Abstract
Synaptic transmission from photoreceptors to all types of ON bipolar cells is primarily mediated by the mGluR6 receptor. This receptor, which is apparently expressed uniquely in the nervous system by ON bipolar cells, couples negatively to a nonselective cation channel. This arrangement results in a sign reversal at photoreceptor/ON bipolar cell synapse, which is necessary in order to establish parallel ON and OFF pathways in the retina. The synapse is an important target for 2nd messenger molecules that are known to modulate synaptic transmission elsewhere in the nervous system, 2nd messengers that act on a time scale ranging from milliseconds to minutes. This review focuses on two of these molecules, Ca2+ and cGMP, summarizing our current knowledge of how they modulate gain at the photoreceptor/ON bipolar cell synapse, as well as their proposed sites of action within the mGluR6 cascade. The implications of plasticity at this synapse for retinal function will also be examined.
Keywords: mGluR6, Ca2+, cGMP, synaptic plasticity, cation channel
1. Introduction
The retina is divided into two functional channels. Cells belonging to the ON channel are depolarized by the onset of light, while members of the OFF channel depolarize when light is turned off. In principle, this could have been accomplished if photoreceptors themselves were organized into two channels, depending upon the polarity of their light response. Instead, all photoreceptor are hyperpolarized by light, and channels are created by differential expression of glutamate receptors on the dendrites of bipolar cells. OFF bipolar cells express ionotropic AMPA/kainate receptors (Gilbertson et al., 1991; DeVries and Schwartz, 1999; DeVries, 2000), and so hyperpolarization of photoreceptors is conserved at this synapse, and the OFF pathway retains the light-induced hyperpolarization characteristic of photoreceptors. ON bipolar cells have instead adapted the use of a novel metabotropic receptor called mGluR6 (Nakajima et al., 1993; Nomura et al., 1994), which is negatively coupled to a cation-selective channel. Here, glutamate acts essentially as an inhibitory transmitter, closing the cation channel that would otherwise keep ON bipolar cells depolarized (Shiells et al., 1981; Slaughter and Miller, 1981). Thus, a major role of the photoreceptor-ON bipolar cell synapse is to invert light-activated hyperpolarizations of photoreceptors into a depolarization (Werblin and Dowling, 1969; Kaneko, 1970).
Although several aspects of the transduction mechanism itself remains elusive, significant progress has been made toward characterizing plasticity at this synapse. Both potentiation and depression of postsynaptic responses have been demonstrated, and an emerging theme is that intracellular Ca2+ induces depression, while cGMP potentiates postsynaptic responses. Not unexpectedly there are differences in both experimental findings and interpretations in the literature regarding the specific roles of these two ubiquitous second messengers. This review focuses on recent findings regarding the mechanisms and sites of modulation by Ca2+ and cGMP, as well as the transduction mechanism itself. It also examines the potential role of plasticity at this synapse in shaping the kinetics of light responses in the ON pathway.
2. A brief history of mGluR6 transduction
2.1 The mGluR6 receptor and its signaling pathway
The primary task of the photoreceptor-ON bipolar cell synapse is to invert the “sign” of the synapse. Glutamate is a canonical excitatory transmitter, but at this synapse, its acts essentially as an inhibitory transmitter by hyperpolarizing ON bipolar cells. Early studies of the glutamate receptor expressed by ON bipolar cells in lower vertebrates revealed that it possessed a novel pharmacological profile, as it was activated by L-2- amino-4-phosphonobutyric acid (L-AP4), and that activation of the receptor was associated with a conductance decrease (Shiells et al., 1981; Slaughter and Miller, 1981). Measurements of the current-voltage relation associated with the response to L-AP4 and to light demonstrated that both L-AP4 and the endogenous photoreceptor transmitter closed a channel with a reversal potential near 0 mV (Nawy and Copenhagen, 1987; Nawy and Jahr, 1990a). Furthermore, patch clamp experiments in which GTP analogs were dialyzed into ON bipolar cells demonstrated that the as yet unidentified receptor operated via a G protein-coupled mechanism (Nawy and Jahr, 1990a). Several years later, the receptor was cloned and termed mGluR6 (Nakajima et al., 1993), a member of the group III family of metabotropic glutamate receptors. Thus it was established that the cascade consisted of a G protein-coupled receptor at one end, and a cation-selective channel at the other, although the route of communication between them was unknown.
Other members of the group III category of metabotropic glutamate receptors include mGluR4, mGluR7, and mGluR8 (Conn and Pin, 1997). In expression systems, group III receptors are coupled to a decrease in stimulated cAMP levels (Okamoto et al., 1994). However, there is no evidence that mGluR6 is coupled to, or in any way modulates cAMP levels in ON bipolar cells. In native systems, group III metabotropic receptors are expressed predominantly at presynaptic terminals, where they couple to pertussis toxin-sensitive G proteins. Their predominant physiological action is to inhibit transmitter release by reducing Ca2+ channel activation (Trombley and Westbrook, 1992; Gereau and Conn, 1995; Martin et al., 2007) via a membrane-delimited binding of G protein βγ subunits to the β subunit of Ca2+ channels (Herlitze et al., 1996; Ikeda, 1996; O'Connor et al., 1999). Coupling of mGluR6, in contrast, is less well understood. Early patch clamp studies of mGluR6 transduction in salamander (Nawy and Jahr, 1990a, 1991) and dogfish (Shiells and Falk, 1990) retina pointed to cGMP as the second messenger that gated the opening of synaptic cation channel. It was hypothesized that, analogous to phototransduction, mGluR6 activated a cGMP phosphodiesterase through a G protein-coupled mechanism, leading to hydrolysis of cGMP and subsequent closure of the cation channel. However more recent experiments, described below, argue against a photoreceptor-like signal transduction mechanism. The G protein downstream of mGluR6 has been identified as Go (Vardi, 1998; Nawy, 1999; Dhingra et al., 2000), but the molecular identity of the synaptic channel is not yet known, and this is clearly a key step toward completely elucidating the transduction mechanism.
Recent progress has also been made toward identifying proteins that increase the rate of GTP hydrolysis of the G protein. Understanding how the mGluR6-Gαo complex is shut off is critical, as the rise time of the light response is determined by the rate at which G protein signaling is terminated. The RGS proteins RGS11, RGS7, and their obligate binding partner Gβ5 have recently been shown to be concentrated at the dendrites of mouse ON bipolar cells along with mGluR6 (Morgans et al., 2007; Rao et al., 2007). To date, however, there is no functional evidence that either RGS11 or RGS7 specifically increase the rate of GTPase activity by Gαo. Another regulator of G protein signaling, ret-RGS1 has been detected in the dendrites and cell bodies of rod ON bipolar cells (Dhingra et al., 2004). Using an expression system, this group demonstrated that coexpression of ret-RGS1, along with Go and mGluR6, sped the rate at which signaling is terminated, although this rate is still quite slow compared to the rising phase of the light response. Further studies will be required to sort out the roles of each RGS in the regulating of the mGluR6 pathway.
2.2 Some ON bipolar cells signal through alternative mechanisms
There is evidence for additional mechanisms of signaling at the photoreceptor/ON bipolar cell synapse, primarily in teleost retina. In this retina, as in the retinas of other lower vertebrates, most classes of ON bipolar cells collect input from both rods and cones. Early studies of the light responses of carp and goldfish retina demonstrated that in these mixed input bipolar cells, light responses arising from rods were associated with a positive reversal potential and a conductance increase, while the light responses originating from cones reversed at negative voltages and were associated with a conductance decrease (Saito et al., 1978; Saito et al., 1979). Note that both conductance mechanisms are functionally similar in that they result in membrane depolarization. It was later demonstrated that the rod, but not the cone component of the light response is mediated by the mGluR6 receptor (Nawy and Copenhagen, 1987). Subsequent studies revealed that the synaptic response generated by the cone pathway is probably not a true glutamate receptor, but instead displays a transporter-like pharmacology. Most likely the transported ion is Cl− (Grant and Dowling, 1995, 1996; Connaughton and Nelson, 2000). However, by activating a Cl- conductance, glutamate released from cones in the teleost still obeys the general rule that photoreceptor transmitter inhibits ON bipolar cells. There is also immunocytochemical evidence that AMPA receptors are localized to the dendrites of some ON bipolar cells (Hughes, 1997), but a physiological response to AMPA application had not yet been reported.
2.3 ON bipolar cells and the cGMP hypothesis
During patch clamp recording, it was observed that the responses induced by glutamate or L-AP4 often ran down, suggesting that dialysis of the internal contents of the cell washed away or blocked the production of a substance that was required to gate the channel open (Nawy and Jahr, 1990b). Inclusion of cGMP in the pipette solution prevented this run down, and often enhanced the amplitude of the agonist-suppressed current (fig. 1) (Nawy and Jahr, 1990a; Shiells and Falk, 1990). This finding was consistent with the hypothesis that run down of the response was due to the wash out of endogenous cGMP from the cytoplasm during patch recording, and that enhancement of the agonist-suppressed cation current resulted from dialysis of ON bipolar cells with a concentration of cGMP that exceeded endogenous levels. These results led two groups to propose that responses to glutamate in ON bipolar cells were transduced in a way that was analogous to phototransduction: Binding of glutamate to mGluR6 turned on a transducin-like G protein which then activated a cGMP-preferring phosphodiesterase (PDE). Hydrolysis of cGMP resulted in closure of cGMP-gated channels, and membrane hyperpolarization. The hypothesis that these two neighboring cells shared a conserved signal transduction mechanism to attain a common goal seemed appealing, and gained rapid acceptance in the literature.
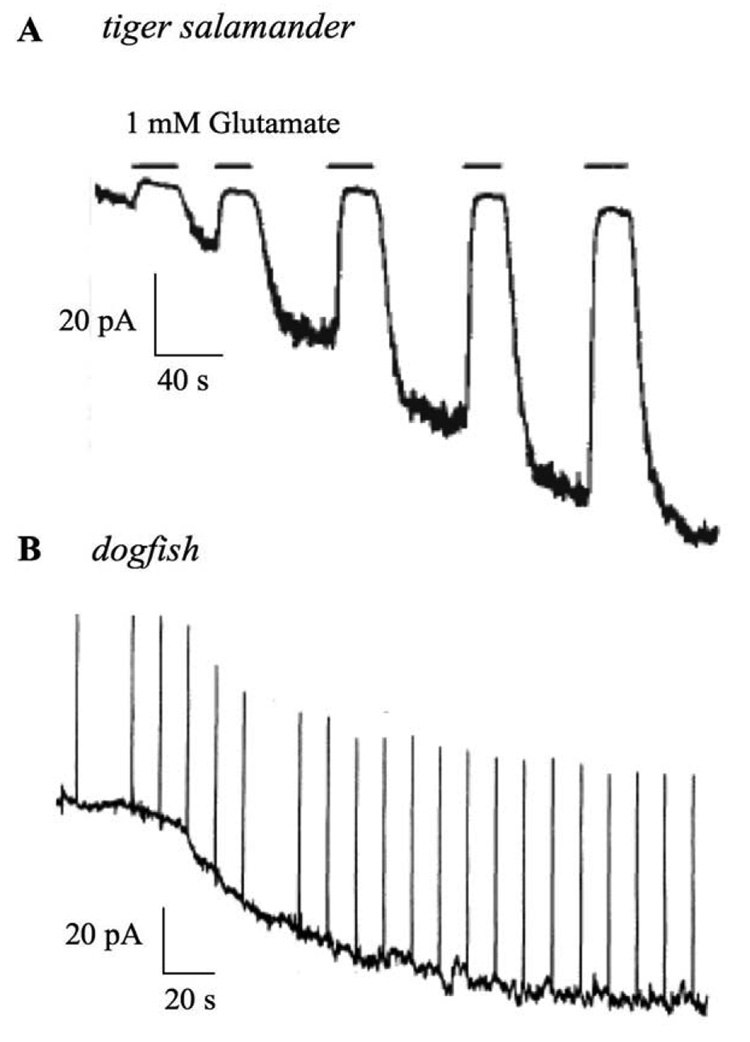
Fig. 1. Summary of the effects of cGMP on holding current in two studies of ON bipolar cells.
