanchez-Mazas A. An apportionment of human HLA diversity. Tissue Antigens. 2007;69 Suppl 1:198. doi: 10.1111/j.1399-0039.2006.00802.x. [DOI] [PubMed] [Google Scholar]
- 8.Garrigan D, Hedrick PW. Perspective: detecting adaptive molecular polymorphism: lessons from the MHC. Evolution Int J Org Evolution. 2003;57:1707. doi: 10.1111/j.0014-3820.2003.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao X, Nelson GW, Karacki P, Martin MP, Phair J, Kaslow R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, O'Brien SJ, Carrington M. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med. 2001;344:1668. doi: 10.1056/NEJM200105313442203. [DOI] [PubMed] [Google Scholar]
- 10.Shiina T, Inoko H, Kulski JK. An update of the HLA genomic region, locus information and disease associations: 2004. Tissue Antigens. 2004;64:631. doi: 10.1111/j.1399-0039.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 11.Mignot E, Lin L, Rogers W, Honda Y, Qiu X, Lin X, Okun M, Hohjoh H, Miki T, Hsu S, Leffell M, Grumet F, Fernández-Viña M, Honda M, Risch N. Complex HLA-DR and - DQ interactions confer risk of narcolepsy-cataplexy in three ethnic groups. Am J Hum Genet. 2001;68:686. doi: 10.1086/318799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oksenberg JR, Barcellos LF, Cree BA, Baranzini SE, Bugawan TL, Khan O, Lincoln RR, Swerdlin A, Mignot E, Lin L, Goodin D, Erlich HA, Schmidt S, Thomson G, Reich DE, Pericak-Vance MA, Haines JL, Hauser SL. Mapping multiple sclerosis susceptibility to the HLA-DR locus in African Americans. Am J Hum Genet. 2004;74:160. doi: 10.1086/380997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mack SJ, Sanchez-Mazas A, Meyer D, Single RM, Tsai Y, Erlich HA. Methods used in the generation and preparation of data for analysis in the 13th International Histocompatibility Workshop. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 564. [Google Scholar]
- 14.Abdennaji Guenounou B, Loueslati BY, Buhler S, Hmida S, Ennafaa H, Khodjet-Elkhil H, Moojat N, Dridi A, Boukef K, Ben Ammar Elgaaied A, Sanchez-Mazas A. HLA class II genetic diversity in southern Tunisia and the Mediterranean area. Int J Immunogenet. 2006;33(2):93. doi: 10.1111/j.1744-313X.2006.00577.x. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal S, Khan F, Bharadwaj U. Human genetic variation studies and HLA class II loci. Int J Immunogenet. 2007;34(4):247. doi: 10.1111/j.1744-313X.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 16.Akisaka M, Suzuki M, Inoko H. Molecular genetic studies on DNA polymorphism of the HLA class II genes associated with human longevity. Tissue Antigens. 1997;50(5):489. doi: 10.1111/j.1399-0039.1997.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 17.al-Daccak R, Wang FQ, Theophille D, Lethielleux P, Colombani J, Loiseau P. Gene polymorphism of HLA-DPB1 and DPA1 loci in caucasoid population: frequencies and DPB1-DPA1 associations. Hum Immunol. 1991;31(4):277. doi: 10.1016/0198-8859(91)90100-n. [DOI] [PubMed] [Google Scholar]
- 18.Aldener-Cannava A, Olerup O. HLA-DPA1 typing by PCR amplification with sequence-specific primers (PCR-SSP) and distribution of DPA1 alleles in Caucasian, African and Oriental populations. Tissue Antigens. 1996;48(3):153. doi: 10.1111/j.1399-0039.1996.tb02623.x. [DOI] [PubMed] [Google Scholar]
- 19.Aldener-Cannava A, Olerup O. HLA-DPB1 typing by polymerase chain reaction amplification with sequence-specific primers. Tissue Antigens. 2001;57(4):287. doi: 10.1034/j.1399-0039.2001.057004287.x. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hussein KA, Rama NR, Butt AI, Meyer B, Rozemuller E, Tilanus MG. HLA class II sequence-based typing in normal Saudi individuals. Tissue Antigens. 2002;60(3):259. doi: 10.1034/j.1399-0039.2002.600308.x. [DOI] [PubMed] [Google Scholar]
- 21.Allen M, Sandberg-Wollheim M, Sjogren K, Erlich HA, Petterson U, Gyllensten U. Association of susceptibility to multiple sclerosis in Sweden with HLA class II DRB1 and DQB1 alleles. Hum Immunol. 1994;39(1):41. doi: 10.1016/0198-8859(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 22.Amirzargar A, Mytilineos J, Farjadian S, Doroudchi M, Scherer S, Opelz G, Ghaderi A. Human leukocyte antigen class II allele frequencies and haplotype association in Iranian normal population. Hum Immunol. 2001;62(11):1234. doi: 10.1016/s0198-8859(01)00320-2. [DOI] [PubMed] [Google Scholar]
- 23.Amirzargar A, Mytilineos J, Yousefipour A, Farjadian S, Scherer S, Opelz G, Ghaderi A. HLA class II (DRB1, DQA1 and DQB1) associated genetic susceptibility in Iranian multiple sclerosis (MS) patients. Eur J Immunogenet. 1998;25(4):297. doi: 10.1046/j.1365-2370.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 24.Arnaiz-Villena A, Dimitroski K, Pacho A, Moscoso J, Gomez-Casado E, Silvera-Redondo C, Varela P, Blagoevska M, Zdravkovska V, Martinez-Laso J. HLA genes in Macedonians and the sub-Saharan origin of the Greeks. Tissue Antigens. 2001;57(2):118. doi: 10.1034/j.1399-0039.2001.057002118.x. [DOI] [PubMed] [Google Scholar]
- 25.Arnaiz-Villena A, Iliakis P, Gonzalez-Hevilla M, Longas J, Gomez-Casado E, Sfyridaki K, Trapaga J, Silvera-Redondo C, Matsouka C, Martinez-Laso J. The origin of Cretan populations as determined by characterization of HLA alleles. Tissue Antigens. 1999;53(3):213. doi: 10.1034/j.1399-0039.1999.530301.x. [DOI] [PubMed] [Google Scholar]
- 26.Arnaiz-Villena A, Martinez-Laso J, Moscoso J, Livshits G, Zamora J, Gomez-Casado E, Silvera-Redondo C, Melvin K, Crawford MH. HLA genes in the Chuvashian population from European Russia: admixture of Central European and Mediterranean populations. Hum Biol. 2003;75(3):375. doi: 10.1353/hub.2003.0040. [DOI] [PubMed] [Google Scholar]
- 27.Arnaiz-Villena A, Vargas-Alarcon G, Granados J, Gomez-Casado E, Longas J, Gonzales-Hevilla M, Zuniga J, Salgado N, Hernandez-Pacheco G, Guillen J, Martinez-Laso J. HLA genes in Mexican Mazatecans, the peopling of the Americas and the uniqueness of Amerindians. Tissue Antigens. 2000;56(5):405. doi: 10.1034/j.1399-0039.2000.560503.x. [DOI] [PubMed] [Google Scholar]
- 28.Ayed K, Ayed-Jendoubi S, Sfar I, Labonne MP, Gebuhrer L. HLA class-I and HLA class-II phenotypic, gene and haplotypic frequencies in Tunisians by using molecular typing data. Tissue Antigens. 2004;64(4):520. doi: 10.1111/j.1399-0039.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 29.Bannai M, Ohashi J, Harihara S, Takahashi Y, Juji T, Omoto K, Tokunaga K. Analysis of HLA genes and haplotypes in Ainu (from Hokkaido, northern Japan) supports the premise that they descent from Upper Paleolithic populations of East Asia. Tissue Antigens. 2000;55(2):128. doi: 10.1034/j.1399-0039.2000.550204.x. [DOI] [PubMed] [Google Scholar]
- 30.Bannai M, Tokunaga K, Imanishi T, Harihara S, Fujisawa K, Juji T, Omoto K. HLA class II alleles in Ainu living in Hidaka District, Hokkaido, northern Japan. Am J Phys Anthropol. 1996;101(1):1. doi: 10.1002/(SICI)1096-8644(199609)101:1<1::AID-AJPA1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Begovich AB, Klitz W, Moonsamy PV, Van de Water J, Peltz G, Gershwin ME. Genes within the HLA class II region confer both predisposition and resistance to primary biliary cirrhosis. Tissue Antigens. 1994;43(2):71. doi: 10.1111/j.1399-0039.1994.tb02303.x. [DOI] [PubMed] [Google Scholar]
- 32.Begovich AB, McClure GR, Suraj VC, Helmuth RC, Fildes N, Bugawan TL, Erlich HA, Klitz W. Polymorphism, recombination, and linkage disequilibrium within the HLA class II region. J Immunol. 1992;148(1):249. [PubMed] [Google Scholar]
- 33.Begovich AB, Moonsamy PV, Mack SJ, Barcellos LF, Steiner LL, Grams S, Suraj-Baker V, Hollenbach J, Trachtenberg E, Louie L, Zimmerman P, Hill AV, Stoneking M, Sasazuki T, Konenkov VI, Sartakova ML, Titanji VP, Rickards O, Klitz W. Genetic variability and linkage disequilibrium within the HLA-DP region: analysis of 15 different populations. Tissue Antigens. 2001;57(5):424. doi: 10.1034/j.1399-0039.2001.057005424.x. [DOI] [PubMed] [Google Scholar]
