Abstract
Inorganic arsenic (iAs) is a well-established carcinogen and human exposure has been associated with a variety of cancers including those of skin, lung, and bladder. High expression of transforming growth factor alpha (TGF-α) has associated with local relapses in early stages of urinary bladder cancer. iAs exposures are at least in part determined by the rate of formation and composition of iAs metabolites (MAsIII, MAsV, DMAsIII, DMAsV). This study examines the relationship between TGF-α concentration in exfoliated bladder urothelial cells (BUC) separated from urine and urinary arsenic species in 72 resident women (18-51 years old) from areas exposed to different concentrations of iAs in drinking water (2-378 ppb) in central Mexico. Urinary arsenic species, including trivalent methylated metabolites were measured by hydride generation atomic absorption spectrometry method. The concentration of TGF-α in BUC was measured using an ELISA assay. Results show a statistically significant positive correlation between TGF-α concentration in BUC and each of the six arsenic species present in urine. The multivariate linear regression analyses show that the increment of TGF-α levels in BUC was importantly associated with the presence of arsenic species after adjusting by age, and presence of urinary infection. People from areas with high arsenic exposure had a significantly higher TGF-α concentration in BUC than people from areas of low arsenic exposure (128.8 vs. 64.4 pg/mg protein; p<0.05). Notably, exfoliated cells isolated from individuals with skin lesions contained significantly greater amount of TGF-α than cells from individuals without skin lesions: 157.7 vs. 64.9 pg/mg protein (p=0.003). These results suggest that TGF-α in exfoliated BUC may serve as a susceptibility marker of adverse health effects on epithelial tissue in arsenic-endemic areas.
Keywords: Transforming growth factor alpha, Susceptibility marker, Bladder urothelial cells, Arsenic, Trivalent arsenic, Urine metabolites, Arsenic-skin lesions, Hyperkeratosis, Hypo-hyperpigmentation
Introduction
Inorganic arsenic (iAs) is naturally occurring and ubiquitously present in the environment. iAs in drinking water is the main source of human exposure. Epidemiologic data have shown that chronic exposure of humans to iAs compounds is associated with cardiovascular diseases, liver and kidney injury, diabetes mellitus, neurologic disorders and an increased incidence of liver, lung, skin and bladder cancer (ATSDR, 1999).
The relationship between iAs exposure and bladder cancer has been well documented (National Research Council, 1999). However, the mechanism by which iAs induces cancer has not been thoroughly characterized. Laboratory studies have shown that the metabolic conversions of iAsIII and iAsV in biological systems yield metabolites for which toxic and carcinogenic potentials differ significantly from those of the parent compounds. iAs is enzymatically methylated in human tissues to mono- and dimethylated metabolites that contain trivalent arsenic (AsIII) or pentavalent arsenic (AsV). A recently identified arsenic methyltransferase (AS3MT) is the key enzyme in this pathway (Waters et al., 2004). Several studies have shown that methylated trivalent arsenicals are more acutely toxic than iAsIII and are more potent than iAsIII as enzyme inhibitors, cytotoxins, and genotoxins (Styblo et al., 2000; Mass et al., 2001; Gomez et al., 2005). In addition, exposures to low concentrations of either MAsIII or DMAsIII induce cell proliferation and production of growth promoting cytokines in normal human keratinocytes (Vega et al., 2001). Moreover, exposure of iAs and its metabolites has been linked to activation of AP-1 and NF-kappaB (nuclear factor kappa B) signal transduction pathways, which in turn lead to the transcription of genes involved in cell growth regulatory pathways (Drobna et al., 2003; Hughes and Kitchin, 2006).
Increased transforming growth factor alpha (TGF-α) production has previously been reported in normal human keratinocytes treated with micromolar concentration of iAsIII (Germolec et al., 1996, 1997) and in the skin of mice exposed to iAsIII in drinking water (Germolec et al., 1998).
In a population study in Bangladesh, urinary levels of TGF-α correlated with both urinary total As (TAs) and the occurrence of iAs-associated skin lesions (Do et al., 2001). In addition, the appearance of high TGF-α expression has been associated with local relapses in early stages of bladder cancer (Thogersen et al., 2001).
Therefore, in the present study, we analyzed the relationship among TGF-α concentration in exfoliated bladder urothelial cell (BUC) separated from urine and urinary arsenic species in people environmentally exposed to iAs in an arsenic-endemic area of Mexico. Furthermore, we investigated the association between the TGF-α levels and the presence of arsenic-related skin lesions.
Materials and methods
Chemicals and reagents
Arsenic acid disodium salt (Na2HAsVO4), sodium m-arsenite (NaAsIIIO2), both >99% pure, and dimethylarsinic acid [DMAsV;as (CH3)2AsvO(OH); 98% pure] were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Methylarsonic acid (MAsV) disodium salt [CH3AsVO (ONa)2; 99% pure] was obtained from Ventron (Danvers, MA, USA). The trivalent methylated arsenicals methyloxoarsine (MAsIIIO; CH3AsIIIO) and iododimethylarsine of DMAsIII [DMAsIII; (CH3)2AsIII] were synthesized by W. R. Cullen (University of British Columbia, Vancouver, British Columbia, Canada) using previously described methods (Styblo and Thomas, 1997). Working standards of these arsenicals that contained 1 μg of As/ml were prepared daily from stock solutions. Sodium borohydride (NaBH4) was obtained from EM Science (Gibbstown, NJ, USA). Tris hydrochloride was purchased from J.T. Baker (Phillipsburg, NJ, USA). Creatinine kits were purchased from Randox (San Diego CA, USA). TGF-α ELISA kits were purchased from Oncogene systems (Baltimore, USA). All other chemicals used were at least analytical grade. Standard reference material (SRM) water (SRM 1643e) and urine [SRM 2670; National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA] were used for quality control of TAs in water and urine analysis, respectively.