(A) Recording of a tiger salamander ON bipolar cell dialyzed with a solution containing 1 mM cGMP. Note the development of a standing inward current which can be suppressed by application of glutamate. Modified from (Nawy and Jahr, 1990a). (B) Recording from an ON bipolar cell in dogfish retina. The cell was recorded with a pipet solution containing 200 µM cGMP. Note again the development of a standing inward current. Upward deflections are responses to 2 mV voltage steps, used to monitor changes in input resistance. Modified from (Shiells and Falk, 1990). See text for details.
The central dogma that phototransduction was recapitulated in bipolar cells drove a concerted effort by a number of groups to identify and clone the elements of the cascade, all of which were unsuccessful. For example, immunocytochemical identification of cyclic nucleotide-gated channels was negative (Wassle et al., 1992), although detection of mRNA from ON bipolar cells has been reported (Henry et al., 2003). Studies of mGluR6 G-protein coupling in expression systems showed that mGluR6 couples poorly to transducin compared with the Go family of G proteins (Weng et al., 1997; Tian and Kammermeier, 2007). In addition Go, rather than transducin, has been detected immunocytochemically on the tips of ON bipolar cell dendrites (Vardi, 1998). Furthermore, dialysis via patch pipette of the Gαo subunit, or an antibody directed against the subunit, functionally disrupted the cascade (Nawy, 1999). Most convincingly, the b wave component of the ERG was found to be completely absent in mice lacking a specific Gαo splice variant, Gαo1, indicating a loss of ON bipolar cell function (Dhingra et al., 2002). These findings strongly implicate Go rather than transducin in mGluR6 transduction. However they do not address the possibility that a PDE is still the effector enzyme for mGluR6 transduction, and that Go might couple to the PDE in a novel way. An obligatory role for PDE in the cascade seems unlikely for several reasons. First, dialysis of ON bipolar cells with poorly hydrolyzed analogs of cGMP, does not inhibit or alter the kinetics of the glutamate response (Nawy, 1999). Furthermore, when exogenous glutamate is present in the bath solution to ensure that all the cation channels are closed, cGMP application has no effect on holding current, indicating that it cannot gate the opening of channels on its own (Snellman and Nawy, 2004). These findings call into question the notion that hydrolysis of cGMP is required in order for the cation channel to open. Instead, they point toward a modulatory, rather than an obligatory role for cGMP in transduction. This will be discussed in more detail in the next section.
2.4 If not a cGMP-gated channel, then what?
If the phototransduction hypothesis is to be rejected, then a compelling argument for an alternative pathway has yet to be advanced. What is required is a model that can account for how the channel is gated open, and how it is closed by activation of Go. Perhaps the simplest model is that the channel does not require a second messenger to be gated open, but is opened either constitutively, or by phosphorylation. Closure would then be achieved by a direct interaction of the channel with one or more G protein subunits. Pertussis toxin-sensitive G proteins, including Go, interact directly with certain classes of voltage-gated Ca2+ channel through a membrane delimited pathway mediated by Gβγ (Dolphin, 2003). Here, channel closure is achieved by shifting voltage activation to more positive potentials. However, closure of voltage-independent channels by Gβγ has not been reported, making such a mechanism less likely (although in the retina, anything is possible). More likely is a model where gating of the channel is accomplished by a second messenger, and closure of the channel is achieved either by G protein-mediated breakdown of the second messenger, or by direct interaction with the channel. Lipid-based molecules such as PIP2 may be the most likely candidates here, as they have been implicated in gating of a variety of different channels (Suh and Hille, 2005).
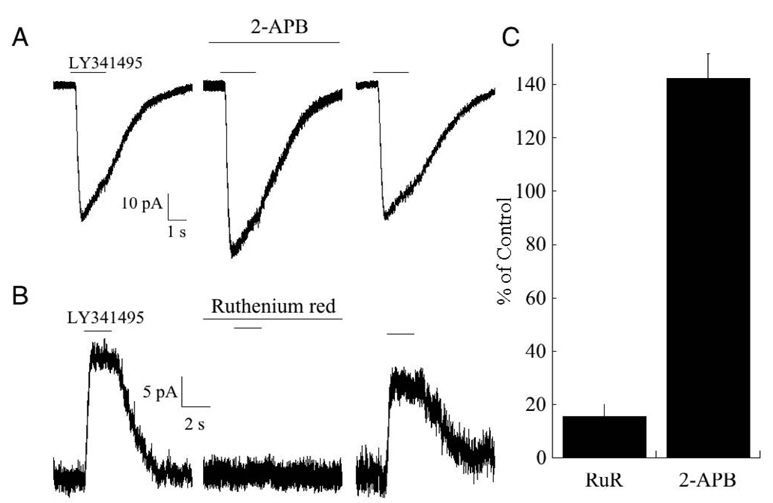
Recent pharmacological experiments performed in our lab suggest that the cation-selective transduction channel expressed in mouse ON bipolar cells possesses several of the basic pharmacological properties of the TRP family of channels. In particular, the transduction current is inhibited by ruthenium red at micromolar concentrations, but potentiated by 2-aminoethoxydiphenyl borate (2-APB), consistent with the pharmacological profile of several classes of TRP channels (Ramsey et al., 2006; Clapham, 2007). A TRP-like channel has been implicated in signal transduction in melanopsin-containing light-sensitive ganglion cells (Warren et al., 2006; Hartwick et al., 2007). Furthermore, TRP channels are strongly desensitized by Ca2+ (Cholewinski et al., 1993; Liu and Simon, 1996; Koplas et al., 1997), as are the cation-selective transduction channels expressed in ON bipolar cells (see below).
3. ON bipolar cell signaling and cGMP
3.1 Elevated cGMP levels improves signal detection near threshold
There is convincing evidence from a number of studies and species that cGMP modulates the mGluR6 pathway. Dialysis of salamander ON bipolar cells with cGMP potentiates the standing inward current, but still allows glutamate to suppress this current completely, thus increasing the size of the response to exogenously applied glutamate and to light (Nawy and Jahr, 1990a; Shiells and Falk, 1990; Nawy and Jahr, 1991). Noise analysis of the cation current in dissociated ON bipolar cells from cat retina suggested that dialysis with cGMP dramatically increases the open channel probability (de la Villa et al., 1995). In all of these studies, cGMP was applied under conditions where a fraction of the currently was tonically activated. Modulation of the activated component of the current by cGMP was, at that time, interpreted as a recruitment of closed channels, and led to the hypothesis that cGMP might play a role in channel gating. As noted in the previous section, cGMP has no effect on the membrane current or voltage of ON bipolar cells when the cation current is completely suppressed in the presence of a saturating concentration of mGluR6 agonist (Snellman and Nawy, 2004). Thus, cGMP appears to act as a true modulator of the current (but see Shiells and Falk, 2002).
Two groups have studied cGMP modulation in detail. In the all rod retina of the dogfish, intracellular dialysis of cGMP produced a leftward shift in the intensity-response function (Shiells and Falk, 2002). Rather than remaining parallel to the control intensity-response function, the cGMP-induced shift was associated with a significant decrease in the slope. As a result, the potentiating effects of cGMP were most prominent near the threshold for light detection. Light stimuli too weak to elicit detectable events in ON bipolar cells often produced reliable responses after dialysis of ON bipolar cells with cGMP. Working in the mammalian retina, our lab used pressure ejection of the mGluR6 antagonist (RS)-a-Cyclopropyl-4-phosphonophenylglycine (CPPG) while flooding the retina with L-AP4 to mimic light flashes, varying the simulated flash intensity by changing the duration of CPPG application. As reported in the dogfish retina, addition of cGMP also caused a leftward shift in the pharmacologically simulated intensity-response function without significantly changing the maximum response (Snellman and Nawy, 2004). Similarly, puffs of CPPG that were too brief to evoke a measurable response in the absence of cGMP could be reliably detected after cGMP application. Similar results were obtained using activators of guanylate cyclase, as well as inhibitors of cGMP phosphodiesterases (PDE), indicating that rod bipolar cells can generate cGMP at concentration that is sufficient to induce potentiation (Snellman and Nawy, 2004). These studies suggest that cGMP may play a role in setting the threshold for the postsynaptic detection of photons that are absorbed within the rod network. The relationship between cGMP and the threshold for event detection in ON bipolar cells will be discussed below. The role of cGMP in mGluR6 transduction appears to be highly conserved, as it found in the animals as divergent as dogfish and mouse.
3.2 Mechanism of cGMP potentiation
cGMP acts on three main classes of effectors, including CNG channels, PDEs and cGMP dependent kinase (cGK) (Beavo and Brunton, 2002; Feil and Kleppisch, 2008). As discussed above, there is currently no evidence for expression of CNG channels in the dendrites of ON bipolar cells. Furthermore, PDEs activated by cGMP belong to the type-2 family which can be found in a subset of olfactory neurons and in certain cell types of the hippocampus, but have no known expression in the retina (Beavo, 1995). However, there is evidence that the third effector of cGMP, cGK, is involved in modulation of the mGluR6 cascade. In mouse retina, inhibitors of cGK completely reverse potentiation of the cation current by exogenous cGMP (Snellman and Nawy, 2004). Similarly, stimulation of endogenous cGMP production by application of the NO analog SNAP also potentiates mGluR6 responses, (Shiells and Falk, 1992; Snellman and Nawy, 2004), and this potentiation is blocked by cGK inhibitors (Snellman and Nawy, 2004).