- 34.Bera O, Cesaire R, Quelvennec E, Quillivic F, de Chavigny V, Ribal C, Semana G. HLA class I and class II allele and haplotype diversity in Martinicans. Tissue Antigens. 2001;57(3):200. doi: 10.1034/j.1399-0039.2001.057003200.x. [DOI] [PubMed] [Google Scholar]
- 35.Blagitko N, O'HUigin C, Figueroa F, Horai S, Sonoda S, Tajima K, Watkins D, Klein J. Polymorphism of the HLA-DRB1 locus in Colombian, Ecuadorian, and Chilean Amerinds. Hum Immunol. 1997;54(1):74. doi: 10.1016/s0198-8859(97)00005-0. [DOI] [PubMed] [Google Scholar]
- 36.Briceno I, Gomez A, Bernal JE, Papiha SS. HLA-DPB1 polymorphism in seven South American Indian tribes in Colombia. Eur J Immunogenet. 1996;23(3):235. doi: 10.1111/j.1744-313x.1996.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 37.Bugawan TL, Chang JD, Klitz W, Erlich HA. PCR/oligonucleotide probe typing of HLA class II alleles in a Filipino population reveals an unusual distribution of HLA haplotypes. Am J Hum Genet. 1994;54(2):331. [PMC free article] [PubMed] [Google Scholar]
- 38.Bugawan TL, Klitz W, Blair A, Erlich HA. High-resolution HLA class I typing in the CEPH families: analysis of linkage disequilibrium among HLA loci. Tissue Antigens. 2000;56(5):392. doi: 10.1034/j.1399-0039.2000.560502.x. [DOI] [PubMed] [Google Scholar]
- 39.Buhler S, Megarbane A, Lefranc G, Tiercy JM, Sanchez-Mazas A. HLA-C molecular characterization of a Lebanese population and genetic structure of 39 populations from Europe to India-Pakistan. Tissue Antigens. 2006;68(1):44. doi: 10.1111/j.1399-0039.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 40.Cechova E, Fazekasova H, Ferencik S, Shawkatova I, Buc M. HLA-DRB1, -DQB1 and -DPB1 polymorphism in the Slovak population. Tissue Antigens. 1998;51(5):574. doi: 10.1111/j.1399-0039.1998.tb02996.x. [DOI] [PubMed] [Google Scholar]
- 41.Cerna M, Falco M, Friedman H, Raimondi E, Maccagno A, Fernandez-Vina M, Stastny P. Differences in HLA class II alleles of isolated South American Indian populations from Brazil and Argentina. Hum Immunol. 1993;37(4):213. doi: 10.1016/0198-8859(93)90504-t. [DOI] [PubMed] [Google Scholar]
- 42.Cerna M, Fernandez-Vina M, Ivaskova E, Stastny P. Comparison of HLA class II alleles in Gypsy and Czech populations by DNA typing with oligonucleotide probes. Tissue Antigens. 1992;39(3):111. doi: 10.1111/j.1399-0039.1992.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen BH, Chiang CH, Lin SR, Chao MG, Tsai ST. The influence of age at onset and gender on the HLA-DQA1, DQB1 association in Chinese children with insulin dependent diabetes mellitus. Hum Immunol. 1999;60(11):1131. doi: 10.1016/s0198-8859(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Hong W, Shao H, Fu Y, Liu X, Chen D, Xu A. Allelic distribution of HLA class I genes in the Tibetan ethnic population of China. Int J Immunogenet. 2006;33(6):439. doi: 10.1111/j.1744-313X.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Hu Q, Xie Y, Zhou L, Xiao C, Wu Y, Xu A. Origin of Tibeto-Burman speakers: evidence from HLA allele distribution in Lisu and Nu inhabiting Yunnan of China. Hum Immunol. 2007;68(6):550. doi: 10.1016/j.humimm.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Chen S, Li W, Hu Q, Liu Z, Xu Y, Xu A. Polymorphism of HLA class I genes in Meizhou Han population of Guangdong, China. Int J Immunogenet. 2007;34(2):131. doi: 10.1111/j.1744-313X.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 47.Chowdari KV, Xu K, Zhang F, Ma C, Li T, Xie BY, Wood J, Trucco M, Tsoi WF, Saha N, Rudert WA, Nimgaonkar VL. Immune related genetic polymorphisms and schizophrenia among the Chinese. Hum Immunol. 2001;62(7):714. doi: 10.1016/s0198-8859(01)00256-7. [DOI] [PubMed] [Google Scholar]
- 48.Comas D, Mateu E, Calafell F, Perez-Lezaun A, Bosch E, Martinez-Arias R, Bertranpetit J. HLA class I and class II DNA typing and the origin of Basques. Tissue Antigens. 1998;51(1):30. doi: 10.1111/j.1399-0039.1998.tb02944.x. [DOI] [PubMed] [Google Scholar]
- 49.Congia M, Frau F, Lampis R, Frau R, Mele R, Cucca F, Muntoni F, Porcu S, Boi F, Contu L, et al. A high frequency of the A30, B18, DR3, DRw52, DQw2 extended haplotype in Sardinian celiac disease patients: further evidence that disease susceptibility is conferred by DQ A1*0501, B1*0201. Tissue Antigens. 1992;39(2):78. doi: 10.1111/j.1399-0039.1992.tb01911.x. [DOI] [PubMed] [Google Scholar]
- 50.Cortes LM, Baltazar LM, Perea FJ, Gallegos-Arreola MP, Flores SE, Sandoval L, Olivares N, Lorenz MG, Xu H, Barton SA, Chakraborty R, Rivas F. HLA-DQB1, - DQA1, -DRB1 linkage disequilibrium and haplotype diversity in a Mestizo population from Guadalajara, Mexico. Tissue Antigens. 2004;63(5):458. doi: 10.1111/j.0001-2815.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 51.Dharakul T, Vejbaesya S, Chaowagul W, Luangtrakool P, Stephens HA, Songsivilai S. HLA-DR and -DQ associations with melioidosis. Hum Immunol. 1998;59(9):580. doi: 10.1016/s0198-8859(98)00052-4. [DOI] [PubMed] [Google Scholar]
- 52.Djoulah S, Sanchez-Mazas A, Khalil I, Benhamamouch S, Degos L, Deschamps I, Hors J. HLA-DRB1, DQA1 and DQB1 DNA polymorphisms in healthy Algerian and genetic relationships with other populations. Tissue Antigens. 1994;43(2):102. doi: 10.1111/j.1399-0039.1994.tb02308.x. [DOI] [PubMed] [Google Scholar]
- 53.Doherty DG, Vaughan RW, Donaldson PT, Mowat AP. HLA DQA, DQB, and DRB genotyping by oligonucleotide analysis: distribution of alleles and haplotypes in British caucasoids. Hum Immunol. 1992;34(1):53. doi: 10.1016/0198-8859(92)90085-2. [DOI] [PubMed] [Google Scholar]
- 54.Ellis JM, Hoyer RJ, Costello CN, Mshana RN, Quakyi IA, Mshana MN, Diaby B, Traore M, Johnson AH, Hurley CK. HLA-B allele frequencies in Cote d'Ivoire defined by direct DNA sequencing: identification of HLA-B*1405, B*4410, and B*5302. Tissue Antigens. 2001;57(4):339. doi: 10.1034/j.1399-0039.2001.057004339.x. [DOI] [PubMed] [Google Scholar]
- 55.Ellis JM, Mack SJ, Leke RF, Quakyi I, Johnson AH, Hurley CK. Diversity is demonstrated in class I HLA-A and HLA-B alleles in Cameroon, Africa: description of HLA-A*03012, *2612, *3006 and HLA-B*1403, *4016, *4703. Tissue Antigens. 2000;56(4):291. doi: 10.1034/j.1399-0039.2000.560401.x. [DOI] [PubMed] [Google Scholar]
- 56.Erlich HA, Rotter JI, Chang JD, Shaw SJ, Raffel LJ, Klitz W, Bugawan TL, Zeidler A. Association of HLA-DPB1*0301 with IDDM in Mexican-Americans. Diabetes. 1996;45(5):610. doi: 10.2337/diab.45.5.610. [DOI] [PubMed] [Google Scholar]
- 57.Erlich HA, Zeidler A, Chang J, Shaw S, Raffel LJ, Klitz W, Beshkov Y, Costin G, Pressman S, Bugawan T, et al. HLA class II alleles and susceptibility and resistance to insulin dependent diabetes mellitus in Mexican-American families. Nat Genet. 1993;3(4):358. doi: 10.1038/ng0493-358. [DOI] [PubMed] [Google Scholar]
- 58.Evseeva I, Spurkland A, Thorsby E, Smerdel A, Tranebjaerg L, Boldyreva M, Groudakova E, Gouskova I, Alexeev LL. HLA profile of three ethnic groups living in the North-Western region of Russia. Tissue Antigens. 2002;59(1):38. doi: 10.1034/j.1399-0039.2002.590107.x. [DOI] [PubMed] [Google Scholar]
- 59.Fainboim L, Marcos Y, Pando M, Capucchio M, Reyes GB, Galoppo C, Badia I, Remondino G, Ciocca M, Ramonet M, et al. Chronic active autoimmune hepatitis in children. Strong association with a particular HLA-DR6 (DRB1*1301) haplotype. Hum Immunol. 1994;41(2):146. doi: 10.1016/0198-8859(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 60.Farjadian S, Naruse T, Kawata H, Ghaderi A, Bahram S, Inoko H. Molecular analysis of HLA allele frequencies and haplotypes in Baloch of Iran compared with related populations of Pakistan. Tissue Antigens. 2004;64(5):581. doi: 10.1111/j.1399-0039.2004.00302.x. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Vina M, Lazaro AM, Sun Y, Miller S, Forero L, Stastny P. Population diversity of B-locus alleles observed by high-resolution DNA typing. Tissue Antigens. 1995;45(3):153. doi: 10.1111/j.1399-0039.1995.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 62.Fernandez-Vina MA, Lazaro AM, Marcos CY, Nulf C, Raimondi E, Haas EJ, Stastny P. Dissimilar evolution of B-locus versus A-locus and class II loci of the HLA region in South American Indian tribes. Tissue Antigens. 1997;50(3):233. doi: 10.1111/j.1399-0039.1997.tb02867.x. [DOI] [PubMed] [Google Scholar]