Study area
Three towns (Llano Norte, Calvario, and Aguacatito) in Zimapan, Hidalgo located in the central part of Mexico were selected for the study on the basis of a survey of arsenic in water, Aguacatito with lower iAs concentrations in water than Mexican Reference Value (MRV) of 25 μg/l (Diario Oficial de la Federación, 2000) and Llano Norte and El Calvario with iAs levels >MRV. In this area high concentrations of naturally occurring iAs are frequently found in bedrock and as a consequence in underground and surface waters (Armienta and Rodríguez, 1996). Although a significant effort has been made to provide safe drinking water for people residing in this area, many communities still depend entirely on drinking water containing high levels of iAs. In Zimapan region, historical iAs exposure is known since 1992 when iAs concentrations in wells and the potable supply was between 21 and 1100 μg As/l (Armienta et al., 1993). In 1999, the most contaminated well was closed by the local authorities, and the mean of iAs concentration in water decreased significantly; however, the iAs concentration in this region is still significantly higher than the recommended standard of 25 μg/l for drinking water, with mean values of 120 μg iAs/l (Valenzuela et al., 2005; Soto-Pena et al., 2006). In recent time many of the residents have been warned about the iAs water contamination and now they drink bottled water with normal values of iAs instead of drinking water from the municipal source.
Subjects selection and recruitment
A cross-sectional study was conducted with 72 local residents of Zimapan region in accordance with the regulations of the Ethical Committee of the Cinvestav. Subjects were recruited through door-to-door contact. They had to be at least 15 years old and lived in the town for the previous 2 years. Before enrollment in the study each participant read and signed an informed consent form. Subjects were interviewed by trained interviewers regarding general characteristics with emphasis on liters of water that each participant consumes daily, the duration of water consumption including the water source, detailed residential, medical and occupational histories. They underwent physical examination looking for typical dermatological signs of the chronic exposure to iAs. Approximately 50% of the iAs-high exposed group presented at least one skin sign of chronic arsenicism, such as hypo/hyperpigmentation, palmoplantar hyperkeratosis, and ulcerative lesions as described by Yeh (1973).
Individuals who had received drugs with well-defined organ toxicity within the past 4 months or suffering chronic alcoholism were not included. Each family’s drinking water was analyzed for As concentration.
Arsenic in water
Data collection methods have been previously described (Valenzuela et al., 2005). Briefly, tap water samples were collected in the homes of each potential participants using acid-washed containers transported to the site of the study by the investigator. We collected 70 water samples from 62 households (more than one sample was obtained from each households if participants used different water sources to drink and cook). Water samples for each participant were stored at -20 °C until their assay. TAs was measured as previously described (Del Razo et al., 1990) by HG-AAS using a PerkinElmer 3100 spectrophotometer (PerkinElmer, Norwalk, CT, USA), equipped with a FIAS-200 flow injection atomic spectroscopy system. SRM 1643e was used for quality control of TAs in water analysis. The certified TAs concentration in SRM 1643e is 60.45±0.72 μg/l. We obtained concentrations of 61.3±0.68 μg/l in replicate analyses of this SRM using the method described above, which is in good agreement with the certified value.
Cumulative As exposure
Cumulative As exposure or time-weighted exposure (TWE) serves as an indicator for evaluation of dose-response relationships of the adverse health effect appearing after long period of iAs exposure. In subjects, where drinking water iAs level has large variations or where there has been a long period of low iAs exposure years, the index of TWE may be suitable. Therefore, the cumulative iAs exposure was calculated as the product of multiplying iAs concentration of the drinking water source for each individual of the study by the duration of consumption and the liters of water that each participant consumed daily. As a result, the TWE is expressed in milligrams of iAs.
Analytical detection of urinary As species
A spot urine sample was collected from the participants after clinical examination. Spot urines were collected in 250-ml polypropylene containers by a procedure that minimized the chance for contamination. A 50 ml aliquot for each urine sample was immediately frozen on dry ice to prevent oxidation of unstable trivalent methylated arsenicals; the remainder of the sample was stored at 4 °C. Then, urine samples were immediately transported to Cinvestav-IPN laboratory to analyze the trivalent As species within 6 h after collection.
All urinary arsenical metabolites (iAsIII, iAsV, MAsIII, MAsV, DMAsIII, and DMAsV) were evaluated by a pH-specific HG-ASS optimized by Del Razo et al. (2001). We used SMR 2670 to validate analysis of TAs in urine samples with elevated As concentrations (480±100 μg/l). The low As concentration urine sample in SRM 2670 has a reference value of 60 μg/l. Replicate analyses of these SMRs made by pH-specific HG-AAS gave values of 507±17 μg/l and 64±5 μg/l, respectively, for the high and low standards. In this paper, the urinary TAs is reported as the sum of iAsIII, iAsV, MAsIII, MAsV, DMAsIII, and DMAsV concentrations.
The As species were evaluated in one spot urine sample and would thus be dependent on urine dilution. To correct for differences in urine dilution in spot urine samples, the urinary As species were adjusted for urinary creatinine. This parameter was measured by the Jaffe reaction using a Randox commercial kit.
Isolated urothelial cells (extraction and quantification of protein)
Exfoliated BUC were collected from spontaneously voided urine which was stored for less than 4 h at 4 °C before processing. Urine was transferred aseptically to several 50-ml tubes and centrifuged at 2000 rpm for 10 min at 4 °C. Supernates from each tube were discarded, but sediments were combined to one 1.5-ml tube and centrifuged again for 5 min.