Likely intracellular targets of phosphorylation by cGK include the mGluR6 receptor and the synaptic cation channel, but at the present time there is no direct evidence favoring one or the other. There is circumstantial evidence that cGK could potentially phosphorylate mGluR6: G protein-coupled receptors are regulated by phosphorylation of sites within the intracellular loops and the carboxyl terminus (Dale et al., 2002). A consensus cGK phosphorylation site, RKRS, is present in the carboxyl terminus of mGluR6 (NCBI nucleotide sequence NM_1733772). Furthermore, cGK has been shown to phosphorylate the closely related group III mGluR7 receptor (Sorensen et al., 2002). Phosphorylation of mGluR7 by cGK prevents binding of calmodulin to the carboxyl terminus. Binding of calmodulin and Gβγ, in turn, are mutually exclusive (O'Connor et al., 1999), and so phosphorylation by cGK effectively increases the capacity of receptor binding to Gβγ, preventing Gβγ from interacting with its effector. One possible scenario is that cGK phosphorylation of the mGluR6 C-terminus increases the strength of its interaction with Gβγ. If Gβγ is responsible for closing the synaptic channel, then phosphorylated mGluR6 could buffer free Gβγ, preventing it from interacting with the channel, and resulting in an upregulation of channel function.
Another possibility is that cGK phosphorylates the transduction channel itself, thereby changing its open probability, as has been shown for TRPC channels (Kwan et al., 2004). Phosphorylation by cGK could either increase the affinity of the synaptic channel for an intracellular agonist that is required for channel opening, or it could lower channel affinity for a substance that closes the channel (as is discussed in the following section). The mGluR6 receptor has been expressed in oocytes (Dhingra et al., 2004) and in superior cervical ganglion cells (Tian and Kammermeier, 2007). Thus, the question of whether cGK can functionally modulate mGluR6 can be addressed by coexpression of the receptor and cGK along with a readout channel (such as a G protein-regulated inwardly rectifying K+ channel). Addressing the potential role of phosphorylation of the synaptic channel expressed in ON bipolar cells will have to await the cloning of the channel.
While cGMP-mediated potentiation of mouse ON bipolar cell responses is dependent upon the activation of cGK, it should be mentioned that possible species differences exist. It has been reported that inhibition of tyrosine kinase potentiates dogfish ON bipolar cell responses in a way analogous to that of cGMP, and that activators of tyrosine kinase block cGMP-mediated increases in sensitivity (Shiells and Falk, 2002). This is similar to a mechanism reported in photoreceptors, where phosphorylation of the CNG channel by tyrosine kinase favors channel closing and decreases sensitivity to cGMP, while dephosphorylation favors channel opening (Molokanova et al., 1999; Krajewski et al., 2003). This mechanism does not appear to be present in mouse bipolar cells since addition of the general tyrosine kinase inhibitor genestein in the pipette had no potentiating effect (Snellman and Nawy, unpublished observations).
3.3 cGMP and the threshold model of mGluR6 transduction
Several groups (Shiells and Falk, 1994; van Rossum and Smith, 1998; Sampath and Rieke, 2004) have proposed variations on a threshold model to account for the nonlinear relationship between the concentration of mGluR6 agonist and the fraction of open transduction channels. An essential feature of the model is that there is an excess number of molecules within the dendrites of the ON bipolar cell that serve to keep the synaptic channels closed in the dark. Random fluctuations in the rod membrane potential, due to the occurrence of spontaneous events in the phototransduction cascade (Baylor et al., 1980; Rieke and Baylor, 1996) lead to small decreases in the concentration of glutamate in the cleft. As proposed by Sampath and Rieke (2004), a drop in transmitter concentration results in an increase in the number of unoccupied mGluR6 receptors, and a concomitant drop in the concentration of the channel closing substance (S*). However, because of the excess in the levels of S*, the synaptic channels do not open. Such a thresholding mechanism is therefore potentially important for reducing the transfer of voltage noise across the synapse, but the penalty to be paid is a loss of sensitivity to small single photon responses. In an alternative and equally valid version of the model, also proposed by Sampath and Rieke (2004), there is linear relationship between the concentration of S* and the channel open probability, and the nonlinearity occurs upstream of the channel, perhaps at the level of the G protein.
The role of cGK can be viewed within the context of the threshold model (fig. 3). Phosphorylation of the channel by cGK decreases the affinity for the channel for S*. A higher concentration of S* is now needed to close the channel, and so there is no longer an excess amount of S* in the dark. As a result, small perturbations in transmitter levels at the synapse are now sufficient to open the channel. Of course, as discussed in the previous section, cGK might instead phosphorylate the mGluR6 receptor. This would result in a decrease in the levels of free S*, once again allowing for small perturbations in transmitter levels to open synaptic channels. Regardless of the site of action, the net result of activation of the cGMP pathway is an amplification of small changes in transmitter release that accompany the detection of a sparse number of photons.
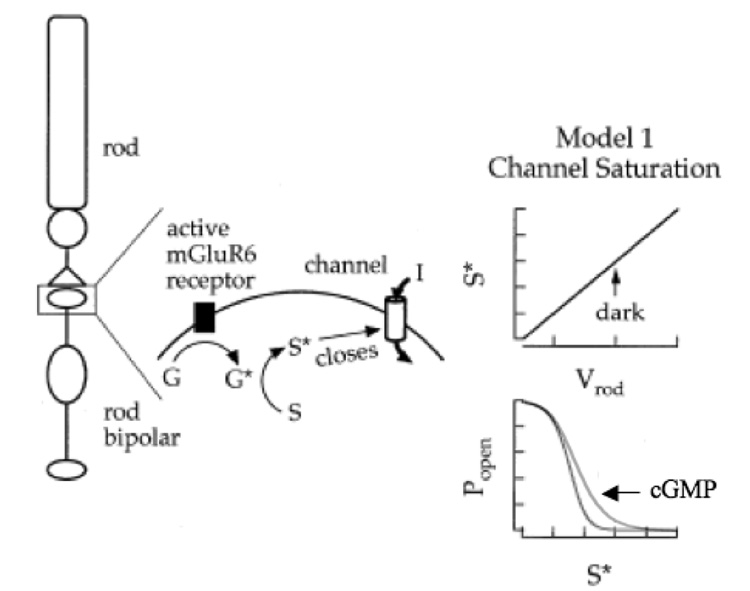
Fig. 3. Model depicting the proposed role of cGMP in mGluR6 transduction.
Left: Binding of glutamate, the rod transmitter, to mGluR6 activates Go (G*), which in turn catalyzes the production of a substance (S*) that closes the synaptic channel. Right: In this model, there is a linear relationship between the rod membrane potential (which controls transmitter release) and the production of S* (top). At depolarized rod membrane potentials (i.e., in the dark) the production of S* is in excess compared with the number of channels, allowing for negative-going excursions of the rod membrane potential without significant channel opens (bottom). cGMP/cGK functionally shifts this curve to the right, allowing for small changes in membrane potential to open channels, but does not change the maximum open probability. Modified from (Sampath and Rieke, 2004).
Interestingly, in both dogfish and mouse rod bipolar cells, dim backgrounds have essentially the same effect as cGMP on the ON bipolar cell intensity response function, decreasing the slope of the intensity response function, and shifting the function to the left (Shiells and Falk, 2002; Sampath and Rieke, 2004). In dogfish retina, it has been postulated that the cation channel is gated by cGMP, and that glutamate closes the channel by hydrolyzing cGMP. In this scenario, dim backgrounds would increase endogenous intracellular cGMP by lowering the glutamate concentration in the synaptic cleft sufficiently to slow the rate of cGMP hydrolysis. Thus cGMP would serve two roles, both gating and modulating of the cation channel (Shiells and Falk, 2002). However, a more parsimonious explanation is that dim backgrounds relieve saturation of the mGluR6 cascade by imposing a small hyperpolarization of the rod membrane voltage (Sampath and Rieke, 2004). Further hyperpolarization of the rod by dim flashes superimposed upon the background readily moves the cascade into a non-saturated state, and the synaptic channels can now open. Yet to be tested experimentally is the prediction that both cGMP and dim backgrounds should augment the detection of rod noise in the ON bipolar cell, as the threshold behavior of the mGluR6 cascade would be removed.
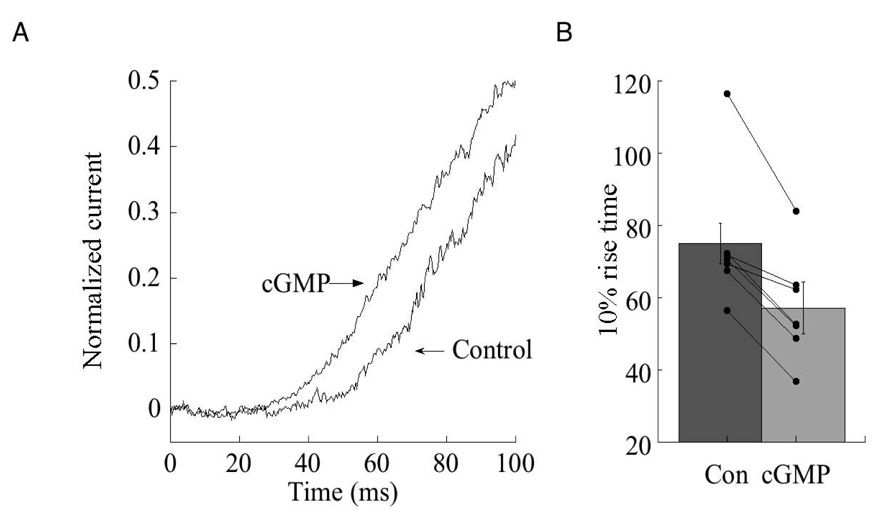
Perhaps as a consequence of lowering the threshold for channel opening, cGMP also reduces the latency of channel opening (fig. 4). In rod bipolar cells dialyzed with 1 mM cGMP, the latency of the response to puffs of CPPG was consistently reduced compared with the latency after break in. In cells that were not dialyzed with cGMP, no change in the latency was observed over the same time period (data not shown). The response latency following a puff of CPPG reflects not only the transduction time, but also the time required for CPPG to reach the dendrites after it is ejected from the puffer pipette, as well as the time required for the unbinding of agonist. However, the difference in the latency (17.8±3.2 ms) in the presence and absence of cGMP most likely reflects a change in transduction time, as the position of the puffer pipette, and the pressure of ejection were not changed during experiments. This may represent the savings in time gained by reducing the steady state levels of S*, or decreasing the affinity of S* for the channel, and thus decreasing the change in concentration of S* required to initiate channel opening.
Fig. 4. Cyclic GMP reduces the latency of channel opening following removal of agonist.
(A) Comparison of the latency of the response to a 200 ms puff CPPG immediately after break in and after 10 minutes of recording in a rod bipolar cell dialyzed with an internal solution containing 1 mM cGMP. Response amplitudes have been normalized to unity. Holding potential: −50 mV. (B) Summary of response latency, defined as the time required to reach 10% of the peak response after break in (Con) and after 10 minutes of dialysis with 1 mM cGMP (n=7 cells). Shaded histogram indicates mean and standard error. Connected points indicate individual cells before and after dialysis. p<.01 (Student’s t test).