- 63.Fort M, de Stefano GF, Cambon-Thomsen A, Giraldo-Alvarez P, Dugoujon JM, Ohayon E, Scano G, Abbal M. HLA class II allele and haplotype frequencies in Ethiopian Amhara and Oromo populations. Tissue Antigens. 1998;51(4 Pt 1):327. doi: 10.1111/j.1399-0039.1998.tb02971.x. [DOI] [PubMed] [Google Scholar]
- 64.Fu Y, Liu Z, Lin J, Jia Z, Chen W, Pan D, Liu Y, Zhu Y, Chen R, Xu A. HLA-DRB1, DQB1 and DPB1 polymorphism in the Naxi ethnic group of South-western China. Tissue Antigens. 2003;61(2):179. doi: 10.1034/j.1399-0039.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 65.Gao X, Veale A, Serjeantson SW. HLA class II diversity in Australian aborigines: unusual HLA-DRB1 alleles. Immunogenetics. 1992;36(5):333. doi: 10.1007/BF00215663. [DOI] [PubMed] [Google Scholar]
- 66.Gao XJ, Sun YP, An JB, Fernandez-Vina M, Qou JN, Lin L, Stastny P. DNA typing for HLA-DR, and -DP alleles in a Chinese population using the polymerase chain reaction (PCR) and oligonucleotide probes. Tissue Antigens. 1991;38(1):24. doi: 10.1111/j.1399-0039.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 67.Gendzekhadze K, Herrera F, Montagnani S, Balbas O, Witter K, Albert E, Layrisse Z. HLA-DP polymorphism in Venezuelan Amerindians. Hum Immunol. 2004;65(12):1483. doi: 10.1016/j.humimm.2004.07.238. [DOI] [PubMed] [Google Scholar]
- 68.Geng L, Imanishi T, Tokunaga K, Zhu D, Mizuki N, Xu S, Geng Z, Gojobori T, Tsuji K, Inoko H. Determination of HLA class II alleles by genotyping in a Manchu population in the northern part of China and its relationship with Han and Japanese populations. Tissue Antigens. 1995;46(2):111. doi: 10.1111/j.1399-0039.1995.tb02486.x. [DOI] [PubMed] [Google Scholar]
- 69.Grahovac B, Sukernik RI, O'HUigin C, Zaleska-Rutczynska Z, Blagitko N, Raldugina O, Kosutic T, Satta Y, Figueroa F, Takahata N, Klein J. Polymorphism of the HLA class II loci in Siberian populations. Hum Genet. 1998;102(1):27. doi: 10.1007/s004390050650. [DOI] [PubMed] [Google Scholar]
- 70.Grubic Z, Zunec R, Cecuk-Jelicic E, Kerhin-Brkljacic V, Kastelan A. Polymorphism of HLA-A, -B, -DRB1, -DQA1 and -DQB1 haplotypes in a Croatian population. Eur J Immunogenet. 2000;27(1):47. doi: 10.1046/j.1365-2370.2000.00193.x. [DOI] [PubMed] [Google Scholar]
- 71.Guedez YB, Layrisse Z, Dominguez E, Rodriguez-Larralde A, Scorza J. Molecular analysis of MHC class II alleles and haplotypes (DRB1, DQA1 and DQB1) in the Bari Amerindians. Tissue Antigens. 1994;44(2):125. doi: 10.1111/j.1399-0039.1994.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 72.Haas JP, Nevinny-Stickel C, Schoenwald U, Truckenbrodt H, Suschke J, Albert ED. Susceptible and protective major histocompatibility complex class II alleles in early-onset pauciarticular juvenile chronic arthritis. Hum Immunol. 1994;41(3):225. doi: 10.1016/0198-8859(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 73.Hajjej A, Kaabi H, Sellami MH, Dridi A, Jeridi A, El borgi W, Cherif G, Elgaaied A, Almawi WY, Boukef K, Hmida S. The contribution of HLA class I and II alleles and haplotypes to the investigation of the evolutionary history of Tunisians. Tissue Antigens. 2006;68(2):153. doi: 10.1111/j.1399-0039.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 74.Haras D, Silicani-Amoros P, Amoros JP. HLA and susceptibility to insulin-dependent diabetes mellitus on the island of Corsica. Tissue Antigens. 1994;44(2):129. doi: 10.1111/j.1399-0039.1994.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 75.Hmida S, Gauthier A, Dridi A, Quillivic F, Genetet B, Boukef K, Semana G. HLA class II gene polymorphism in Tunisians. Tissue Antigens. 1995;45(1):63. doi: 10.1111/j.1399-0039.1995.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 76.Hong W, Chen S, Shao H, Fu Y, Hu Z, Xu A. HLA class I polymorphism in Mongolian and Hui ethnic groups from Northern China. Hum Immunol. 2007;68(5):439. doi: 10.1016/j.humimm.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 77.Hong W, Fu Y, Chen S, Wang F, Ren X, Xu A. Distributions of HLA class I alleles and haplotypes in Northern Han Chinese. Tissue Antigens. 2005;66(4):297. doi: 10.1111/j.1399-0039.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 78.Horiki T, Ichikawa Y, Moriuchi J, Hoshina Y, Yamada C, Wakabayashi T, Jackson K, Inoko H. HLA class II haplotypes associated with pulmonary interstitial lesions of polymyositis/dermatomyositis in Japanese patients. Tissue Antigens. 2002;59(1):25. doi: 10.1034/j.1399-0039.2002.590105.x. [DOI] [PubMed] [Google Scholar]
- 79.Hu W, Wang J, Wang B, Lu J, Li H, Zhang J, Cun Y, Tang W, Xiao C. Sequencingbased analysis of the HLA-DPB1 polymorphism in Nu ethnic group of south-west China. Int J Immunogenet. 2006;33(6):397. doi: 10.1111/j.1744-313X.2006.00638.x. [DOI] [PubMed] [Google Scholar]
- 80.Hu WH, Lu J, Dong YL, Cheng BW, Tang WR, Cun YN, Lei YP, Tan SJ, Xiao CJ. Polymorphism of the DPB1 locus in Hani ethnic group of south-western China. Int J Immunogenet. 2005;32(6):421. doi: 10.1111/j.1744-313X.2005.00551.x. [DOI] [PubMed] [Google Scholar]
- 81.Huang FY, Chang TY, Chen MR, Hsu CH, Lee HC, Lin SP, Kao HA, Chiu NC, Chi H, Liu TY, Liu HF, Dang CW, Chu CC, Lin M, Sung TC, Lee YJ. Genetic variations of HLA-DRB1 and susceptibility to Kawasaki disease in Taiwanese children. Hum Immunol. 2007;68(1):69. doi: 10.1016/j.humimm.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 82.Hviid TV, Madsen HO, Morling N. HLA-DPB1 typing with polymerase chain reaction and restriction fragment length polymorphism technique in Danes. Tissue Antigens. 1992;40(3):140. doi: 10.1111/j.1399-0039.1992.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 83.Itoh Y, Mizuki N, Shimada T, Azuma F, Itakura M, Kashiwase K, Kikkawa E, Kulski JK, Satake M, Inoko H. High-throughput DNA typing of HLA-A, -B, -C, and -DRB1 loci by a PCR-SSOP-Luminex method in the Japanese population. Immunogenetics. 2005;57(10):717. doi: 10.1007/s00251-005-0048-3. [DOI] [PubMed] [Google Scholar]
- 84.Ivanova M, Rozemuller E, Tyufekchiev N, Michailova A, Tilanus M, Naumova E. HLA polymorphism in Bulgarians defined by high-resolution typing methods in comparison with other populations. Tissue Antigens. 2002;60(6):496. doi: 10.1034/j.1399-0039.2002.600605.x. [DOI] [PubMed] [Google Scholar]
- 85.Just JJ, King MC, Thomson G, Klitz W. African-American HLA class II allele and haplotype diversity. Tissue Antigens. 1997;49(5):547. doi: 10.1111/j.1399-0039.1997.tb02801.x. [DOI] [PubMed] [Google Scholar]
- 86.Kapustin S, Lyshchov A, Alexandrova J, Imyanitov E, Blinov M. HLA class II molecular polymorphisms in healthy Slavic individuals from North-Western Russia. Tissue Antigens. 1999;54(5):517. doi: 10.1034/j.1399-0039.1999.540509.x. [DOI] [PubMed] [Google Scholar]
- 87.Kitawaki J, Obayashi H, Kado N, Ishihara H, Koshiba H, Maruya E, Saji H, Ohta M, Hasegawa G, Nakamura N, Yoshikawa T, Honjo H. Association of HLA class I and class II alleles with susceptibility to endometriosis. Hum Immunol. 2002;63(11):1033. doi: 10.1016/s0198-8859(02)00438-x. [DOI] [PubMed] [Google Scholar]
- 88.Klitz W, Maiers M, Spellman S, Baxter-Lowe LA, Schmeckpeper B, Williams TM, Fernandez-Vina M. New HLA haplotype frequency reference standards: high-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens. 2003;62(4):296. doi: 10.1034/j.1399-0039.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 89.Klitz W, Stephens JC, Grote M, Carrington M. Discordant patterns of linkage disequilibrium of the peptide-transporter loci within the HLA class II region. Am J Hum Genet. 1995;57(6):1436. [PMC free article] [PubMed] [Google Scholar]
- 90.Kocova M, Blagoevska M, Bogoevski M, Konstantinova M, Dorman J, Trucco M. HLA class II molecular typing in an European Slavic population with a low incidence of insulin-dependent diabetes mellitus. Tissue Antigens. 1995;45(3):216. doi: 10.1111/j.1399-0039.1995.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 91.Krokowski M, Bodalski J, Bratek A, Boitard C, Caillat-Zucman S. HLA class II-associated predisposition to insulin-dependent diabetes mellitus in a Polish population. Hum Immunol. 1998;59(7):451. doi: 10.1016/s0198-8859(98)00036-6. [DOI] [PubMed] [Google Scholar]
