Abstract
Retinoic acid, a derivative of vitamin A, is an essential component of cell-cell signaling during vertebrate organogenesis. In early development retinoic acid functions as a trunk organizer by providing an instructive signal for posterior neuroectoderm and foregut endoderm and a permissive signal for trunk mesoderm differentiation. At later stages, retinoic acid contributes to the development of the eye and other organs. Recent efforts suggest that retinoic acid acts primarily in a paracrine manner and provide insight into the cell-cell signaling networks that control differentiation of pluripotent cells.
Introduction
Retinoic acid (RA) is a small lipophilic molecule (M.W. 300) derived from vitamin A that stands apart from other diffusible cell-cell signaling factors that direct developmental processes for many reasons. In stark contrast with protein factors, such as fibroblast growth factor (FGF), WNT, hedgehog, or the transforming growth factor-beta (TGFβ) superfamily, that bind cell-surface receptors and initiate intracellular signaling pathways, RA enters the nucleus and directly binds to target genes via nuclear receptors. RA signaling also differs from the major classes of protein growth factors in that it appears to be a chordate invention (Marlétaz et al., 2006). The most convincing evidence that RA signaling is limited to chordates is the observation that only chordates appear to possess the retinaldehyde dehydrogenase (RALDH) enzymes needed to synthesize RA. Thus, during chordate evolution RA signaling was layered on top of many other pre existing cell-cell signaling pathways. RA regulates many of the same developmental processes that are controlled by protein growth factors including neurogenesis, cardiogenesis, body axis extension, and development of the forelimb buds, foregut, and eye. In addition, recent studies indicate that RA signaling represses several of these growth factor signaling pathways.
Retinoic Acid Synthesis, Degradation, and Signaling
The ability of vitamin A to influence development is made possible by enzymes controlling the conversion of the alcohol form of vitamin A (retinol) first to an aldehyde (retinaldehyde) and then to a carboxylic acid (retinoic acid; RA) (Figure 1). The first step of RA synthesis, oxidation of retinol to retinaldehyde, is catalyzed by several alcohol dehydrogenases (ADHs) and retinol dehydrogenases (RDHs). Genetic studies suggest that at least three ADHs (ADH1, ADH3, and ADH4) and two RDHs (RDH1 and RDH10) play a physiological role in RA synthesis (Table 1). Expression of these retinol-oxidizing enzymes is widespread and overlapping (Ang et al., 1996; Zhang et al., 2001; Sandell et al., 2007). The second step of RA synthesis, oxidation of retinaldehyde to RA, is catalyzed by three retinaldehyde dehydrogenases (RALDH1, RALDH2, and RALDH3), which display non-overlapping tissue-specific patterns of expression during embryogenesis (Mic et al., 2002) (Table 1). Oxidation of RA, which leads to its degradation, is carried out by three cytochrome P450 (CYP) enzymes known as CYP26A1 (Abu-Abed et al., 2001), CYP26B1 (Yashiro et al., 2004), and CYP26C1 (Uehara et al., 2007). These enzymes also display unique tissue-specific patterns of expression during mouse embryogenesis, suggesting that they influence where RA signaling is able to occur in the embryo.
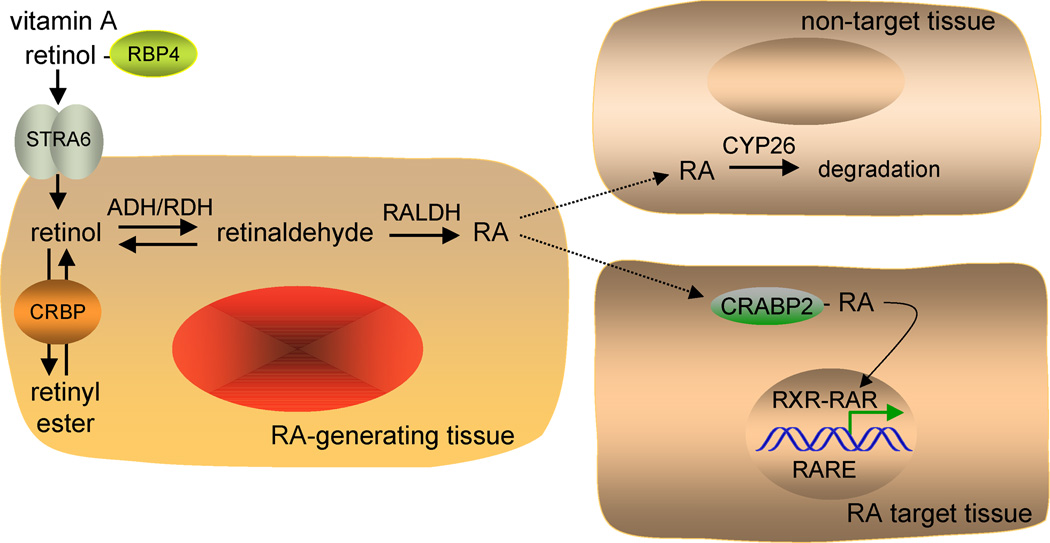
Figure 1. Retinoic acid synthesis and signaling.
Depicted is the paracrine mechanism of retinoic acid (RA) signaling. Retinol is carried in the serum by retinol-binding protein (RBP4) secreted from the liver. Retinol enters cells via a specific receptor STRA6, and cellular retinol-binding protein (CRBP) facilitates conversion of retinol to retinyl esters for storage. In an RA-generating tissue, retinol is oxidized to retinaldehyde by either alcohol dehydrogenase (ADH) or retinol dehydrogenase (RDH), and retinaldehyde is oxidized to RA by retinaldehyde dehydrogenase (RALDH). RA is then released and taken up by surrounding cells. Cells that express cytochrome P450 (CYP26) initiate the further oxidation of RA for degradation and excretion and are not RA target cells. Some RA target cells express cellular-RA binding protein (CRABP) that facilitates uptake of RA and transport to the nucleus where RA binds the RA receptor (RAR). The ternary complex of ligand bound-RAR with RXR and a retinoic acid response element (RARE) regulates transcription of RA target genes by altering the binding of corepressors and coactivators.
Table 1.
Enzymes involved in RA synthesis during early organogenesis in mouse.
| Gene | Major sites of expression | Knockout phenotype |
|---|---|---|
| Retinaldehyde Dehydrogenases (oxidation of retinaldehyde to RA) | ||
| Raldh1 | dorsal retina at E9.5 | non-lethal; adults fertile; perioptic mesenchyme defect when Raldh3 also null; protects against obesity in adult |
| Raldh2 | paraxial mesoderm at E7.5; somites; lateral plate mesoderm; optic vesicle mesenchyme; mesonephros; ventral spinal cord | lethal at E9.5; hindbrain and spinal cord defects; small somites with left-right asymmetry; forelimb bud absent; abnormal heart with medial distended cavity; lung and pancreas agenesis; ventral retina defect when Raldh3 also null |
| Raldh3 | lens and nasal placodes at E8.75; retinal pigment epithelium; ventral retina; mesonephros; ventral forebrain | lethal at birth (blockage of nasal passages); ventral retina defect; forebrain striatal defect; perioptic mesenchyme defect when Raldh1 also null |
| Alcohol/Retinol Dehydrogenases (oxidation of retinol to retinaldehyde) | ||
| Adh1 | mesonephros at E9.5; liver; kidney | non-lethal; adults fertile; hypersensitive to vitamin A toxicity; increased retinyl esters |
| Adh3 | ubiquitous from E6.5 to adult | postnatal growth deficiency; adults fertile but smaller litters; hypersensitive to vitamin A deficiency |
| Adh4 | posterior mesoderm at E7.5; craniofacial mesenchyme; stomach | non-lethal; adults fertile; hypersensitive to vitamin A deficiency |
| Rdh1 | widespread at E7.5; liver; kidney | non-lethal; adults fertile; increased weight and adiposity; increased retinyl esters |
| Rdh10 | lateral plate mesoderm at E7.5; somites; floor plate of neural tube | lethal at E13.0; small forelimbs; craniofacial defects; lung agenesis |
RA serves as a ligand for two families of nuclear receptors that bind DNA and directly regulate transcription: (1) the RA receptors (RARα, RARβ, and RARγ) which bind the abundant form of RA known as all-trans-RA, and (2) the retinoid X receptors (RXRα, RXRβ, and RXRγ) which bind an isomer known as 9-cis-RA (Chawla et al., 2001). However, 9-cis-RA is normally undetectable except when vitamin A is present in excess (Arnhold et al., 1996; Mic et al., 2003). Hence, it may play a pharmacological but not a physiological role as an RXR ligand. As RXR forms heterodimers with RAR and several other nuclear receptors when bound to DNA, this suggests that RXR functions as a scaffold protein to facilitate DNA-binding for several different types of nuclear receptors (Chawla et al., 2001). In vivo studies have demonstrated that ligand binding to just the RAR portion of RAR/RXR heterodimers is sufficient and necessary to rescue a lethal defect in RA synthesis, whereas ligand binding to RXR does not rescue the defect and is unnecessary (Mic et al., 2003). When RA binds to the RAR partner of RAR/RXR heterodimers bound to a regulatory DNA element, this stimulates a cascade of events resulting in recruitment of transcriptional coactivators and initiation of transcription (Germain et al., 2002).