The presence of leukocytes in urine was evaluated in the sediment by microscopic leukocyte count. The appearance of leukocytes in urine had been associated with the presence of urinary infection.
The remaining urothelial cells were washed twice with PBS and harvested in cell lysis buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% SDS), containing a protease inhibitor cocktail (Complete-mini, Roche), and phosphatase inhibitors (25 mM NaF, 1 mM orthovanadate). After 30 min of incubation at 4 °C, cell lysates were clarified at 12,000 rpm for 10 min at 4 °C. Finally, the supernatant was collected as the whole cell extract and stored at -70 °C until analysis. Protein concentration was determined with Bio-Rad (Hercules, CA) protein assay reagent. A standard curve was drawn for each plate using bovine serum albumin for reference.
TGF-α determination
TGF-α concentrations in urothelial cells extracts were measured using a commercially available enzyme test (TGF-α ELISA kit, Oncogene systems, Baltimore, USA). For detection of soluble TGF-α in epithelial cells, 96-well flexible pre-coated microtiter plates were incubated for 2 h with protein samples. After washing with PBS (pH 7.4) containing 0.05% Tween 20, the wells were incubated for 2 h with 100 ng/ml biotin-conjugated polyclonal anti-TGF-α antibody (Oncogene) followed by reaction with avidin-conjugated peroxidase (Oncogene) using a substrate reagent (Oncogene). The color reaction was stopped by addition of 2 N sulfuric acid. Color intensity was estimated by a photometer at a wavelength of 450 nm with a reference wavelength of 630. A standard curve was drawn for each plate using recombinant TGF-α proteins for reference. Minimum detection limit of the assays for urothelial cells TGF-α was 3.1 pg/ml.
Statistical analysis
Data analysis included crude comparisons of high and low exposure groups as well as regression models to assess the association of urinary iAs metabolites and TGF-α concentrations. The logarithmic value of TGF-α levels was used to compare differences between low and high arsenic exposure groups as well as individuals with and without skin lesions by Student’s t-test. We categorized the TGF-α concentrations based on percentiles 0-33%, 34-66% and 67-100%. Because percentile 0-33% and 34-66% had similar mean and standard deviation, we decided to join them in one group called low TGF-α concentrations in BUC. Thus, percentile 67-100% represents the high TGF-α levels group, in this way the cut point established randomly in bases of percentiles was 100 pg/mg of protein. TGF-α concentrations as a continuous variable (pg/mg protein) with respect to different parameters: urinary TAs, urinary arsenic species (iAsIII, iAsV, MAsIII, MAsV, DMAsIII, DMAsV, iAsIII+V, MAsIII+V, and DMAsIII+V) were analyzed by bivariate analysis on log-transformed TGF-α levels stratified as people with skin lesions and without cutaneous lesions. Furthermore, TGF-α level-associated exposure and effect variables were further evaluated in multiple linear regression models. In these models each of the As metabolites was evaluated in relation to TGF-α urothelial concentrations and confounder/covariates. We evaluated potential confounding factors based on their influence on the urinary arsenic and TGF-α urothelial levels. These included the following variables age, TWE as a continuous variable, and categorized variables like: skin lesion (yes/no), melanosis (yes/no), keratosis (yes/no), and presence of leukocytes in urine (yes/no). We evaluated the heteroscedasticity of the multivariate linear regression models using the Breusch-Pagan/Cook-Weisberg test. Comparisons with p-value <0.05 were considered significant. All statistical analyses were performed Stata 8.0 (Stata, Corp. College Station, TX).
Results
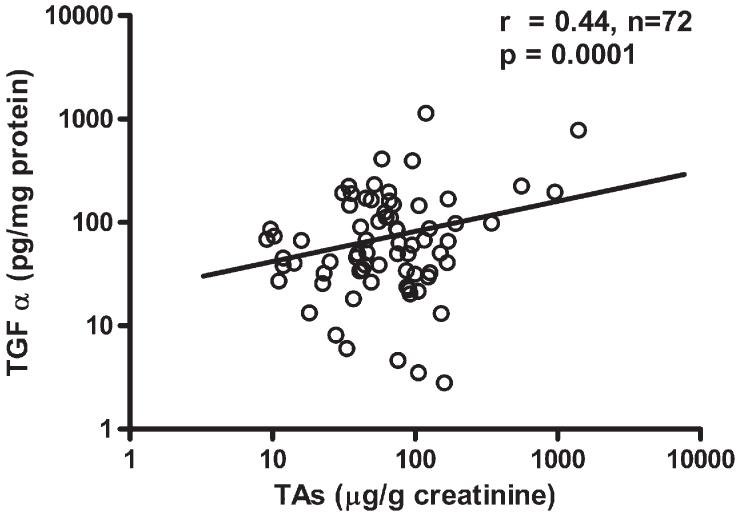
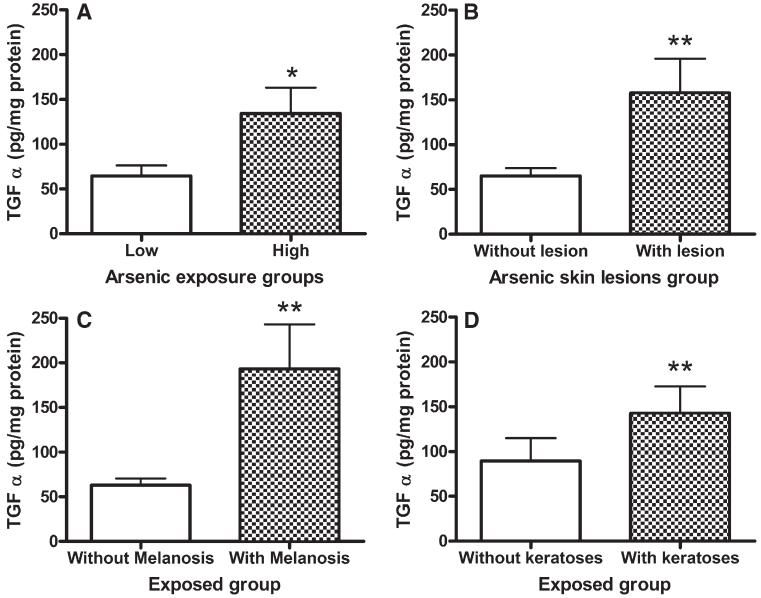
The study population characteristics, exposure variables, and effect markers are presented in Table 1. In this population (n=72) all the subjects were women between 18 and 51 years old. A statistically significant positive correlation was found between the log-transformed TGF-α concentration in exfoliated cells and TAs concentration in urine collected from Zimapan residents (Fig. 1; r=0.44, p<0.0001). The average TGF-α concentration was significantly higher in individuals with high iAs exposure compared to low iAs exposure group (means 128.8 vs. 64.4 pg/mg protein; p<0.05; respectively Fig. 2A). Notably, exfoliated cells isolated from individuals with skin lesions contained significantly greater amount of TGF-α than cells from people without skin lesions (157.7 vs. 64.9 pg/mg protein, p=0.003; Fig. 2B). Melanosis and keratosis were the main skin lesions present in iAs-exposed participants, with TGF-α concentrations significantly higher in the group with melanosis (p<0.003; Fig. 2C) relative to keratosis (p<0.01; Fig. 2D).