3.4 Improved ON bipolar cell signal detection is reflected in postsynaptic cells
For cGMP/cGK-mediated potentiation of ON bipolar cell responses to be relevant to retina signalling, potentiation must be passed across the synapse to the downstream neuron. In the mouse (Tsukamoto et al., 2001), as in other mammalian retinas (reviewed in Bloomfield and Dacheux, 2001), rod bipolar cells synapse onto the AII amacrine cell (Kolb and Famiglietti, 1974). At the rod bipolar cell terminal, the predominant Ca2+ channel, the L-type channel (Protti, 1998; Satoh et al., 1998; Hartveit, 1999; Pan, 2000), begins to activate between −40 mV and −45 mV (Protti, 1998; Hartveit, 1999; Singer and Diamond, 2003). In darkness, when rod bipolar cell synaptic channels are mostly closed, the resting potential of the rod bipolar cell has been measured to be between −59 mV and −47 mV (Berntson and Taylor, 2000; Euler and Masland, 2000; Pang et al., 2004; Wu et al., 2004). Our own measurements of rod membrane potential, made in the presence of L-AP4, indicate an average value of −56.4 ± 0.4 mV (n=24). Thus, in the absence of light, the membrane potential of the rod bipolar cell is too negative to support transmitter release. Direct evidence for this comes from the observation that bath application of the mGluR6 agonist L-AP4 suppresses spontaneous EPSCs in AII amacrine cells (Singer and Diamond, 2003; Tamalu and Watanabe, 2007). Because most bipolar cells lack Na+ or other regenerative voltage-gated channels to drive depolarization, the light-evoked opening of synaptic cation channels in the dendrites must therefore be sufficient to depolarize the axon terminal into the range of Ca2+ channel activation.
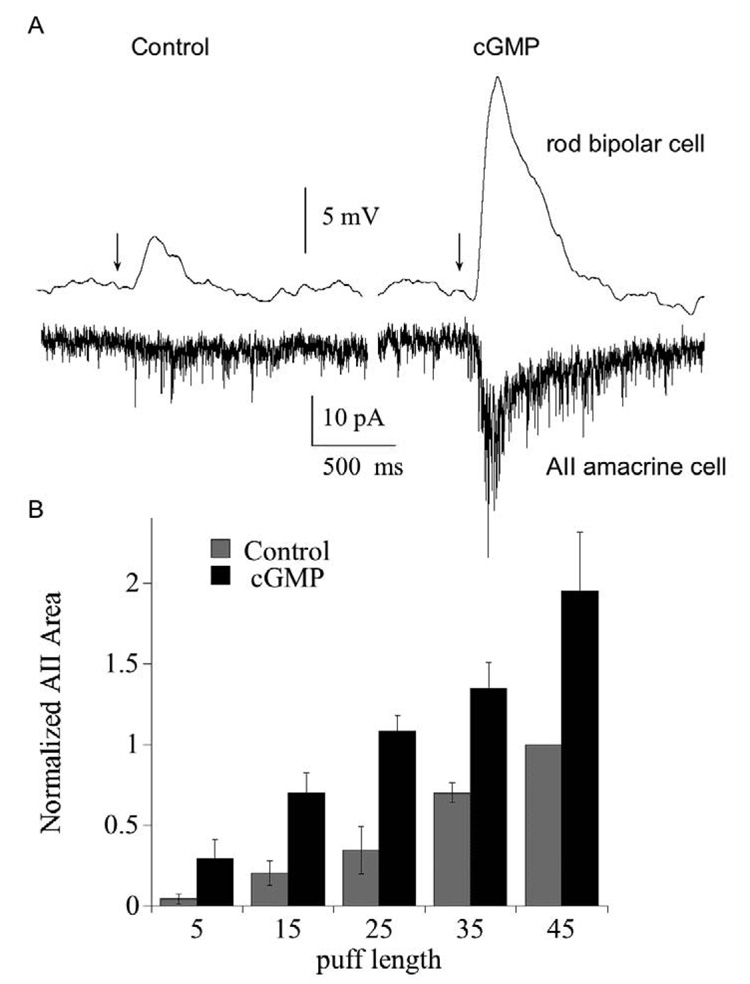
To determine whether cGMP can enhance synaptic transmission from rod bipolar to AII amacrine by amplifying presynaptic events, we recorded from synaptically connected rod bipolar and AII amacrine cell pairs and puffed CPPG onto the dendrites of the rod bipolar cell (fig. 5). The rod bipolar cell, recorded in current clamp mode, was depolarized by a brief puff of CPPG, whose duration was adjusted so as to be too brief to produce a significant response in the voltage-clamped AII amacrine cell. After 10 minutes of dialysis with cGMP, the response to the same length puff of CPPG in the rod bipolar cell was considerably potentiated, and was now large enough to evoke an EPSC in the AII amacrine cell. Fig. 5B summarizes the results of this experiment as a function of puff length for all of the rod bipolar-AII amacrine cell pairs that we tested. Cyclic GMP increased the postsynaptic response, expressed here as integrated current, at every puff length. Thus, potentiation by cGMP of the response in the presynaptic cell can be transmitted across the synapse.
Fig. 5. Potentiation of rod bipolar cell responses is passed forward to the AII amacrine cell.
(A) Paired recordings of a rod bipolar cell (current clamp) and an AII amacrine cell (voltage clamp). Traces on the left show the response of the rod bipolar and amacrine cell to a 15 ms puff of the mGluR6 antagonist CPPG immediately after breaking into the rod bipolar cell. Traces on the right show the responses 10 minutes later. Bipolar cell pipet contained 1 mM cGMP. Bath solution contained 4 µM L-AP4. Each trace is the average of 3 responses. (B) Summary of the effect of rod bipolar cell cGMP on the postsynaptic response of the AII amacrine cell, plotted as a function of puff length. Amacrine cell responses were integrated over the first 500 ms following the CPPG puff, and each was normalized to the current elicited by the 45 ms control puff (i.e., prior to dialysis with cGMP). For each puff, n=6 (15 and 25 ms puff) or 8 cells (5, 35, 45 ms puffs). P<.05 for 15–45 ms puff.
The size of the presynaptic response produced by brief puffs of CPPG might be an underestimate of the average voltage response to a photon of light absorbed by a rod. In 7 rod bipolar cell-AII amacrine cell pairs, the average size of the response to CPPG that failed to elicit an EPSC in the AII was 3.4±0.6 mV. By comparison, the single photon response, measured in voltage clamp, has been estimated to be between 5 and 10 pA in mouse (Field and Rieke, 2002; Berntson et al., 2004b), similar to the estimate of 8 pA in dogfish, obtained 20 years earlier (Ashmore and Falk, 1982) using sharp electrode recording and noise analysis. For a rod bipolar cell with an input resistance of 2 GΩ (Zhou et al., 2006; Oltedal et al., 2007), the single photon response would produce a depolarization of approximately 10–20 mV in the rod bipolar cell. A response of this magnitude, rising from a baseline membrane potential of −55 mV is well matched to the voltage required for L-type Ca2+ channel activation. However, it is unclear to what extent these values of the single photon response in rod bipolar cells reflect cGMP-mediated potentiation. In the dark-adapted retina, it has been reported that addition of exogenous cGMP through the recording pipette does not enhance the amplitude of the flash response (Sampath and Rieke, 2004), possibly because levels of endogenous cGMP are already high. It would be of interest to know whether application of cGK antagonist in the dark-adapted retina would reduce the amplitude of the dim flash response.
3.5 Localization of cGMP and NOS in the retina
cGMP is produced by the enzyme guanylate cyclase. In mammals, there are two families of guanylate cyclase: Particulate guanylate cyclases are membrane bound and activated by natriuretic peptides, while soluble guanylate cyclases are activated by nitric oxide (NO) (Israel et al., 1990; de Vente and Steinbusch, 1992). Studies have demonstrated that the NO-activated form of soluble guanylate cyclase is expressed in ON bipolar cells (Ahmad and Barnstable, 1993; Spreca et al., 1999; Haberecht et al., 2000). Furthermore, there is strong evidence for up-regulation of cGMP levels in ON bipolar cells in response to NO stimulation (Koistinaho et al., 1993; Gotzes et al., 2000; Baldridge and Fischer, 2002). As mentioned earlier, studies in ON bipolar cells have shown that NO donors increase response amplitudes to light flashes in a way similar to that of cGMP (Shiells and Falk, 1992; Snellman and Nawy, 2004). ON bipolar cell responses potentiate in the presence of SNAP, an activator of soluble guanylate cyclase, suggesting that production of endogenous cGMP is sufficient for response amplification. This potentiation is readily reversible, indicating that ON bipolar cells contain an endogenous PDE capable of restricting the duration of the signal.
Physiological studies indicate multiple roles for NO in retinal modulation. In cone photoreceptors, NO has been found to increase gain and affect release by extending the voltage range over which exocytosis occurs (Rieke and Schwartz, 1994). In ganglion cells, NO facilitates the activation of N-type calcium channels through activation of cGK (Hirooka et al., 2000). In horizontal cells, NO decreases the affinity of AMPA/Kainate receptors, and increases the maximal current, a possible mechanism for preserving response range during light adaptation (McMahon and Schmidt, 1999). Furthermore, NO closes gap junctions between horizontal cells (DeVries and Schwartz, 1989; Pottek et al., 1997). NO stimulated increases in cGMP have also been reported to close the gap junction between amacrine cells and cone bipolar cells (Mills and Massey, 1995) and depress GABAA currents in amacrine cells (Wexler et al., 1998).
4. Role of calcium ions in mGluR6 transduction
Synaptic transmission from photoreceptors to OFF bipolar cells is mediated by ionotropic AMPA and kainate receptors (Gilbertson et al., 1991; DeVries and Schwartz, 1999; DeVries, 2000). Following the binding of glutamate, AMPA/kainate receptors move rapidly from an open to a desensitized, poorly conducting state (Jonas et al., 1993; Paternain et al., 1998). The specific types of AMPA/kainate receptors that are expressed in OFF bipolar also desensitize, although there are significant differences in desensitization rates between them (DeVries and Schwartz, 1999; DeVries, 2000; DeVries et al., 2006). This means that receptors on the OFF bipolar cell dendrites are rapidly trapped in a desensitized state as a result of the continuous bombardment of transmitter that is released from photoreceptors in darkness. It is only during illumination, when release is suppressed, that AMPA/kainate receptors have an opportunity to recover from desensitization. If the period of illumination is longer than the time required to recover from desensitization, then the response to renewed transmitter release (i.e., a decrements in illumination) will be robust. Conversely, if release lasts longer than the time required for AMPA/kainate receptors to enter the desensitized state, then the response to suppression of transmitter release (i.e., increments in light) will be weak. Hence, the photoreceptor-OFF bipolar cell synapse is well constructed to signal decrements in illumination, but responds poorly to an increase in light intensity. Instead, this is the function of the ON pathway.
4.1 Ca2+ depresses synaptic transmission
The ON bipolar mGluR6 cascade undergoes a functional desensitization in the presence of steady illumination, as described below, but the mGluR6 receptor itself does not desensitize in the classic sense, as there is no evidence that agonist-bound mGluR6 receptors enter a desensitized state. Instead, ON bipolar cells display desensitization in response to withdrawal of transmitter, thus allowing the ON pathway to respond most strongly to increments in light. This phenomenon has been studied by three different groups, including this laboratory, and has been given a different name by each. Although there are differences in the experimental approach and species used, all the laboratories agree that Ca2+ plays an essential role.