RA is not produced by all cells of the body at all stages of development, but is instead produced in a unique spatiotemporal pattern. Retinol is secreted by the liver and transported in the blood at micromolar levels via serum retinol-binding protein (RPB4) and is made available to all cells (including embryonic cells by maternal transfer) for potential conversion to RA (Quadro et al., 1999). Many cells possess STRA6, which functions as a membrane receptor for RBP4 to facilitate retinol uptake (Kawaguchi et al., 2007). Many cells also contain cellular retinol-binding proteins (CRBPs) that bind retinol inside the cell (Noy, 2000). CRBP1 has been proposed to facilitate conversion of retinol to retinyl esters for storage and to facilitate oxidation of retinol to retinaldehyde by RDHs, but not ADHs, for RA synthesis. These findings suggested that RDHs but not ADHs are important in endogenous RA synthesis. However, recent comparisons of ADH and RDH retinol enzymatic activity in the presence or absence of CRBP1 concluded that free retinol but not CRBP1-bound retinol is the substrate for both enzyme families (Gallego et al., 2006). Also, mice lacking CRBP1 do not exhibit decreased RA synthesis but do have greatly reduced stores of liver retinyl esters, and are thus very sensitive to vitamin A deficiency (Ghyselinck et al., 1999). Interestingly, mice lacking ADH1 have higher than normal levels of liver retinyl esters, and mice deficient in both ADH1 and CRBP1 have relatively normal levels of liver retinyl esters and reduced sensitivity to vitamin A deficiency (Molotkov et al., 2004). Thus, ADH1 and CRBP1 have opposing roles in the liver that prevent toxic accumulation of retinol but still enable a large fraction of retinol to become esterifed as a stored form, thus providing a continuous source of retinol to be secreted into the blood for use in peripheral tissues including embryos.
Studies on mice carrying null mutations in ADH and RDH enzymes suggest that oxidation of retinol to retinaldehyde may be controlled in vivo by multiple genes (Table 1). Mice lacking ADH1 are susceptible to retinol toxicity, yet are otherwise phenotypically normal, indicating that oxidation of retinol by ADH1 contributes to removal of excess retinol (via further metabolism to RA and oxidized forms of RA) rather than contributing to RA needed for signaling (Molotkov et al., 2002a). In contrast, loss of ADH3 and ADH4 impairs postnatal survival during vitamin A deficiency suggesting potential roles in RA synthesis designed for RA signaling (Molotkov et al., 2002b). However, given that ADH3 has very low activity for retinol oxidation (Molotkov et al., 2002b), the effect of its knockout may be unrelated to RA synthesis. In comparison, ADH4 and RDH1 are very efficient in retinol oxidation (Zhang et al., 2001; Gallego et al., 2006), yet mice lacking ADH4 and RDH1 have not been shown to display detectable alterations in embryonic RA signaling (Molotkov et al., 2002b; Zhang et al., 2007). Among these genes, only mutants in Rdh10 have a serious defect in embryonic RA signaling resulting in embryonic lethality at embryonic day 13 (E13) (Sandell et al., 2007). Rdh10 mutant embryos still maintain RA signaling in some embryonic tissues, suggesting that ADH1, ADH3, ADH4, or RDH1, which are all expressed in embryos, may also function to generate retinaldehyde for RA synthesis.
Retinol oxidation is a reversible reaction. Hence, the ability to convert retinaldehyde back to retinol, a reaction that multiple enzymes can accomplish, may provide further control over RA synthesis (Gallego et al., 2006). In contrast, oxidation of retinaldehyde to RA is irreversible. Given the widespread access to retinol via the circulatory system, it is possible that all cells establish an equilibrium between retinol and retinaldehyde, but only cells expressing one of the RALDHs can oxidize the available retinaldehyde to RA.
Conversion of retinol to RA occurs at relatively low levels, but RA has been detected in mouse embryos using sensitive RA-reporter assays. Evidence from a mouse strain that bears a transgene expressing lacZ under the control of a retinoic acid response element (RARE) indicates that RA signaling activity is first observed at E7.5, the late primitive streak stage, and is localized to the trunk from E7.5–E8.5 (Rossant et al., 1991). An RA-reporter cell line was used to demonstrate that mouse embryo explants from E7.5, but not E6.5, have detectable RA activity (Ang et al., 1996). The RA biosynthetic enzyme RALDH2 is first expressed at E7.5 in trunk paraxial mesoderm and by E8.5 displays expression in paraxial and lateral plate mesoderm that appears quite similar to the pattern of RA localization (Sirbu et al., 2005; Molotkova et al., 2005). Studies on mice lacking RALDH2 have shown that it is responsible for all RA signaling activity in the embryo from E7.5 (Sirbu et al., 2005) to E8.5 (Molotkova et al., 2005), but that immediately after this stage RALDH1 and RALDH3 also contribute to RA synthesis in the eye and olfactory pit (Molotkov et al., 2006).
RA released by RA-generating cells can enter adjacent cells where it has two main fates (Figure 1). In cells expressing one of the Cyp26 genes, RA is degraded and RA signaling is prevented (Hernandez et al., 2007; Uehara et al., 2007) . In cells not expressing Cyp26, RA can enter the nucleus and bind to RAR receptors leading to transcription of target genes. Additionally, some cells express cellular RA-binding protein-2 (CRABP2), which greatly facilitates cellular uptake of RA and transfer to the nucleus (Sessler and Noy, 2005; Schug et al., 2007).
Retinoic Acid Influences Induction and Patterning of Embryonic Tissues
Vitamin A is unique among the vitamins in that its concentration must be within a very narrow range in order to avoid both deficiency and toxicity. Thus, adding vitamin A or RA to embryos can easily induce teratogenic effects including major alterations in organogenesis. However studying the teratogenic effects of RA are often not useful for determining its physiological role given that high levels of RA may induce or repress genes not normally regulated by endogenous RA. Thus, loss-of-function studies are necessary to determine the normal function of RA during organogenesis.