Table 1.
Participant characteristic and data of markers in a population environmentally exposed to inorganic arsenic in Zimapan, Mexico
| Variable (Unit) |
Low arsenic exposure group |
High arsenic exposure group |
|---|---|---|
| Population characteristics | Geometric mean (range) | Geometric mean (range) |
| Women (n) | 21a | 51a |
| Age (years) | 36 (18-50) | 35 (18-51) |
| Leukocytes in urine: no/yes (%) | 52/48b | 63/37b |
| Exposure variables | ||
| Water arsenic (μg/l) | 7 (2-10) | 104 (23-378)** |
| Arsenatec(iAsV) | 2.5 (0.5-10.5) | 5.8 (1.3-65.5)** |
| Arsenitec(iAsIII) | 1.7 (0.3-8.7) | 3.9 (0.3-172)* |
| Monomethylarsenatec(MAsV) | 0.6 (0.2-5.5) | 1.7 (0.2-28.3)** |
| Monomethylarsonousc(MAsIII) | 2.1 (0.6-9.3) | 5.1 (0.7-101.9)** |
| Dimethylarsinatec(DMAsV) | 7.1 (1.8-19.7) | 13.9 (0.6-710)** |
| Dimethylarsonousc(DMAsIII) | 7.8 (0.3-28.0) | 31.9 (1.4-506)** |
| Total arsenicc(TAs) | 31.7 (9.1-55.8) | 81.5 (9.7-1,398)** |
| Time-weighted exposure (mg) | 0.4 (0.7-2.2) | 103.7 (2.1-497)** |
| Biomarker | ||
| Skin lesions: none/at least one (%) | 100/0b | 31/69b |
| % Melanosis (hipo- or/and hyperpigmentation) | 0b | 51b |
| % Keratosis (palm, sole or/and trunk) | 0b | 55b |
| TGF-α in exfoliated BUC (pg/mg protein) | 42.1 (4.6-189) | 68.2 (2.8-1135)* |
BUC, bladder urothelial cells.
Data are presented as number of subjects.
Data are presented as percent.
Urinary concentration expressed in μg/g creatinine.
p<0.05, statistically significant difference between low and high arsenic exposure groups.
p<0.01, statistically significant difference between low and high arsenic exposure groups.
Fig. 1.
The relationship between TAs in urine with TGF-α concentrations in exfoliated bladder urothelial cells of subjects environmentally exposed to iAs in Zimapan, Mexico.
Fig. 2.
Comparative associations of TGF-α concentrations in exfoliated BUC between low and high arsenic exposure group (A), skin lesions (B), melanosis, as changes in the skin pigmentation (C) and the presence of keratosis (D) n=72. Graphic bars indicate mean and standard deviation of TGF-α among comparison groups. *p<0.05, **p<0.01 (Student’s t-test).
Comparison of urinary arsenic species based on different TGF-α expression groups is shown in Table 2. Table 2 showed that participants with high TGF-α concentrations (>100 pg/mg protein) in BUC had elevated urinary TAs, iAsIII+V, MAsIII+V, and DMAsIII+V (mean 196.8, 29.6, 18.2, and 149) respectively compared to low TGF-α concentrations group (mean 67.7, 9.2, 6.5, and 51.9, respectively). Furthermore, the high TGF-α concentrations group in BUC had higher concentrations in all arsenic metabolites, being the trivalent species the highest.
Table 2.
Comparisons of TGF-α concentrations in exfoliated BUC with urinary arsenic metabolites in the study population (n=72)
| Low TGF-α concentration group (n=48) | High TGF-α concentration group (n=24) | |
|---|---|---|
| TGF-α (pg/mg protein) | 41.8±24.1 | 246.4±237.9* |
| iAsIII | 3.9±4.3 | 18.9±38.9* |
| iAsV | 5.2±4.3 | 10.7±14.1* |
| MAsIII | 4.9±4.7 | 14.0±23.0* |
| MAsV | 1.7±1.7 | 4.2±6.3* |
| DMAsIII | 38.2±33.8 | 94.9±148.9* |
| DMAsV | 13.7±14.1 | 54.2±142.4 |
| iAsIII+V | 9.2±6.9 | 29.6±47.7* |
| MAsIII+V | 6.5±5.1 | 18.2±25.9* |
| DMAsIII+V | 51.9±38.3 | 149.0±265* |
| ΣAs speciesIII | 47.0±39 | 127.7±206* |
| ΣAs speciesV | 20.6±17 | 69.1±160* |
| TAs | 67.7±47.4 | 196.8±330.3* |
P<0.05, high vs. low group using as a cut point, the value of 100 pg/mg of protein of TGF-α.