- 92.Lampis R, Morelli L, Congia M, Macis MD, Mulargia A, Loddo M, De Virgiliis S, Marrosu MG, Todd JA, Cucca F. The inter-regional distribution of HLA class II haplotypes indicates the suitability of the Sardinian population for case-control association studies in complex diseases. Hum Mol Genet. 2000;9(20):2959. doi: 10.1093/hmg/9.20.2959. [DOI] [PubMed] [Google Scholar]
- 93.Layrisse Z, Guedez Y, Dominguez E, Herrera F, Soto M, Balbas O, Matos M, Alfonzo JC, Granados J, Scorza J. Extended HLA haplotypes among the Bari Amerindians of the Perija Range. Relationship to other tribes based on four-loci haplotype frequencies. Hum Immunol. 1995;44(4):228. doi: 10.1016/0198-8859(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 94.Layrisse Z, Guedez Y, Dominguez E, Paz N, Montagnani S, Matos M, Herrera F, Ogando V, Balbas O, Rodriguez-Larralde A. Extended HLA haplotypes in a Carib Amerindian population: the Yucpa of the Perija Range. Hum Immunol. 2001;62(9):992. doi: 10.1016/s0198-8859(01)00297-x. [DOI] [PubMed] [Google Scholar]
- 95.Lazaro AM, Moraes ME, Marcos CY, Moraes JR, Fernandez-Vina MA, Stastny P. Evolution of HLA-class I compared to HLA-class II polymorphism in Terena, a South-American Indian tribe. Hum Immunol. 1999;60(11):1138. doi: 10.1016/s0198-8859(99)00092-0. [DOI] [PubMed] [Google Scholar]
- 96.Leal CA, Mendoza-Carrera F, Rivas F, Rodriguez-Reynoso S, Portilla-de Buen E. HLA-A and HLA-B allele frequencies in a mestizo population from Guadalajara, Mexico, determined by sequence-based typing. Tissue Antigens. 2005;66(6):666. doi: 10.1111/j.1399-0039.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- 97.Lee KW, Jung YA, Oh DH. Diversity of HLA-DQA1 gene in the Korean population. Tissue Antigens. 2007;69 Suppl 1:82. doi: 10.1111/j.1399-0039.2006.761_2.x. [DOI] [PubMed] [Google Scholar]
- 98.Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, - DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005;65(5):437. doi: 10.1111/j.1399-0039.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 99.Leffell MS, Fallin MD, Hildebrand WH, Cavett JW, Iglehart BA, Zachary AA. HLA alleles and haplotypes among the Lakota Sioux: report of the ASHI minority workshops, part III. Hum Immunol. 2004;65(1):78. doi: 10.1016/j.humimm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 100.Lienert K, Krishnan R, Fowler C, Russ G. HLA DPB1 genotyping in Australian aborigines by amplified fragment length polymorphism analysis. Hum Immunol. 1993;36(3):137. doi: 10.1016/0198-8859(93)90116-i. [DOI] [PubMed] [Google Scholar]
- 101.Lin JH, Liu ZH, Lv FJ, Fu YG, Fan XL, Li SY, Lu JM, Liu XY, Xu AL. Molecular analyses of HLA-DRB1, -DPB1, and -DQB1 in Jing ethnic minority of Southwest China. Hum Immunol. 2003;64(8):830. doi: 10.1016/s0198-8859(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 102.Lin L, Jin L, Kimura A, Carrington M, Mignot E. DQ microsatellite association studies in three ethnic groups. Tissue Antigens. 1997;50(5):507. doi: 10.1111/j.1399-0039.1997.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 103.Little AM, Scott I, Pesoa S, Marsh SG, Arguello R, Cox ST, Ramon D, Vullo C, Madrigal JA. HLA class I diversity in Kolla Amerindians. Hum Immunol. 2001;62(2):170. doi: 10.1016/s0198-8859(00)00248-2. [DOI] [PubMed] [Google Scholar]
- 104.Liu Y, Liu Z, Fu Y, Jia Z, Chen S, Xu A. Polymorphism of HLA class II genes in Miao and Yao nationalities of Southwest China. Tissue Antigens. 2006;67(2):157. doi: 10.1111/j.1399-0039.2006.00510.x. [DOI] [PubMed] [Google Scholar]
- 105.Liu ZH, Lin J, Pan D, Chen W, Tao H, Xu A. HLA-DPB1 allelic frequency of the Pumi ethnic group in south-west China and evolutionary relationship of Pumi with other populations. Eur J Immunogenet. 2002;29(3):259. doi: 10.1046/j.1365-2370.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 106.Lou H, Li HC, Kuwayama M, Yashiki S, Fujiyoshi T, Suehara M, Osame M, Yamashita M, Hayami M, Gurtsevich V, Ballas M, Imanishi T, Sonoda S. HLA class I and class II of the Nivkhi, an indigenous population carrying HTLV-I in Sakhalin, Far Eastern Russia. Tissue Antigens. 1998;52(5):444. doi: 10.1111/j.1399-0039.1998.tb03071.x. [DOI] [PubMed] [Google Scholar]
- 107.Lulli P, Grammatico P, Brioli G, Catricala C, Morellini M, Roccella M, Mariani B, Pennesi G, Roccella F, Cappellacci S, Trabace S. HLA-DR and -DQ alleles in Italian patients with melanoma. Tissue Antigens. 1998;51(3):276. doi: 10.1111/j.1399-0039.1998.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 108.Machulla HK, Batnasan D, Steinborn F, Uyar FA, Saruhan-Direskeneli G, Oguz FS, Carin MN, Dorak MT. Genetic affinities among Mongol ethnic groups and their relationship to Turks. Tissue Antigens. 2003;61(4):292. doi: 10.1034/j.1399-0039.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 109.Mack SJ, Bugawan TL, Moonsamy PV, Erlich JA, Trachtenberg EA, Paik YK, Begovich AB, Saha N, Beck HP, Stoneking M, Erlich HA. Evolution of Pacific/Asian populations inferred from HLA class II allele frequency distributions. Tissue Antigens. 2000;55(5):383. doi: 10.1034/j.1399-0039.2000.550501.x. [DOI] [PubMed] [Google Scholar]
- 110.Magzoub MM, Stephens HA, Sachs JA, Biro PA, Cutbush S, Wu Z, Bottazzo GF. HLA-DP polymorphism in Sudanese controls and patients with insulin-dependent diabetes mellitus. Tissue Antigens. 1992;40(2):64. doi: 10.1111/j.1399-0039.1992.tb01961.x. [DOI] [PubMed] [Google Scholar]
- 111.Main P, Attenborough R, Chelvanayagam G, Bhatia K, Gao X. The peopling of New Guinea: evidence from class I human leukocyte antigen. Hum Biol. 2001;73(3):365. doi: 10.1353/hub.2001.0036. [DOI] [PubMed] [Google Scholar]
- 112.Martinovic I, Bakran M, Chaventre A, Janicijevic B, Jovanovic V, Smolej-Narancic N, Kastelan A, Grubic Z, Zunec R, Roberts DF, Rudan P. Application of HLA class II polymorphism analysis to the study of the population structure of the Island of Krk, Croatia. Hum Biol. 1997;69(6):819. [PubMed] [Google Scholar]
- 113.May J, Mockenhaupt FP, Loliger CC, Ademowo GO, Falusi AG, Jenisch S, Dippmann K, Schnittger L, Kremsner PG, Bienzle U, Meyer CG. HLA DPA1/DPB1 genotype and haplotype frequencies, and linkage disequilibria in Nigeria, Liberia, and Gabon. Tissue Antigens. 1998;52(3):199. doi: 10.1111/j.1399-0039.1998.tb03033.x. [DOI] [PubMed] [Google Scholar]
- 114.Mazzola G, Berrino M, Bersanti M, D'Alfonso S, Cappello N, Bottaro A, Curtoni ES, Fusco P, Vallati M, Bundino S, et al. Immunoglobulin and HLA-DP genes contribute to the susceptibility to juvenile dermatitis herpetiformis. Eur J Immunogenet. 1992;19(3):129. doi: 10.1111/j.1744-313x.1992.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 115.Middleton D, Williams F, Meenagh A, Daar AS, Gorodezky C, Hammond M, Nascimento E, Briceno I, Perez MP. Analysis of the distribution of HLA-A alleles in populations from five continents. Hum Immunol. 2000;61(10):1048. doi: 10.1016/s0198-8859(00)00178-6. [DOI] [PubMed] [Google Scholar]
- 116.Mitsunaga S, Kuwata S, Tokunaga K, Uchikawa C, Takahashi K, Akaza T, Mitomi Y, Juji T. Family study on HLA-DPB1 polymorphism: linkage analysis with HLA-DR/DQ and two "new" alleles. Hum Immunol. 1992;34(3):203. doi: 10.1016/0198-8859(92)90113-2. [DOI] [PubMed] [Google Scholar]
- 117.Mitsunaga S, Oguchi T, Moriyama S, Tokunaga K, Akaza T, Tadokoro K, Juji T. Multiplex ARMS-PCR-RFLP method for high-resolution typing of HLA-DRB1. Eur J Immunogenet. 1995;22(5):371. doi: 10.1111/j.1744-313x.1995.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 118.Mitsunaga S, Oguchi T, Tokunaga K, Akaza T, Tadokoro K, Juji T. High-resolution HLA-DQB1 typing by combination of group-specific amplification and restriction fragment length polymorphism. Hum Immunol. 1995;42(4):307. doi: 10.1016/0198-8859(94)00118-a. [DOI] [PubMed] [Google Scholar]