Early studies on the embryonic effects of a loss of RA were performed using vitamin A deficiency (Clagett-Dame and DeLuca, 2002; Dersch and Zile, 1993; Dickman et al., 1997) or compound RAR mutations (Lohnes et al., 1994; Mendelsohn et al., 1994). These early studies indicated that RA was essential for development of several organs including the hindbrain, spinal cord, heart, eye, skeleton, forelimb buds, lung, pancreas and genitourinary tract. More recently, the use of RALDH mutations that completely eliminate RA synthesis in specific tissues at early stages of development has made it possible to examine the mechanism of RA action in detail as described below. Cumulatively, these studies show that RA provides an instructive signal for posterior neuroectoderm (hindbrain, spinal cord) and posterior foregut endoderm (pancreas, lung), and a permissive signal for trunk mesoderm (somites, heart, forelimb) in early development. At later stages RA contributes to the development of the eye, and other organs. Defects in the forelimb bud, lung, and pancreas can be characterized as defects in induction of these tissues, as a loss of RA inhibits organogenesis. In contrast, defects in hindbrain, spinal cord, heart, somites, and eye occur after organogenesis has been induced and can be characterized as defects in patterning or morphogenesis of these tissues. Many important RA target genes in these tissues have been found (Table 2).
Table 2.
Key target genes regulated by retinoic acid during early organogenesis
| DEVELOPMENTAL PROCESS | KEY TARGET GENES |
|---|---|
| Hindbrain anteroposterior patterning | ↑Hoxa1, Hoxb1, Hoxa3, Hoxd4, vHnf1 |
| Spinal cord motor neuron differentiation | ↑Pax6, Olig2 |
| Formation of early somites | ↓Fgf8 ↑Cdx1 |
| Heart anteroposterior patterning | ↓Fgf8 |
| Forelimb induction | ↓Fgf8? |
| Pancreas induction | ↑ Pdx1 |
| Lung induction | ↑Hoxa5 ↓TGFβ1 |
| Optic cup formation | ? |
| Anterior eye formation | ↑ Pitx2 |
| Kidney formation | ↑ Ret |
| Induction of meiosis | ↑ Stra8 |
Expression (↑)induced or repressed (↓).
Retinoic Acid Acts as a Trunk Organizer
RA generated by RALDH2 in the presomitic mesoderm is released and travels to the adjacent posterior neuroectoderm and posterior foregut endoderm where it induces various homeobox genes in posterior hindbrain and trunk tissues. This instructive mechanism of RA action is widely accepted as the main role of RA signaling for early organogenesis. However, recent findings indicate RA can also influence trunk development in a permissive fashion through its ability to repress caudal Fgf8 expression to ensure proper spinal cord neuronal differentiation (Del Corral et al., 2003) and somitogenesis (Vermot et al., 2005; Sirbu and Duester, 2006) during body axis extension. A major target of RA repression during body axis extension has been found at the junction of the epiblast and neuroectoderm where RA restricts Fgf8 expression to epiblast (primitive ectoderm), thus preventing it from extending anteriorly into node ectoderm and neuroectoderm (Sirbu and Duester, 2006). RA also represses caudal Wnt8 expression which plays a role in regulating Raldh2 expression in presomitic mesoderm, suggesting that Wnt signaling may control the timing of the caudal RA-FGF differentiation switch (Olivera-Martinez and Storey, 2007). Additionally, RA represses Fgf8 in the posterior mesoderm of the cardiac field (Ryckebusch et al., 2008; Sirbu et al., 2008). Thus, in the absence of RA signaling the epiblast Fgf8 domain moves further to the anterior and the cardiac Fgf8 domain moves further to the posterior. As RA signaling restricts the anteroposterior limits of each of the two early Fgf8 expression domains (cardiac and epiblast), it creates an FGF8-free zone in between where the trunk develops (Figure 2). Overall, instructive and permissive actions of RA signaling during late gastrulation function to organize the embryonic trunk by allowing trunk progenitor cells to properly differentiate. Further evidence that RA functions as a trunk organizer has come from studies on embryos treated with excess RA, which transforms head structures such as forebrain/midbrain and anterior foregut endoderm to trunk neuroectoderm (hindbrain/spinal cord) and trunk foregut endoderm fates, respectively (Conlon and Rossant, 1992; Stafford and Prince, 2002).
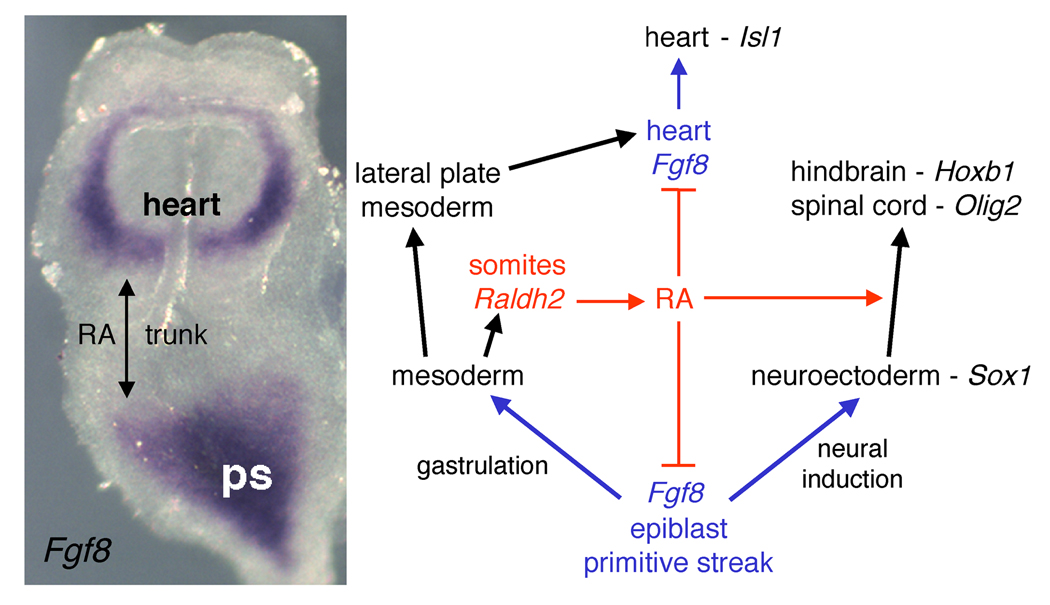
Figure 2. Retinoic acid functions as a trunk organizer by repressing Fgf8.
Shown on the left is an E8.0 mouse embryo stained for fibroblast growth factor 8 (Fgf8) mRNA by in situ hybridization. At this stage Fgf8 mRNA is expressed in an anterior cardiac domain where it induces Isl1, plus a posterior domain encompassing the epiblast and primitive streak (ps) where FGF8 is needed during gastrulation and neural induction; retinaldehyde dehydrogenase 2 (Raldh2) is expressed in between these two domains in the somitic mesoderm where the future trunk will form. The diagram depicts the function of retinoic acid (RA) as a repressor of both Fgf8 domains and as an inducer of neural posteriorization in the trunk to allow hindbrain and spinal cord differentiation.
As Fgf8 is expressed prior to Raldh2 , FGF8 signaling is already underway prior to the advent of RA signaling at E7.5 in mouse embryos (Sirbu et al., 2005). RA antagonism of FGF8 signaling along the anteroposterior axis then allows cells at the boundaries of these two signaling pathways to quickly transition away from FGF8 signaling and begin responding to RA signaling. Fgf8 repression in the developing trunk during late gastrulation can thus be considered a fundamental permissive function of RA needed for trunk development, which is simultaneous to the well-established instructive role of RA as an inducer of homeobox genes in the posterior neuroectoderm and posterior foregut endoderm.
Neuroectoderm-derived tissues
The endogenous concentration of RA in mouse embryos is approximately 25 nM (Mic et al., 2003). Treatment of embryonic stem cells or embryonal carcinoma cells with levels of RA above 100 nM can induce a neural fate (Cai and Grabel, 2007), although studies on the normal function of RA in mouse embryos have demonstrated that neural induction does not require RA (Molotkova et al., 2005). The fact that RA is not synthesized in mouse embryos until E7.5 (well after induction of forebrain and midbrain neuroectoderm) is one indication that RA is not required for neural induction (Sirbu et al., 2005). Also, RA generated in the somitic mesoderm (E7.5–E8.5) stimulates RA signaling only in posterior neural tissues, and a loss of RA signaling does not effect expression of the neural induction markers Sox1 and Sox2 (Molotkova et al., 2005).