Bivariate analyses
The association of TGF-α levels with each of the arsenic urinary species, and some confounding variables were analyzed as a bivariate linear regression shown in Table 3. TGF-α concentrations in exfoliated BUC were associated with each urinary arsenic species. Interestingly, urinary arsenicals in pentavalency (Σ of pentavalent species: iAsV+MAsV+DMAsV) were better associated with TGF-α concentration than Σ of trivalent species: iAsIII+MAsIII+DMAsIII (p=0.006 vs. 0.027, respectively). In addition, a significant positive relationship between TGF-α concentration in individuals whose skin have the signs of the chronic arsenic exposure was observed (r=0.33, p=0.004), being higher for melanosis presence than for keratosis (r=0.40, p=0.001 vs. r=0.32, p=0.007, respectively).
Table 3.
Bivariate linear regression analyses between log TGF alpha levels with urinary iAs metabolites and other potential confounding variables (n=72)
| Independent variable | β | 95% CI | r | P |
|---|---|---|---|---|
| Water TAs | 0.00004 | -0.001-0.001 | 0.01 | 0.93 |
| iAsIII+V | 0.005 | 0.0016-0.009 | 0.32 | 0.006 |
| MAsIII+V | 0.008 | 0.0013-0.015 | 0.27 | 0.021 |
| DMAsIII+V | 0.001 | 0.0002-0.0016 | 0.31 | 0.009 |
| TAs | 0.0007 | 0.0002-0.0013 | 0.33 | 0.001 |
| iAsV | 0.015 | 0.002-0.027 | 0.28 | 0.016 |
| iAsIII | 0.006 | 0.001-0.011 | 0.29 | 0.013 |
| MAsV | 0.042 | 0.015-0.069 | 0.32 | 0.003 |
| MAsIII | 0.007 | -0.001-0.015 | 0.21 | 0.073 |
| DMAsV | 0.001 | 0.0005-0.003 | 0.31 | 0.008 |
| DMAsIII | 0.001 | 0.0001-0.0025 | 0.25 | 0.034 |
| ΣAs speciesIII | 0.001 | 0.0001-0.0018 | 0.26 | 0.027 |
| ΣAs speciesv | 0.001 | 0.0005-0.0028 | 0.31 | 0.006 |
| Age | 0.001 | -0.011-0.013 | 0.02 | 0.87 |
| TWEa | 0.001 | -0.0004-0.002 | 0.15 | 0.20 |
| Leukocytesb | 0.11 | -0.12-0.35 | 0.11 | 0.35 |
| Skin lesionc | 0.33 | 0.11-0.55 | 0.33 | 0.004 |
| Melanosisd | 0.40 | 0.18-0.62 | 0.39 | 0.001 |
| Keratosise | 0.32 | 0.09-0.55 | 0.32 | 0.007 |
Abbreviations: β, regression coefficient; 95% CI, 95% confidence interval; r, correlation coefficient.
TWE, time-weighted exposure.
Leukocytes in urine, yes=1.
Lesion, yes=1.
Melanosis, yes=1
Keratosis, yes=1.
There was no significant association among TGF-α concentrations in exfoliated BUC with age, level of As in drinking water, TWE, and presence of leukocytes in urine.
Multivariate analyses
Multiple linear regression analysis examined the association of the concentration of each of the urinary arsenic species with TGF-α levels in exfoliated BUC (n=72) after adjustment by age in two models stratified according to the presence of cutaneous lesions that were analyzed separately (Table 4). In the group of people without skin lesions we only observed a marginal association of TGF-α in BUC with DMAsIII (R2=0.14, p<0.10, n=37) when the model is adjusted. Moreover, in this model the association between TGF-α and TAs in urine was not observed. However, in the group of individuals with skin lesions (n=35), TGF-α levels in urothelial cells were significantly associated with TAs, iAsV, MAsV, DMAsIII, DMAsV, iAsIII+V, DMAsIII+V, ΣAs speciesIII and ΣAs speciesV. It is important to mention that each one of the arsenic species previously described was analyzed separately as an individual model, adjusted by age.
Table 4.