Shiells and Falk (1999) first showed that during continuous exposure to dim illumination, the amplitude of the ON bipolar cell response declines precipitously. In theory, this form of synaptic depression could have been presynaptic (i.e., adaptation in the rods). However, the authors also showed that it was absent in OFF bipolar cells, making a presynaptic mechanism less likely. Furthermore, the decline in the response was prevented by dialyzing cells with the rapid Ca2+ chelator BAPTA, indicating not only a postsynaptic locus, but a role for Ca2+ as well. They also found that during the plateau phase of the response to dim backgrounds, the response to flashes superimposed on the background were strongly reduced in amplitude, as compared with the flash response in the absence of a steady background. This was not due to occlusion of the flash response by steady illumination, as the summed flash and background response was still smaller than the flash response alone. The authors argued that the suppressive effect of dim backgrounds on the flash response was mediated by a rise in intracellular Ca2+. The cation channel itself was later shown to be permeable to Ca2+ (Nawy, 2000), thus providing a potential route for its entry. It seems plausible that influx of Ca2+ through the channel would be sufficient to significantly raise intracellular Ca2+. However, conformation of this hypothesis will require direct measurements of intracellular Ca2+ before and after channel opening, and this has yet to be accomplished.
The effects of continuous illumination on flash sensitivity have been proposed be an adaptive mechanism, whereby light lowers the gain of the photoreceptor-bipolar cell synapse (Shiells and Falk, 1999, 2000). A lowering of synaptic gain would help prevent saturation of the postsynaptic response at low light intensities, and would extend the operating range of the synapse. However, backgrounds do not induce a rightward shift of the intensity-response function, as would be expected for an adaptive process. Instead, the depressive effects of dim steady backgrounds on the flash response cannot be overcome by increasing flash intensity, resulting in a compression of the intensity response function. These findings are more consistent with an inhibitory or desensitizing process.
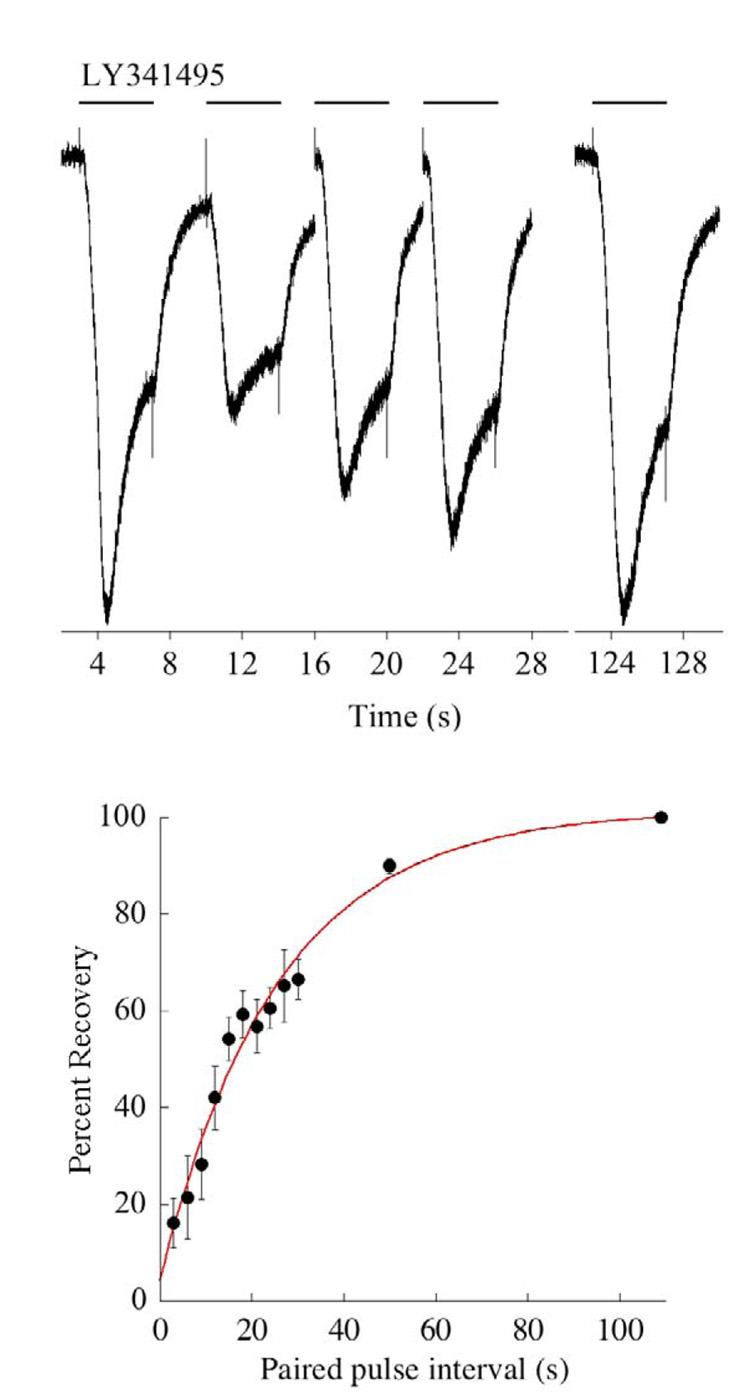
As a complimentary approach to studying the properties of the photoreceptor-ON bipolar cell synapse, light responses were simulated pharmacologically (Nawy, 2004). This approach is similar to that described earlier in this review, except that rather than using a puffer pipette, drugs are applied to bipolar cells using a series of “flowpipes” which were originally designed for drug application to neurons in culture (Johnson and Ascher, 1987). This approach allows for the delivery of a constant stream of antagonist for an extended period of time. Following the application of the mGluR6 antagonist CPPG, the response reaches a peak and then decays to a plateau response (fig. 6), a phenomenon that was termed desensitization. As had been reported earlier in the dogfish retina (Shiells and Falk, 1999), desensitization was dependent upon a rise in intracellular Ca2+, and was blocked by BAPTA. This requirement for Ca2+ confers a voltage dependence to desensitization, which is absent at positive voltages, presumably due to the lack of driving force for Ca2+, but is initiated by stepping the voltage to negative potentials (fig. 6). The time constant of desensitization, when measured using this voltage step protocol, is within a range of 300–1000 ms. Responses to steps of light in salamander ON bipolar cells have similarly been shown to be transient (Awatramani and Slaughter, 2000). The time constants of the decay of the response have been reported to fall into two classes, one with an average of 800 ms, and the other about 300 ms, perhaps corresponding to different subtypes of ON bipolar cells (Awatramani and Slaughter, 2000). Thus, when the synaptic current is activated by either light or application of an mGluR6 antagonist, the peak decays to a steady state with a rate constant of 300–1000 ms. Recovery from the desensitized state, measured using a paired pulse protocol, is surprisingly slow, having a time constant of 27 seconds (fig. 7). Thus, at least in tiger salamander, a light stimulus that opens all of the synaptic channels long enough to drive them into the desensitized state will decrease the sensitivity to further light stimulation for about 1 minute.
Fig. 6. Ca2+-dependent depression of synaptic cation current in tiger salamander On bipolar cells.
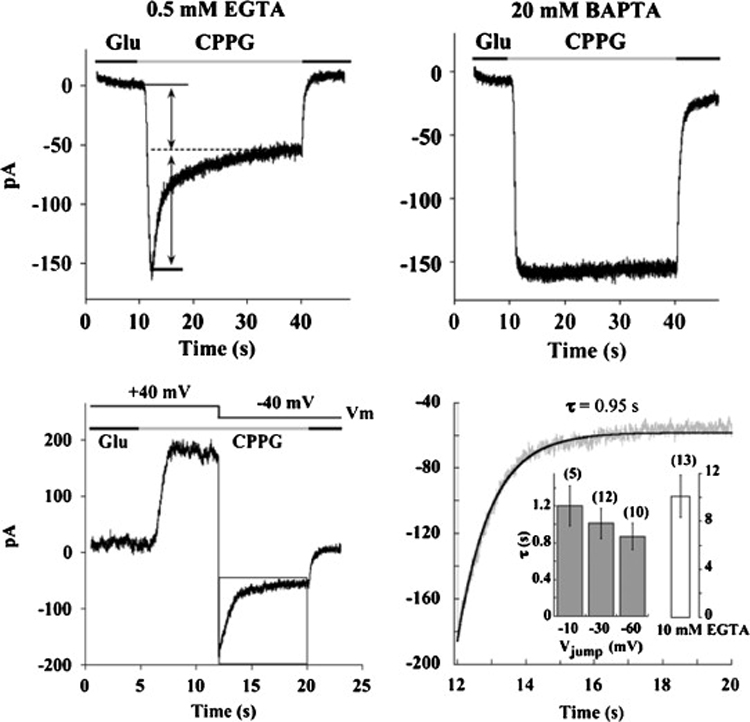
Top: Representative responses to application of the mGluR6 antagonist CPPG, one from a cell recorded with a solution containing 0.5 mM EGTA (left) and the other with a solution containing 20 mM BAPTA. Holding potential of each cell was −40 mV. Bottom: Left panel is the response of another cell to the application of CPPG at +40 mV. Note the absence of desensitization. A jump from a holding potential of +40 mV to −40 mV results in an exponential decay in the cation current. Right panel is an expansion of the decay of the transduction current indicated by the box on the left. Dark line is the fit with a single time constant of 0.95 seconds. Inset: Filled bars represent the mean ± s.e.m. of the time constant at each voltage in cells that were buffered with 0.5 mM EGTA. Open bar is the time constant measured in cells buffered with 10 mM EGTA when stepped to −40 mV. From Nawy, 2004.
Fig. 7. The timecourse of recovery from desensitization.
Top; Example of a paired pulse experiment in which a conditioning pulse of the mGluR6 antagonist LY341495 was first applied to induce desensitization, followed by a second test application at variable times to measure the amount of recovery. Only the responses at the three shortest intervals are shown, followed by the response after complete recovery (note break in x-axis). Holding potential was −40 mV, and cells were dialyzed with 0.5 mM EGTA. Bottom: Summary of the experiment conducted in 5 cells. Smooth line is a single exponential fit with a time constant of 25.4 seconds.
Recordings from rod bipolar cells of the mouse retina similarly reveal a sag in the response to light steps (Berntson et al., 2004a), which they termed inactivation (fig. 8). This was unlikely to be presynaptic as the sag was eliminated either by buffering Ca2+ with BAPTA or by holding the membrane potential at positive voltages so as to reduce the driving force for Ca2+ influx through the synaptic channel. Thus, both mammals and lower vertebrates share a Ca2+-mediated negative feedback that limits the duration of signaling in On bipolar cells. In mouse, the time constant of Ca2+ feedback in mouse was found to be considerably faster than in tiger salamander, approximately 60 ms. As the 10–90% time for channel opening was found to be approximately 30 ms, negative feed back is rapid enough to attenuate the peak of the flash response (Berntson et al., 2004a). As pointed out by the authors, there is considerable variability in the amplitude and shape of the single photon response in rod bipolar cells. One function of Ca2+ feedback may be to improve the reproducibility of the single photon response: If the strength of feedback inhibition is correlated with the size of the flash response, then larger than average quantal responses will be reduced more than smaller responses.