Instead, RA signaling acts upon neuroectoderm to influence its further differentiation. Investigation of embryos from single, double, and triple knockout mice lacking expression of Raldh1, Raldh2, and/or Raldh3 indicates that the only action of RA required during early stages of neural development (up to E10.5) is in the posterior neuroectoderm (hindbrain and spinal cord) and eye (an outpocketing of the forebrain), but not in the forebrain itself (Molotkov et al., 2006; Molotkova et al., 2007). However, at later stages (after E12.5) Raldh3 expression in the ventral forebrain is required for differentiation within the striatum, particularly for induction of dopamine receptor D2 in the nucleus accumbens (Molotkova et al., 2007).
RA treatment of amphibian embryos results in a loss of forebrain/midbrain accompanied by an anterior advance of hindbrain suggesting that RA may be one of the factors regulating posterior transformation of the nervous system (Durston et al., 1989; Sive et al., 1990). Indeed, vitamin A deficient quail embryos and mouse embryos completely lacking RA signaling exhibit hindbrain defects, indicating that endogenous RA transforms the posterior neuroectoderm after neural induction occurs (Maden et al., 1996; Niederreither et al., 2000; Molotkova et al., 2005; Sirbu et al., 2005).
Hindbrain Patterning
Hox genes exhibit differential expression along the anteroposterior axis of the developing hindbrain and are intimately involved in rhombomere formation and identity (Krumlauf, 1993). A major function of RA signaling in the hindbrain involves control of Hox gene expression (Maden et al., 1996; Dupé and Lumsden, 2001). Several members of the Hox gene family are direct targets of RA signaling including Hoxb1 (Simeone et al., 1990), which is required for facial motor neuron differentiation in rhombomere 4 (r4). Prior to rhombomere formation, Hoxb1 is expressed throughout the posterior hindbrain up to the presumptive r3/r4 border, but soon becomes restricted to r4 (Wilkinson et al., 1989). Hoxb1 is regulated by a retinoic acid response element (RARE) located 3’ to the promoter. This element is required for early widespread induction in the posterior hindbrain up to the presumptive r3/4 boundary (Marshall et al., 1994). Interestingly, another RARE located 5’ to the promoter is required for repression of Hoxb1 in r3 and r5 to limit its expression to r4 (Studer et al., 1994). Additionally, repression of Hoxb1 in r5 also depends upon the homeodomain protein encoded by vHnf1 (Hnf1b), which is expressed in response to RA in the posterior hindbrain up to the r4/r5 boundary (Wiellette and Sive, 2003; Sirbu et al., 2005) (Hernandez et al., 2004; Sirbu et al., 2005).
During establishment of Hoxb1 expression, RA generated by RALDH2 in paraxial mesoderm initially travels as far anterior as presumptive r3 forming an early RA signaling boundary at r2/r3 just posterior to the forebrain/midbrain, which expresses the RA-degrading enzyme Cyp26a1. However, this boundary soon shifts posteriorly to the r4/r5 border due to expression of Cyp26c1 in r4. Hence the hindbrain utilizes the RA-degrading functions of Cyp26a1 and Cyp26c1 to establish shifting boundaries of RA activity that induce both Hoxb1 and vHnf1, a repressor of Hoxb1 (Sirbu et al., 2005). Supporting this model, knockout and knockdown of Cyp26a1 and Cyp26c1 in mouse and zebrafish results in an anterior extension of Hoxb1 expression into territory that normally develops into midbrain or forebrain (Hernandez et al., 2007; Uehara et al., 2007).
Motor Neuron Lineage Specification
In the spinal cord, sonic hedgehog (SHH) and RA are needed to establish a ventral fate that generates motor neurons (Sockanathan and Jessell, 1998; Del Corral et al., 2003; Novitch et al., 2003; Molotkova et al., 2005). Transcription factors required for motor neuron differentiation include Pax6 (expressed dorsally) Nkx6.1 (expressed ventrally) and Olig2 expressed in the region where Pax6 and Nkx6.1 overlap and where motor neurons develop (Marquardt and Pfaff, 2001). RA synthesized by RALDH2 in the adjacent somitic mesoderm travels to the spinal cord neuroectoderm. Mouse embryos lacking this source of RA fail to express Pax6 and Olig2 in the spinal cord, whereas Nkx6.1 expression is unaffected (Molotkova et al., 2005). In the absence of RA signaling, undifferentiated spinal cord neuroectoderm does not acquire a ventral motor neuron cell fate (Novitch et al., 2003; Molotkova et al., 2005). RA has been used as a differentiation agent along with SHH to generate motor neurons from both mouse (Wichterle et al., 2002) and human (Li et al., 2005) embryonic stem cells, providing a potential source of replacement cells for motor neuron diseases or spinal cord injuries.
Mesoderm-derived tissues and organs
Bilateral Symmetry of Somites
Somitogenesis is the process whereby trunk paraxial mesoderm is sequentially segmented along the anteroposterior axis into bilaterally-paired epithelial structures known as somites on the left and right sides of the embryonic axis. Rhythmic somite formation relies on a "clock and wavefront" mechanism in which a molecular oscillator dependent upon Notch and Wnt signaling controls rhythmic expression of genes along the presomitic mesoderm (Pourquié 2003). A moving wavefront of Fgf8 gene expression in the primitive streak regresses posteriorly as the body axis extends, thus generating a somite determination front just anterior to the Fgf8 expression domain (Dubrulle et al., 2001). A role for RA in somite development was suggested by studies showing that RA is required for the caudal expression of Cdx1, which is needed for proper development of the axial skeleton (Houle et al., 2003). The anteroposterior position of the determination front is also dependent upon RA generated in presomitic mesoderm, which represses caudal Fgf8 expression (Del Corral et al., 2003).
Experiments in mouse, chick, and zebrafish embryos demonstrated that RALDH2 generates the RA required to maintain bilateral symmetry of the left and right columns of somites (Kawakami et al., 2005; Vermot et al., 2005; Vermot and Pourquié 2005; Sirbu and Duester, 2006). Loss of RA signaling leads to a loss of left-right bilateral symmetry such that one side has fewer somites than the other. Presomitic mesoderm in RA-deficient embryos displays abnormal left-right asymmetric expression of Hes7 and Lfng, which are required for Notch-dependent oscillator function during somitogenesis (Kawakami et al., 2005; Vermot et al., 2005; Vermot and Pourquié 2005). Thus, a loss of RA allows left-right asymmetry to occur in presomitic mesoderm where it normally does not occur, but does not alter left-right asymmetry normally observed in lateral plate mesoderm.
Expression of Fgf8 mRNA in epiblast (primitive ectoderm) is shifted anteriorly in RALDH2 deficient embryos so that it enters the node ectoderm and neuroectoderm; thus it has been proposed that excessive anterior FGF signaling from ectoderm to mesoderm may be responsible for the somite defect (Sirbu and Duester, 2006). This hypothesis is supported by two observations. First, the somite determination front normally occurs in presomitic mesoderm just anterior to the Fgf8 expression domain in the primitive streak, and an anterior shift in FGF signaling results in an anterior shift in somite position along the anteroposterior axis (Dubrulle et al., 2001). Second, as the FGF8 signal to the node is required for left-right asymmetry in lateral plate mesoderm (for instance, heart tube looping) (Meyers and Martin, 1999), it is possible that excessive FGF8 signaling to the node during RA deficiency may result in left-right asymmetry occurring also in the presomitic mesoderm where it should normally not occur.