- 119.Mizuki N, Ohno S, Tanaka H, Sugimura K, Seki T, Mizuki N, Kera J, Inaba G, Tsuji K, Inoko H. Association of HLA-B51 and lack of association of class II alleles with Behcet's disease. Tissue Antigens. 1992;40(1):22. doi: 10.1111/j.1399-0039.1992.tb01953.x. [DOI] [PubMed] [Google Scholar]
- 120.Mohyuddin A, Williams F, Mansoor A, Mehdi SQ, Middleton D. Distribution of HLA-A alleles in eight ethnic groups from Pakistan. Tissue Antigens. 2003;61(4):286. doi: 10.1034/j.1399-0039.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 121.Monsalve MV, Edin G, Devine DV. Analysis of HLA class I and class II in Na-Dene and Amerindian populations from British Columbia, Canada. Hum Immunol. 1998;59(1):48. doi: 10.1016/s0198-8859(97)00251-6. [DOI] [PubMed] [Google Scholar]
- 122.Morel PA, Chang HJ, Wilson JW, Conte C, Saidman SL, Bray JD, Tweardy DJ, Medsger JTA. Severe systemic sclerosis with anti-topoisomerase I antibodies is associated with an HLA-DRw11 allele. Hum Immunol. 1994;40(2):101. doi: 10.1016/0198-8859(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 123.Munkhbat B, Sato T, Hagihara M, Sato K, Kimura A, Munkhtuvshin N, Tsuji K. Molecular analysis of HLA polymorphism in Khoton-Mongolians. Tissue Antigens. 1997;50(2):124. doi: 10.1111/j.1399-0039.1997.tb02851.x. [DOI] [PubMed] [Google Scholar]
- 124.Muro M, Marin L, Torio A, Moya-Quiles MR, Minguela A, Rosique-Roman J, Sanchis MJ, Garcia-Calatayud MC, Garcia-Alonso AM, Alvarez-Lopez MR. HLA polymorphism in the Murcia population (Spain): in the cradle of the archaeologic Iberians. Hum Immunol. 2001;62(9):910. doi: 10.1016/s0198-8859(01)00290-7. [DOI] [PubMed] [Google Scholar]
- 125.Nathalang O, Tatsumi N, Hino M, Prayoonwiwat W, Yamane T, Suwanasophon C, Sriphaisal T. HLA class II polymorphism in Thai patients with non-Hodgkin's lymphoma. Eur J Immunogenet. 1999;26(6):389. doi: 10.1046/j.1365-2370.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- 126.Ogata S, Shi L, Matsushita M, Yu L, Huang XQ, Shi L, Sun H, Ohashi J, Muramatsu M, Tokunaga K, Chu JY. Polymorphisms of human leucocyte antigen genes in Maonan people in China. Tissue Antigens. 2007;69(2):154. doi: 10.1111/j.1399-0039.2006.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ohashi J, Yoshida M, Ohtsuka R, Nakazawa M, Juji T, Tokunaga K. Analysis of HLA-DRB1 polymorphism in the Gidra of Papua New Guinea. Hum Biol. 2000;72(2):337. [PubMed] [Google Scholar]
- 128.Ohta H, Takahashi I, Kojima T, Takamatsu J, Shima M, Yoshioka A, Saito H, Kamiya T. Histocompatibility antigens and alleles in Japanese haemophilia A patients with or without factor VIII antibodies. Tissue Antigens. 1999;54(1):91. doi: 10.1034/j.1399-0039.1999.540111.x. [DOI] [PubMed] [Google Scholar]
- 129.Papassavas EC, Spyropoulou-Vlachou M, Papassavas AC, Schipper RF, Doxiadis IN, Stavropoulos-Giokas C. MHC class I and class II phenotype, gene, and haplotype frequencies in Greeks using molecular typing data. Hum Immunol. 2000;61(6):615. doi: 10.1016/s0198-8859(00)00115-4. [DOI] [PubMed] [Google Scholar]
- 130.Park MH, Kim HS, Kang SJ. HLA-A,-B,-DRB1 allele and haplotype frequencies in 510 Koreans. Tissue Antigens. 1999;53(4 Pt 1):386. doi: 10.1034/j.1399-0039.1999.530412.x. [DOI] [PubMed] [Google Scholar]
- 131.Pedron B, Yakouben K, Guerin V, Borsali E, Auvrignon A, Landman J, Alberti C, Leverger G, Baruchel A, Sterkers G. HLA alleles and haplotypes in French North African immigrants. Hum Immunol. 2006;67(7):540. doi: 10.1016/j.humimm.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 132.Perdriger A, Semana G, Quillivic F, Chales G, Chardevel F, Legrand E, Meadeb J, Fauchet R, Pawlotsky Y. DPB1 polymorphism in rheumatoid arthritis: evidence of an association with allele DPB1 0401. Tissue Antigens. 1992;39(1):14. doi: 10.1111/j.1399-0039.1992.tb02149.x. [DOI] [PubMed] [Google Scholar]
- 133.Perez-Bravo F, Araya M, Mondragon A, Rios G, Alarcon T, Roessler JL, Santos JL. Genetic differences in HLA-DQA1* and DQB1* allelic distributions between celiac and control children in Santiago, Chile. Hum Immunol. 1999;60(3):262. doi: 10.1016/s0198-8859(98)00119-0. [DOI] [PubMed] [Google Scholar]
- 134.Perez-Miranda AM, Alfonso-Sanchez MA, Pena JA, Calderon R. HLA-DQA1 polymorphism in autochthonous Basques from Navarre (Spain): genetic position within European and Mediterranean scopes. Tissue Antigens. 2003;61(6):465. doi: 10.1034/j.1399-0039.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 135.Perez-Miranda AM, Alfonso-Sanchez MA, Vidales MC, Calderon R, Pena JA. Genetic polymorphism and linkage disequilibrium of the HLA-DP region in Basques from Navarre (Spain) Tissue Antigens. 2004;64(3):264. doi: 10.1111/j.1399-0039.2004.00284.x. [DOI] [PubMed] [Google Scholar]
- 136.Perri F, Piepoli A, Quitadamo M, Quarticelli M, Merla A, Bisceglia M. HLA-DQA1 and -DQB1 genes and Helicobacter pylori infection in Italian patients with gastric adenocarcinoma. Tissue Antigens. 2002;59(1):55. doi: 10.1034/j.1399-0039.2002.590112.x. [DOI] [PubMed] [Google Scholar]
- 137.Petlichkovski A, Efinska-Mladenovska O, Trajkov D, Arsov T, Strezova A, Spiroski M. High-resolution typing of HLA-DRB1 locus in the Macedonian population. Tissue Antigens. 2004;64(4):486. doi: 10.1111/j.1399-0039.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 138.Petrone A, Battelino T, Krzisnik C, Bugawan T, Erlich H, Di Mario U, Pozzilli P, Buzzetti R. Similar incidence of type 1 diabetes in two ethnically different populations (Italy and Slovenia) is sustained by similar HLA susceptible/protective haplotype frequencies. Tissue Antigens. 2002;60(3):244. doi: 10.1034/j.1399-0039.2002.600306.x. [DOI] [PubMed] [Google Scholar]
- 139.Piancatelli D, Canossi A, Aureli A, Oumhani K, Del Beato T, Di Rocco M, Liberatore G, Tessitore A, Witter K, El Aouad R, Adorno D. Human leukocyte antigen-A, -B, and -Cw polymorphism in a Berber population from North Morocco using sequence-based typing. Tissue Antigens. 2004;63(2):158. doi: 10.1111/j.1399-0039.2004.00161.x. [DOI] [PubMed] [Google Scholar]
- 140.Pickl WF, Fae I, Fischer GF. Detection of established and novel alleles of the HLA-DPB1 locus by PCR-SSO. Vox Sang. 1993;65(4):316. doi: 10.1111/j.1423-0410.1993.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 141.Pimtanothai N, Charoenwongse P, Mutirangura A, Hurley CK. Distribution of HLA-B alleles in nasopharyngeal carcinoma patients and normal controls in Thailand. Tissue Antigens. 2002;59(3):223. doi: 10.1034/j.1399-0039.2002.590308.x. [DOI] [PubMed] [Google Scholar]
- 142.Pimtanothai N, Hurley CK, Leke R, Klitz W, Johnson AH. HLA-DR and -DQ polymorphism in Cameroon. Tissue Antigens. 2001;58(1):1. doi: 10.1034/j.1399-0039.2001.580101.x. [DOI] [PubMed] [Google Scholar]
- 143.Ploski R, Ek J, Thorsby E, Sollid LM. On the HLA-DQ(alpha 1*0501, beta 1*0201)-associated susceptibility in celiac disease: a possible gene dosage effect of DQB1*0201. Tissue Antigens. 1993;41(4):173. doi: 10.1111/j.1399-0039.1993.tb01998.x. [DOI] [PubMed] [Google Scholar]
- 144.Pratsidou-Gertsi P, Kanakoudi-Tsakalidou F, Spyropoulou M, Germenis A, Adam K, Taparkou A, Siamopoulou A, Drakou C, Konstantinidis T, Prieur AM, Stavropoulos-Giokas C. Nationwide collaborative study of HLA class II associations with distinct types of juvenile chronic arthritis (JCA) in Greece. Eur J Immunogenet. 1999;26(4):299. doi: 10.1046/j.1365-2370.1999.00156.x. [DOI] [PubMed] [Google Scholar]
- 145.Raguenes O, Mercier B, Cledes J, Whebe B, Ferec C. HLA class II typing and idiopathic IgA nephropathy (IgAN): DQB1*0301, a possible marker of unfavorable outcome. Tissue Antigens. 1995;45(4):246. doi: 10.1111/j.1399-0039.1995.tb02447.x. [DOI] [PubMed] [Google Scholar]
- 146.Rajalingam R, Krausa P, Shilling HG, Stein JB, Balamurugan A, McGinnis MD, Cheng NW, Mehra NK, Parham P. Distinctive KIR and HLA diversity in a panel of north Indian Hindus. Immunogenetics. 2002;53(12):1009. doi: 10.1007/s00251-001-0425-5. [DOI] [PubMed] [Google Scholar]