Fig. 8. Ca2+-dependent depression is also present in the light responses of mammalian rod bipolar cells.
A: Response to a step of saturating light in a rod-driven bipolar cell (left), and a cone-driven ON bipolar cell (right). The light response has the characteristic transient peak in the rod-driven bipolar cell, but not in the cell driven by cones. B: Response to a light step as a function of holding voltage. At positive potentials, when the driving force for Ca2+ is reduced, the depression is less pronounced. C. Averaged I-V relation measured at the times indicated by the symbols in (B). The I-V relation measured at the peak of the response is linear, but the I-V measured at steady-state rectifies outwardly as would be expected if Ca2+ influx depresses the steady-state response. From Berntson et al., 2004a.
4.2 Mechanisms of Ca2+-mediated depression
Based on the studies summarized above, there is good consensus that Ca2+ provides a feedback signal that depresses the synaptic current. However, there is little or no agreement regarding the downstream targets of Ca2+. In dogfish, inhibitors of CaMKII block desensitization, as well as Ca2+-mediated outward rectification of the cation channel, implying that CaMKII-mediated phosphorylation of the channel induces desensitization (Shiells and Falk, 2000, 2001). In salamander, inhibition of neither CaMKII nor calcineurin, a Ca2+-activated phosphatase, prevents the type of fast desensitization discussed above (Nawy, 2004). Perhaps this is due to differences in the nature of the synaptic channels in the two species, as the channel in dogfish retina is thought to be gated by cGMP, while the tiger salamander channel is not. Another common mechanism by which Ca2+ modulates channel function is via direct interaction of Ca2+ with calmodulin that is tightly bound to the target channel. A well-studied example of this is the gating by Ca2+ of Ca2+-activated K+ channels (Levitan, 1999; Maylie et al., 2004). Although our lab has failed to block desensitization with inhibitors of calmodulin, this is a common finding in cases where calmodulin is constitutively bound to the targeted channel (i.e., even in the absence of Ca2+) (Levitan, 1999). Interesting, Ca2+-mediated TRP channel desensitization has been reported to be mediated by calmodulin (Numazaki et al., 2003; Lishko et al., 2007). One possible way to distinguish between direct and indirect modes of Ca2+ action on the channel would be to measure the latency of desensitization following the induction of a rapid increase in intracellular Ca2+, perhaps by flash photolysis of caged Ca2+. Such experiments have yet to be carried out.
In salamander, there is evidence for at least two separate forms of Ca2+-dependent depression of the mGluR6 cascade. In addition to the fast type discussed above, a second, slower form has also been described (Nawy, 2000). The time constant of this form of depression is about 2 minutes (Nawy, 2000; Snellman and Nawy, 2002). Unlike the faster type of depression, this slow form is poorly reversed, at least in whole cell patch clamp recording, suggesting the loss of an enzyme that is responsible for reversing the effects of Ca2+. Several lines of evidence suggest that the slower form of depression is mediated by calcineurin. First, it is prevented by intracellular dialysis of the calcineurin inhibitor cyclosporine A. Second, it is induced by intracellular application of a truncated form of constitutively active calcineurin under conditions where slow synaptic depression would normally not occur, such as when the cell is dialyzed with BAPTA (Snellman and Nawy, 2002). As mentioned above, inhibitors of calcineurin have been reported to have no effect on the fast component of depression, suggesting that each form of depression works to downregulate channel function via an independent pathway. In mouse, the molecular mechanism underlying fast Ca2+- mediated depression has not been investigated, and there is as yet no evidence for a slower component of depression. Yet the obvious advantages of genetic manipulations would seem to make mouse the most likely preparation for working out the specific details of these pathways in the future.
4.3 Role of desensitization
Thus in the outer plexiform layer, the influx of Ca2+ into ON bipolar cell dendrites is responsible for the conversion of a sustained input from photoreceptors into a more transient output. A number of mechanisms exist for performing this task in the inner retina, including feed-forward and feedback inhibition mediated by amacrine cells (Hartveit, 1999; Shields et al., 2000; Matsui et al., 2001), desensitization of postsynaptic AMPA receptors (Lukasiewicz et al., 1995; Matsui et al., 1998), and removal of transmitter from the synaptic cleft by glutamate transporters (Higgs and Lukasiewicz, 1999). It would seem that there are a number of ways to terminate signaling in the inner retina, so why are additional mechanism required in the outer retina? The large degree of convergence of photoreceptor input onto bipolar cells, particularly in lower vertebrates, may require that the voltage response from local portions of the dendritic tree be “reset” so that illumination detected elsewhere in the receptive field is able to further depolarize the bipolar cell. L-type Ca2+ channels expressed in ON bipolar cell terminals begin to turn on at around −50 mV, and the activation curve is steepest 23 between −45 mV and −35 mV (Protti, 1998; Pan, 2000). In darkness, bipolar cell membrane potential is at the foot of the L-type channel activation curve. Light that falls on a portion of the receptive field will depolarize the membrane sufficiently to move it into, or even beyond the steepest portion of the Ca2+ channel activation curve. Thus the light signal is passed on to the postsynaptic cell. However, if signaling were to continue (i.e., the bipolar cell membrane remains depolarized), then further increases in light intensity in other regions of the dendritic tree would go undetected. Limiting the duration of ON bipolar cell responses at individual synaptic sites helps to prevent saturation of the ON bipolar cell membrane potential due to spatial summation across the dendritic tree.
It is interesting to note that Berntson et al. (2004) observed desensitization in ON bipolar cells that receive input from rods, but not in those driven by cones. It will be important to determine if this dichotomy is observed in other species. A mechanism that avoids saturation due to spatial summation might be less important for cone bipolar cells, which tend to collect input from fewer photoreceptors than rod bipolar cells. In mouse, rod bipolar cells each receive input from about 22 rods, while cone bipolar cells gather input from 4–7 cones (Tsukamoto et al., 2001). Furthermore, the integration time of mammalian cones is about 25 msec (Schneeweis and Schnapf, 1999), underlying the cone pathway’s ability to respond at higher temporal frequencies compared with the rod pathway. Assuming a Ca2+ feedback system with the same time constant of recovery of 375 ms, as has been measured in rods (Berntson et al., 2004a), then the time required to recover from even a brief light stimulus would strongly compromise the sensitivity of cone bipolar cells for an order of magnitude longer than the integration time of the cone. There may be physical constraints imposed on the minimum time required to return synaptic channels to the non-desensitized, highly conducting state (i.e., buffering of Ca2+, or possibly phosphorylation/dephosphorylation of the channel) which make it impractical for the Ca2+ feedback pathway to be adapted to the cone system. Alternatively, rod and cone ON bipolar cells may express different synaptic channels, distinguished by their susceptibility to regulation by Ca2+.
5 Conclusions and future directions
This review summarizes our knowledge of the mGluR6 cascade in ON bipolar cells, focusing on its regulation by 2nd messengers, particularly Ca2+ and cGMP. These two molecules have opposing effects on the cascade, as Ca2+ reduces the synaptic current, while cGMP potentiates it. Although Ca2+ has multiple actions on the mGluR6 cascade, operating over a range of time scales, its physiological role seems straightforward, as it acts as a signal for the initiation of feedback inhibition, thus preventing prolonged signalling of the ON bipolar cell. Future studies will need to address more directly the relationship between the number of synaptic channels opened by light and the strength of the Ca2+ feedback. Is the amount of inhibition (i.e., the ratio of the peak to steady-state response) constant over the entire range of channel openings? This is what would be predicted if the domain for Ca2+-mediated inhibition encompasses only a single channel. The same fraction of inhibition would then be observed if one channel opened, or if 100 channels opened. An alternative model, for which there is more evidence, is that the response to dim illumination, which only opens a few channels in each dendrite, is insufficient for the induction of inhibitory feedback. This implies a larger Ca2+ domain, and the presence of a threshold for Ca2+. This issue has implications for the broader issue of whether Ca2+ feedback operates continuously at all light levels, or whether its main function is as a safety mechanism, activated only during strong illumination. Likewise, there is a need for further studies on the manner in which cGMP levels are regulated in ON bipolar cells. One issue which will need to be addressed is the range of ambient light intensities over which cGMP-mediated gain amplification operates. On the one hand, it would make sense for cGMP levels to be high under scotopic conditions, when photons are scarce, as a high synaptic gain would make it more likely that single photon responses of rods will be transmitted across the rod bipolar cell synapse. On the other hand, a high gain would also contribute to the amplification of noise, potentially swamping the signal.
Undoubtedly the central question that needs to be solved by future studies is the molecular identity of the synaptic cation channel(s) in the mGluR6 cascade. Aside from the inherent importance of identifying the channel, the ability to express it in cell lines will allow us to address key questions of synaptic modulation raised in this review. For example, the identification and subsequent mutation of putative Ca2+/calmodulin binding domains are essential for determining the role of Ca2+ feedback in retinal function. Ultimately, generation of a mice with a cell specific mutation in a putative Ca2+/calmodulin binding domain would allow for the study of Ca2+ feedback at the level of a single bipolar cell, or using behavioral approaches, at the level of the entire visual system.
As discussed previously in this review, cloning strategies based on the assumption that the mGluR6 channel shares a conserved cGMP-binding domain with cyclic nucleotide gated channels proved to be unsuccessful. Preliminary studies aimed at defining the pharmacology of the channel point instead toward a TRP-like channel (see fig. 2 of this review). Future experiments in this vein, albeit with the use of pharmacological reagents that are less than ideal, should be able to further narrow the identity of the channel, at least to its closest relatives. Such information should help guide future cloning strategies. Once this is accomplished, all of the major known components in this exceedingly elusive transduction cascade will have been identified molecularly. Then, the real work of understanding how the parts interact with one another, and how these interactions are regulated to control the gain of the postsynaptic response can begin.
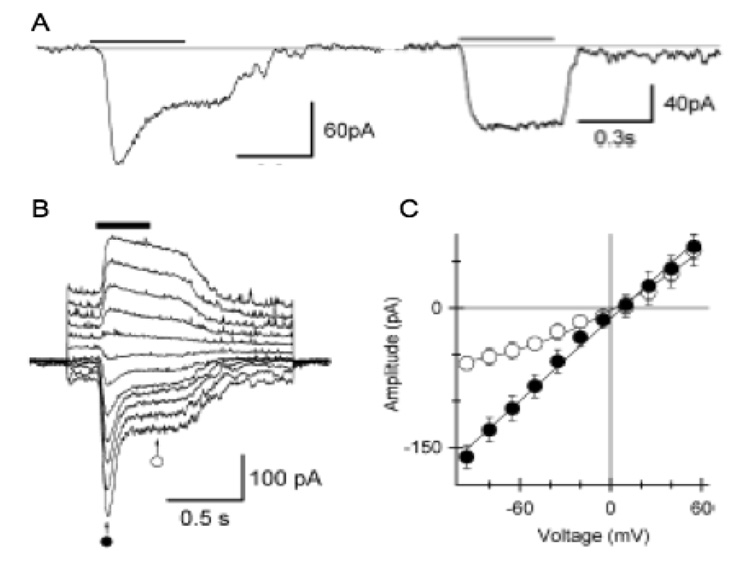
Fig. 2. Pharmacological properties of the cationic transduction channel.