Regulation of Heart Patterning
RA is required for anteroposterior patterning of the heart tube. Loss of RA synthesis leads to a severe reduction in the atria/inflow tract domain and the outflow tract/ventricular domain forms an abnormal cavity that is distended medially rather than undergoing rightward looping and septation into right and left ventricles (Niederreither et al., 2001). In both mouse and chick embryos, Raldh2 is first expressed in the presomitic mesoderm, and then during the early stages of somitogenesis a caudorostral wave of Raldh2 expression occurs in the lateral plate mesoderm up to a location just posterior to the cardiac crescent (Hochgreb et al., 2003). RA generated by RALDH2 travels into posterior heart mesoderm and is able to induce expression of an RA-reporter in the mouse heart from E7.5–E8.5.
Studies in zebrafish have shown that RA restricts the size of the cardiac progenitor pool (Keegan et al., 2005) and studies in chick demonstrate that RA limits the location of ventricular progenitors along the anteroposterior axis (Hochgreb et al., 2003). Analysis of cardiac genes in mouse embryos lacking RALDH2 suggests that the effect of RA on early heart development is not mediated through induction of target genes (Niederreither et al., 2001), but rather through repression of Fgf8 expression in the posterior region of the heart (Ryckebusch et al., 2008; Sirbu et al., 2008).
During early heart tube organogenesis, studies in chick and mouse embryos have identified distinct progenitor cell populations in the splanchnic lateral plate mesoderm of the cardiac crescent that express unique combinations of transcription factors essential for heart development (Cai et al., 2003; Buckingham et al., 2005). For instance, Tbx5 is expressed more laterally (sometimes referred to as first heart field), whereas Isl1 and Fgf8 are expressed more medially (sometimes referred to as second heart field). In mice, loss of RA signaling results in a loss of Tbx5 expression in the posterior-lateral region of the cardiac crescent, but not the anterior region (Niederreither et al., 2001; Sirbu et al., 2008). This effect on Tbx5 is indirect as RA signaling is required only in the posterior-medial domain where Fgf8 and Isl1 (but not Tbx5) are expressed (Sirbu et al., 2008). Mouse embryos lacking RALDH2 exhibit an increase in Fgf8 and Isl1 cardiac expression posteriorly (Ryckebusch et al., 2008; Sirbu et al., 2008) as well as in increase in FGF8 signaling marked by Sprouty2 expression (Sirbu et al., 2008). Studies on embryos with cardiac-specific loss of Fgf8 indicate that Fgf8 is required for expression of Isl1 in splanchnic mesodermal progenitors (Ilagan et al., 2006; Park et al., 2006). Due to the requirement of FGF8 signaling for Isl1 induction, RA downregulation of Fgf8 may thus normally limit the posterior extent of Isl1 expression in cardiac mesoderm (Figure 3). Alternative promoters for Fgf8 are controlled by a unique retinoic acid response element that allows expression of the major isoform (Fgf8b) when RAR is unliganded, but represses Fgf8b when RA is present (Brondani et al., 2002). Thus, RA appears to function in a repressive rather than an inductive fashion during early cardiac organogenesis.
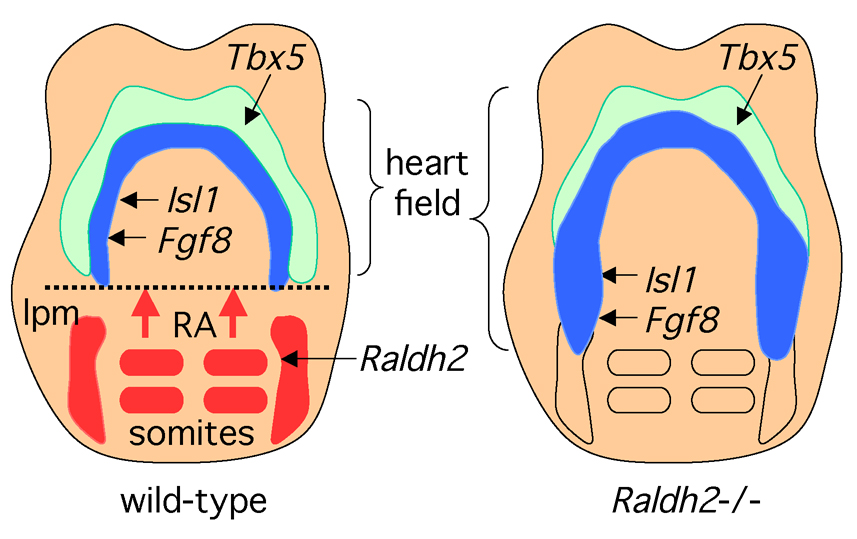
Figure 3. Retinoic acid signaling during early heart organogenesis.
Retinoic acid (RA) generated by retinaldehyde dehydrogenase 2 (Raldh2) in the somites and lateral plate mesoderm (lpm) travels anteriorly where it provides a signal that helps establish the posterior border of the heart field. The lateral domain of the heart field expresses Tbx5 whereas the medial domain expresses Isl1 as well as Fgf8 which is required for cardiac Isl1 expression. Embryos from Raldh2 knockout mice exhibit posterior expansion of Fgf8 and Isl1 into lateral plate mesoderm that normally is not part of the heart field. As the Fgf8 promoter has been reported to contain an RA response element, RA may function as a repressor of cardiac Fgf8.
Forelimb Induction
Introduction of exogenous RA alters anteroposterior patterning of chick limb buds (Tickle et al., 1982) or proximodistal patterning of regenerating axolotl limbs (Maden, 1982). Later, it was demonstrated that Shh controls limb anteroposterior patterning and that RA-bead implants ectopically induce Shh (Riddle et al., 1993). Genetic studies in mice and zebrafish demonstrated that RA synthesis in the vicinity of limb buds is controlled by RALDH2 expressed in flank mesoderm lying next to the limb bud (Niederreither et al., 1999; Mic et al., 2002; Gibert et al., 2006). Further studies in mice demonstrated that RA activity in early limb buds is uniform across the anteroposterior axis but decreases from proximal to distal (Mic et al., 2004). It was proposed that opposing signals of RA, which is generated proximally in the flank, and Fgf8, which is expressed distally in the apical ectodermal ridge, may be important to control establishment of the limb proximodistal axis (Mercader et al., 2000). Whereas genetic support for Fgf8 as a distal signal has been obtained, there is no genetic evidence that a proximal RA signaling center is required to establish the proximodistal axis of the limb (Tabin and Wolpert, 2007). In addition, studies on mouse embryos lacking the RA-degrading enzyme CYP26B1, which in wild-type embryos is expressed in the distal region of both forelimbs and hindlimbs, have shown that RA degradation is necessary for proper outgrowth of limb buds (Yashiro et al., 2004). Although loss of CYP26B1 results in an abnormal distal expansion of RA signaling in the forelimbs and hindlimbs that correlates with RA-induced limb teratogenesis, these findings do not provide evidence that a normal level of endogenous RA signaling is necessary for proximodistal patterning of the limbs.
In the absence of RA synthesis by RALDH2, forelimb buds do not develop and embryonic growth ceases prior to the stage when hindlimb buds are initiated (Niederreither et al., 2002; Mic et al., 2004); in zebrafish the absence of RA synthesis blocks induction of pectoral fin buds (Gibert et al., 2006). Thus, RA is required for induction of forelimb development, but whether RA also plays a later role in patterning of the limbs remains controversial. Rescue of Raldh2 mutant embryos by low-dose maternal dietary RA supplementation rescues lethality and results in limb bud induction despite no detection of RA activity in forelimbs or hindlimbs; in this model, forelimb buds are undersized whereas hindlimb buds appear normal (Niederreither et al., 2002; Mic et al., 2004) suggesting that RA may not have a role in hindlimb induction and patterning. Further support for this conclusion has come from Rdh10 mutant embryos (lacking an enzyme upstream of RALDH2 for RA synthesis), which survive long enough to exhibit small forelimbs and normal hindlimbs similar to RA-rescued Raldh2 mutants (Sandell et al., 2007). However, hindlimb buds may be affected by RA signaling occurring in the mesonephros which expresses Raldh3 (Mic et al., 2002). Thus, studies examining whether low-dose dietary RA can rescue limb development in embryos lacking both RALDH2 and RALDH3 will be needed to assess whether hindlimbs require RA for induction or for patterning along either the proximodistal or anteroposterior axes.