- 147.Rani R, Marcos C, Lazaro AM, Zhang Y, Stastny P. Molecular diversity of HLA-A, -B and -C alleles in a North Indian population as determined by PCR-SSOP. Int J Immunogenet. 2007;34(3):201. doi: 10.1111/j.1744-313X.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 148.Reed E, Ho E, Lupu F, McManus P, Vasilescu R, Foca-Rodi A, Suciu-Foca N. Polymorphism of HLA in the Romanian population. Tissue Antigens. 1992;39(1):8. doi: 10.1111/j.1399-0039.1992.tb02148.x. [DOI] [PubMed] [Google Scholar]
- 149.Reil A, Bein G, Machulla HK, Sternberg B, Seyfarth M. High-resolution DNA typing in immunoglobulin A deficiency confirms a positive association with DRB1*0301, DQB1*02 haplotypes. Tissue Antigens. 1997;50(5):501. doi: 10.1111/j.1399-0039.1997.tb02906.x. [DOI] [PubMed] [Google Scholar]
- 150.Renquin J, Sanchez-Mazas A, Halle L, Rivalland S, Jaeger G, Mbayo K, Bianchi F, Kaplan C. HLA class II polymorphism in Aka Pygmies and Bantu Congolese and a reassessment of HLA-DRB1 African diversity. Tissue Antigens. 2001;58(4):211. doi: 10.1034/j.1399-0039.2001.580401.x. [DOI] [PubMed] [Google Scholar]
- 151.Reveille JD, Arnett FC, Olsen ML, Moulds JM, Papasteriades CA, Moutsopoulos HM. HLA-class II alleles and C4 null genes in Greeks with systemic lupus erythematosus. Tissue Antigens. 1995;46(5):417. doi: 10.1111/j.1399-0039.1995.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 152.Ronningen KS, Spurkland A, Markussen G, Iwe T, Vartdal F, Thorsby E. Distribution of HLA class II alleles among Norwegian Caucasians. Hum Immunol. 1990;29(4):275. doi: 10.1016/0198-8859(90)90041-m. [DOI] [PubMed] [Google Scholar]
- 153.Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, Pandey JP, Newman LS, Magira E, Beznik-Cizman B, Monos D. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet. 2003;73(4):720. doi: 10.1086/378097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sage DA, Evans PR, Cawley MI, Smith JL, Howell WM. HLA DPB1 alleles and susceptibility to rheumatoid arthritis. Eur J Immunogenet. 1991;18(4):259. doi: 10.1111/j.1744-313x.1991.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 155.Sage DA, Evans PR, Howell WM. HLA DPA1-DPB1 linkage disequilibrium in the British caucasoid population. Tissue Antigens. 1994;44(5):335. doi: 10.1111/j.1399-0039.1994.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 156.Saito S, Ota S, Yamada E, Inoko H, Ota M. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens. 2000;56(6):522. doi: 10.1034/j.1399-0039.2000.560606.x. [DOI] [PubMed] [Google Scholar]
- 157.Sanchez-Velasco P, Escribano de Diego J, Paz-Miguel JE, Ocejo-Vinyals G, Leyva-Cobian F. HLA-DR, DQ nucleotide sequence polymorphisms in the Pasiegos (Pas valleys, Northern Spain) and comparison of the allelic and haplotypic frequencies with those of other European populations. Tissue Antigens. 1999;53(1):65. doi: 10.1034/j.1399-0039.1999.530107.x. [DOI] [PubMed] [Google Scholar]
- 158.Sanchez-Velasco P, Gomez-Casado E, Martinez-Laso J, Moscoso J, Zamora J, Lowy E, Silvera C, Cemborain A, Leyva-Cobian F, Arnaiz-Villena A. HLA alleles in isolated populations from North Spain: origin of the Basques and the ancient Iberians. Tissue Antigens. 2003;61(5):384. doi: 10.1034/j.1399-0039.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 159.Sanchez-Velasco P, Leyva-Cobian F. The HLA class I and class II allele frequencies studied at the DNA level in the Svanetian population (Upper Caucasus) and their relationships to Western European populations. Tissue Antigens. 2001;58(4):223. doi: 10.1034/j.1399-0039.2001.580402.x. [DOI] [PubMed] [Google Scholar]
- 160.Sanjeevi CB, Kanungo A, Shtauvere A, Samal KC, Tripathi BB. Association of HLA class II alleles with different subgroups of diabetes mellitus in Eastern India identify different associations with IDDM and malnutrition-related diabetes. Tissue Antigens. 1999;54(1):83. doi: 10.1034/j.1399-0039.1999.540109.x. [DOI] [PubMed] [Google Scholar]
- 161.Savage DA, Middleton D, Trainor F, Taylor A, McKenna PG, Darke C. Frequency of HLA-DPB1 alleles, including a novel DPB1 sequence, in the Northern Ireland population. Hum Immunol. 1992;33(4):235. doi: 10.1016/0198-8859(92)90330-p. [DOI] [PubMed] [Google Scholar]
- 162.Sawitzke AD, Sawitzke AL, Ward RH. HLA-DPB typing using co-digestion of amplified fragments allows efficient identification of heterozygous genotypes. Tissue Antigens. 1992;40(4):175. doi: 10.1111/j.1399-0039.1992.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 163.Shankarkumar U, Ghosh K, Mohanty D. Molecular diversity of HLA-Cw alleles in the Maratha community of Mumbai, Maharashtra, western India. Int J Immunogenet. 2005;32(4):223. doi: 10.1111/j.1744-313X.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 164.Shankarkumar U, Sridharan B, Pitchappan RM. HLA diversity among Nadars, a primitive Dravidian caste of South India. Tissue Antigens. 2003;62(6):542. doi: 10.1046/j.1399-0039.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 165.Shi L, Xu SB, Ohashi J, Sun H, Yu JK, Huang XQ, Tao YF, Yu L, Horai S, Chu JY, Tokunaga K. HLA-A, HLA-B, and HLA-DRB1 alleles and haplotypes in Naxi and Han populations in southwestern China (Yunnan province) Tissue Antigens. 2006;67(1):38. doi: 10.1111/j.1399-0039.2005.00526.x. [DOI] [PubMed] [Google Scholar]
- 166.Sirikong M, Tsuchiya N, Chandanayingyong D, Bejrachandra S, Suthipinittharm P, Luangtrakool K, Srinak D, Thongpradit R, Siriboonrit U, Tokunaga K. Association of HLA-DRB1*1502-DQB1*0501 haplotype with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 2002;59(2):113. doi: 10.1034/j.1399-0039.2002.590206.x. [DOI] [PubMed] [Google Scholar]
- 167.Song EY, Park H, Roh EY, Park MH. HLA-DRB1 and -DRB3 allele frequencies and haplotypic associations in Koreans. Hum Immunol. 2004;65(3):270. doi: 10.1016/j.humimm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 168.Spinola H, Brehm A, Bettencourt B, Middleton D, Bruges-Armas J. HLA class I and II polymorphisms in Azores show different settlements in Oriental and Central islands. Tissue Antigens. 2005;66(3):217. doi: 10.1111/j.1399-0039.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 169.Spinola H, Bruges-Armas J, Middleton D, Brehm A. HLA polymorphisms in Cabo Verde and Guine-Bissau inferred from sequence-based typing. Hum Immunol. 2005;66(10):1082. doi: 10.1016/j.humimm.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 170.Spinola H, Middleton D, Brehm A. HLA genes in Portugal inferred from sequence-based typing: in the crossroad between Europe and Africa. Tissue Antigens. 2005;66(1):26. doi: 10.1111/j.1399-0039.2005.00430.x. [DOI] [PubMed] [Google Scholar]
- 171.Spurkland A, Sollid LM, Ronningen KS, Bosnes V, Ek J, Vartdal F, Thorsby E. Susceptibility to develop celiac disease is primarily associated with HLA-DQ alleles. Hum Immunol. 1990;29(3):157. doi: 10.1016/0198-8859(90)90111-2. [DOI] [PubMed] [Google Scholar]
- 172.Taneja V, Giphart MJ, Verduijn W, Naipal A, Malaviya AN, Mehra NK. Polymorphism of HLA-DRB, -DQA1, and -DQB1 in rheumatoid arthritis in Asian Indians: association with DRB1*0405 and DRB1*1001. Hum Immunol. 1996;46(1):35. doi: 10.1016/0198-8859(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 173.Tokunaga K, Ishikawa Y, Ogawa A, Wang H, Mitsunaga S, Moriyama S, Lin L, Bannai M, Watanabe Y, Kashiwase K, Tanaka H, Akaza T, Tadokoro K, Juji T. Sequence-based association analysis of HLA class I and II alleles in Japanese supports conservation of common haplotypes. Immunogenetics. 1997;46(3):199. doi: 10.1007/s002510050262. [DOI] [PubMed] [Google Scholar]
- 174.Torimiro JN, Carr JK, Wolfe ND, Karacki P, Martin MP, Gao X, Tamoufe U, Thomas A, Ngole EM, Birx DL, McCutchan FE, Burke DS, Carrington M. HLA class I diversity among rural rainforest inhabitants in Cameroon: identification of A*2612-B*4407 haplotype. Tissue Antigens. 2006;67(1):30. doi: 10.1111/j.1399-0039.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 175.Tracey MC, Carter JM. Class II HLA allele polymorphism: DRB1, DQB1 and DPB1 alleles and haplotypes in the New Zealand Maori population. Tissue Antigens. 2006;68(4):297. doi: 10.1111/j.1399-0039.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- 176.Tsuneto LT, Probst CM, Hutz MH, Salzano FM, Rodriguez-Delfin LA, Zago MA, Hill K, Hurtado AM, Ribeiro-dos-Santos AK, Petzl-Erler ML. HLA class II diversity in seven Amerindian populations. Clues about the origins of the Ache. Tissue Antigens. 2003;62(6):512. doi: 10.1046/j.1399-0039.2003.00139.x. [DOI] [PubMed] [Google Scholar]