(A) Response of a mouse rod bipolar cell to application of the mGluR6 antagonist LY341495 (100 µM) before, during and after application of 200 µM 2-APB. Holding potential was −40 mV. (B) Response of another cell before, during, and after application of 10 µM ruthenium red. Holding potential was +40 mV, and thus the responses were outward. (C) Summary of the effects of both compounds on the transduction current. Ruthenium red strongly reduced the current (n=8; p<.01, Student’s t test), while 2-APB (200 µM) potentiated the current (n=5; p<.02, Student’s t test).
Acknowledgements
We are grateful for the continued support by the National Eye Institute of our studies of mGluR6 transduction over the years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad I, Barnstable CJ. Differential Laminar Expression of Particulate and Soluble Guanylate Cyclase Genes in Rat Retina. Experimental Eye Research. 1993;56:51–62. doi: 10.1006/exer.1993.1008. [DOI] [PubMed] [Google Scholar]
- Ashmore JF, Falk G. An analysis of voltage noise in rod bipolar cells of the dogfish retina. J Physiol. 1982;332:273–297. doi: 10.1113/jphysiol.1982.sp014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Slaughter MM. Origin of Transient and Sustained Responses in Ganglion Cells of the Retina. J. Neurosci. 2000;20:7087–7095. doi: 10.1523/JNEUROSCI.20-18-07087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge WH, Fischer AJ. Nitric oxide donor stimulated increase of cyclic GMP in the goldfish retina. Cambridge Journals Online. 2002 [PubMed] [Google Scholar]
- Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. Journal of Physiology. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiological reviews. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- Beavo JA, Brunton LL. Cyclic nucleotide research - still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Postsynaptic calcium feedback between rods and rod bipolar cells in the mouse retina. Vis Neurosci. 2004a;21:913–924. doi: 10.1017/S095252380421611X. [DOI] [PubMed] [Google Scholar]
- Berntson A, Smith RG, Taylor WR. Transmission of single photon signals through a binary synapse in the mammalian retina. Vis Neurosci. 2004b;21:693–702. doi: 10.1017/S0952523804215048. [DOI] [PubMed] [Google Scholar]
- Berntson A, Taylor WR. Response characteristics and receptive field widths of on-bipolar cells in the mouse retina. J Physiol. 2000;524(Pt 3):879–889. doi: 10.1111/j.1469-7793.2000.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Eye Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Cholewinski A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993;55:1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- Clapham DE. SnapShot: Mammalian TRP Channels. Cell. 2007;129:220.e221–220.e222. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and Functions of Metabotropic Glutamate Receptors. Annual Review of Pharmacology and Toxicology. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Connaughton VP, Nelson R. Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J Physiol. 2000;524:135–146. doi: 10.1111/j.1469-7793.2000.t01-1-00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Babwah AV, Ferguson SS. Mechanisms of metabotropic glutamate receptor desensitization: role in the patterning of effector enzyme activation. Neurochemistry international. 2002;41:319–326. doi: 10.1016/s0197-0186(02)00073-6. [DOI] [PubMed] [Google Scholar]
- de la Villa P, Kurahashi T, Kaneko A. L-glutamate-induced responses and cGMP-activated channels in three subtypes of retinal bipolar cells dissociated from the cat. Journal of Neuroscience. 1995;15:3571–3582. doi: 10.1523/JNEUROSCI.15-05-03571.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vente J, Steinbusch H. On the stimulation of soluble and particulate guanylate cyclase in the rat brain and the involvement of nitric oxide as studied cGMP immunohistochemistry. Acta Histochem. 1992;92:13–38. doi: 10.1016/S0065-1281(11)80138-8. [DOI] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Li W, Saszik S. Parallel Processing in Two Transmitter Microenvironments at the Cone Photoreceptor Synapse. Neuron. 2006;50:735–748. doi: 10.1016/j.neuron.2006.04.034. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. Journal of Physiology. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and 'Off' bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Faurobert E, Dascal N, Sterling P, Vardi N. A Retinal-Specific Regulator of G-Protein Signaling Interacts with G{alpha}o and Accelerates an Expressed Metabotropic Glutamate Receptor 6 Cascade. J. Neurosci. 2004;24:5684–5693. doi: 10.1523/JNEUROSCI.0492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Jiang M, Wang TL, Lyubarsky A, Savchenko A, Bar-Yehuda T, Sterling P, Birnbaumer L, Vardi N. Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o) J Neurosci. 2002;22:4878–4884. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolphin AC. G Protein Modulation of Voltage-Gated Calcium Channels. Pharmacol Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Feil R, Kleppisch T. Pharmacology of Neurotransmitter Release. 2008. NO/cGMP-Dependent Modulation of Synaptic Transmission; pp. 529–560. [DOI] [PubMed] [Google Scholar]
- Field GD, Rieke F. Nonlinear Signal Transfer from Mouse Rods to Bipolar Cells and Implications for Visual Sensitivity. Neuron. 2002;34:773–785. doi: 10.1016/s0896-6273(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Gereau RWt, Conn PJ. Multiple presynaptic metabotropic glutamate receptors modulate excitatory and inhibitory synaptic transmission in hippocampal area CA1. J. Neurosci. 1995;15:6879–6889. doi: 10.1523/JNEUROSCI.15-10-06879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson TA, Scobey R, Wilson M. Permeation of calcium ions through non-NMDA glutamate channels in retinal bipolar cells. Science. 1991;251:1613–1615. doi: 10.1126/science.1849316. [DOI] [PubMed] [Google Scholar]
- Gotzes S, de Vente JAN, Uuml and Ller F. Nitric oxide modulates cGMP levels in neurons of the inner and outer retina in opposite ways. Cambridge Journals Online. 2000 doi: 10.1017/s0952523898155141. [DOI] [PubMed] [Google Scholar]
- Grant GB, Dowling JE. A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. Journal of Neuroscience. 1995;15:3852–3862. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GB, Dowling JE. On bipolar cell responses in the teleost retina are generated by two distinct mechanisms. J Neurophysiol. 1996;76:3842–3849. doi: 10.1152/jn.1996.76.6.3842. [DOI] [PubMed] [Google Scholar]
- Haberecht MF, Schmidt HHHW, Mills SL, Massey SC, Nakane M, Redburn-Johnson DA. Localization of nitric oxide synthase, NADPH diaphorase and soluble guanylate cyclase in adult rabbit retina. Cambridge Journals Online. 2000 doi: 10.1017/s0952523898155104. [DOI] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal Synaptic Interactions Between Rod Bipolar Cells and Amacrine Cells in the Rat Retina. J. Neurophys. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Hartwick ATE, Bramley JR, Yu J, Stevens KT, Allen CN, Baldridge WH, Sollars PJ, Pickard GE. Light-Evoked Calcium Responses of Isolated Melanopsin-Expressing Retinal Ganglion Cells. J. Neurosci. 2007;27:13468–13480. doi: 10.1523/JNEUROSCI.3626-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry D, Burke S, Shishido E, Matthews G. Retinal bipolar neurons express the cyclic nucleotide-gated channel of cone photoreceptors. J Neurophysiol. 2003;89:754–761. doi: 10.1152/jn.00771.2002. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Higgs MH, Lukasiewicz PD. Glutamate Uptake Limits Synaptic Excitation of Retinal Ganglion Cells. J. Neurosci. 1999;19:3691–3700. doi: 10.1523/JNEUROSCI.19-10-03691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka K, Kourennyi DE, Barnes S. Calcium channel activation facilitated by nitric oxide in retinal ganglion cells. J Neurophysiol. 2000;83:198–206. doi: 10.1152/jn.2000.83.1.198. [DOI] [PubMed] [Google Scholar]
- Hughes T. Are there ionotropic glutamate receptors on the rod bipolar cell of the mouse retina? Vis Neurosci. 1997;14:103–109. doi: 10.1017/s0952523800008804. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Israel A, del Rosario Garrido M, Mathison Y, Barbella Y, Becemberg I. Brain natriuretic peptide stimulates particulate guanylate cyclase activity in selected areas of the rat brain. Neuroscience Letters. 1990;114:107–112. doi: 10.1016/0304-3940(90)90436-d. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jonas P, Major G, Sakmann B. Quantal components of unitary EPSCs at the mossy fibre synapse on CA3 pyramidal cells of rat hippocampus. J Physiol. 1993;472:615–663. doi: 10.1113/jphysiol.1993.sp019965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970;207:623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho J, Swanson RA, de Vente J, Sagar SM. NADPH-diaphorase (nitric oxide synthase)-reactive amacrine cells of rabbit retina: Putative target cells and stimulation by light. Neuroscience. 1993;57:587–597. doi: 10.1016/0306-4522(93)90008-4. [DOI] [PubMed] [Google Scholar]
- Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The Role of Calcium in the Desensitization of Capsaicin Responses in Rat Dorsal Root Ganglion Neurons. J. Neurosci. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski JL, Luetje CW, Kramer RH. Tyrosine Phosphorylation of Rod Cyclic Nucleotide-Gated Channels Switches Off Ca2+/Calmodulin Inhibition. J. Neurosci. 2003;23:10100–10106. doi: 10.1523/JNEUROSCI.23-31-10100.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2625–2630. doi: 10.1073/pnas.0304471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB. It Is Calmodulin After All! Mediator of the Calcium Modulation of Multiple Ion Channels. Neuron. 1999;22:645–648. doi: 10.1016/s0896-6273(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The Ankyrin Repeats of TRPV1 Bind Multiple Ligands and Modulate Channel Sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Liu L, Simon SA. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J Neurophysiol. 1996;75:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Lawrence JE, Valentino TL. Desensitizing glutamate receptors shape excitatory synaptic inputs to tiger salamander retinal ganglion cells. J. Neurosci. 1995;15:6189–6199. doi: 10.1523/JNEUROSCI.15-09-06189.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R, Torres M, Sanchez-Prieto J. mGluR7 inhibits glutamate release through a PKC-independent decrease in the activity of P/Q-type Ca2+ channels and by diminishing cAMP in hippocampal nerve terminals. European Journal of Neuroscience. 2007;26:312–322. doi: 10.1111/j.1460-9568.2007.05660.x. [DOI] [PubMed] [Google Scholar]
- Matsui K, Hasegawa J, Tachibana M. Modulation of Excitatory Synaptic Transmission by GABAC Receptor-Mediated Feedback in the Mouse Inner Retina. J Neurophysiol. 2001;86:2285–2298. doi: 10.1152/jn.2001.86.5.2285. [DOI] [PubMed] [Google Scholar]