The rescue of Raldh2 null mutant embryos with low-dose maternal dietary supplementation of RA provides less RA than normally synthesized by RALDH2 (Mic et al., 2003), resulting in conditions where RA activity is absent in forelimb mesoderm but present nearby in the body axis, particularly the neuroectoderm (Niederreither et al., 2002; Mic et al., 2004); neuroectoderm expresses very high levels of CRABP2 and RARβ and can presumably respond to lower RA levels than somitic and limb mesoderm . This observation suggests that although RA signaling is unnecessary in forelimb mesoderm for forelimb budding, RA may act in the neuroectoderm to permit limb induction. Given neuroectoderm is unnecessary for induction of forelimbs (Rong et al., 1992), RA activity in neuroectoderm may instead down-regulate expression of a diffusible factor in the body axis that inhibits forelimb induction. Three observations suggest this factor, if it exists, could be FGF8. (1) RA represses caudal Fgf8 expression during body axis extension (Del Corral et al., 2003; Vermot et al., 2005); (2) ectopic FGF signaling from beads implanted in the chick wing field impairs wing development (Cohn et al., 1995; Mercader et al., 2000); (3) RA repression of Fgf8 expression during mouse body axis extension occurs at the junction of the neuroectoderm/epiblast during the somite stages when forelimb induction occurs (Sirbu and Duester, 2006). Thus, rather than playing an instructive role in forelimb budding, recent findings suggest that RA generated in somites may play a permissive role in forelimb induction through its action in the body axis near the forelimb field. At later steps when limb patterning occurs, it is doubtful that RA has a role.
Endoderm-derived organs
Pancreas Induction
Many studies of pancreas development have focused on the homeobox transcription factor Pdx1, which is required for pancreatic specification in the posterior foregut. Pdx1 is highly expressed in the dorsal and ventral endodermal buds that give rise to the mature pancreas. Evidence suggests that RA may be the mesodermal signal required for initiation of Pdx1 expression (Stafford and Prince, 2002). Zebrafish embryos deficient in RA lack expression of Pdx1 and consequently fail to induce pancreas development. Similar results have also been obtained in mouse (Martin et al., 2005; Molotkov et al., 2005) and frog (Chen et al., 2004) embryos. Studies on avian embryos revealed that signals from the lateral plate mesoderm can drive endodermal cells to express Pdx1 and thus generate a pancreatic fate (Kumar et al., 2003). Examination of mouse embryos carrying the RA-reporter transgene demonstrated that the pancreas is normally exposed to RA generated in the surrounding splanchnic lateral plate mesoderm (Molotkov et al., 2005).
In RALDH2-deficient mouse embryos (Molotkov et al., 2005) and RAR antagonist-treated Xenopus embryos (Chen et al., 2004), dorsal endodermal pancreatic tissue is not correctly specified. In contrast ventral endodermal pancreatic tissue and liver are still specified, demonstrating that only dorsal endoderm requires RA activity for pancreatic development. Specification of dorsal pancreatic tissue can be rescued in RALDH2-deficient embryos by low-dose maternal administration of RA, a treatment that preferentially restores RA activity to the dorsal endoderm but not the surrounding dorsal mesoderm (Molotkov et al., 2005). This suggests that RA acts directly in the dorsal endoderm for pancreas specification rather than in the lateral plate mesoderm where RA is synthesized. In zebrafish, transplantation studies have shown that RAR needs to act only in the endoderm for pancreas induction and the RA is derived from the surrounding mesoderm (Stafford et al., 2006).
Lung Induction
The lung is another organ derived from the endoderm of the posterior foregut that requires RA signaling for organogenesis. RA from the splanchnic mesoderm surrounding the endoderm has been found to be important for stimulation of posterior foregut endoderm to a lung fate at E9.5 in the mouse (Malpel et al., 2000). RALDH2-deficient embryos rescued from early lethality by maternal dietary RA between E7.5 and E8.5 fail to develop lungs and lack RA signaling in the foregut (Wang et al., 2006). In RA-deficient embryos the primary lung bud is specified, but it does not express the RA-inducible Hoxa5 gene and it is unable to achieve outgrowth or branching due to a loss of both Fgf10 expression and FGF10 signaling in the lung epithelium; treatment of RA-deficient embryos in vitro with FGF10 can restore lung budding and branching (Wang et al., 2006). Further studies demonstrated that a loss of RA signaling in the foregut results in upregulation of TGFβ1 and TGFβ target genes, and treatment of wild-type embryos with exogenous TGFβ1 can reproduce the lung bud defect seen in RA-deficient embryos (Chen et al., 2007). Therefore, it appears that RA functions during lung budding both as an inducer of Hoxa5 and an inhibitor of TGFβ1 signaling, which then permits local expression of Fgf10 needed for expansion of the lung bud and branching.
Morphogenetic Movements during Formation of the Eye
As all three mouse Raldh genes are expressed in the developing eye, it is of interest to discuss in more detail their expression patterns and what their gene knockouts have revealed so far about the mechanism of RA signaling during eye development. Vitamin A deficiency and reduced RA receptor function in RAR null mice (Lohnes et al., 1994) both result in incomplete closure of the choroid fissure (ocular coloboma) as well as microphthalmia (small eyes) and abnormalities of the cornea, eyelids, and conjunctiva observed during late fetal stages. However, due to remaining RA activity these studies could not determine at what developmental stage(s) RA is required for eye development.
The three RALDHs are expressed in distinct tissues during development of the mouse eye, and in each case RA signaling activity has been detected in nearby tissues and found to be required for eye development (Molotkov et al., 2006). Invagination of the optic vesicle epithelium from E9.5–E10.5 results in formation of an optic cup in which the epithelium has folded to form separate layers for neural retina and retinal pigment epithelium, with both being folded around the lens vesicle which has developed by invagination of the surface ectoderm (Figure 4). RALDH2 generates RA at E9.0 in the perioptic mesenchyme next to the temporal side of the optic vesicle whereas RALDH3 generates RA in the dorsal retinal pigment epithelium starting at E9.5 just prior to invagination; a lack of both RALDH2 and RALDH3 results in a failure of ventral optic cup invagination (Molotkov et al., 2006). During optic cup formation the following changes in Raldh function occur: (1) Raldh2 expression near the optic cup terminates at E10.0; (2) Raldh3 expression terminates in the dorsal retinal pigment epithelium and initiates in the ventral neural retina at E10.5; (3) RALDH1 begins to generate RA in the dorsal neural retina at E10.5. At E11.5 when the ventral portion of the optic cup forms the choroid fissure, expression of Raldh1 and Raldh3 continues in the dorsal and ventral neural retina, respectively, and endures even after closure of the optic fissure at E13.5. However, RALDH1 and RALDH3 do not generate RA for retinal dorsoventral patterning, but instead they have completely redundant functions in generating RA that travels from the retina to the perioptic mesenchyme where it stimulates apoptosis; a lack of both RALDH1 and RALDH3 results in mesenchymal overgrowth in the cornea and eyelids (Matt et al., 2005; Molotkov et al., 2006). Pitx2 is a potential target of ocular RA signaling as its expression is severely down-regulated in perioptic mesenchyme of mutant embryos lacking both RALDH1 and RALDH3 (Matt et al., 2005).