Multivariate linear regression models relating the TGF-α concentrations in exfoliated BUC with markers of arsenic biomethylation in Mexican population environmentally exposed to iAs, stratified for the presence of arsenic-skin lesions
| Independent variable | Without skin lesions group (n=37) |
With skin lesions group (n=35) |
||||
|---|---|---|---|---|---|---|
| β (95% CI) | p | R2 | β (95% CI) | p | R2 | |
| TAs | -0.003 (-0.008-0.001) | 0.11 | 0.09 | 0.001 (0.1e-3-0.001) | 0.013 | 0.19** |
| iAsIII | 0.034 (-0.016-0.08) | 0.17 | 0.07 | 0.004 (-0.4e-3-0.008) | 0.074 | 0.12 |
| iAsV | -0.016 (-0.06-0.031) | 0.48 | 0.03 | 0.013 (0.001-0.025) | 0.025 | 0.16* |
| MAsIII | -0.03 (-0.08-0.012) | 0.13 | 0.08 | 0.005 (-0.002-0.013) | 0.19 | 0.08 |
| MAsV | 0.02 (-0.028-0.08) | 0.32 | 0.04 | 0.04 (0.010-0.069) | 0.009 | 0.16** |
| DMAsIII | -0.006 (-0.01-0.006) | 0.032 | 0.14* | 0.001 (0.02e-3-0.002) | 0.046 | 0.14* |
| DMAsV | -0.002 (-0.014-0.01) | 0.76 | 0.02 | 0.001 (0.4e-3-0.002) | 0.009 | 0.16** |
| iAsIII+V | 0.006 (-0.02-0.04) | 0.70 | 0.02 | 0.003 (0.0003-0.007) | 0.033 | 0.15* |
| MAsIII+V | -0.008 (-0.04-0.031) | 0.65 | 0.02 | 0.005 (-0.001-0.012) | 0.085 | 0.11 |
| DMAsIII+V | -0.004 (-0.009-0.0002) | 0.064 | 0.11 | 0.001 (0.2e-3-0.001) | 0.011 | 0.20** |
| ΣAs speciesIII | -0.005 (-0.01-0.4e-4) | 0.052 | 0.12 | 0.001 (-0.1e-4-0.001) | 0.054 | 0.13* |
| ΣAs speciesv | -0.001 (-0.011-0.009) | 0.82 | 0.02 | 0.001 (0.3e-3-0.002) | 0.009 | 0.16** |
Abbreviations: β, regression coefficient; 95% CI, 95% confidence interval; p, value for each metabolite; adjusted R2, explained variance. All the analyses were adjusted by age.
p<0.1, mean p-value of each model.
p<0.05 mean p-value of each model.
Discussion
A variety of studies indicate that the TGF-α plays an important role in the modulation of differentiation and proliferation cells. Moreover, altered cellular responsiveness to growth factors is one of the factors involved in carcinogenesis. Epidemiological studies had shown that humans develop tumors in urinary bladder after chronic drinking water iAs exposure (Chiou et al., 1995; Hopenhayn-Rich et al., 1996; Steinmaus et al., 2006).
The goal of this study was to characterize the urinary pattern of iAs metabolites and their relationship with concentration of TGF-α in BUC of people from an arsenic-endemic area. A positive linear regression between urinary TAs and TGF-α concentration in BUC was observed (Fig. 1). Additionally, we found a statistically significant difference between the low and high As exposure groups in which we observed higher concentrations of TGF-α in BUC from the high exposure group than in the low exposure group (Fig. 2A). The association between TGF-α levels and urinary As was also observed by Do et al. (2001), in a population of an iAs endemic area of Bangladesh; although in this study the TGF-α levels in urine were evaluated.
Moreover, the present study shows that individuals with skin lesions, which have long been known to be the hallmark signs of chronic As exposure, present higher concentration of TGF-α in the urothelial cells (Fig. 2B). Recent evidence suggests that the development of skin lesions from arsenic exposure may be mediated by increases in the expression of various growth factors, including TGF-α (Hsu et al., 2006). This may suggest that individuals with skin lesions are more sensitive to present alterations in urothelial cells. This association resembled those found in skin samples obtained from residents of Taiwan, in where Germolec et al. (1998) observed that TGF-α mRNA transcripts were highly expressed in hyperpigmentation and hyperkeratosis areas in arsenic-exposed individuals. Elevated levels of TGF-α may play an essential role in mitogenic stimulation during tumor promotion by diverse mechanisms.
It is difficult to explain why in our study we observed a better correlation of TGF-α with pentavalent metabolites rather than trivalent arsenic species (Tables 3 and 4), which had been suggested to be the more toxic species in the arsenic metabolism (Styblo et al., 2000). However, it is important to consider that trivalent methylated arsenic metabolites are mainly accumulated in the urothelial cells (Drobna et al., 2005), so this can increase their toxicity whereas pentavalent forms are readily excreted. Further studies including the analysis of As species in BUC may provide more information for risk analysis of diseases associated with iAs exposure.
This study has several methodological advantages, including individual exposure assessment, and inclusion of all arsenic metabolites in human urine in order to determine whether a particular urinary metabolite is associated with increased TGF-α levels in BUC from individuals chronically exposed to iAs. The significant associations among arsenic exposure with TGF-α levels in BUC support, but do not specifically define causality. Importantly in this study, we found multivariate associations among all the urinary As species (trivalent and pentavalent species) with TGF-α levels in BUC after adjusting by age, in which we observed a strong correlation only in the models of the As skin lesions group, but not in the non-As skin lesions group (Table 3). These results suggest that the induction of TGF-α concentrations in BUC by As species is only present in people who are susceptible to present the toxic effects after chronic As exposure. Therefore, the presence of TGF-α in exfoliated epithelial cells could be used as an early marker of human susceptibility before the development of the classical arseniasis associated with chronic iAs exposure. Furthermore, this hypothesis is consistent with the finding that the TWE to iAs is an indicator of the effects of chronic exposure to iAs that is closely linked to the hyper-hypopigmentation and keratosis but not associated with the increased TGF-α concentrations in BUC.
In the multivariate analyses we observed the relation between age and TGF-α concentrations in BUC. Because of the small number of subjects, this association was not significant. Moreover, smoking is one of the principal confounding factors in the development of bladder cancer (Di Menza et al., 1992; Murata et al., 1996). Because our study population included only a small number of smokers, we were unable to estimate the association between TGF-α concentrations and smoking habits. An additional limitation of this study was that we cannot evaluate the difference in response of TGF-α and urinary arsenic metabolites between genders, because this study only includes women. However, in the literature it has been reported that women had more risk of bladder cancer than men following arsenic exposure (Chen et al., 1992).