- Matsui K, Hosoi N, Tachibana M. Excitatory Synaptic Transmission in the Inner Retina: Paired Recordings of Bipolar Cells and Neurons of the Ganglion Cell Layer. J. Neurosci. 1998;18:4500–4510. doi: 10.1523/JNEUROSCI.18-12-04500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J, Bond CT, Herson PS, Lee W-S, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554:255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon DG, Schmidt KF. Horizontal cell glutamate receptor modulation by NO: mechanisms and functional implications for the first visual synapse. Vis Neurosci. 1999;16:425–433. doi: 10.1017/s0952523899163041. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Molokanova E, Maddox F, Luetje CW, Kramer RH. Activity-Dependent Modulation of Rod Photoreceptor Cyclic Nucleotide-Gated Channels Mediated by Phosphorylation of a Specific Tyrosine Residue. J. Neurosci. 1999;19:4786–4795. doi: 10.1523/JNEUROSCI.19-12-04786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Wensel TG, Brown RL, Perez-Leon JA, Bearnot B, Duvoisin RM. Gβ5-RGS complexes co-localize with mGluR6 in retinal ON-bipolar cells. European Journal of Neuroscience. 2007;26:2899–2905. doi: 10.1111/j.1460-9568.2007.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. Journal of Biological Chemistry. 1993;268:11868–11873. [PubMed] [Google Scholar]
- Nawy S. The Metabotropic Receptor mGluR6 May Signal Through Go, but not Phosphodiesterase, in Retinal Bipolar Cells. J. Neurosci. 1999;19:2938–2944. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Regulation of the On bipolar cell mGluR6 pathway by Ca2+ J. Neurosci. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Desensitization of the mGluR6 transduction current in tiger salamander On bipolar cells. J Physiol. 2004;558:137–146. doi: 10.1113/jphysiol.2004.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Copenhagen DR. Multiple classes of glutamate receptor on depolarizing bipolar cells in retina. Nature. 1987;325:56–58. doi: 10.1038/325056a0. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990a;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Time-dependent reduction of glutamate current in retinal bipolar cells. Neuroscience Letters. 1990b;108:279–283. doi: 10.1016/0304-3940(90)90654-r. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;7:677–683. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- Nomura A, Shigemoto R, Nakamura Y, Okamoto N, Mizuno N, Nakanishi S. Developmentally regulated postsynaptic localization of a metabotropic glutamate receptor in rat rod bipolar cells. Cell. 1994;77:361–369. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proceedings of the National Academy of Sciences. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor V, Far OE, Bofill-Cardona E, Nanoff C, Freissmuth M, Karschin A, Airas J, eacute M, Betz H, Boehm S. Calmodulin Dependence of Presynaptic Metabotropic Glutamate Receptor Signaling. Science. 1999;286:1180–1184. doi: 10.1126/science.286.5442.1180. [DOI] [PubMed] [Google Scholar]
- Okamoto N, Hori S, Akazawa C, Hayashi Y, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a new metabotropic glutamate receptor mGluR7 coupled to inhibitory cyclic AMP signal transduction. J. Biol. Chem. 1994;269:1231–1236. [PubMed] [Google Scholar]
- Oltedal L, Morkve SH, Veruki ML, Hartveit E. Patch-Clamp Investigations and Compartmental Modeling of Rod Bipolar Axon Terminals in an In Vitro Thin-Slice Preparation of the Mammalian Retina. J Neurophysiol. 2007;97:1171–1187. doi: 10.1152/jn.01010.2006. [DOI] [PubMed] [Google Scholar]
- Pan Z-H. Differential Expression of High- and Two Types of Low-Voltage-Activated Calcium Currents in Rod and Cone Bipolar Cells of the Rat Retina. J Neurophysiol. 2000;83:513–527. doi: 10.1152/jn.2000.83.1.513. [DOI] [PubMed] [Google Scholar]
- Pang J-J, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004;558:897–912. doi: 10.1113/jphysiol.2003.059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Pottek M, Schultz K, Weiler R. Effects of nitric oxide on the horizontal cell network and dopamine release in the carp retina. Vision research. 1997;37:1091–1102. doi: 10.1016/s0042-6989(96)00298-2. [DOI] [PubMed] [Google Scholar]
- Protti DA, Llano I. Calcium current and calcium signaling in rod bipolar cells of rat retina slices. Journal of Neuroscience. 1998;18:3715–3724. doi: 10.1523/JNEUROSCI.18-10-03715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. AN INTRODUCTION TO TRP CHANNELS. Annual Review of Physiology. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Rao A, Dallman R, Henderson S, Chen C-K. G 5 Is Required for Normal Light Responses and Morphology of Retinal ON-Bipolar Cells. J. Neurosci. 2007;27:14199–14204. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–2572. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Saito T, Kondo H, Toyoda J-i. Rod and cone signals in the on-center bipolar cell: Their different ionic mechanisms. Vision research. 1978;18:591–595. doi: 10.1016/0042-6989(78)90208-0. [DOI] [PubMed] [Google Scholar]
- Saito T, Kondo H, Toyoda JI. Ionic mechanisms of two types of on-center bipolar cells in the carp retina. I. The responses to central illumination. J. Gen. Physiol. 1979;73:73–90. doi: 10.1085/jgp.73.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Satoh H, Aoki K, Watanabe SI, Kaneko A. L-type calcium channels in the axon terminal of mouse bipolar cells. Neuroreport. 1998;9:2161–2165. doi: 10.1097/00001756-199807130-00002. [DOI] [PubMed] [Google Scholar]
- Schneeweis DM, Schnapf JL. The Photovoltage of Macaque Cone Photoreceptors: Adaptation, Noise, and Kinetics. J. Neurosci. 1999;19:1203–1216. doi: 10.1523/JNEUROSCI.19-04-01203.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong ROL, Lukasiewicz PD. Distinct Ionotropic GABA Receptors Mediate Presynaptic and Postsynaptic Inhibition in Retinal Bipolar Cells. J. Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells R, Falk G. Retinal on-bipolar cells contain a nitric oxide-sensitive guanylate cyclase. Neuroreport. 1992;3:845–848. doi: 10.1097/00001756-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proceedings of the Royal Society of London - Series B: Biological Sciences. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Responses of rod bipolar cells isolated from dogfish retinal slices to concentration-jumps of glutamate. Visual Neuroscience. 1994;11:1175–1183. doi: 10.1017/s0952523800006970. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Ca(2+)-induced light adaptation in retinal ON-bipolar cells. Keio J Med. 1999;48:140–146. doi: 10.2302/kjm.48.140. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Activation of Ca2+--calmodulin kinase II induces desensitization by background light in dogfish retinal 'on' bipolar cells. J Physiol. 2000;528(Pt 2):327–338. doi: 10.1111/j.1469-7793.2000.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Rectification of cGMP-activated channels induced by phosphorylation in dogfish retinal 'on' bipolar cells. J Physiol. 2001;535:697–702. doi: 10.1111/j.1469-7793.2001.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Potentiation of 'on' bipolar cell flash responses by dim background light and cGMP in dogfish retinal slices. J Physiol. 2002;542:211–220. doi: 10.1113/jphysiol.2002.019752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiells RA, Falk G, Naghshineh S. Action of glutamate and aspartate analogues on rod horizontal and bipolar cells. Nature. 1981;294:592–594. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Miller RF. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Snellman J, Nawy S. Regulation of the Retinal Bipolar Cell mGluR6 Pathway by Calcineurin. J Neurophysiol. 2002;88:1088–1096. doi: 10.1152/jn.2002.88.3.1088. [DOI] [PubMed] [Google Scholar]
- Snellman J, Nawy S. cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. J Neurosci. 2004;24:6621–6628. doi: 10.1523/JNEUROSCI.1474-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen SD, Macek TA, Cai Z, Saugstad JA, Conn PJ. Dissociation of Protein Kinase-Mediated Regulation of Metabotropic Glutamate Receptor 7 (mGluR7) Interactions with Calmodulin and Regulation of mGluR7 Function. Mol Pharmacol. 2002;61:1303–1312. doi: 10.1124/mol.61.6.1303. [DOI] [PubMed] [Google Scholar]
- Spreca A, Giamanco I, Rambotti M. Ultracytochemical study of guanlylate cyclases A and B in light- and dark-adapted retinas. Histochem J. 1999;31:477–483. doi: 10.1023/a:1003712110751. [DOI] [PubMed] [Google Scholar]
- Suh B-C, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Current Opinion in Neurobiology. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tamalu F, Watanabe S-I. Glutamatergic input is coded by spike frequency at the soma and proximal dendrite of AII amacrine cells in the mouse retina. European Journal of Neuroscience. 2007;25:3243–3252. doi: 10.1111/j.1460-9568.2007.05596.x. [DOI] [PubMed] [Google Scholar]
- Tian L, Kammermeier PJ. G protein coupling profile of mGluR6 and expression of G[alpha] proteins in retinal ON bipolar cells. Visual Neuroscience. 2007;23:909–916. doi: 10.1017/S0952523806230268. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. L-AP4 inhibits calcium currents and synaptic transmission via a G- protein-coupled glutamate receptor. J. Neurosci. 1992;12:2043–2050. doi: 10.1523/JNEUROSCI.12-06-02043.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for Night Vision in Mouse Retina. J. Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum MC, Smith RG. Noise removal at the rod synapse of mammalian retina. Vis Neurosci. 1998;15:809–821. doi: 10.1017/s0952523898155037. [DOI] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. Journal of Comparative Neurology. 1998;395:43–52. [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. European Journal of Neuroscience. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H, Grunert U, Cook NJ, Molday RS. The cGMP-gated channel of rod outer segments is not localized in bipolar cells of the mammalian retina. Neuroscience Letters. 1992;134:199–202. doi: 10.1016/0304-3940(92)90516-a. [DOI] [PubMed] [Google Scholar]
- Weng K, Lu CC, Daggett LP, Kuhn R, Flor PJ, Johnson EC, Robinson PR. Functional Coupling of a Human Retinal Metabotropic Glutamate Receptor (hmGluR6) to Bovine Rod Transducin and Rat Go in an in Vitro Reconstitution System. J. Biol. Chem. 1997;272:33100–33104. doi: 10.1074/jbc.272.52.33100. [DOI] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Stanton PK, Nawy S. Nitric Oxide Depresses GABAA Receptor Function via Coactivation of cGMP-Dependent Kinase and Phosphodiesterase. Journal of Neuroscience. 1998:18. doi: 10.1523/JNEUROSCI.18-07-02342.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Gao F, Pang J-J. Synaptic circuitry mediating light-evoked signals in dark-adapted mouse retina. Vision research. 2004;44:3277–3288. doi: 10.1016/j.visres.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Zhou Z-Y, Wan Q-F, Thakur P, Heidelberger R. Capacitance Measurements in the Mouse Rod Bipolar Cell Identify a Pool of Releasable Synaptic Vesicles. J Neurophysiol. 2006;96:2539–2548. doi: 10.1152/jn.00688.2006. [DOI] [PubMed] [Google Scholar]