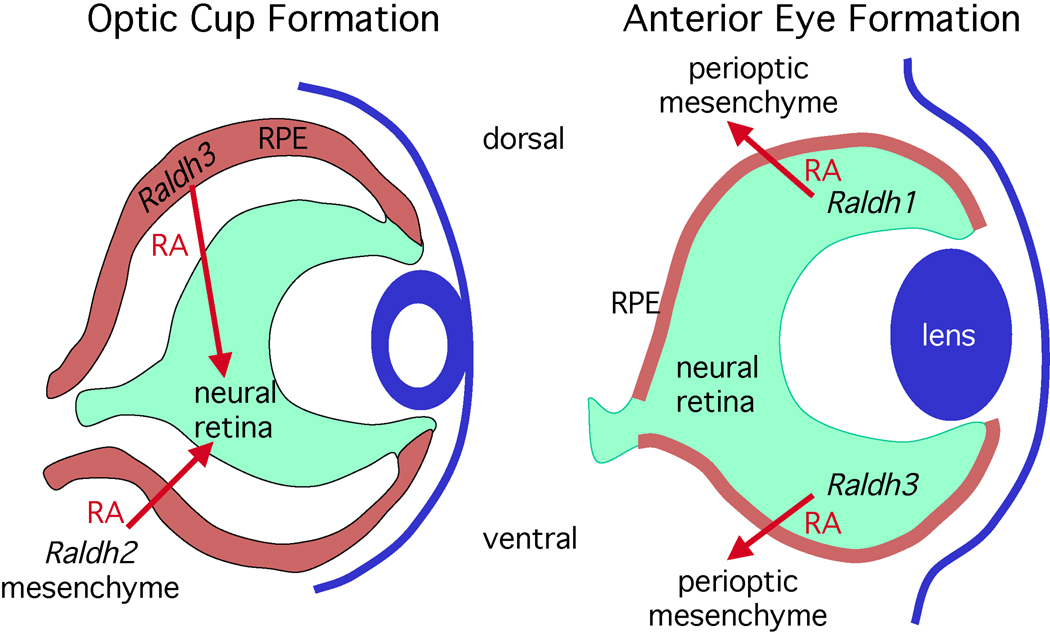
Figure 4. Retinoic acid regulates eye development.
Paracrine retinoic acid (RA) signaling controls two distinct phases of eye morphogenetic movements and involves all three retinaldehyde dehydrogenase (Raldh) genes. During invagination of the optic vesicle to form an optic cup (E9.5–E10.5 in mouse), RA generated by Raldh2 in the perioptic mesenchyme as well as Raldh3 in tissue fated to become the dorsal retinal pigment epithelium (RPE) travels to the neural retina where it is required for ventral invagination during optic cup formation. After optic cup formation (E10.5-birth), Raldh2 is no longer expressed in the perioptic mesenchyme and Raldh3 expression ends in the RPE, but Raldh1 and Raldh3 are now expressed in the dorsal and ventral neural retina, respectively. RA generated by Raldh1 and Raldh3 is not required for patterning the neural retina, but this RA travels outside the retina where it limits invasion of perioptic mesenchyme during anterior eye development (cornea and eyelid formation).
These findings suggest the existence of two distinct phases of RA signaling required for eye development: an early phase for optic cup formation and a late phase for anterior eye formation (Figure 4). In both cases, cells expressing Raldh genes provide an RA signal that functions to control morphogenetic movements in neighboring cells. Also, in both cases two Raldh genes function as RA sources, providing functional redundancy. The target of RA action changes during eye morphogenesis. The initial target is the invaginating neural retina at the optic vesicle stage with the sources of RA being Raldh2 expressed in mesenchyme located temporally to the optic vesicle and Raldh3 expressed in the retinal pigment epithelium. After optic cup formation the target switches to the perioptic mesenchyme with the sources being Raldh1 expressed in the dorsal retina and Raldh3 expressed in the ventral retina. These targets are distinct from but adjacent to locations of RA synthesis, thus demonstrating that RA functions in a paracrine fashion to guide morphogenetic movements of neighboring cells.
Genitourinary Tract Development
RA controls some aspects of genitourinary tract development as Raldh2 is expressed in mesenchymal cells of the mesonephros and stromal cells of the developing kidney, and Raldh3 is expressed in the ureteric bud (Batourina et al., 2001; Mic et al., 2002). Through analysis of RA receptor knockout mice, RA signaling has been found to play a key role in controlling epithelial/mesenchymal interactions during kidney development through induction of Ret expression (Batourina et al., 2001). Also, RA generated in the urogenital sinus stimulates apoptosis in the common nephric duct needed for establishing the connections between the ureters and bladder (Batourina et al., 2005).
RA has recently been found to stimulate sex-specific onset of meiosis in germ cells of mice. The onset of meiosis occurs earlier in the ovary (E13.5) than in the testis (after birth) (Bowles et al., 2006; Koubova et al., 2006). RA (likely from the mesonephros) induces expression of Stra8 in germ cells, which is needed for the transition into meiosis in both the ovary and testis. In the testis the RA-degrading enzyme CYP26B1 is expressed and prevents induction of Stra8 expression. Premature onset of meiosis in the testis occurs in mice lacking CYP26B1 (Bowles et al., 2006) and in organ cultures treated with a CYP26 inhibitor (Koubova et al., 2006) .
Paracrine Retinoic Acid Signaling
In summary, there are many examples of paracrine RA signaling, but so far there is surprisingly no genetic support for autocrine RA signaling (Table 3). The existence of autocrine RA signaling is not yet ruled out, but further studies will be needed to determine if this actually occurs. Future investigations of this type should provide a more complete understanding of when and where RA is generated in embryos, how far it can travel from its site of synthesis, what the target tissues are, what developmental processes it affects, and what genes it regulates. Such knowledge will be essential for understanding mammalian organogenesis and will facilitate the development of rational strategies for optimal use of RA with other reagents to reliably differentiate stem cells into specific cell types that can be used to develop stem cell-based treatments for disease.
Table 3.
Examples of the paracrine function of retinoic acid.
| RETINOIC ACID SOURCE | RA TARGET |
|---|---|
| somitic mesoderm(Raldh2) | hindbrain neuroectoderm (anteroposterior patterning) |
| somitic mesoderm(Raldh2) | spinal cord neuroectoderm (motor neuron formation) |
| somitic mesoderm(Raldh2) | posterior neuroectoderm & node ectoderm (somitogenesis) |
| somitic mesoderm(Raldh2) | posterior body axis (forelimb induction) |
| somitic/lateral plate mesoderm(Raldh2) | cardiac mesoderm (anteroposterior patterning) |
| lateral plate mesoderm(Raldh2) | foregut endoderm (pancreas & lung induction) |
| optic vesicle mesenchyme (Raldh2) | neural retina (ventral optic cup formation) |
| retinal pigment epithelium(Raldh3) | neural retina (ventral optic cup formation) |
| neural retina (Raldh1 and Raldh3) | perioptic mesenchyme (eyelid and cornea development) |
| mesonephros(Raldh2) | ureteric bud (kidney induction) |
| mesonephros(Raldh2) | ovary (meiotic induction) |
Acknowledgements
I am grateful to past and present members of my laboratory for provocative discussions. Funding was provided by NIH grants GM062848 and EY013969 as well as California Institute of Regenerative Medicine grant RS1-00193.