Another important confounding factor that had been reported to increase the TGF-α expression is the presence of urinary infection (Tungekar and Linehan, 1998). In our studies this was evaluated as a categorical variable measuring the presence of leukocytes in urine by a semiquantitative technique. As a result, we adjusted for this factor in multivariate analyses that examined the association between the urinary arsenical concentrations and TGF-α. The presence of inflammatory urologic diseases and urothelial carcinoma has also been associated with increased TGF-α levels in the urine (Chow et al., 1998), emphasizing need for this adjustment.
In previous studies, we observed the patterns of methylated metabolites in people exposed to iAs, and noted that individuals with skin lesions have higher urinary concentrations of MAsIII, despite the fact that DMAsIII was the major metabolite present in the urine (49%) (Valenzuela et al., 2005). It has been documented that the trivalent methylated species are the most toxic metabolites in the arsenic pathway in several target tissues (Mass et al., 2001; Styblo et al., 2000, 2002). In this work we observed that people exposed to iAs have increased concentrations of TGF-α in BUC, and that these significantly higher levels of TGF-α showed a positive and linear association with urinary TAs. After adjusting by age, alcohol consumption, and urinary infection we found an association between TGF-α in BUC and urinary levels of MAsIII and DMAsIII, which have higher uptakes and retention in urothelial cells.
Interestingly, statistically significant differences were found between the group with and without skin lesions, with a stronger association in the presence of melanosis. One problem, common in the interpretation of data from cross-sectional studies, is that the exposure is measured at the same time as the effects, which may not be the etiologically relevant period. This may be problematic for TGF-α concentrations in urothelial cells, because it largely reflects recent exposures. This is not the case for arsenic-induced skin lesions, which are a good estimate of the chronic arsenic exposure over decades. Our data suggest that TGF-α in BUC of individuals exposed to iAs can be used as an early human biomarker of the toxicity of this metalloid in bladder epithelium, and may be useful to detect susceptible individuals prior to the development of bladder cancer.
Acknowledgments
This study was supported by Mexican Council for Science and Technology (Conacyt), grant 50097-M. We greatly appreciate the personnel from the Sanitary Jurisdiction in the Municipality of Zimapán, Hidalgo, Mexico for their help in the field study. The authors acknowledge Becton Dickinson for their generous donation of sterile containers for urine. The technical assistance of Eliud A. Garcia-Montalvo, Araceli Hernandez-Zavala, Luz C. Sánchez-Peña, Rachel Patterson and Angel Barrera-Hernández is deeply appreciated. Olga L. Valenzuela was the recipient of a scholarship from the Conacyt. This work was supported, in part, by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Abbreviations
- AS3MT
arsenic methyltransferase
- BUC
bladder urothelial cell
- iAs
inorganic arsenic
- iAsV
arsenate
- iAsIII
arsenite
- MAsIII
mono-methylarsonous acid
- MAsV
monomethylarsonic acid
- DMAsIII
dimethylarsinous acid
- DMAsV
dimethylarsinic acid
- TMAsVO
trimethylarsine oxide
- TAs
total arsenic
- SAM
sulfo-adenosilmethionine
- HG-AAS
hydride generation atomic absorption spectrophotometry
- TGF-α
transforming growth factor alpha
- TWE
time-weighted exposure
- β
regression coefficient
- CI
confidence interval
- r
correlation coefficient
- R2
determination coefficient
Footnotes
Portions of the data included in this paper were presented in poster session at the 45th Annual Conference of The Society of Toxicology, March 2006, San Diego, CA.
References
- Armienta MA, Rodríguez R. Arsénico en el Valle de Zimapán, México. Problemática Ambiental Rev. MAPFRE Seguridad. 1996;63:33–43. [Google Scholar]
- Armienta MA, Rodríguez R, Villaseñor G, Aguayo A, Ceniceros N, Juárez F. Estudio de reconocimiento de la contaminación por arsénico en la zona de Zimapán Hgo. 1993. p. 60. Reporte Técnico, Informe del IGF al Municipio de Zimapán, Hgo. [Google Scholar]
- ATSDR . Toxicological Profile for Arsenic. Agency for Toxic Substances and Disease Registry; Atlanta, GA: 1999. [PubMed] [Google Scholar]
- Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br. J. Cancer. 1992;66:888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, Lin JS, Huang CH, Chen CJ. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [PubMed] [Google Scholar]
- Chow NH, Liu HS, Chang CJ, Chi YC, Tzai TS, Li EI, Lin JS. Urinary excretion of transforming growth factor-alpha in patients with transitional cell carcinoma. Anticancer Res. 1998;18:2053–2057. [PubMed] [Google Scholar]
- Del Razo LM, Arellano MA, Cebrian ME. The oxidation states of arsenic in well-water from a chronic arsenicism area of northern Mexico. Environ. Pollut. 1990;64:143–153. doi: 10.1016/0269-7491(90)90111-o. [DOI] [PubMed] [Google Scholar]
- Del Razo LM, Styblo M, Cullen WR, Thomas DJ. Determination of trivalent methylated arsenicals in biological matrices. Toxicol. Appl. Pharmacol. 2001;174:282–293. doi: 10.1006/taap.2001.9226. [DOI] [PubMed] [Google Scholar]
- Di Menza L, Baron JC, Vieillefond A, Choudat D, Boccon-Gibod L, Zummer K. Risk factors for tumors of the bladder. Epidemiological study of 701 patients in Ile-de-France. Presse Med. 1992;21:153–156. [PubMed] [Google Scholar]