- 177.Tu B, Mack SJ, Lazaro A, Lancaster A, Thomson G, Cao K, Chen M, Ling G, Hartzman R, Ng J, Hurley CK. HLA-A, -B, -C, -DRB1 allele and haplotype frequencies in an African American population. Tissue Antigens. 2007;69(1):73. doi: 10.1111/j.1399-0039.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- 178.Tuysuz B, Dursun A, Kutlu T, Sokucu S, Cine N, Suoglu O, Erkan T, Erginel-Unaltuna N, Tumay G. HLA-DQ alleles in patients with celiac disease in Turkey. Tissue Antigens. 2001;57(6):540. doi: 10.1034/j.1399-0039.2001.057006540.x. [DOI] [PubMed] [Google Scholar]
- 179.Uinuk-Ool TS, Takezaki N, Derbeneva OA, Volodko NV, Sukernik RI. Variation of HLA class II genes in the Nganasan and Ket, two aboriginal Siberian populations. Eur J Immunogenet. 2004;31(1):43. doi: 10.1111/j.1365-2370.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 180.Uinuk-Ool TS, Takezaki N, Sukernik RI, Nagl S, Klein J. Origin and affinities of indigenous Siberian populations as revealed by HLA class II gene frequencies. Hum Genet. 2002;110(3):209. doi: 10.1007/s00439-001-0668-0. [DOI] [PubMed] [Google Scholar]
- 181.Vambergue A, Fajardy I, Bianchi F, Cazaubiel M, Verier-Mine O, Goeusse P, Fontaine P, Danze PM. Gestational diabetes mellitus and HLA class II (-DQ, -DR) association: The Digest Study. Eur J Immunogenet. 1997;24(5):385. doi: 10.1046/j.1365-2370.1997.d01-114.x. [DOI] [PubMed] [Google Scholar]
- 182.Velickovic ZM, Carter JM. HLA-DPA1 and DPB1 polymorphism in four Pacific Islands populations determined by sequencing based typing. Tissue Antigens. 2001;57(6):493. doi: 10.1034/j.1399-0039.2001.057006493.x. [DOI] [PubMed] [Google Scholar]
- 183.Velickovic ZM, Delahunt B, Carter JM. HLA-DRB1 and HLA-DQB1 polymorphisms in Pacific Islands populations. Tissue Antigens. 2002;59(5):397. doi: 10.1034/j.1399-0039.2002.590506.x. [DOI] [PubMed] [Google Scholar]
- 184.Vidal S, Morante MP, Moga E, Mosquera AM, Querol S, Garcia J, Rodriguez-Sanchez J. Molecular analysis of HLA-DRB1 polymorphism in north-east Spain. Eur J Immunogenet. 2002;29(1):75. doi: 10.1046/j.0960-7420.2001.00293.x. [DOI] [PubMed] [Google Scholar]
- 185.Volpini WM, Testa GV, Marques SB, Alves LI, Silva ME, Dib SA, Guerra JG, Paulino MF, Marini SH, Persoli LB, Caillat-Zucman S. Family-based association of HLA class II alleles and haplotypes with type I diabetes in Brazilians reveals some characteristics of a highly diversified population. Hum Immunol. 2001;62(11):1226. doi: 10.1016/s0198-8859(01)00323-8. [DOI] [PubMed] [Google Scholar]
- 186.Vullo CM, Delfino L, Angelini G, Ferrara GB. HLA polymorphism in a Mataco South American Indian tribe: serology of class I and II antigens. Molecular analysis of class II polymorphic variants. Hum Immunol. 1992;35(4):209. doi: 10.1016/0198-8859(92)90001-4. [DOI] [PubMed] [Google Scholar]
- 187.Wang FQ, al-Daccak R, Ju LY, Lethielleux P, Charron D, Loiseau P, Colombani J. HLA-DP distribution in Shanghai Chinese--a study by polymerase chain reaction--restriction fragment length polymorphism. Hum Immunol. 1992;33(2):129. doi: 10.1016/0198-8859(92)90063-s. [DOI] [PubMed] [Google Scholar]
- 188.Williams F, Meenagh A, Darke C, Acosta A, Daar AS, Gorodezky C, Hammond M, Nascimento E, Middleton D. Analysis of the distribution of HLA-B alleles in populations from five continents. Hum Immunol. 2001;62(6):645. doi: 10.1016/s0198-8859(01)00247-6. [DOI] [PubMed] [Google Scholar]
- 189.Wongsurawat T, Nakkuntod J, Charoenwongse P, Snabboon T, Sridama V, Hirankarn N. The association between HLA class II haplotype with Graves' disease in Thai population. Tissue Antigens. 2006;67(1):79. doi: 10.1111/j.1399-0039.2005.00498.x. [DOI] [PubMed] [Google Scholar]
- 190.Wu Z, Stephens HA, Sachs JA, Biro PA, Cutbush S, Magzoub MM, Becker C, Schwartz G, Bottazzo GF. Molecular analysis of HLA-DQ and -DP genes in caucasoid patients with Hashimoto's thyroiditis. Tissue Antigens. 1994;43(2):116. doi: 10.1111/j.1399-0039.1994.tb02310.x. [DOI] [PubMed] [Google Scholar]
- 191.Yang G, Deng YJ, Hu SN, Wu DY, Li SB, Zhu J, Zhu BF, Liu Y. HLA-A, -B, and -DRB1 polymorphism defined by sequence-based typing of the Han population in Northern China. Tissue Antigens. 2006;67(2):146. doi: 10.1111/j.1399-0039.2006.00529.x. [DOI] [PubMed] [Google Scholar]
- 192.Yao Z, Hartung K, Deicher HG, Brunnler G, Bettinotti MP, Keller E, Paul C, Gawron C, Mikschl S, Albert E. DNA typing for HLA-DPB1-alleles in German patients with systemic lupus erythematosus using the polymerase chain reaction and DIG-ddUTPlabelled oligonucleotide probes Members of SLE Study Group. Eur J Immunogenet. 1993;20(4):259. doi: 10.1111/j.1744-313x.1993.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 193.Yoshida M, Ohtsuka R, Nakazawa M, Juji T, Tokunaga K. HLA-DRB1 frequencies of non-Austronesian-speaking Gidra in south New Guinea and their genetic affinities with Oceanian populations. Am J Phys Anthropol. 1995;96(2):177. doi: 10.1002/ajpa.1330960206. [DOI] [PubMed] [Google Scholar]
- 194.Yunis I, Salazar M, Alosco SM, Gomez N, Yunis EJ. HLA-DQA1 and MLC among HLA (generic)-identical unrelated individuals. Tissue Antigens. 1992;39(4):182. doi: 10.1111/j.1399-0039.1992.tb01934.x. [DOI] [PubMed] [Google Scholar]
- 195.Yunis JJ, Ossa H, Salazar M, Delgado MB, Deulofeut R, de la Hoz A, Bing DH, Ramos O, Yunis EJ, Yunis EJ. Major histocompatibility complex class II alleles and haplotypes and blood groups of four Amerindian tribes of northern Colombia. Hum Immunol. 1994;41(4):248. doi: 10.1016/0198-8859(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 196.Zhao YP, Yang JQ, Ge Y, Fan LA, Loiseau P, Colombani J. HLA-DR and -DQB1 genotyping in a Chinese population. Eur J Immunogenet. 1993;20(4):293. doi: 10.1111/j.1744-313x.1993.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 197.Zhou L, Lin B, Xie Y, Liu Z, Yan W, Xu A. Polymorphism of human leukocyte antigen-DRB1, -DQB1, and -DPB1 genes of Shandong Han population in China. Tissue Antigens. 2005;66(1):37. doi: 10.1111/j.1399-0039.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- 198.Zimdahl H, Schiefenhovel W, Kayser M, Roewer L, Nagy M. Towards understanding the origin and dispersal of Austronesians in the Solomon Sea: HLA class II polymorphism in eight distinct populations of Asia-Oceania. Eur J Immunogenet. 1999;26(6):405. doi: 10.1046/j.1365-2370.1999.00183.x. [DOI] [PubMed] [Google Scholar]
- 199.Mack SJ, Erlich HA. Anthropology / Human Genetic Diversity Joint Report. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 557. [Google Scholar]
- 200.Bodmer J, Cambon-Thomsen A, Hors J, Piazza A, Sanchez-Mazas A. Report of the Anthropology Component. In: Charron D, editor. HLA: Proceedings of the Twelfth International Histocompatibility Workshop and Conference. vol 1. EDK; 1997. p. 269. [Google Scholar]
- 201.Middleton D, Menchaca L, Rood H, Komerofsky R. New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens. 2003;61:403. doi: 10.1034/j.1399-0039.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 202.Lancaster A, Nelson MP, Meyer D, Single RM, Thomson G. PyPop: a software framework for population genomics: analyzing large-scale multi-locus genotype data. Pac Symp Biocomput. 2003;8:514. [PMC free article] [PubMed] [Google Scholar]
- 203.Lancaster AK. Identifying associations between natural selection and molecular function in human MHC genes. Berkeley: University of California; 2006. [Google Scholar]
- 204.Lancaster AK, Nelson MP, Single RM, Meyer D, Thomson G. Software framework for the Biostatistics Core of the International Histocompatibility Working Group. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 510. [Google Scholar]