References
- Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang HL, Deltour L, Hayamizu TF, Zgombic-Knight M, Duester G. Retinoic acid synthesis in mouse embryos during gastrulation and craniofacial development linked to class IV alcohol dehydrogenase gene expression. J. Biol. Chem. 1996;271:9526–9534. doi: 10.1074/jbc.271.16.9526. [DOI] [PubMed] [Google Scholar]
- Arnhold T, Tzimas G, Wittfoht W, Plonait S, Nau H. Identification of 9-cis-retinoic acid, 9,13-di-cis-retinoic acid, and 14-hydroxy-4,14-retro-retinol in human plasma after liver consumption. Life Sci. 1996;59:PL 169–PL 177. doi: 10.1016/0024-3205(96)00408-0. [DOI] [PubMed] [Google Scholar]
- Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nature Genet. 2001;27:74–78. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang FW, Niederreither K, McMahon AP, et al. Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nature Genet. 2005;37:1082–1089. doi: 10.1038/ng1645. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Brondani V, Klimkait T, Egly JM, Hamy F. Promoter of FGF8 reveals a unique regulation by unliganded RARa. J. Mol. Biol. 2002;319:715–728. doi: 10.1016/S0022-2836(02)00376-5. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nature Rev. Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai C, Grabel L. Directing the differentiation of embryonic stem cells to neural stem cells. Dev. Dyn. 2007;236:3255–3266. doi: 10.1002/dvdy.21306. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev. Biol. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisúa-Belmonte JC, Abud H, Heath JK, Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Rossant J. Exogenous retinoic acid rapidly induces anterior ectopic expression of murine Hox-2 genes in vivo. Development. 1992;116:357–368. doi: 10.1242/dev.116.2.357. [DOI] [PubMed] [Google Scholar]
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dersch H, Zile MH. Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev. Biol. 1993;160:424–433. doi: 10.1006/dbio.1993.1318. [DOI] [PubMed] [Google Scholar]
- Dickman ED, Thaller C, Smith SM. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development. 1997;124:3111–3121. doi: 10.1242/dev.124.16.3111. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, McGrew MJ, Pourquié O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- Dupé V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- Dupé V, Matt N, Garnier J-M, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston AJ, Timmermans JPM, Hage WJ, Hendriks HFJ, De Vries NJ, Heideveld M, Nieuwkoop PD. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Fan X, Molotkov A, Manabe S-I, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell. Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego O, Belyaeva OV, Porte S, Ruiz FX, Stetsenko AV, Shabrova EV, Kostereva NV, Farres J, Pares X, Kedishvili NY. Comparative functional analysis of human medium-chain dehydrogenases, short-chain dehydrogenases/reductases and aldo-keto reductases with retinoids. Biochem. J. 2006;399:101–109. doi: 10.1042/BJ20051988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Båvik C, Sapin V, Mark M, Bonnier D, Hindelang C, Dierich A, Nilsson CB, Håkansson H, Sauvant P, et al. Cellular retinol-binding protein I is essential for vitamin A homeostasis. EMBO Journal. 1999;18:4903–4914. doi: 10.1093/emboj/18.18.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert Y, Gajewski A, Meyer A, Begemann G. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RE, Rikhof HA, Bachmann R, Moens CB. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–4520. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- Hochgreb T, Linhares VL, Menezes DC, Sampaio AC, Yan CYI, Cardoso WV, Rosenthal N, Xavier-Neto J. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- Houle M, Sylvestre JR, Lohnes D. Retinoic acid regulates a subset of Cdx1 function in vivo. Development. 2003;130:6555–6567. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. Fgf8 is required for anterior heart field development. Development. 2006;133:2435–2445. doi: 10.1242/dev.02408. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Raya A, Raya RM, Rodriguez-Esteban C, Izpisúa-Belmonte JC. Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature. 2005;435:165–171. doi: 10.1038/nature03512. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–249. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes and pattern formation in the branchial region of the vertebrate head. Trends Genet. 1993;9:106–112. doi: 10.1016/0168-9525(93)90203-t. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev. Biol. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982;295:672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- Maden M, Gale E, Kostetskii I, Zile MH. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Marlétaz F, Holland LZ, Laudet V, Schubert M. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2006;2:38–47. doi: 10.7150/ijbs.2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Pfaff SL. Cracking the transcriptional code for cell specification in the neural tube. Cell. 2001;106:651–654. doi: 10.1016/s0092-8674(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Marshall H, Studer M, Pöpperl H, Aparicio S, Kuroiwa A, Brenner S, Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994;370:567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- Martín M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dollé P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev. Biol. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupé V, Garnier J-M, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, Le Meur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development. (II) Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Martin GR. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science. 1999;285:403–406. doi: 10.1126/science.285.5426.403. [DOI] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc. Natl. Acad. Sci. USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Sirbu IO, Duester G. Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation. J. Biol. Chem. 2004;279:26698–26706. doi: 10.1074/jbc.M401920200. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Deltour L, Foglio MH, Cuenca AE, Duester G. Distinct retinoid metabolic functions for alcohol dehydrogenase genes Adh1 and Adh4 in protection against vitamin A toxicity or deficiency revealed in double null mutant mice. J. Biol. Chem. 2002a;277:13804–13811. doi: 10.1074/jbc.M112039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farrés J, Parés X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously-expressed alcohol dehydrogenase Adh3. Proc. Natl. Acad. Sci. USA. 2002b;99:5337–5342. doi: 10.1073/pnas.082093299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Ghyselinck NB, Chambon P, Duester G. Opposing actions of cellular retinol-binding protein and alcohol dehydrogenase control the balance between retinol storage and degradation. Biochem. J. 2004;383:295–302. doi: 10.1042/BJ20040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev. Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N, Molotkov A, Duester G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 2007;303:601–610. doi: 10.1016/j.ydbio.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N, Molotkov A, Sirbu IO, Duester G. Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation. Mech. Dev. 2005;122:145–155. doi: 10.1016/j.mod.2004.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem. J. 2000;348:481–495. [PMC free article] [PubMed] [Google Scholar]
- Olivera-Martinez I, Storey KG. Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development. 2007;134:2125–2135. doi: 10.1242/dev.000216. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquié O. The segmentation clock: converting embryonic time into spatial pattern. Science. 2003;301:328–330. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol-binding protein. EMBO Journal. 1999;18:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rong PM, Teillet M-A, Ziller C, Le Douarin NM. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992;115:657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin S-C, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic Acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler RJ, Noy N. A ligand-activated nuclear localization signal in cellular retinoic acid binding protein-II. Mol.Cell. 2005;18:343–353. doi: 10.1016/j.molcel.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Arcioni L, Andrews PW, Boncinelli E, Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990;346:763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Draper BW, Harland RM, Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990;4:932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr. Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Studer M, Pöpperl H, Marshall H, Kuroiwa A, Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994;265:1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21:1433–1442. doi: 10.1101/gad.1547407. [DOI] [PubMed] [Google Scholar]
- Tickle C, Alberts BM, Wolpert L, Lee J. Local application of retinoic acid to the limb bud mimics the action of the polarizing region. Nature. 1982;296:564–565. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Uehara M, Yashiro K, Mamiya S, Nishino J, Chambon P, Dolle P, Sakai Y. CYP26A1 and CYP26C1 cooperatively regulate anterior-posterior patterning of the developing brain and the production of migratory cranial neural crest cells in the mouse. Dev. Biol. 2007;302:399–411. doi: 10.1016/j.ydbio.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Vermot J, Llamas JG, Fraulob V, Niederreither K, Chambon P, Dollé P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science. 2005;308:563–566. doi: 10.1126/science.1108363. [DOI] [PubMed] [Google Scholar]
- Vermot J, Pourquié O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature. 2005;435:215–220. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dolle P, Cardoso WV, Niederreither K. Retinoic acid regulates morphogenesis and patterning of posterior foregut derivatives. Dev. Biol. 2006;297:433–445. doi: 10.1016/j.ydbio.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wiellette EL, Sive H. vhnf1 and Fgf signals synergize to specify rhombomere identity in the zebrafish hindbrain. Development. 2003;130:3821–3829. doi: 10.1242/dev.00572. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Cook M, Boncinelli E, Krumlauf R. Segmental expression of Hox-2 homeobox-containing genes in the developing mouse hindbrain. Nature. 1989;341:405–409. doi: 10.1038/341405a0. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing limb. Dev. Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen WG, Smith SM, Napoli JL. Molecular characterization of a mouse short chain dehydrogenase/reductase active with all-trans-retinol in intact cells, mRDH1. J. Biol. Chem. 2001;276:44083–44090. doi: 10.1074/jbc.M105748200. [DOI] [PubMed] [Google Scholar]
- Zhang M, Hu P, Krois CR, Kane MA, Napoli JL. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J. 2007;21:2886–2896. doi: 10.1096/fj.06-7964com. [DOI] [PubMed] [Google Scholar]