- Do T, Gambelunghe A, Ahsan H, Graziano J, Perrin M, Slavkovich V, Parvez F, Milton AH, Brandt-Rauf P. Biomarkers. 2001;6:127–132. doi: 10.1080/13547500010017376. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Jaspers I, Thomas DJ, Styblo M. Differential activation of AP-1 in human bladder epithelial cells by inorganic and methylated arsenicals. FASEB J. 2003;17:67–69. doi: 10.1096/fj.02-0287fje. [DOI] [PubMed] [Google Scholar]
- Drobna Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Styblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol. Appl. Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germolec DR, Yoshida T, Gaido K, Wilmer JL, Simeonova PP, Kayama F, Burleson F, Dong W, Lange RW, Luster MI. Arsenic induces overexpression of growth factors in human keratinocytes. Toxicol. Appl. Pharmacol. 1996;141:308–318. doi: 10.1006/taap.1996.0288. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Boorman GA, Wilmer JL, Yoshida T, Simeonova PP, Bruccoleri A, Kayama F, Gaido K, Tennant R, Burleson F, Dong W, Lang RW, Luster MI. Arsenic can mediate skin neoplasia by chronic stimulation of keratinocyte-derived growth factors. Mutat. Res. 1997;386:209–218. doi: 10.1016/s1383-5742(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Yu HS, Chen GS, Simeonova PP, Humble MC, Bruccoleri A, Boorman GA, Foley JF, Yoshida T, Luster MI. Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors. Am. J. Pathol. 1998;153:1775–1785. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez SE, Del Razo LM, Munoz Sanchez JL. Induction of DNA damage by free radicals generated either by organic or inorganic arsenic (AsIII, MMAIII, and DMAIII) in cultures of B and T lymphocytes. Biol. Trace Elem. Res. 2005;108:115–126. doi: 10.1385/bter:108:1-3:115. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Fuchs A, Bergoglio R, Tello EE, Nicolli H, Smith AH. Bladder cancer mortality associated with arsenic in drinking water in Argentina. Epidemiology. 1996;7:117–124. doi: 10.1097/00001648-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Hsu KH, Brandt-Rauf P, Lin TM, Chiou HY, Tseng CH, Chen CJ, Luo JC. Plasma-transforming growth factor-alpha expression in residents of an arseniasis area in Taiwan. Biomarkers. 2006;11:538–546. doi: 10.1080/13547500600881488. [DOI] [PubMed] [Google Scholar]
- Hughes MF, Kitchin KT. Arsenic, oxidative stress and carcinogenesis. In: Singh KK, editor. Oxidative Stress, Disease and Cancer. Imperial College Press; London: 2006. pp. 825–850. [Google Scholar]
- Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD. Methylated trivalent arsenic species are genotoxic. Chem. Res. Toxicol. 2001;14:355–361. doi: 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- Murata M, Takayama K, Choi BC, Pak AW. A nested case-control study on alcohol drinking, tobacco smoking, and cancer. Cancer Detect. Prev. 1996;20:557–565. [PubMed] [Google Scholar]
- National Research Council, NRC . Arsenic in drinking water. 1999. p. 330. [Google Scholar]
- Soto-Pena GA, Luna AL, Acosta-Saavedra L, Conde P, Lopez-Carrillo L, Cebrian ME, Bastida M, Calderon-Aranda ES, Vega L. Assessment of lymphocyte subpopulations and cytokine secretion in children exposed to arsenic. FASEB J. 2006;20:779–781. doi: 10.1096/fj.05-4860fje. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Bates MN, Yuan Y, Kalman D, Atallah R, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Hoang BK, Smith AH. Arsenic methylation and bladder cancer risk in case-control studies in Argentina and the United States. J. Occup. Environ. Med. 2006;48:478–488. doi: 10.1097/01.jom.0000200982.28276.70. [DOI] [PubMed] [Google Scholar]
- Styblo M, Thomas DJ. Binding of arsenicals to proteins in an in vitro methylation system. Toxicol. Appl. Pharmacol. 1997;147:1–8. doi: 10.1006/taap.1997.8256. [DOI] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ. Health Perspect. 2002;110:767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thogersen VB, Sorensen BS, Poulsen SS, Orntoft TF, Wolf H, Nexo E. A subclass of HER1 ligands are prognostic markers for survival in bladder cancer patients. Cancer Res. 2001;61:6227–6233. [PubMed] [Google Scholar]
- Tungekar MF, Linehan J. Patterns of expressions of transforming growth factor and epidermal growth factor receptor in squamous cell lesions of the urinary bladder. J. Clin. Pathol. 1998;51:583–587. doi: 10.1136/jcp.51.8.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzalez MB, Garcia-Montalvo EA, Calderon-Aranda ES, Del Razo LM. Urinary trivalent methylated arsenic species in a population chronically exposed to inorganic arsenic. Environ. Health Perspect. 2005;113:250–254. doi: 10.1289/ehp.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega L, Styblo M, Patterson R, Cullen W, Wang C, Germolec D. Differential effects of trivalent and pentavalent arsenicals on cell proliferation and cytokine secretion in normal human epidermal keratinocytes. Toxicol. Appl. Pharmacol. 2001;172:225–232. doi: 10.1006/taap.2001.9152. [DOI] [PubMed] [Google Scholar]
- Waters SB, Devesa V, Del Razo LM, Styblo M, Thomas DJ. Endogenous reductants support the catalytic function of recombinant rat cyt19, an arsenic methyltransferase. Chem. Res. Toxicol. 2004;17:404–409. doi: 10.1021/tx0342161. [DOI] [PubMed] [Google Scholar]
- Yeh S. Skin cancer in chronic arsenicism. Hum. Pathol. 1973;4:469–485. doi: 10.1016/s0046-8177(73)80060-7. [DOI] [PubMed] [Google Scholar]