- 205.Lancaster AK, Single RM, Solberg OD, Nelson MP, Thomson G. PyPop update--a software pipeline for large-scale multilocus population genomics. Tissue Antigens. 2007;69 Suppl 1:192. doi: 10.1111/j.1399-0039.2006.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Single RM, Meyer D, Mack SJ, Lancaster A, Erlich HA, Thomson G. 14th International HLA and Immunogenetics Workshop: report of progress in methodology, data collection, and analyses. Tissue Antigens. 2007;69 Suppl 1:185. doi: 10.1111/j.1399-0039.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 207.Single RM, Meyer D, Thomson G. Statistical methods for analysis of population genetic data. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 518. [Google Scholar]
- 208.Mack SJ, Tsai Y, Sanchez-Mazas A, Erlich HA. Anthropology / Human Genetic Diversity Population Reports. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 580. [Google Scholar]
- 209.Meyer D, Single RM, Mack SJ, Lancaster AK, Nelson MP, Erlich HA, Fernández-Viña MA, Thomson G. Single locus polymorphism of classical HLA genes. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 653. [Google Scholar]
- 210.Single RM, Meyer D, Mack SJ, Lancaster AK, Nelson MP, Erlich HA, Fernández-Viña MA, Thomson G. Haplotype frequencies and linkage disequilibrium among classical HLA genes. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol I. Seattle: IHWG Press; 2007. p. 705. [Google Scholar]
- 211.Robinson J, Waller MJ, Fail SC, Marsh SG. The IMGT/HLA and IPD databases. Hum Mutat. 2006;27:1192. doi: 10.1002/humu.20406. [DOI] [PubMed] [Google Scholar]
- 212.Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31:311. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cano P, Klitz W, Mack SJ, Maiers M, Marsh SG, Noreen H, Reed EF, Senitzer D, Setterholm M, Smith A, Fernández-Viña M. Common and well-documented HLA alleles: report of the Ad-Hoc committee of the american society for histocompatiblity and immunogenetics. Hum Immunol. 2007;68:392. doi: 10.1016/j.humimm.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 214.Ewens W. The sampling theory of selectively neutral alleles. Theor Popul Biol. 1972;3:87. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- 215.Watterson G. The homozygosity test of neutrality. Genetics. 1978;88:405. doi: 10.1093/genetics/88.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Slatkin M. A correction to the exact test based on the Ewens sampling distribution. Genet Res. 1996;68:259. doi: 10.1017/s0016672300034236. [DOI] [PubMed] [Google Scholar]
- 217.Slatkin M. An exact test for neutrality based on the Ewens sampling distribution. Genet Res. 1994;64:71. doi: 10.1017/s0016672300032560. [DOI] [PubMed] [Google Scholar]
- 218.Salamon H, Klitz W, Easteal S, Gao X, Erlich HA, Fernández-Viña M, Trachtenberg EA, McWeeney SK, Nelson MP, Thomson G. Evolution of HLA class II molecules: Allelic and amino acid site variability across populations. Genetics. 1999;152:393. doi: 10.1093/genetics/152.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tsai Y, Thomson G. Selection intensity differences in seven HLA loci in many populations. In: Hansen JA, editor. Immunobiology of the MHC: Proceedings of the 13th International Histocompatibility Workshop and Conference. vol II. Seattle: IHWG Press; 2007. p. 199. [Google Scholar]
- 220.Felsenstein J. PHYLIP - Phylogeny Inference Package (Version 3.2) Cladistics. 1989;5:164. [Google Scholar]
- 221.Wessel P, Smith W. New, improved version of generic mapping tools released. EOS Transactions. 1998;79:579. [Google Scholar]
- 222.Hedrick P, Thomson G. Evidence for balancing selection at HLA. Genetics. 1983;104:449. doi: 10.1093/genetics/104.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Klitz W, Thomson G, Borot N, Cambon-Thomsen A. Evolutionary and population perspectives of the human HLA complex. Evolutionary Biology. 1992;26:35. [Google Scholar]
- 224.Klitz W, Thomson G, Baur M. Contrasting evolutionary histories among tightly linked HLA loci. Am J Hum Genet. 1986;39:340. [PMC free article] [PubMed] [Google Scholar]
- 225.Markow T, Hedrick PW, Zuerlein K, Danilovs J, Martin J, Vyvial T, Armstrong C. HLA polymorphism in the Havasupai: evidence for balancing selection. Am J Hum Genet. 1993;53:943. [PMC free article] [PubMed] [Google Scholar]
- 226.Valdes AM, McWeeney SK, Meyer D, Nelson MP, Thomson G. Locus and population specific evolution in HLA class II genes. Ann Hum Genet. 1999;63:27. doi: 10.1046/j.1469-1809.1999.6310027.x. [DOI] [PubMed] [Google Scholar]
- 227.Hollenbach JA, Thomson G, Cao K, Fernández-Viña MA, Erlich HA, Bugawan TL, Winkler C, Winter M, Klitz W. HLA diversity, differentiation, and haplotype evolution in Mesoamerican Natives. Hum Immunol. 2001;62:378. doi: 10.1016/s0198-8859(01)00212-9. [DOI] [PubMed] [Google Scholar]
- 228.Renquin J, Sanchez-Mazas A, Halle L, Rivalland S, Jaeger G, Mbayo K, Bianchi F, Kaplan C. HLA class II polymorphism in Aka Pygmies and Bantu Congolese and a reassessment of HLA-DRB1 African diversity. Tissue Antigens. 2001;58:211. doi: 10.1034/j.1399-0039.2001.580401.x. [DOI] [PubMed] [Google Scholar]
- 229.Cao K, Moormann AM, Lyke KE, Masaberg C, Sumba OP, Doumbo OK, Koech D, Lancaster A, Nelson M, Meyer D, Single R, Hartzman RJ, Plowe CV, Kazura J, Mann DL, Sztein MB, Thomson G, Fernández-Viña MA. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens. 2004;63:293. doi: 10.1111/j.0001-2815.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 230.Williams F, Meenagh A, Single R, McNally M, Kelly P, Nelson MP, Meyer D, Lancaster A, Thomson G, Middleton D. High resolution HLA-DRB1 identification of a Caucasian population. Hum Immunol. 2004;65:66. doi: 10.1016/j.humimm.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 231.Trachtenberg E, Vinson M, Hayes E, Hsu YM, Houtchens K, Erlich H, Klitz W, Hsia Y, Hollenbach J. HLA class I (A, B, C) and class II (DRB1, DQA1, DQB1, DPB1) alleles and haplotypes in the Han from southern China. Tissue Antigens. 2007;70:455. doi: 10.1111/j.1399-0039.2007.00932.x. [DOI] [PubMed] [Google Scholar]
- 232.Tishkoff SA, Kidd KK. Implications of biogeography of human populations for 'race' and medicine. Nat Genet. 2004;36:S21. doi: 10.1038/ng1438. [DOI] [PubMed] [Google Scholar]
- 233.Aguilar A, Roemer G, Debenham S, Binns M, Garcelon D, Wayne RK. High MHC diversity maintained by balancing selection in an otherwise genetically monomorphic mammal. Proc Natl Acad Sci U S A. 2004;101:3490. doi: 10.1073/pnas.0306582101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sanchez-Mazas A, Poloni ES, Jacques G, Sagart L. HLA genetic diversity and linguistic variation in East Asia. In: Sagart L, Blench R, Sanchez-Mazas A, editors. The peopling of East Asia : Putting together Archaeology, Linguistics and Genetics. London and New York: RoutledgeCurzon; 2005. p. 273. [Google Scholar]
- 235.Liu H, Prugnolle F, Manica A, Balloux F. A geographically explicit genetic model of worldwide human-settlement history. Am J Hum Genet. 2006;79(2):230. doi: 10.1086/505436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Norman PJ, Abi-Rached L, Gendzekhadze K, Korbel D, Gleimer M, Rowley D, Bruno D, Carrington CV, Chandanayingyong D, Chang YH, Crespi C, Saruhan-Direskeneli G, Fraser PA, Hameed K, Kamkamidze G, Koram KA, Layrisse Z, Matamoros N, Mila J, Park MH, Pitchappan RM, Ramdath DD, Shiau MY, Stephens HA, Struik S, Verity DH, Vaughan RW, Tyan D, Davis RW, Riley EM, Ronaghi M, Parham P. Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat Genet. 2007;39(9):1092. doi: 10.1038/ng2111. [DOI] [PubMed] [Google Scholar]
- 237.Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, Kidd KK, Carrington M. Global diversity and evidence for coevolution of KIR and HLA. Nat Genet. 2007;39(9):1114. doi: 10.1038/ng2077. [DOI] [PubMed] [Google Scholar]
- 238.Handley LJ, Manica A, Goudet J, Balloux F. Going the distance: human population genetics in a clinal world. Trends Genet. 2007;23(9):432. doi: 10.1016/j.tig.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 239.Ramachandran S, Deshpande O, Roseman CC, Rosenberg NA, Feldman MW, Cavalli-Sforza LL. Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proc Natl Acad Sci U S A. 2005;102(44):15942. doi: 10.1073/pnas.0507611102. [DOI] [PMC free article] [PubMed] [Google Scholar]